The National Marrow Donor Program (NMDP) maintains a registry of approximately 4 million volunteer unrelated donors for patients in need of a stem cell transplant. When several comparably HLA-matched volunteers are identified for a patient, various criteria are used to select a donor. A retrospective analysis of 6978 bone marrow transplantations facilitated by the NMDP from 1987 to 1999 was conducted to study the effects of various donor characteristics on recipient outcome. The evaluation addressed possible effects of donor age, cytomegalovirus serologic status, ABO compatibility, race, sex, and parity on overall and disease-free survival, acute and chronic graft-versus-host disease (GVHD), engraftment, and relapse. Age was the only donor trait significantly associated with overall and disease-free survival. Five-year overall survival rates for recipients were 33%, 29%, and 25%, respectively, with donors aged 18 to 30 years, 31 to 45 years, and more than 45 years (P = .0002). A similar effect was observed among HLA-mismatched cases (28%, 22%, and 19%, respectively). A race mismatch between recipient and donor did not affect outcome. The cumulative incidences of grade III or IV acute GVHD were 30%, 34%, and 34%, respectively, with donors aged 18 to 30 years, 31 to 45 years, and more than 45 years (P = .005). The corresponding incidences of chronic GVHD at 2 years were 44%, 48%, and 49% (P = 0.02). Recipients with female donors who had undergone multiple pregnancies had a higher rate of chronic GVHD than recipients with male donors (54% versus 44%;P < .0001). The use of younger donors may lower the incidence of GVHD and improve survival after bone marrow transplantation. Age should be considered when selecting among comparably HLA-matched volunteer donors.
Introduction
The use of marrow from unrelated volunteer donors is an accepted treatment for patients in need of an allogeneic stem cell transplant who do not have an HLA-matched sibling donor.1-7 The National Marrow Donor Program (NMDP) was established in 1986 to recruit and conduct HLA typing of large numbers of unrelated volunteer donors for such patients. By November 1, 2000, approximately 4.2 million volunteers were listed in the NMDP Registry, of whom 2.4 million were typed for the HLA-A, HLA-B, and DR loci. The policies and procedures of the NMDP were described previously.8-10
Searches of the NMDP Registry often identify multiple HLA-A, HLA-B, and DRB1 matches for a patient. Strategies for selecting an unrelated donor vary. Transplant physicians often prefer donors who are seronegative for cytomegalovirus (CMV), male, racially matched with the patient, or younger than other possible donors. Priority may also be given to ABO-compatible, HLA-DQ–, HLA-DP–, and C-matched11-13donors or to female donors who have never been pregnant. If there is concern about obtaining an adequate number of stem cells, a larger donor may be preferred. The purpose of this retrospective study was to examine the effects of various donor characteristics on the outcome in recipients of bone marrow transplants. We here report associations between outcome and donor age, CMV serologic status, race, ABO compatibility, sex, and parity.
Methods
Patients
The data set consisted of records of 6978 unrelated-donor bone marrow transplantations facilitated by the NMDP from December 1987 through June 1999. Mobilized peripheral blood transplants were excluded from this analysis. Transplantations were done at 133 different member centers of the NMDP network. Recipients were excluded from this analysis if they had previously undergone transplantation of allogeneic or autologous stem cells. Characteristics of the recipients, including their diagnoses, are listed in Table 1. The patients received a variety of preparative regimens. Total-body irradiation (median dose, 1320 cGy; range, 200-1600 cGy) was used in 5545 cases (79%). The methods used for prophylaxis for graft-versus-host disease (GVHD) are listed in Table 1.
Recipient and transplant characteristics according to donor age (n = 6978 bone marrow transplantations)
| Characteristic . | Total . | Age of donor . | |||
|---|---|---|---|---|---|
| 18-30 Years . | 31-45 Years . | >46 Years . | P* . | ||
| Disease | |||||
| CML | 2469 (35) | 662 (34) | 1379 (35) | 428 (38) | .62 |
| Chronic phase | 1665 (24) | 448 (23) | 939 (24) | 278 (25) | |
| Accelerated phase | 666 (10) | 177 (9) | 369 (9) | 120 (11) | |
| Blast phase | 138 (2) | 37 (2) | 71 (2) | 30 (3) | |
| AML | 1373 (20) | 401 (21) | 756 (19) | 216 (19) | |
| 1st remission | 268 (4) | 74 (4) | 157 (4) | 37 (3) | |
| 2nd remission | 345 (5) | 102 (5) | 196 (5) | 47 (4) | |
| 3rd or higher remission and relapse | 760 (11) | 225 (12) | 403 (10) | 132 (12) | |
| ALL | 1359 (19) | 397 (21) | 755 (19) | 207 (18) | |
| 1st remission | 282 (4) | 90 (5) | 152 (4) | 40 (4) | |
| 2nd remission | 494 (7) | 134 (7) | 280 (7) | 80 (7) | |
| 3rd or higher remission and relapse | 583 (8) | 173 (9) | 323 (8) | 87 (8) | |
| MDS | 589 (8) | 148 (8) | 356 (9) | 85 (8) | |
| NHL | 205 (3) | 48 (2) | 122 (3) | 35 (3) | |
| Other malignant disease | 235 (3) | 66 (3) | 131 (3) | 38 (3) | |
| SAA | 286 (4) | 82 (4) | 164 (4) | 40 (4) | |
| Other nonmalignant disease | 462 (7) | 119 (6) | 261 (7) | 82 (7) | |
| Female | 2850 (41) | 817 (42) | 1568 (40) | 465 (41) | .27 |
| CMV seropositive† | 3359 (49) | 905 (47) | 1927 (49) | 527 (47) | .62 |
| Match status | |||||
| HLA-A, -B, DRB1 match | 4290 (61) | 1230 (64) | 2385 (61) | 675 (60) | .20 |
| Potential match | 773 (11) | 182 (9) | 475 (12) | 116 (10) | |
| HLA-A mismatch | 719 (10) | 189 (10) | 401 (10) | 129 (11) | |
| HLA-B mismatch | 513 (7) | 140 (7) | 284 (7) | 89 (8) | |
| HLA-DRB1 mismatch | 683 (10) | 182 (9) | 379 (10) | 122 (11) | |
| Race or ethnic group | |||||
| African American | 301 (4) | 97 (5) | 160 (4) | 44 (4) | .009 |
| Asian/Pacific Islander | 180 (3) | 68 (4) | 85 (2) | 27 (2) | |
| White | 6029 (86) | 1609 (84) | 3427 (87) | 993 (88) | |
| Hispanic | 411 (6) | 133 (7) | 217 (6) | 61 (5) | |
| Native American | 16 (<1) | 5 (<1) | 10 (<1) | 1 (<1) | |
| Other | 41 (1) | 11 (1) | 25 (1) | 5 (<1) | |
| Year of infusion | |||||
| 1987-1992 | 1378 (20) | 283 (15) | 865 (22) | 230 (20) | <.0001 |
| 1993-1995 | 2122 (30) | 582 (30) | 1174 (30) | 366 (32) | |
| 1996-1999 | 3478 (50) | 1058 (55) | 1885 (48) | 535 (47) | |
| GVHD prophylaxis | <.0001 | ||||
| T-cell depletion ± other | 1747 (25) | 531 (28) | 965 (25) | 251 (22) | |
| MTX + CsA | 1871 (27) | 535 (28) | 1046 (27) | 290 (26) | |
| MTX + CsA + prednisone | 1178 (17) | 343 (18) | 644 (16) | 191 (17) | |
| MTX ± other | 1563 (22) | 376 (20) | 890 (23) | 297 (26) | |
| Other | 619 (9) | 138 (7) | 379 (10) | 102 (9) | |
| Median age, y (range) | 28.8 (0-66) | 27.4 (0-66) | 29.3 (0-64) | 28.5 (0-66) | .12 |
| Median time, mo (range), from diagnosis to transplant | 15 (0-395) | 14 (0-258) | 15 (0-395) | 15 (0-242) | .04 |
| Median cell dose, ×108/kg (range)‡ | 2.5 (0.1-10) | 2.4 (0.1-10) | 2.5 (0.1-10) | 2.4 (0.1-9.9) | .54 |
| Characteristic . | Total . | Age of donor . | |||
|---|---|---|---|---|---|
| 18-30 Years . | 31-45 Years . | >46 Years . | P* . | ||
| Disease | |||||
| CML | 2469 (35) | 662 (34) | 1379 (35) | 428 (38) | .62 |
| Chronic phase | 1665 (24) | 448 (23) | 939 (24) | 278 (25) | |
| Accelerated phase | 666 (10) | 177 (9) | 369 (9) | 120 (11) | |
| Blast phase | 138 (2) | 37 (2) | 71 (2) | 30 (3) | |
| AML | 1373 (20) | 401 (21) | 756 (19) | 216 (19) | |
| 1st remission | 268 (4) | 74 (4) | 157 (4) | 37 (3) | |
| 2nd remission | 345 (5) | 102 (5) | 196 (5) | 47 (4) | |
| 3rd or higher remission and relapse | 760 (11) | 225 (12) | 403 (10) | 132 (12) | |
| ALL | 1359 (19) | 397 (21) | 755 (19) | 207 (18) | |
| 1st remission | 282 (4) | 90 (5) | 152 (4) | 40 (4) | |
| 2nd remission | 494 (7) | 134 (7) | 280 (7) | 80 (7) | |
| 3rd or higher remission and relapse | 583 (8) | 173 (9) | 323 (8) | 87 (8) | |
| MDS | 589 (8) | 148 (8) | 356 (9) | 85 (8) | |
| NHL | 205 (3) | 48 (2) | 122 (3) | 35 (3) | |
| Other malignant disease | 235 (3) | 66 (3) | 131 (3) | 38 (3) | |
| SAA | 286 (4) | 82 (4) | 164 (4) | 40 (4) | |
| Other nonmalignant disease | 462 (7) | 119 (6) | 261 (7) | 82 (7) | |
| Female | 2850 (41) | 817 (42) | 1568 (40) | 465 (41) | .27 |
| CMV seropositive† | 3359 (49) | 905 (47) | 1927 (49) | 527 (47) | .62 |
| Match status | |||||
| HLA-A, -B, DRB1 match | 4290 (61) | 1230 (64) | 2385 (61) | 675 (60) | .20 |
| Potential match | 773 (11) | 182 (9) | 475 (12) | 116 (10) | |
| HLA-A mismatch | 719 (10) | 189 (10) | 401 (10) | 129 (11) | |
| HLA-B mismatch | 513 (7) | 140 (7) | 284 (7) | 89 (8) | |
| HLA-DRB1 mismatch | 683 (10) | 182 (9) | 379 (10) | 122 (11) | |
| Race or ethnic group | |||||
| African American | 301 (4) | 97 (5) | 160 (4) | 44 (4) | .009 |
| Asian/Pacific Islander | 180 (3) | 68 (4) | 85 (2) | 27 (2) | |
| White | 6029 (86) | 1609 (84) | 3427 (87) | 993 (88) | |
| Hispanic | 411 (6) | 133 (7) | 217 (6) | 61 (5) | |
| Native American | 16 (<1) | 5 (<1) | 10 (<1) | 1 (<1) | |
| Other | 41 (1) | 11 (1) | 25 (1) | 5 (<1) | |
| Year of infusion | |||||
| 1987-1992 | 1378 (20) | 283 (15) | 865 (22) | 230 (20) | <.0001 |
| 1993-1995 | 2122 (30) | 582 (30) | 1174 (30) | 366 (32) | |
| 1996-1999 | 3478 (50) | 1058 (55) | 1885 (48) | 535 (47) | |
| GVHD prophylaxis | <.0001 | ||||
| T-cell depletion ± other | 1747 (25) | 531 (28) | 965 (25) | 251 (22) | |
| MTX + CsA | 1871 (27) | 535 (28) | 1046 (27) | 290 (26) | |
| MTX + CsA + prednisone | 1178 (17) | 343 (18) | 644 (16) | 191 (17) | |
| MTX ± other | 1563 (22) | 376 (20) | 890 (23) | 297 (26) | |
| Other | 619 (9) | 138 (7) | 379 (10) | 102 (9) | |
| Median age, y (range) | 28.8 (0-66) | 27.4 (0-66) | 29.3 (0-64) | 28.5 (0-66) | .12 |
| Median time, mo (range), from diagnosis to transplant | 15 (0-395) | 14 (0-258) | 15 (0-395) | 15 (0-242) | .04 |
| Median cell dose, ×108/kg (range)‡ | 2.5 (0.1-10) | 2.4 (0.1-10) | 2.5 (0.1-10) | 2.4 (0.1-9.9) | .54 |
CML indicates chronic myelogenous leukemia; AML, acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; NHL, non-Hodgkin lymphoma; SAA; severe aplastic anemia; CMV; cytomegalovirus; GVHD, graft-versus-host disease; MTX, methotrexate; and CsA, cyclosporine. Values are numbers (percentages) unless otherwise indicated.
Determined by the Wilcoxon rank sum test for discrete variables and the Spearman rank correlation test for continuous variables.
The recipient's CMV serologic status was not available for 56 cases.
T-cell–replete cases only.
Data collection
Outcome data for recipients of transplants were reported to the NMDP Coordinating Center by the transplant centers on standardized NMDP forms submitted at the time of transplantation (baseline) and at 100 days, 6 months, and annually afterward. Computerized validation was used to check data for consistency at the time of entry into the database. The median follow-up time was 731 days (range, 95-3650 days).
Acute GVHD was reported with use of standardized stages of skin, liver, and intestinal involvement. A severity grade was calculated on the basis of these stages by using standard criteria.14Hematologic relapse was recorded on NMDP forms for patients with leukemia, lymphoma, myelodysplastic syndrome, and other malignant diseases. Analyses of relapse and disease-free survival were restricted to these diseases (n = 6230). Neutrophil engraftment was defined as a neutrophil count of at least 0.5 × 109/L on 3 consecutive laboratory analyses. These data were missing for 50 patients (< 1%) and unevaluable for 410 patients (6%) who died before day 21, leaving 6518 available for analysis.
Race and ethnicity were self-reported by patients and donors. An individual was considered a member of a minority group if he or she was self-identified as African American, Asian or Pacific Islander, Hispanic, or Native American. A minor ABO mismatch was defined as transplantation of marrow from a donor with type O blood into a recipient with a non–O type blood or from a type A or B donor into a type AB recipient. A bidirectional mismatch was defined as transplantation of marrow from a type A donor into a type B recipient or vice versa.
HLA matching and typing
The minimum level of HLA matching required by the NMDP is a 5-of-6 antigen level match at HLA-A, HLA-B, and DR. Transplant centers may have imposed stricter matching criteria depending on their transplant protocols or patient characteristics. HLA typing was done with a variety of methods during the time examined in this study. Serologic typing for HLA antigens was assigned according to the definitions established by the World Health Organization HLA nomenclature committee.15 Molecular-based HLA-A, HLA-B, and DRB1 typing was done with a variety of techniques at differing levels of resolution.16 Cases typed on the basis of serologic findings or a low-resolution molecular method in which an allele-level DRB1 mismatch could not be identified were classified as potential matches in this analysis. Matching at the HLA-A and HLA-B loci was defined at the serologic antigen level regardless of whether the typing was done by molecular-based or serologic methods.
Statistical analysis
Donor characteristics were tested for associations by using the χ2 test for discrete variables, the Spearman rank correlation test for continuous variables, and the Wilcoxon rank sum test for a discrete by continuous comparison. Survival and disease-free survival rates were calculated by using the method of Kaplan and Meier17 and were compared by using the log rank statistic.18 Rates of acute and chronic GVHD, engraftment, and relapse were calculated according to cumulative incidence,19 with death considered a competing risk. Cases were considered evaluable for engraftment if the patient survived at least 21 days and for chronic GVHD if the patient survived at least 80 days. Cumulative incidences were compared at 100 days for acute GVHD and engraftment and at 2 years for chronic GVHD by using a Taylor series linear approximation to estimate the variance.20
Logistic regression was used for multivariate analysis of neutrophil engraftment, and the proportional hazards model21 was used for the other outcomes. Each model included disease and stage (Table1), donor age, sex, parity, CMV serologic status, and race, regardless of significance. Other factors were included in the model if they showed a significant (P ≤ .05 on Wald χ2testing) association with outcome. Factors considered for the model were HLA-A, HLA-B, and DRB1 matching; transplant center; cell dose; time from diagnosis to transplantation; year of transplantation; age of recipient; sex; CMV serologic status; and race. The time from diagnosis to transplantation was modeled separately for each disease group. Because of nonlinear effects, the continuous variables recipient age and time from diagnosis to transplantation were divided into discrete categories. The effect of donor CMV serologic status was modeled separately for CMV-seropositive and CMV-seronegative recipients, the effect of donor race was modeled separately for white and minority recipients, and the effect of cell dose was modeled for T-cell–replete cases only.
Results
Donor characteristics
Donor characteristics are shown in Table2. The median age of donors was 37 years (range, 18-60 years). CMV-seronegative donors tended to be younger (median age, 36 years versus 38 years; P < .0001) as did donors from minority groups (35 years versus 37 years; P< .0001), women who had never been pregnant (30 years versus 37 years; P < .0001), and donors in more recent transplantations (P < .0001). Transplantations with T-cell–depleted marrow tended to use younger donors (36 years versus 37 years; P < 0.0001). Donor age also differed significantly according to transplant centers (P < .0001), but was not associated with recipient diagnosis, recipient age, or level of HLA matching.
Donor characteristics according to age
| Characteristic . | Total no. (%) . | Age of donor . | P* . | ||
|---|---|---|---|---|---|
| 18-30 years, no. (%) . | 31-45 years, no. (%) . | >46 years, no (%) . | |||
| No. (%) of donors | 6978 (100) | 1923 (28) | 3924 (56) | 1131 (16) | |
| CMV seropositive† | 2552 (38) | 557 (30) | 1488 (40) | 507 (47) | <.0001 |
| Sex | |||||
| Male | 4033 (58) | 1048 (54) | 2318 (59) | 667 (59) | <.0001 |
| Female (no pregnancies) | 1140 (16) | 591 (31) | 457 (12) | 92 (8) | |
| Female (1 pregnancy) | 416 (6) | 145 (8) | 217 (6) | 54 (5) | |
| Female (≥2 pregnancies) | 1358 (19) | 133 (7) | 913 (23) | 312 (28) | |
| Female (parity unknown) | 31 (<1) | 6 (<1) | 19 (<1) | 6 (<1) | |
| Race or ethnic group | |||||
| African American | 260 (4) | 83 (4) | 140 (4) | 37 (3) | <.0001 |
| Asian/Pacific Islander | 180 (3) | 80 (4) | 78 (2) | 22 (2) | |
| White | 5349 (77) | 1365 (71) | 3056 (78) | 928 (82) | |
| Hispanic | 358 (5) | 124 (6) | 190 (5) | 44 (4) | |
| Native American | 74 (1) | 30 (2) | 35 (1) | 9 (1) | |
| Other | 56 (1) | 18 (1) | 31 (1) | 7 (1) | |
| Unknown | 701 (10) | 223 (12) | 394 (10) | 84 (7) | |
| Match status | |||||
| ABO match | 2860 (41) | 762 (40) | 1656 (42) | 442 (39) | .56 |
| Minor ABO mismatch‡ | 1802 (26) | 486 (25) | 1014 (26) | 302 (27) | |
| Bidirectional ABO mismatch2-153 | 587 (8) | 163 (8) | 328 (8) | 96 (8) | |
| Other ABO mismatch | 1670 (24) | 489 (25) | 893 (23) | 288 (25) | |
| ABO unknown | 59 (1) | 23 (1) | 33 (1) | 3 (<1) | |
| Characteristic . | Total no. (%) . | Age of donor . | P* . | ||
|---|---|---|---|---|---|
| 18-30 years, no. (%) . | 31-45 years, no. (%) . | >46 years, no (%) . | |||
| No. (%) of donors | 6978 (100) | 1923 (28) | 3924 (56) | 1131 (16) | |
| CMV seropositive† | 2552 (38) | 557 (30) | 1488 (40) | 507 (47) | <.0001 |
| Sex | |||||
| Male | 4033 (58) | 1048 (54) | 2318 (59) | 667 (59) | <.0001 |
| Female (no pregnancies) | 1140 (16) | 591 (31) | 457 (12) | 92 (8) | |
| Female (1 pregnancy) | 416 (6) | 145 (8) | 217 (6) | 54 (5) | |
| Female (≥2 pregnancies) | 1358 (19) | 133 (7) | 913 (23) | 312 (28) | |
| Female (parity unknown) | 31 (<1) | 6 (<1) | 19 (<1) | 6 (<1) | |
| Race or ethnic group | |||||
| African American | 260 (4) | 83 (4) | 140 (4) | 37 (3) | <.0001 |
| Asian/Pacific Islander | 180 (3) | 80 (4) | 78 (2) | 22 (2) | |
| White | 5349 (77) | 1365 (71) | 3056 (78) | 928 (82) | |
| Hispanic | 358 (5) | 124 (6) | 190 (5) | 44 (4) | |
| Native American | 74 (1) | 30 (2) | 35 (1) | 9 (1) | |
| Other | 56 (1) | 18 (1) | 31 (1) | 7 (1) | |
| Unknown | 701 (10) | 223 (12) | 394 (10) | 84 (7) | |
| Match status | |||||
| ABO match | 2860 (41) | 762 (40) | 1656 (42) | 442 (39) | .56 |
| Minor ABO mismatch‡ | 1802 (26) | 486 (25) | 1014 (26) | 302 (27) | |
| Bidirectional ABO mismatch2-153 | 587 (8) | 163 (8) | 328 (8) | 96 (8) | |
| Other ABO mismatch | 1670 (24) | 489 (25) | 893 (23) | 288 (25) | |
| ABO unknown | 59 (1) | 23 (1) | 33 (1) | 3 (<1) | |
Determined by the Wilcoxon rank sum test.
The donor's CMV serologic status was not available for 339 cases.
Marrow from a donor with type O blood was transplanted into a recipient with non–type O blood or marrow from a type A or B donor was transplanted into an AB recipient.
Marrow from a type A donor was transplanted into a type B recipient or vice versa.
Among adult recipients (≥ 18 years old) weighing at least 50 kg for whom the marrow was not modified by T-cell depletion, volume reduction, red-cell depletion, or plasma depletion (n = 1830), older donors were associated with slightly lower nucleated cell doses (P = .03). The median infused cell doses were 3.23 × 108/kg of body weight, 3.18 × 108/kg, and 2.99 × 108/kg, respectively, with donors aged 18 to 30 years, 31 to 45 years, and older than 45 years. Among the 1766 cases for which donor weight was available, larger donors were associated with significantly higher infused cell doses (P < .0001); median cell doses were 2.95 × 108/kg, 3.14 × 108/kg, and 3.37 × 108/kg, respectively, for donors who weighed less than 70 kg, 70 to 90 kg, and more than 90 kg.
Neutrophil engraftment
The cumulative incidence of engraftment among evaluable cases was 65% ± 1% by day 21 and 94% ± 1% by day 100. There was no association between engraftment and donor age, race, or CMV serologic status.
Logistic regression analysis adjusted for the significant effects of disease, transplant center, HLA matching, cell dose, T-cell depletion, recipient CMV status, and race. None of the donor factors examined, except for the level of HLA matching, was significantly associated with engraftment (Table 3). In a Cox regression that incorporated speed of engraftment as well as overall incidence, patients with a male donor had significantly faster engraftment (relative risk [RR], 1.12; 95% confidence interval [CI], 1.06-1.18; P < .0001) than those with a female donor, regardless of parity.
Logistic regression model for primary neutrophil engraftment in evaluable patients surviving at least 21 days (n = 6518)
| Factor . | Primary neutrophil engraftment . | |||
|---|---|---|---|---|
| Odds ratio . | 95% CI . | P . | Favorable . | |
| Donor age (per decade) | 1.04 | 0.92-1.18 | .53 | NS |
| Donor CMV positive (CMV-negative recipient)3-150 | 0.75 | 0.56-1.02 | .07 | NS |
| Donor CMV positive (CMV-positive recipient)3-151 | 0.98 | 0.72-1.34 | .91 | NS |
| Male donor | 1.00 | — | — | — |
| Female donor (no pregnancies) | 0.86 | 0.65-1.14 | .30 | NS |
| Female donor (1 pregnancy) | 1.09 | 0.69-1.74 | .70 | NS |
| Female donor (≥2 pregnancies) | 0.99 | 0.75-1.31 | .95 | NS |
| ABO match | 1.00 | — | — | — |
| ABO minor mismatch | 1.12 | 0.86-1.48 | .40 | NS |
| ABO major mismatch | 0.95 | 0.74-1.21 | .68 | NS |
| Donor race mismatch (white recipient) | 0.90 | 0.67-1.20 | .47 | NS |
| Donor race mismatch (minority recipient) | 1.03 | 0.59-1.77 | .93 | NS |
| HLA-A, -B, DRB1 match3-152 | 1.00 | — | — | Matched |
| HLA-A mismatch3-150 | 0.58 | 0.42-0.80 | .0008 | — |
| HLA-B mismatch | 0.52 | 0.37-0.73 | .0002 | — |
| HLA-DRB1 mismatch | 0.52 | 0.38-0.72 | <.0001 | — |
| Factor . | Primary neutrophil engraftment . | |||
|---|---|---|---|---|
| Odds ratio . | 95% CI . | P . | Favorable . | |
| Donor age (per decade) | 1.04 | 0.92-1.18 | .53 | NS |
| Donor CMV positive (CMV-negative recipient)3-150 | 0.75 | 0.56-1.02 | .07 | NS |
| Donor CMV positive (CMV-positive recipient)3-151 | 0.98 | 0.72-1.34 | .91 | NS |
| Male donor | 1.00 | — | — | — |
| Female donor (no pregnancies) | 0.86 | 0.65-1.14 | .30 | NS |
| Female donor (1 pregnancy) | 1.09 | 0.69-1.74 | .70 | NS |
| Female donor (≥2 pregnancies) | 0.99 | 0.75-1.31 | .95 | NS |
| ABO match | 1.00 | — | — | — |
| ABO minor mismatch | 1.12 | 0.86-1.48 | .40 | NS |
| ABO major mismatch | 0.95 | 0.74-1.21 | .68 | NS |
| Donor race mismatch (white recipient) | 0.90 | 0.67-1.20 | .47 | NS |
| Donor race mismatch (minority recipient) | 1.03 | 0.59-1.77 | .93 | NS |
| HLA-A, -B, DRB1 match3-152 | 1.00 | — | — | Matched |
| HLA-A mismatch3-150 | 0.58 | 0.42-0.80 | .0008 | — |
| HLA-B mismatch | 0.52 | 0.37-0.73 | .0002 | — |
| HLA-DRB1 mismatch | 0.52 | 0.38-0.72 | <.0001 | — |
The model also adjusted for disease, transplant center, cell dose, T-cell depletion, recipient CMV status, and race (results not shown). An odds ratio greater than 1.00 indicates a favorable outcome for engraftment.
CI indicates confidence interval; CMV, cytomegalovirus; and NS, not significant.
Comparing CMV-seropositive donor with seronegative donor among seronegative recipients.
Comparing CMV-seropositive donor with seronegative donor among seropositive recipients.
Includes potential matches.
In univariate analysis, male donor was a predictor of a higher incidence of engraftment by day 21 in both male recipients (65% ± 2% versus 59% ± 3%) and female recipients (70% ± 2% versus 65% ± 3%). Incidences at day 100 were 89% ± 1%, 90% ± 1%, and 88% ± 1%, respectively, with donors aged 18 to 30 years, 31 to 45 years, and more than 45 years.
Acute GVHD
Multivariate analysis adjusted for the significant effects of disease, transplant center, HLA matching, T-cell depletion, year of infusion, recipient age, and race. Age and HLA matching were the only donor characteristics significantly associated with severe (grade III-IV) acute GVHD (Table 4). No significant interactions of donor age with disease or recipient age were detected. There was a possible interaction of donor age with HLA matching (P = .04), but this finding should be viewed with caution because of the multiple comparisons done with these data.
Proportional hazards regression models for grade III or IV acute graft-versus-host disease (GVHD) (n = 6978) and chronic GVHD (n = 4819 evaluable patients surviving at least 80 days)
| Factor . | Grade III-IV acute GVHD . | Chronic GVHD . | ||||||
|---|---|---|---|---|---|---|---|---|
| RR . | 95% CI . | P . | Favorable factor . | RR . | 95% CI . | P . | Favorable factor . | |
| Donor age (per decade) | 1.08 | 1.03-1.14 | .002 | Younger | 1.08 | 1.02-1.14 | .005 | Younger |
| Donor CMV positive (CMV-negative recipient)4-150 | 0.99 | 0.88-1.12 | .90 | NS | 1.02 | 0.90-1.15 | .77 | NS |
| Donor CMV positive (CMV-positive recipient)4-151 | 0.99 | 0.87-1.11 | .81 | NS | 0.95 | 0.83-1.09 | .46 | NS |
| Male donor | 1.00 | — | — | — | 1.00 | — | — | Male |
| Female donor (no pregnancies) | 1.04 | 0.92-1.17 | .53 | NS | 1.09 | 0.96-1.24 | .17 | Never |
| Female donor (1 pregnancy) | 0.94 | 0.78-1.12 | .48 | NS | 1.19 | 1.00-1.43 | .05 | Pregnant |
| Female donor (≥2 pregnancies) | 1.04 | 0.93-1.16 | .46 | NS | 1.40 | 1.25-1.57 | <.0001 | — |
| ABO match | 1.00 | — | — | — | 1.00 | — | — | — |
| ABO minor mismatch | 1.11 | 1.00-1.23 | .05 | NS | 1.01 | 0.90-1.12 | .92 | NS |
| ABO major mismatch | 1.02 | 0.93-1.13 | .67 | NS | 0.97 | 0.88-1.08 | .58 | NS |
| Donor race mismatch (white recipient) | 1.06 | 0.94-1.19 | .38 | NS | 0.98 | 0.86-1.12 | .74 | NS |
| Donor race mismatch (minority recipient) | 1.17 | 0.94-1.46 | .16 | NS | 1.16 | 0.90-1.51 | .25 | NS |
| HLA-A, -B, DRB1 match‡ | 1.00 | — | — | Matched | N/A | N/A | N/A | NS |
| HLA-A mismatch | 1.22 | 1.06-1.41 | .006 | — | N/A | N/A | N/A | NS |
| HLA-B mismatch | 1.47 | 1.26-1.71 | <.0001 | — | N/A | N/A | N/A | NS |
| HLA-DRB1 mismatch | 1.46 | 1.27-1.67 | <.0001 | — | N/A | N/A | N/A | NS |
| Factor . | Grade III-IV acute GVHD . | Chronic GVHD . | ||||||
|---|---|---|---|---|---|---|---|---|
| RR . | 95% CI . | P . | Favorable factor . | RR . | 95% CI . | P . | Favorable factor . | |
| Donor age (per decade) | 1.08 | 1.03-1.14 | .002 | Younger | 1.08 | 1.02-1.14 | .005 | Younger |
| Donor CMV positive (CMV-negative recipient)4-150 | 0.99 | 0.88-1.12 | .90 | NS | 1.02 | 0.90-1.15 | .77 | NS |
| Donor CMV positive (CMV-positive recipient)4-151 | 0.99 | 0.87-1.11 | .81 | NS | 0.95 | 0.83-1.09 | .46 | NS |
| Male donor | 1.00 | — | — | — | 1.00 | — | — | Male |
| Female donor (no pregnancies) | 1.04 | 0.92-1.17 | .53 | NS | 1.09 | 0.96-1.24 | .17 | Never |
| Female donor (1 pregnancy) | 0.94 | 0.78-1.12 | .48 | NS | 1.19 | 1.00-1.43 | .05 | Pregnant |
| Female donor (≥2 pregnancies) | 1.04 | 0.93-1.16 | .46 | NS | 1.40 | 1.25-1.57 | <.0001 | — |
| ABO match | 1.00 | — | — | — | 1.00 | — | — | — |
| ABO minor mismatch | 1.11 | 1.00-1.23 | .05 | NS | 1.01 | 0.90-1.12 | .92 | NS |
| ABO major mismatch | 1.02 | 0.93-1.13 | .67 | NS | 0.97 | 0.88-1.08 | .58 | NS |
| Donor race mismatch (white recipient) | 1.06 | 0.94-1.19 | .38 | NS | 0.98 | 0.86-1.12 | .74 | NS |
| Donor race mismatch (minority recipient) | 1.17 | 0.94-1.46 | .16 | NS | 1.16 | 0.90-1.51 | .25 | NS |
| HLA-A, -B, DRB1 match‡ | 1.00 | — | — | Matched | N/A | N/A | N/A | NS |
| HLA-A mismatch | 1.22 | 1.06-1.41 | .006 | — | N/A | N/A | N/A | NS |
| HLA-B mismatch | 1.47 | 1.26-1.71 | <.0001 | — | N/A | N/A | N/A | NS |
| HLA-DRB1 mismatch | 1.46 | 1.27-1.67 | <.0001 | — | N/A | N/A | N/A | NS |
These models also adjusted for disease, transplant center, T-cell depletion, year of infusion, and recipient age and race (results not shown).
RR indicates relative risk; CI, confidence interval; CMV, cytomegalovirus; NS, not significant; and N/A, not applicable (ie, factors excluded from the model because they were not statistically significant).
Comparing CMV-seropositive donor with seronegative donor among seronegative recipients.
Comparing CMV-seropositive donor with seronegative donor among seropositive recipients.
Includes potential matches.
In univariate analysis, younger donors were associated with a significant decrease in severe acute GVHD among HLA-mismatched pairs. Incidences at day 100 were 30% ± 4%, 41% ± 3%, and 40% ± 5%, respectively, with donors aged 18 to 30 years, 31 to 45 years, and more than 45 years (P < 0.0001). However, no trend was apparent among HLA-matched pairs (29% ± 3%, 30% ± 2%, and 30% ± 3%, respectively). Female donors were not associated with more frequent acute GVHD in either male recipients (33% ± 2% versus 33% ± 2%) or female recipients (31% ± 2% versus 32% ± 2%). Parity among female donors was not associated with frequency of acute GVHD (data not shown).
CMV seronegativity of donors was not a predictor of lower rates of acute GVHD in either CMV-seronegative recipients (31% ± 2% versus 33% ± 3%) or seropositive recipients (32% ± 2% versus 34% ± 2%). Among pairs matched for HLA-A, HLA-B, and DRB1, a racially matched donor was not a predictor of a lower incidence in minority recipients (32% ± 6% versus 40% ± 7%) or white recipients (29% ± 2% versus 32% ± 6%). An ABO match was not associated with a lower incidence than either a minor or a major mismatch (32% ± 2% versus 34% ± 2% versus 33% ± 2%).
Chronic GVHD
Multivariate analysis adjusted for the significant effects of disease, transplant center, T-cell depletion, year of infusion, recipient age, and race. The analysis found that a younger donor, male donor, and female donor without previous pregnancies were all predictors of less chronic GVHD (Table 4). There were no detectable interactions of donor age or parity with disease, HLA matching, recipient age, or recipient sex.
In univariate analysis among evaluable patients surviving at least 80 days, a younger donor was associated with a decreased frequency of chronic GVHD. Cumulative incidences at 2 years were 44% ± 3%, 48% ± 2%, and 50% ± 3%, respectively, with donors aged 18 to 30 years, 31 to 45 years, and more than 45 years (P = .02).
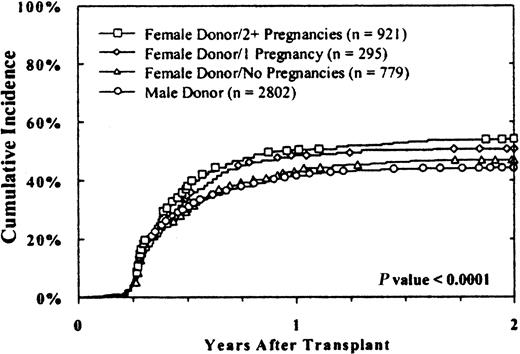
Donor parity was also associated with chronic GVHD (Figure1). Cumulative rates among evaluable cases were 44% ± 2%, 47% ± 3%, 51% ± 5%, and 54% ± 3%, respectively, with male donors, female donors who had never been pregnant, female donors with one previous pregnancy, and female donors with 2 or more previous pregnancies (P < .0001). Results were similar in male and female recipients.
The cumulative incidence of chronic GVHD was higher with multiparous female donors.
Results with male donors and female donors without pregnancies were similar, whereas an increasing incidence of chronic GVHD was associated with female donors with one or more pregnancies. Results were similar in female and male recipients. Twenty-two cases that were evaluable for chronic GVHD were excluded from this analysis because parity data were unavailable.
The cumulative incidence of chronic GVHD was higher with multiparous female donors.
Results with male donors and female donors without pregnancies were similar, whereas an increasing incidence of chronic GVHD was associated with female donors with one or more pregnancies. Results were similar in female and male recipients. Twenty-two cases that were evaluable for chronic GVHD were excluded from this analysis because parity data were unavailable.
A CMV-seronegative donor was a predictor of a lower rate of chronic GVHD in CMV-seronegative patients (46% ± 2% versus 51% ± 3%;P = .02) but not in seropositive recipients (46% ± 3% versus 47% ± 3%). However, the effect of donor CMV status was not significant in multivariate analysis after adjustment for other risk factors.
An ABO match was not associated with a lower incidence of GVHD than a minor or major mismatch (47% ± 2% versus 48% ± 3% versus 46% ± 2%). Among pairs matched for HLA-A, HLA-B, and DRB1, a racially matched donor did not reduce the rate of chronic GVHD in either a white recipient (49% ± 2% versus 45% ± 8%) or a minority recipient (49% ± 7% versus 42% ± 8%).
Relapse
The overall cumulative incidence of hematologic relapse at 2 years among patients with a malignant disease was 15% ± 2%. Relapse was not significantly associated with donor age, CMV serologic status, race, sex, or parity. We did not identify any subpopulation of recipients classified according to disease, HLA matching, recipient age, CMV serologic status, or race in which any of these donor characteristics had a significant effect on relapse.
Survival
Multivariate analysis adjusted for the significant effects of disease, transplant center, HLA matching, cell dose, time from diagnosis to transplantation (chronic-phase chronic myelogenous leukemia [CML] and severe aplastic anemia), and recipient age, sex, and race. Age and HLA matching were the only donor characteristics significantly associated with overall or disease-free survival (P < .0001). No significant effects of donor CMV serologic status, race, sex, or parity were detected (Table5). The effect of donor age on overall survival was similar among HLA-mismatched cases (RR, 1.10 per decade; 95% CI, 1.02-1.19; P = .01). No interactions of donor age with recipient diagnosis or recipient age were detected.
Proportional hazards regression models for overall survival (n = 6978) and disease-free survival (n = 6230).
| Factor . | Overall Survival . | Disease-free Survival . | ||||||
|---|---|---|---|---|---|---|---|---|
| RR . | 95% CI . | P . | Favorable . | RR . | 95% CI . | P . | Favorable . | |
| Donor age (per decade) | 1.10 | (1.06, 1.14) | < 0.0001 | Younger | 1.09 | (1.05, 1.13) | < 0.0001 | Younger |
| Donor CMV positive (CMV negative recipient)5-150 | 0.95 | (0.87, 1.04) | 0.28 | NS | 0.94 | (0.85, 1.03) | 0.17 | NS |
| Donor CMV positive (CMV positive recipient)5-151 | 1.03 | (0.95, 1.12) | 0.51 | NS | 1.03 | (0.94, 1.12) | 0.58 | NS |
| Male donor | 1.00 | 1.00 | ||||||
| Female donor—no pregnancies | 1.04 | (0.95, 1.13) | 0.43 | NS | 1.03 | (0.95, 1.13) | 0.50 | NS |
| Female donor—1 pregnancy | 0.91 | (0.80, 1.03) | 0.14 | NS | 0.92 | (0.80, 1.05) | 0.19 | NS |
| Female donor—2+ pregnancies | 0.99 | (0.92, 1.08) | 0.85 | NS | 1.00 | (0.92, 1.09) | 0.97 | NS |
| ABO match | 1.00 | 1.00 | ||||||
| ABO minor mismatch | 0.98 | (0.91, 1.05) | 0.54 | NS | 1.01 | (0.94, 1.09) | 0.76 | NS |
| ABO major mismatch | 1.01 | (0.94, 1.09) | 0.70 | NS | 1.01 | (0.94, 1.09) | 0.80 | NS |
| Donor race match (white recipient) | 1.05 | (0.96, 1.15) | 0.26 | NS | 1.05 | (0.96, 1.15) | 0.27 | NS |
| Donor race match (minority recipient) | 1.11 | (0.94, 1.31) | 0.23 | NS | 1.05 | (0.88, 1.25) | 0.56 | NS |
| HLA-A,B,DRB1 match5-152 | 1.00 | Matched | 1.00 | Matched | ||||
| HLA-A mismatch | 1.40 | (1.26, 1.54) | < 0.0001 | 1.38 | (1.24, 1.53) | < 0.0001 | ||
| HLA-B mismatch | 1.46 | (1.31, 1.64) | < 0.0001 | 1.43 | (1.27, 1.61) | < 0.0001 | ||
| HLA-DRB1 mismatch | 1.25 | (1.13, 1.38) | < 0.0001 | 1.20 | (1.08, 1.33) | 0.0007 | ||
| Factor . | Overall Survival . | Disease-free Survival . | ||||||
|---|---|---|---|---|---|---|---|---|
| RR . | 95% CI . | P . | Favorable . | RR . | 95% CI . | P . | Favorable . | |
| Donor age (per decade) | 1.10 | (1.06, 1.14) | < 0.0001 | Younger | 1.09 | (1.05, 1.13) | < 0.0001 | Younger |
| Donor CMV positive (CMV negative recipient)5-150 | 0.95 | (0.87, 1.04) | 0.28 | NS | 0.94 | (0.85, 1.03) | 0.17 | NS |
| Donor CMV positive (CMV positive recipient)5-151 | 1.03 | (0.95, 1.12) | 0.51 | NS | 1.03 | (0.94, 1.12) | 0.58 | NS |
| Male donor | 1.00 | 1.00 | ||||||
| Female donor—no pregnancies | 1.04 | (0.95, 1.13) | 0.43 | NS | 1.03 | (0.95, 1.13) | 0.50 | NS |
| Female donor—1 pregnancy | 0.91 | (0.80, 1.03) | 0.14 | NS | 0.92 | (0.80, 1.05) | 0.19 | NS |
| Female donor—2+ pregnancies | 0.99 | (0.92, 1.08) | 0.85 | NS | 1.00 | (0.92, 1.09) | 0.97 | NS |
| ABO match | 1.00 | 1.00 | ||||||
| ABO minor mismatch | 0.98 | (0.91, 1.05) | 0.54 | NS | 1.01 | (0.94, 1.09) | 0.76 | NS |
| ABO major mismatch | 1.01 | (0.94, 1.09) | 0.70 | NS | 1.01 | (0.94, 1.09) | 0.80 | NS |
| Donor race match (white recipient) | 1.05 | (0.96, 1.15) | 0.26 | NS | 1.05 | (0.96, 1.15) | 0.27 | NS |
| Donor race match (minority recipient) | 1.11 | (0.94, 1.31) | 0.23 | NS | 1.05 | (0.88, 1.25) | 0.56 | NS |
| HLA-A,B,DRB1 match5-152 | 1.00 | Matched | 1.00 | Matched | ||||
| HLA-A mismatch | 1.40 | (1.26, 1.54) | < 0.0001 | 1.38 | (1.24, 1.53) | < 0.0001 | ||
| HLA-B mismatch | 1.46 | (1.31, 1.64) | < 0.0001 | 1.43 | (1.27, 1.61) | < 0.0001 | ||
| HLA-DRB1 mismatch | 1.25 | (1.13, 1.38) | < 0.0001 | 1.20 | (1.08, 1.33) | 0.0007 | ||
Analysis of disease-free survival was restricted to malignant diseases. These models also adjusted for disease, transplant center, T-cell depletion, cell dose, interval from diagnosis to transplant (chronic phase CML and SAA) recipient age, sex (overall survival only) and race (results not shown). NS indicates not significant.
Comparing CMV-seropositive donor vs seronegative donor among seronegative recipients.
Comparing CMV-seropositive donor vs seronegative donor among seropositive recipients.
Includes potential matches.
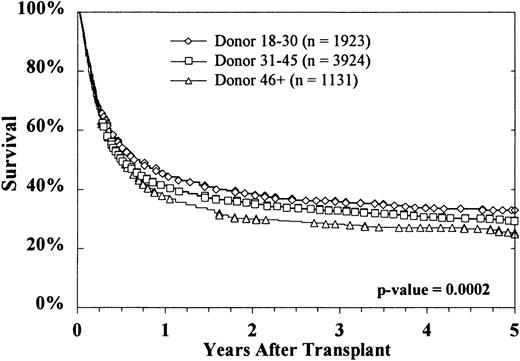
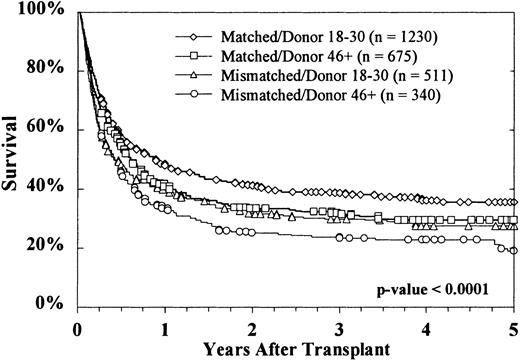
Kaplan-Meier survival curves based on donor age are shown in Figure2. At 5 years, survival rates were 33% ± 2%, 29% ± 2%, and 25% ± 3%, respectively, with donors aged 18 to 30 years, 31 to 45 years, and more than 45 years (P = .0002 on log rank testing). Survival according to both donor age (18-30 years versus > 45 years) and HLA matching is shown in Figure 3. The effect of donor age was evident in both HLA-matched pairs (36% ± 3% versus 29% ± 4%) and HLA-mismatched pairs (28% ± 4% versus 19% ± 5%). The reported causes of death did not vary significantly according to donor age, either overall or when deaths were stratified into early (by day 30), intermediate (day 31-100 and day 101-365), and late (> 1 year) (data not shown).
Overall survival decreased with increasing donor age.
This effect was highly significant.
Overall survival decreased with increasing donor age.
This effect was highly significant.
Increasing donor age reduced survival for both HLA-matched and HLA-mismatched transplants.
HLA-matched donors aged 18 to 30 years were associated with the greatest survival of recipients. Survival results in HLA-mismatched donors aged 18 to 30 years were similar to those in HLA-matched donors aged 46 years or older. The poorest survival was observed with HLA-mismatched donors older than 46 years.
Increasing donor age reduced survival for both HLA-matched and HLA-mismatched transplants.
HLA-matched donors aged 18 to 30 years were associated with the greatest survival of recipients. Survival results in HLA-mismatched donors aged 18 to 30 years were similar to those in HLA-matched donors aged 46 years or older. The poorest survival was observed with HLA-mismatched donors older than 46 years.
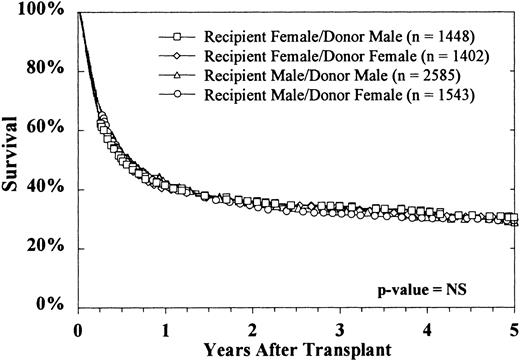
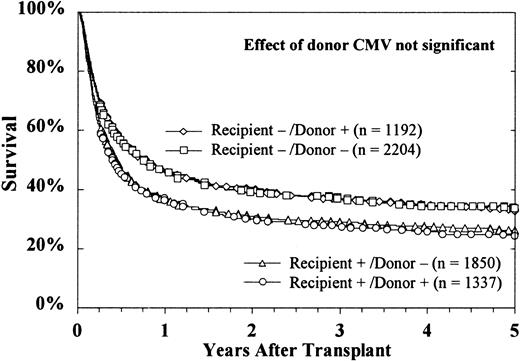
The sex of the donor had no effect on survival, regardless of the sex of the recipient (Figure 4). Donor parity also did not predict survival in either male or female recipients (data not shown). Figure 5 shows that the CMV status of the recipient at the time of transplantation was highly predictive of survival, but the CMV status of the donor was not. The use of marrow from CMV-seronegative donors did not improve 5-year survival in either CMV-seronegative recipients (33% ± 2% versus 34% ± 3%) or CMV-seropositive recipients (26% ± 2% versus 24% ± 3%).
The sex of neither donor nor recipient affected survival.
Survival rates for each of the 4 donor-recipient sex combinations were similar.
The sex of neither donor nor recipient affected survival.
Survival rates for each of the 4 donor-recipient sex combinations were similar.
Donor CMV serologic status did not affect survival of either seronegative or seropositive recipients.
CMV-seropositive recipients had decreased survival, regardless of the donor's CMV serologic status. Cases were excluded from these curves if the CMV serologic status of either the recipient (n = 56) or the donor (n = 339) was unknown.
Donor CMV serologic status did not affect survival of either seronegative or seropositive recipients.
CMV-seropositive recipients had decreased survival, regardless of the donor's CMV serologic status. Cases were excluded from these curves if the CMV serologic status of either the recipient (n = 56) or the donor (n = 339) was unknown.
Among pairs matched for HLA-A, HLA-B, and DRB1, white recipients did not have significantly better survival when the donor was also white (34% ± 2% versus 32% ± 7%). Similarly, survival was not different in racial or ethnic minority recipients given marrow from racially matched donors and those given marrow from donors of a different race (25% ± 10% versus 33% ± 8%). An ABO match was not associated with better survival compared with either a minor or major mismatch (30% ± 2% versus 31% ± 2% versus 28% ± 2%).
Results were similar for disease-free survival. Rates at 5 years were 29% ± 3%, 26% ± 2%, and 21% ± 3%, respectively, with donors aged 18 to 30 years, 31-45 years, and more than 45 years (P = .001 on log rank testing). No significant effects of donor sex, parity, CMV serologic status, or race were detected in either univariate or multivariate analyses.
Subgroup analyses
Because this analysis was retrospective, the patient population was quite heterogeneous. Therefore, separate analyses were done on more homogeneous subgroups to investigate whether the effect of donor age was consistent among different cohorts of patients.
Unlike results of analyses of transplantations of marrow from sibling donors, the age of the unrelated donors in this study did not vary according to recipient age. Median donor ages were 37 years, 36 years, 37 years, and 37 years, respectively, with recipients aged 0 to 17 years, 18 to 30 years, 31 to 45 years, and more than 45 years. Similarly, the effect of donor age on survival did not vary significantly according to the age of the recipient. Among recipients younger than 18 years, 5-year overall survival rates were 39% ± 5%, 38% ± 3%, and 32% ± 6%, respectively, in those with donors aged 18 to 30 years, 31 to 45 years, and more than 46 years. Trends were similar in adult recipients aged 18 to 30 years (30% ± 5% versus 26% ± 4% versus 22% ± 6%) and 31 to 45 years (34% ± 4% versus 26% ± 3% versus 21% ± 5%). No trend was apparent in recipients older than 45 years (19% ± 7% versus 21% ± 5% versus 23% ± 8%), but the statistical margins of error do not allow ruling out a comparable effect in this cohort as well.
Multivariate regression analyses restricted to the various recipient-age cohorts produced similar results. The RR per decade of donor age was comparable in recipients under 18 years (RR, 1.08; 95% CI, 1.01-1.16; P = .03), recipients aged 18 to 30 years (RR, 1.15; 95% CI, 1.07-1.25; P = .0004), recipients aged 31 to 45 years (RR, 1.11; 95% CI, 1.04-1.19; P = .003) and recipients older than 46 years (RR, 1.09; 95% CI, 0.98-1.21;P = .13). These data show no evidence that the effect of donor age varies according to the age of the recipient.
Among patients with CML who underwent transplantation during the first chronic phase, recipients of marrow from younger donors had significantly better survival (48% ± 6% versus 40% ± 4% versus 35% ± 7%; P = .009). Results were similar in patients with acute leukemia who underwent transplantation during the first remission (41% ± 9% versus 36% ± 6% versus 13% ± 19%;P = .009). Similar effects of donor age on survival were observed during separate examinations of cohorts with CMV-seronegative and CMV-seropositive donors; male, female, and multiparous donors; HLA-matched and HLA-mismatched pairs; T-cell–depleted and T-cell–replete cases; and low, medium, and high cell doses (data not shown).
The effect of donor age on acute GVHD may be limited to HLA-mismatched pairs. In multivariate analysis, the RR per decade of donor age was 1.04 (95% CI, 0.97-1.12; P = .24) in the HLA-matched pairs and 1.20 (95% CI, 1.09-1.32; P = .0002) in the mismatched pairs. The test for statistical interaction (ie, that the effect of donor age was different for HLA-matched and HLA-mismatched cases) yielded a borderline result (P = .04). The effect of donor age on chronic GVHD was comparable in HLA-matched pairs (45% ± 3% versus 50% ± 2% versus 51% ± 4%; RR, 1.07; 95% CI, 1.00-1.15; P = .04) and HLA-mismatched pairs (41% ± 5% versus 43% ± 4% versus 50% ± 6%; RR, 1.15; 95% CI, 1.02-1.29;P = .02) pairs. No other subgroups in which the effect of donor age on acute or chronic GVHD varied significantly were identified.
Discussion
Our large set of data on unrelated-donor transplantations allows an opportunity to evaluate the effects of donor characteristics, especially age, on recipient outcome. Although large cohorts of sibling-donor transplantations were described previously,22-26 any effect of donor age was masked by that of recipient age, since siblings tend to be born only a few years apart. One study of related donors other than HLA-identical siblings did identify donor age as associated with leukemia-free survival.27 In the current study of transplants from unrelated donors, having a younger-adult donor was a predictor of less GVHD, better survival, and better disease-free survival in recipients of transplants.
The effect of donor age was observed for both pediatric and adult recipients, with the possible exception of recipients older than 45 years. The sample size in that older cohort, however, was considerably smaller than that in younger cohorts and the data do not allow conclusive ruling out of an effect of donor age in these patients. The effect of donor age overall was comparable to that observed in several other subgroups examined, including patients with chronic-phase CML, patients with acute leukemia in first remission, T-cell–depleted cases, cases without T-cell depletion, male donors, female donors, multiparous donors, CMV-seronegative donors, and CMV-seropositive donors. For acute GVHD, the effect of donor age may be limited to HLA-mismatched pairs; for chronic GVHD and for survival, the effect was apparent in both matched and mismatched pairs.
Transplant physicians often prefer CMV-seronegative donors, especially when the patient is seronegative. Only 38% of donors in this study were CMV seropositive, whereas about half of the recipients were CMV seropositive. Because CMV-seropositive recipients have significantly lower survival rates,28 it seems reasonable to suspect that the possibility of a seropositive donor's transferring the virus to a seronegative recipient poses an additional risk of death. However, we did not detect any adverse effects of seropositive donors in either seronegative or seropositive recipients. After adjustment for other donor characteristics and relevant risk factors, donor CMV serologic status was not associated with survival, GVHD, engraftment, or relapse.
These results are consistent with an earlier study reporting that a CMV-seropositive donor is not a predictor for the development of CMV infection in the recipient after transplantation.29 The use of drugs such as ganciclovir appears to have been largely successful in preventing CMV infections in recipients of transplants. A study of unrelated-donor transplantation in patients with CML showed that use of ganciclovir in CMV-seropositive patients was associated with increased survival.30
Priority is also frequently given to male donors because of their larger size and the increase in GVHD associated with parous female donors. Approximately 60% of those in the NMDP Registry of volunteers are female, but only 42% of those who actually donate are women. Previous studies involving primarily HLA-matched sibling donors reported an increased incidence of acute and chronic GVHD with female or multiparous donors, particularly when the recipient is male.31-33 However, a more recent study found no effect of donor sex on acute GVHD.34 In evaluating our data set, we found that multiparous donors were associated with a higher incidence of chronic GVHD in both male and female recipients. Female donors, regardless of their parity or the sex of the recipient, were also associated with slower neutrophil engraftment. However, these donor sex and parity factors did not result in any difference in overall or disease-free survival.
A previous study of the NMDP Registry identified differences in HLA polymorphism among self-defined racial groups.35 This finding suggests that recruitment of a racially diverse mix of volunteers may improve the likelihood of providing HLA-matched donors for a wider group of patients. This does not mean, however, that race (as available in the NMDP database) should be used to select among comparably HLA-matched potential donors. With acknowledgment of the limitations of these data,36 37 there is no evidence that outcome is any different among cases matched for HLA-A, HLA-B, and DRB1, regardless of whether the donor self-reports the same race as the patient or a different one.
ABO mismatching was previously identified as a risk factor for increased mortality in a single-center study of transplantation in a cohort of patients with acute myelogenous leukemia (AML) and myelodysplastic syndrome (MDS).38 Other studies found no effect of ABO incompatibility on outcome.39 40 We also observed no effect of ABO mismatching in either the overall data set or the subsets of transplantations in patients with AML or MDS.
Among adult recipients of unmodified marrow, larger donors were associated with higher nucleated cell doses, which is in turn predictive of better survival.41 However, the increase in median cell dose with larger donors in our analysis was less than 0.5 × 108/kg, and no direct association between larger donors and improved survival of recipients was detected.
The median age of donors in this data set was 37 years, which is comparable to that in the NMDP Registry as a whole. This finding suggests that age is given relatively less priority in donor selection. Therefore, survival rates might be improved if greater emphasis were placed on selection of younger donors. These data also suggest that use of younger donors could help mitigate the harmful effects of a partial HLA mismatch. Even for rare HLA phenotypes, the likelihood of finding a donor mismatched for only a single HLA determinant is high.35 Thus, many searches that do not identify a full match for a patient on the NMDP Registry have multiple volunteers mismatched for only a single determinant. This typically allows the opportunity to select a young mismatched donor. The use of younger donors to improve outcomes in patients receiving an HLA-mismatched transplant may help expand availability of this therapy to a larger and more diverse group of patients.
Current NMDP policy requires that donors must be between the ages of 18 and 60 years. On the basis of our data, it might be argued that the upper limit for donors should be lowered. However, there are patients whose only HLA match on the NMDP Registry is an older donor. If the upper age limit were lowered, these patients could be denied access to a potentially life-saving therapy. Emphasizing age as a criterion when multiple donors are available is far better for patients than is restricting the pool of potential donors.
Despite the fact that growth in the NMDP Registry of volunteers has led to a higher percentage of transplants matched for HLA-A, HLA-B and DRB1 in recent years, there has been no detectable improvement in survival rates over time. Rates of acute and chronic GVHD have decreased significantly, presumably because of better HLA matching and prophylaxis regimens, but it is unclear why this does not appear to have resulted in better overall or disease-free survival. Our multivariate analyses adjusted for HLA matching to the extent that it was known. However, variations in techniques for HLA typing used by transplant centers and evolving technologies during the period examined in this study make it difficult to assess trends over time in this multicenter study.
The biologic mechanisms for the effect of donor age are not well understood. The increased incidence of GVHD with older donors suggests that tolerance may decrease over time as the immune system is exposed to a greater variety of foreign antigens. This might be due to an increased replacement of naive T cells with memory T cells with age.42 Several studies showed changes in hematopoiesis with age in mice43-45 and humans.46 Other investigations found that younger donors are associated with a lower incidence of B-cell lymphoproliferative disorders,47 obstructive lung disease,48 and secondary graft failure49 after allogeneic marrow transplantation.
It is well known that marrow cellularity decreases with age, and this has been confirmed in studies of older marrow donors.50 We also observed a moderate decline in nucleated cell dose with older donors but only with unmanipulated marrow and only in donors older than 45 years. In our multivariate analysis, the effect of donor age on survival was still apparent after adjustment for cell dose.
The optimal strategy for selecting an unrelated donor remains controversial. Matching the patient for HLA-A, HLA-B, and DRB1 remains the top priority.51 52 The data presented here strongly suggest that in unrelated transplantation, donor age has more effect on overall mortality than donor CMV serologic status, sex, parity, ABO incompatibility, and race. However, the relative importance of such risk factors as matching at other HLA loci is still unknown. It is unclear, for example, if and at what point an HLA-DQ–mismatched younger donor should be preferred over an HLA-DQ–matched older donor. Further research is required to determine the relative importance of the many risk factors that can be influenced by the choice of a donor.
We express our deep gratitude to those who have volunteered to donate stem cells to offer someone a second chance at life. We also appreciate the efforts of physicians and staff at NMDP-affiliated institutions who make transplantations possible. We thank Nancy Morgan for assistance in the preparation of the manuscript.
Supported by contract 240-97-0036 from the Health Resources and Services Administration; grants N00014-93-1-0658 and N00014-95-1-0055 and cooperative agreements N00014-96-2-0016 and N00014-99-2-0006 from the Office of Naval Research; and grant CA18029 from the National Cancer Institute.
The views expressed in this article do not reflect the official policy or position of the Department of the Navy, the Department of Defense, or the United States government.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Craig Kollman, National Marrow Donor Program, Research Department, 3001 Broadway St NE, Suite 500, Minneapolis, MN 55413; e-mail: ckollman@nmdp.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal