Peripheral blood cell (PBC) rescue has become the mainstay for autologous transplantation in patients with lymphoma, multiple myeloma, and solid tumors. Different methods of hematopoietic progenitor cell (HPC) mobilization are in use without an established standard. Forty-seven patients with relapsed or refractory lymphoma received salvage chemotherapy and were randomized to have HPC mobilization using filgrastim [granulocyte–colony-stimulating factor (G-CSF)] alone for 4 days at 10 μg/kg per day (arm A) or cyclophosphamide (5 g/m2) and G-CSF at 10 μg/kg per day until hematologic recovery (arm B). Engraftment and ease of PBC collection were primary outcomes. All patients underwent the same high-dose chemotherapy followed by reinfusion of PBCs. There were no differences in median time to neutrophil engraftment (11 days in both arms;P = .5) or platelet engraftment (14 days in arm A, 13 days in arm B; P = .35). Combined chemotherapy and G-CSF resulted in higher CD34+ cell collection than G-CSF alone (median, 7.2 vs 2.5 × 106 cells/kg;P = .004), but this did not impact engraftment. No differences were found in other PBC harvest outcomes or resource utilization measures. A high degree of tumor contamination, as studied by consensus CDR3 polymerase chain reaction of the mobilized PBCs, was present in both arms (92% in arm A vs 90% in arm B;P = 1). No differences were found in overall survival or progression-free survival at a median follow-up of 21 months. This randomized trial provides clinical evidence that the use of G-CSF alone is adequate for HPC mobilization, even in heavily pretreated patients with relapsed lymphoma.
Introduction
High-dose chemotherapy with autologous hematopoietic progenitor cell (HPC) support has an established role in the treatment of patients with relapsed non-Hodgkin lymphoma (NHL) and Hodgkin disease (HD). Peripheral blood cell (PBC) rescue is increasingly used for both autologous and allogeneic transplantation. It has several advantages over the use of bone marrow, such as ease of collection, speed of hematopoietic recovery, and potentially lower degree of tumor contamination.1 2
The mobilization of HPCs is a multifactorial process that is poorly understood at the molecular level. Interactions between progenitors, stromal cells, endothelial cells, and extracellular matrix through adhesion molecules and indirect effects of cytokines are important for the homing and the mobilization of HPCs.3Hematopoietic progenitors comprise heterogeneous subpopulations of cells committed to different hematopoietic lineages. In humans, they can be enriched using the CD34 cell surface glycoprotein marker.4
Subsequent to the initial demonstration of increased peripheral blood HPC number with the use of granulocyte macrophage–colony-stimulating factor (GM-CSF) or G-CSF5,6 and with successful hematopoietic reconstitution using mobilized autologous HPCs,7 additional studies examined the combined use of chemotherapy and cytokines for the mobilization of HPCs.8,9 The optimal mobilization strategy remains controversial.10 Options include the use of a single growth factor, a combination of cytokines such as G-CSF and GM-CSF or G-CSF and stem cell factor, or the combination of high-dose chemotherapy and a growth factor.
Chemotherapy and cytokines have been shown to have a synergistic effect in mobilizing CD34+ progenitor cells.11,12However, retrospective and prospective single-arm trials have not suggested superiority in clinical outcomes.13 14 There have been no published randomized trials comparing clinical endpoints in HPC mobilization using a single growth factor with combined use of the same growth factor and chemotherapy.
Whichever the mobilization method, the parameters of interest are feasibility and clinical applicability to a large group of patients, ease of collection, engraftment, resource utilization, mobilization-related complications, and, ultimately, relapse and survival. With this goal, we performed a randomized trial comparing the use of G-CSF alone against high-dose cyclophosphamide with G-CSF for HPC mobilization in patients with relapsed lymphoma undergoing autologous transplantation.
Several studies have been performed to detect minimal residual disease in leukemia, NHL, and myeloma to identify and predict patients at risk for future relapse. The use of polymerase chain reaction (PCR) assay with primers to the framework 3 region and J chain of the immunoglobulin heavy-chain gene to detect clonal populations of B cells has been reported for patients with NHL.19-21 To study the incidence of PBC tumor contamination, we conducted PCR analysis for the presence of tumor DNA sequences in both arms.
Patients and methods
Patient selection
Patients with relapsed or refractory NHL or HD eligible to undergo autologous transplantation were enrolled between November 1997 and November 2000 at a single institution. Patients ranging in age from18 to 65 years, with clinical and radiographic evidence of first or subsequent relapse or refractory NHL (working formulation histology B, C, D, E, F, G, H, I, J)15 or HD that was biopsy proven if relapse occurred 1 year after previous chemotherapy, were included. The study protocol was approved by the Human Investigation Review Committee, and all patients gave written informed consent. Pathologic material was reviewed and classified according to the International Working Formulation Project,15 and patients were evaluated using computed tomography (CT) scanning and bone marrow examination. Staging evaluation was performed according to the Ann Arbor staging system. Adequate renal function with a creatinine level less than 2.0 mg/dL; liver function with bilirubin, aspartate aminotransferase, and alanine transferase less than 2 times normal (unless caused by tumor involvement); DLCO greater than 50% predicted; FEV1greater than 75% predicted; and normal left ventricular ejection fraction were required. Patients with central nervous system lymphoma, Eastern Cooperative Oncology Group performance status 3 or 4, human immunodeficiency virus infection, active cardiac disease, or concurrent malignancy were excluded. Randomization was performed at the time of enrollment.
Study design
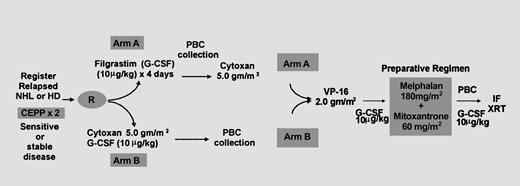
Patients received salvage chemotherapy with 2 cycles of CEPP (cyclophosphamide 600 mg/m2 intravenously [IV] days 1 and 8; etoposide 70 mg/m2 IV day 1, 140 mg/m2 by mouth [PO] days 2 and 3; procarbazine and prednisone 60 mg/m2 PO days 1 through 10). Patients with clear clinical or radiographic evidence of progression during salvage therapy were to be removed from the study. Protocol treatment began a minimum of 4 weeks after the last dose of salvage therapy (Figure1, schema). Patients randomized to arm A received G-CSF at 10 μg/kg per day subcutaneously (SC) for 4 days, following which PBCs were collected. High-dose cyclophosphamide was then administered, no earlier than 1 day and no later than 7 days after complete PBC collection, at 5 g/m2 (2.5 g/m2 IV over 1 hour at a 3-hour interval for 2 doses). Patients randomized to arm B received the same dose of cyclophosphamide (5 g/m2), followed by G-CSF at 10 μg/kg per day SC starting 24 hours later; PBCs were collected on hematologic recovery. After recovery from the previous phase, patients received etoposide at 2 g/m2 IV (undiluted by continuous infusion at 200 mg/m2 per hour), and 60 mg methylprednisolone sodium succinate was administered IV every 8 hours for 1 day starting 6 hours before etoposide administration. The transplantation phase began no earlier than day 20 and no later than day 40 after etoposide administration. Absolute neutrophil count (ANC) was required to be greater than 1500/μL, and platelet count was required to be greater than 100 000/μL. All chemotherapy was based on corrected ideal body weight. Preparative regimen consisted of 60 mg/m2 mitoxantrone (20 mg/m2 IV over 1 hour every 2 hours for 3 doses) on day −4, followed by 2 days of rest and 180 mg/m2 melphalan (60 mg/m2 IV over 1 hour every 2 hours for 3 doses). Autologous PBCs were rapidly thawed at 37°C and infused intravenously through a central venous catheter, without further filtering or washing, 24 hours after the last dose of chemotherapy. Radiation therapy to sites of previous bulk disease (greater than 5 cm) and residual disease (greater than 1 cm) was started only after complete hematologic recovery (ANC greater than 2000/μL and platelet count greater than 50 000/μL), between day +30 and day +100. The radiation dose was planned between 3000 and 3420 cGy in 15 to 19 fractions over 19 to 27 days, as tolerated.
Schematic representation of treatment protocol.
Patients with relapsed or refractory NHL or HD who were eligible for autologous transplantation were randomized to receive G-CSF alone or cyclophosphamide (5 g/m2) and G-CSF for HPC mobilization after salvage therapy with CEPP. All patients received the same dose of cyclophosphamide, followed by etoposide, before autologous transplantation. Involved field radiotherapy was given for bulky disease (mass greater than 5 cm).
Schematic representation of treatment protocol.
Patients with relapsed or refractory NHL or HD who were eligible for autologous transplantation were randomized to receive G-CSF alone or cyclophosphamide (5 g/m2) and G-CSF for HPC mobilization after salvage therapy with CEPP. All patients received the same dose of cyclophosphamide, followed by etoposide, before autologous transplantation. Involved field radiotherapy was given for bulky disease (mass greater than 5 cm).
Mobilization and collection of peripheral blood cells
Patients in arm A began PBC collection on day 5, after G-CSF began. Patients in arm B had PBC collection within 3 days of white blood cell (WBC) recovery to more than 1000/μL and platelet recovery to more than 75 000/μL. Apheresis was continued daily in both arms until a target of 1 × 109 total WBC/kg was achieved. PBCs were collected by a similar procedure in both arms—large-volume leukapheresis using central or peripheral venous access and the COBE spectra machine, as previously described.16,17 Cryopreservation was performed by rate-controlled freezing with 10% dimethyl sulfoxide as cryoprotectant. Cells were frozen at a final concentration of 2 × 108 cells/mL and stored in the vapor phase of liquid nitrogen.18 Aliquots of PBC product were saved at the time of collection for tumor-sequence PCR analysis and for enumeration of CD34+ cells. The percentage of CD34+ cells in a sample of PBC harvest was measured using flow cytometry, with immunofluorescence to identify the CD34 cell surface antigen (Cytometry Associates, San Diego, CA).
Molecular studies
Specimens.
All patients with NHL participated in a laboratory-based companion trial designed to determine the frequency of PBC contamination by occult lymphoma cells using tumor complementarity determining region 3 (CDR3) analysis. Samples of frozen or paraffin-embedded diagnostic lymph node biopsy and bone marrow aspirate were obtained at the time of study registration. After mobilization treatment, aliquots of harvested PBCs were saved for PCR analysis and comparison with the above samples. Molecular analysis for the presence of tumor-sequence DNA was based on the detection of clonal CDR3 regions from the immunoglobulin heavy-chain gene unique for a patient's tumor, using consensus PCR.19 20 Bcl-2/immunoglobulin (Ig) H PCR was used if there was no predominant CDR3 clone, based on previously described methods.
DNA extraction.
Consensus IgH polymerase chain reaction.
CDR3 region of the IgH gene was amplified using semi–nested PCR.20 The first round consisted 30 cycles using consensus VH (Vcon) and JH(LJH2),20 followed by a second round of 20 to 30 cycles using Vcon and the nested JH(Jcon) primers. Cycling conditions were identical in both rounds of PCR. The reaction mixture (40 μL, final volume) contained DNA (1 μg), primers (250 ng), MgCl2 (2.5 mM), Taq polymerase (1 unit; Amplitaq Gold, Perkin Elmer, Emeryville, CA), and dNTPs (250 μM each) in its standard buffer. Initial denaturation for 10 minutes at 95°C was followed by 30 cycles, each consisting of 30 seconds at 95°C, 30 seconds at 55°C, and 30 seconds at 72°C; it was finally subjected to extension at 72°C for 10 minutes. PCR was carried out in a GeneAmp PCR system 2400 (Perkin Elmer). PCR products were analyzed using denaturing polyacrylamide gel (8%) in Tris-Borate-EDTA (TBE) buffer. Positive monoclonal controls were prepared from Raji and Nalm6 cells. The size of the PCR product varied from 80 to 120 bp.
Bcl-2 polymerase chain reaction.
Lymph node DNA lacking predominant CDR3 rearrangement was subjected to bcl-2–nested amplification, as previously described.21The first amplification was carried out for 30 cycles using bcl-2 and JHA outer primers, and the second-round PCR was with bcl-2 and JHA inner primers. PCR conditions in both rounds were identical to the IgH gene PCR detailed above. PCR products ranged from 140 to 250 bp.
β-globin polymerase chain reaction.
β-globin PCR was performed to ensure the suitability of extracted DNA for amplification. The procedure was performed using BG101 and BG102 primers, as described, and the expected product measured approximately 110 bp.22
Controls and sensitivity.
All PCR experiments were performed with appropriate negative and positive controls, including normal lymph node or marrow DNA and lymph node DNA with previously identified tumor clones. Sample contamination was avoided by receiving and processing all samples in a separate work place. All samples were processed using designated laboratory instruments and plugged pipettes. The sensitivity of CDR3 analysis was determined to be 1 in 105 cells through log dilutions of positive control DNA in normal DNA.
Supportive management
Supportive care measures were adopted according to standard procedure in both arms. It included prophylaxis for hemorrhagic cystitis using aggressive hydration and IV Mesna, antibacterial and antifungal prophylactic antibiotics until ANC was greater than 2000/μL, mouth care for prophylaxis of mucositis, and G-CSF was 10 μg/kg per day to support high-dose therapy until ANC reached more than 1000/μL.
Follow-up evaluation
On completion of the transplantation phase and discharge from the hospital, patients were clinically assessed each week for 1 month, each month for the first year, every 3 months for the second year, and every 6 months after that. All patients were restaged by CT on day 100, every 3 months for the first year, every 6 months for the second year, and then yearly, unless clinical evidence warranted earlier evaluation. Complete response was defined as no evidence of disease by CT or physical examination or no change in residual abnormalities smaller than 2 cm over a 6-month period. Partial remission was defined as a greater than 50% decrease in the sum of products of all measurable lesions. Progressive disease was defined as a greater than 25% increase in any pre-existing tumor or the appearance of new lesions. Stable disease was defined as no change in the sum of products of all measurable disease.
Study endpoints and definitions
Primary endpoints of this study were to compare the time to neutrophil and platelet engraftment and the number of apheresis sessions required for target PBC collection. Time to neutrophil and platelet engraftment were, respectively, defined as the number of days after the infusion of PBCs to achieve an ANC greater than 500 /μL and a platelet count greater than 20 000 /μL, independent of platelet transfusions for more than 3 consecutive days.
Secondary endpoints included a comparison of overall survival (OS) and progression-free survival (PFS) in the treatment groups and the frequency of tumor sequence (CDR3) detection in the harvested PBCs. PFS and OS were calculated from the day of PBC infusion (day 0). Treatment-related death was defined as death from causes related to the protocol treatment, from the time mobilization treatment was started until day 60 after PBC infusion.
Statistical methods
All analyses were based on intention to treat. Student t test was used to compare normally distributed continuous variables such as age, length of stay, and time to collection. The Mann-Whitney U test was used for analyzing differences in medians for nonnormal continuous variables such as time to engraftment and CD34+ count. Comparison of categorical variables, such as proportion of NHL patients in each arm and number of apheresis sessions required for collection, were performed using the χ2 test. Tumor sequence contamination of PBCs was compared using Fisher exact test. Survival analyses were performed on an intent-to-treat basis using the Kaplan and Meier method23 and were compared using the log rank test for a difference in curves. Cox proportional hazards regression model24 was used to perform multivariate survival analysis. All statistical tests were 2-sided, and differences were considered significant if P < .05 (the α error). SPSS 10.0 software (SPSS, Cary, NC) was used for all analyses.
Results
Patient characteristics
Forty-seven patients, 32 with NHL and 15 with HD, were enrolled and randomized between November 1997 and November 2000. All patients with relapsed NHL or HD who were eligible to undergo autologous transplantation were invited to participate in the study. Data are not available on the small number of eligible patients who declined to enter the study. Twenty-three patients received G-CSF alone (arm A), and 24 received both cyclophosphamide and G-CSF for HPC mobilization (arm B). One patient in arm A died of a ruptured abdominal aortic aneurysm after randomization and did not receive any treatment on protocol. Forty-six patients underwent HPC mobilization treatment and are evaluable for harvest parameters, and 42 of them have completed all protocol treatment, including transplantation, and are evaluable for all outcomes. One patient in arm B did not receive etoposide chemotherapy per protocol (because of previous treatment with the drug) but did undergo the remainder of protocol therapy. All other patients were treated per protocol. No patients were removed from the study because of disease progression during salvage therapy. Patient characteristics including age, gender, type of lymphoma, prior therapy, type of relapse, chemoresistance, and presence or absence of cytopenias before transplantation were similar in both arms and are summarized in Table 1.
Patient characteristics
| Characteristic . | G-CSF (arm A) n = 23 . | Chemo + G-CSF (arm B) n = 24 . | Significance (95% CI) . |
|---|---|---|---|
| Age at BMT | 41 y | 46 y | 0.2 (− 12, 3) |
| Female gender | 11 (48%) | 7 (29%) | 0.24 |
| NHL | 15 (65%) | 17 (71%) | 0.7 |
| No. prior chemotherapy | |||
| 2 | 15 | 18 | 0.86 |
| 3 | 5 | 4 | |
| 4 | 2 | 2 | |
| Leukopenia* | 7 (33.3%) | 10 (47.6%) | 0.35 |
| Thrombocytopenia† | 8 (38%) | 8 (38%) | 1.0 |
| Chemoresistance at transplantation (n = 43) | 4 (19%) | 6 (28.6%) | 0.47 |
| Primary refractory disease | 8 (38%) | 4 (19%) | 0.4 |
| Characteristic . | G-CSF (arm A) n = 23 . | Chemo + G-CSF (arm B) n = 24 . | Significance (95% CI) . |
|---|---|---|---|
| Age at BMT | 41 y | 46 y | 0.2 (− 12, 3) |
| Female gender | 11 (48%) | 7 (29%) | 0.24 |
| NHL | 15 (65%) | 17 (71%) | 0.7 |
| No. prior chemotherapy | |||
| 2 | 15 | 18 | 0.86 |
| 3 | 5 | 4 | |
| 4 | 2 | 2 | |
| Leukopenia* | 7 (33.3%) | 10 (47.6%) | 0.35 |
| Thrombocytopenia† | 8 (38%) | 8 (38%) | 1.0 |
| Chemoresistance at transplantation (n = 43) | 4 (19%) | 6 (28.6%) | 0.47 |
| Primary refractory disease | 8 (38%) | 4 (19%) | 0.4 |
Low WBC count (< 3.5 × 109/L) immediately before transplantation.
Low platelet count (< 150 × 109/L) immediately before transplantation.
Peripheral blood cell harvest outcomes
Forty-six patients underwent PBC harvest and were evaluable for these outcomes. PBC harvest outcome data are summarized in Table 2. There was a significant difference (P = .004) in the total CD34+ cells collected, with a median of 7.2 × 106 cells/kg in the combined arm (arm B) versus 2.5 × 106 cells/kg in the G-CSF–only arm (arm A). The number of apheresis sessions required for target collection was similar between arms (P = .86). All patients were collected in either 1, 2, or 3 apheresis sessions. Approximately 50% of patients in both arms were collected in one session; 8% of patients in the G-CSF–only arm and 5% in the combined arm required 3 sessions for collection. Total time to collection, liters of blood processed, type of access required, and mean total WBC/kg were similar between the 2 arms (Table 2).
PBC harvest parameters
| PBC harvest parameter . | G-CSF (arm A) n = 22 . | Chemo + G-CSF (arm B) n = 24 . | Significance 2-sided (95% CI) . |
|---|---|---|---|
| Peripheral access | 12 (54.5%) | 10 (42%) | 0.38 |
| Vascath access | 10 (45.5%) | 14 (58%) | |
| Liters blood processed (mean) | 35.7 | 39.96 | 0.42 (− 15, 6.4) |
| Time to complete collection (mean) | 5 h, 53 min | 6 h, 21 min | 0.56 (− 2, 1) |
| WBC × 108/kg (mean)* | 11.97 | 11.92 | 0.95 (− 1.6, 1.7) |
| CD34+ cells × 106/kg* | (n = 17) | (n = 18) | |
| Median | 2.5 | 7.2 | 0.004 |
| Mean | 3.9 | 11.9 | |
| Range | 0.3-12.4 | 0.3-44.8 | |
| No. apheresis sessions | |||
| 1 | 11 (50%) | 12 (50%) | 0.86 |
| 2 | 10 (46%) | 10 (42%) | |
| 3 | 1 (4%) | 2 (8%) |
| PBC harvest parameter . | G-CSF (arm A) n = 22 . | Chemo + G-CSF (arm B) n = 24 . | Significance 2-sided (95% CI) . |
|---|---|---|---|
| Peripheral access | 12 (54.5%) | 10 (42%) | 0.38 |
| Vascath access | 10 (45.5%) | 14 (58%) | |
| Liters blood processed (mean) | 35.7 | 39.96 | 0.42 (− 15, 6.4) |
| Time to complete collection (mean) | 5 h, 53 min | 6 h, 21 min | 0.56 (− 2, 1) |
| WBC × 108/kg (mean)* | 11.97 | 11.92 | 0.95 (− 1.6, 1.7) |
| CD34+ cells × 106/kg* | (n = 17) | (n = 18) | |
| Median | 2.5 | 7.2 | 0.004 |
| Mean | 3.9 | 11.9 | |
| Range | 0.3-12.4 | 0.3-44.8 | |
| No. apheresis sessions | |||
| 1 | 11 (50%) | 12 (50%) | 0.86 |
| 2 | 10 (46%) | 10 (42%) | |
| 3 | 1 (4%) | 2 (8%) |
One patient in each arm had fewer than 1 × 106CD34+ cells/kg.
All PBCs harvested were transplanted.
Engraftment
Forty-two patients, 21 in each arm, underwent all protocol treatment and were evaluable for engraftment, which was the primary outcome of the study. There were no engraftment failures. There were no differences found in time to neutrophil (median, 11 days in both arms; P = .5) or to platelet engraftment (median, 14 days in arm A, 13 days in arm B; P = .4) between the 2 arms. Two patients had fewer than 1 × 106CD34+ cells/kg, whose time to ANC and platelet engraftment were delayed (20, 68 days and 21, 61 days, respectively), compared with the median.
Resource utilization measures
Forty-two patients, 21 in each arm, were evaluable for resource utilization measures. PBC harvest outcomes were similar between the 2 arms, as shown in Table 2. Transplantation-related outcomes were similar in the 2 arms—length of hospital stay was 21.4 days in arm A and 20.6 days in arm B (P = .5), median packed red cell (PRBC) units transfused was 7 in arm A and 8 in arm B (P = .13), and median single-donor platelet (SDP) units transfused was 7 in arm A and 6 in arm B (P = 1).
Patients in the G-CSF–only arm began apheresis a median of 4 days after starting G-CSF, whereas patients in the combined arm began apheresis a median of 15 days after starting chemotherapy. Two patients in the combined arm (B) had delays (by 6 days because of neutropenic fever, 1 day because of thrombocytopenia) in their scheduled PBC harvest, as opposed to none in the G-CSF–only arm (A). All other patients underwent harvest on their scheduled dates. Transfusion requirements at harvest time was higher in arm B; 14 patients required PRBC transfusion on or near the harvest date, 8 of them more than 2 units; in arm A, 5 patients required PRBC transfusion, but only 1 of them required more than 2 units. Similarly, the number of patients requiring SDP transfusion in arm B was 16 (10 of them required more than 2 SDP units), whereas the number was 5 patients in arm A (none required more than 2 units). Statistical comparison was not performed on these results because they were expected outcomes.
Toxicity
There were no treatment-related deaths or life-threatening infectious complications. With a median follow-up of 21 months, there has been no long-term serious organ dysfunction such as pulmonary, cardiac, liver, or renal failure. Myelodysplasia and acute myelogenous leukemia developed 21 months after transplantation in 1 patient with HD who had been heavily pretreated with chemotherapy and chest radiotherapy. Seven patients in arm B were admitted to the hospital near the scheduled harvest time; 1 of them required 2 admissions (9 days). In arm A, only 1 patient was admitted. Reasons for admission in arm B were neutropenic fever (n = 7), dehydration (n = 1), and hemorrhagic cystitis (n = 1). In arm A, the reason was catheter-related thrombosis.
Tumor contamination of peripheral blood cells
Harvested PBC product was studied using PCR-based CDR3 analysis to identify the presence of tumor-sequence DNA contamination. Data are available on 22 of 29 patients with NHL, 12 in the G-CSF arm and 10 in the combined arm. Ninety-two percent of patients in the G-CSF–only group and 90% of patients in the combined group had positive tumor contamination (P = 1) of PBCs after the mobilization treatment was administered. Of 14 patients with histologic bone marrow involvement, 13 were positive for PCR; of 8 patients without marrow disease, 7 were positive for PCR.
Survival
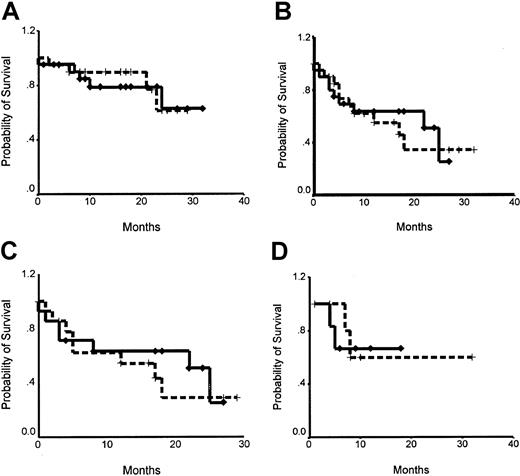
Survival data are available on all 42 patients who completed the protocol treatment, including transplantation. Median follow-up time was 21 months. OS was 79% in the G-CSF–only arm A and 77% in the combined arm B (P = .85). Median PFS time was 17 months for arm A and 25 months for arm B (P = .8). When survival curves were adjusted for type of lymphoma, OS was 82.5% in arm A and 90% in arm B for NHL patients (P = .89) and 100% for HD patients. Median PFS time was 17 months for NHL patients in arm A and 25 months for NHL patients in arm B (P = .77), and PFS was 60% for HD patients in arm A and 67% for HD patients in arm B at 21 months (P = .77). Survival curves are shown in Figure2. In a Cox proportional hazards regression model, chemoresistance at bone marrow transplantation (BMT) was the only independent prognostic variable predicting PFS and OS (Table 3). Type of mobilization did not contribute significantly to predicting either PFS or OS.
Kaplan-Meier curves for OS and PFS.
Broken lines denote the G-CSF only arm A, and solid lines denote Chemo+G-CSF arm B. (A) OS: 79% in arm A, 77% in arm B at a median follow-up of 21 months (P = .85). (B) PFS: median 17 months in arm A, 25 months in arm B (P = .8). (C) PFS in patients with NHL: median 17 months in arm A, 25 months in arm B (P = .77). (D) PFS in patients with HD: 60% in arm A, 67% in arm B at 21 months (P = .77).
Kaplan-Meier curves for OS and PFS.
Broken lines denote the G-CSF only arm A, and solid lines denote Chemo+G-CSF arm B. (A) OS: 79% in arm A, 77% in arm B at a median follow-up of 21 months (P = .85). (B) PFS: median 17 months in arm A, 25 months in arm B (P = .8). (C) PFS in patients with NHL: median 17 months in arm A, 25 months in arm B (P = .77). (D) PFS in patients with HD: 60% in arm A, 67% in arm B at 21 months (P = .77).
Cox proportional hazards model for survival: multivariate analysis
| Prognostic factor . | PFS . | OS . | ||||
|---|---|---|---|---|---|---|
| Hazard ratio . | 95% CI . | P . | Hazard ratio . | 95% CI . | P . | |
| Chemoresistance at BMT | 9.2 | 3, 31 | .0003 | 8.9 | 1.4, 59 | .02 |
| Type of lymphoma | ||||||
| Large-cell B NHL | 0.8 | 0.2, 3.6 | .8 | 2.2 | 0.2, 22 | .5 |
| Follicular NHL | 0.4 | 0, 2 | .3 | 0.35 | 0, 7 | .5 |
| Hodgkin | 0.3 | 0, 1.7 | .2 | 1 | 0.1, 13 | .99 |
| Relapse category | ||||||
| Primary refractory | 0.5 | 0.1, 2 | .3 | 1.1 | 0.1, 9 | .9 |
| Relapse less than 1 y | 0.6 | 0.2, 2 | .4 | 0.64 | 0.1, 5.5 | .7 |
| Mobilization arm (G-CSF) | 1.2 | 0.4, 3.5 | .7 | 1.1 | 0.2, 5.4 | .9 |
| Prognostic factor . | PFS . | OS . | ||||
|---|---|---|---|---|---|---|
| Hazard ratio . | 95% CI . | P . | Hazard ratio . | 95% CI . | P . | |
| Chemoresistance at BMT | 9.2 | 3, 31 | .0003 | 8.9 | 1.4, 59 | .02 |
| Type of lymphoma | ||||||
| Large-cell B NHL | 0.8 | 0.2, 3.6 | .8 | 2.2 | 0.2, 22 | .5 |
| Follicular NHL | 0.4 | 0, 2 | .3 | 0.35 | 0, 7 | .5 |
| Hodgkin | 0.3 | 0, 1.7 | .2 | 1 | 0.1, 13 | .99 |
| Relapse category | ||||||
| Primary refractory | 0.5 | 0.1, 2 | .3 | 1.1 | 0.1, 9 | .9 |
| Relapse less than 1 y | 0.6 | 0.2, 2 | .4 | 0.64 | 0.1, 5.5 | .7 |
| Mobilization arm (G-CSF) | 1.2 | 0.4, 3.5 | .7 | 1.1 | 0.2, 5.4 | .9 |
Patients with chemoresistant disease at transplantation had a 9-fold increased risk for disease progression (P = .0003) and a 9-fold risk for death (P = .02), compared with those with chemosensitive disease. This is after adjusting for other variables such as the type of lymphoma, presence of refractory disease, and type of mobilization. These other factors were not significant predictors of disease progression or survival.
Discussion
We report the results of a randomized study examining the role of 2 different hematopoietic progenitor cell mobilization methods for use in autologous transplantation. We found no advantage to combined chemotherapy and G-CSF mobilization compared with the use of G-CSF alone in clinical endpoints of time to engraftment, apheresis sessions required for target collection, and time and resources used for transplantation. Certain resources used around time of PBC collection, such as transfusions and hospital admissions, were higher with the combined use of chemotherapy and G-CSF.
There is considerable debate regarding a superior hematopoietic progenitor cell mobilization strategy. Numerous studies have examined the efficiency of various high-dose chemotherapy regimens in HPC mobilization and have reported that the combination of chemotherapy and G-CSF or GM-CSF is synergistic and often mobilizes higher numbers of CD34+ cells than growth factors alone.11-13Results from our study are consistent with this observation that higher numbers of CD34+ cells were mobilized by the combination. Using cyclophosphamide along with G-CSF mobilized 3 times as much median CD34+ cells obtained using G-CSF alone. However, this difference did not impact clinical endpoints. Time to neutrophil and platelet engraftment were closely similar in the 2 arms and were comparable to those reported in other studies, despite one patient in each arm having fewer than 1 × 106CD34+ cells/kg.
Numerous mobilization studies have used CD34+ content as the primary endpoint. Quality of progenitor cells may be more important than quantity, as suggested by our results. CD34 antigen is a marker for progenitor cells; it includes lymphoid, megakaryocyte, and myeloid progenitor cells, and several subsets of CD34+ cells have different abilities for mobilization, adhesion, migration, and homing.3,25 Although CD34+ cell content has been correlated with time to engraftment, there is a threshold effect beyond which cell number does not appear to make a difference.25,26 Recent data suggest that CD34+ cells mobilized by G-CSF alone may have better in vitro migration and homing potential than CD34+ cells mobilized using chemotherapy and G-CSF.27 Better in vitro migratory ability of mobilized CD34+ cells has been found to correlate with shorter time to engraftment.27,28 The above findings may provide an explanation for the fact that a higher number of CD34+ cells mobilized by chemotherapy and G-CSF in our study did not translate into faster engraftment. Molecular events leading to HPC mobilization are rapidly being unraveled. A recent report implicates proteolytic cleavage of VCAM-1 by neutrophil elastase as a mechanism of cytokine-induced HPC mobilization.29 Better understanding of the processes of HPC mobilization and homing will provide insight into physiological differences between mobilization strategies.
Resource utilization measures such as number of apheresis sessions required for target PBC collection, time taken for collection, length of hospital stay, and transfusions required after transplantation were similar with the 2 mobilization methods. As expected, hospital admission, time between mobilization treatment and first apheresis, delays in scheduled collection, and transfusions at or near harvest time were all higher in the combined arm. Many investigators now use disease-specific high-dose chemotherapy mobilization strategies. Although this has the advantage of avoiding a separate step for mobilization, it may not be universally applicable to all patients. This strategy also poses significant practical difficulties. Timing of PBC collection after recovery from high-dose chemotherapy has interpatient variability and can make PBC collection schedules problematic, with the potential for wasted resources. Collecting PBCs after 4 days of G-CSF is easy to schedule and is unlikely to be delayed or canceled because of complications. Mobilization with G-CSF alone also allows for effective tumor-specific chemotherapy to be delivered at sites other than the transplantation facility.
The use of high-dose chemotherapy has the theoretical potential for reducing tumor burden and contamination of the harvested PBCs, though increased mobilization of tumor cells has also been reported.30-32 In this study, we did not find a qualitative difference in tumor-sequence DNA contamination of PBCs between the 2 mobilization methods in patients with NHL. There was a high frequency (approximately 90%) of contamination in both arms, and this was true for patients with and without histologic bone marrow involvement. Quantitative differences were not examined. At a median follow-up time of 21 months, there is as yet no difference in the clinical endpoint of median PFS, though the study was not powered to detect small differences.
Overall survival was similar in the 2 arms—77% in arm A and 79% in arm B, with a median follow-up time of 21 months. There were no survival differences after adjusting for the type of lymphoma (NHL or HD). In Cox regression analysis of survival, the type of mobilization was not a significant predictor for either PFS or OS, before or after accounting for chemoresistant disease, type of lymphoma, and type of relapse. Chemoresistance at BMT was the only independent prognostic factor impacting survival.
Multiple cytokines and chemotherapy may be needed to effectively mobilize certain heavily pretreated patients. Our study population, though heavily pretreated, mobilized well with the use of G-CSF alone or the combination. Fewer than 1 × 106 CD34+cells/kg were mobilized in one patient in each group; both were heavily pretreated, and both had relatively delayed engraftment of ANC and platelets. It has been shown that using G-CSF for mobilization twice in a row provides an adequate number of CD34+ cells for transplantation.33 However, using chemotherapy appears to impair the future mobilization of adequate numbers of CD34+cells.12 34
We have provided evidence from a randomized study that G-CSF alone is adequate for progenitor cell mobilization. This was true, even in heavily pretreated patients with relapsed lymphoma. Using G-CSF alone provides a simple strategy that could be broadly applicable to a wide range of diseases, age groups, and clinical settings. These results will translate into easier scheduling, more effective cost control, better resource utilization, and less chemotherapy for patients effectively treated before transplantation referral, and they may decrease long-term complications. The combined strategy may be beneficial if the primary goal is to collect CD34+ cells for manipulation, such as for gene therapy or for in vitro expansion or selection.
We thank the nurses, coordinators, fellows, nurse practitioners, and house staff for their exceptional care of patients.
Supported in part by a research grant from Amgen.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
David P. Schenkein, Division of Hematology-Oncology, Cancer Center and Tupper Research Institute, New England Medical Center, Box 542, 750 Washington St, Boston, MA 02111; e-mail: dschenkein@lifespan.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal