Abstract
Prostaglandin D2 (PGD2) is released following exposure of asthmatics to allergen and acts via the adenylyl cyclase–coupled receptor for PGD2 (DP receptor). In this study, it is reported that human eosinophils possess this receptor, which would be expected to inhibit their activation. In contrast, it was found that prostaglandin D2 is a potent stimulator of eosinophil chemotaxis, actin polymerization, CD11b expression, and L-selectin shedding. These responses are specific for eosinophils, as neutrophils display little or no response to prostaglandin D2. They were not due to interaction with receptors for other prostanoids, as prostaglandins E2 and F2α, U46619 (a thromboxane A2 analogue), and carbaprostacyclin (a prostacyclin analogue) displayed little or no activity. Furthermore, they were not shared by the selective DP receptor agonist BW245C and were not prevented by the selective DP receptor antagonist BWA868C, indicating that they were not mediated by DP receptors. In contrast, the prostaglandin D2 metabolite 13,14-dihydro-15-oxoprostaglandin D2 induced eosinophil activation but did not stimulate DP receptor–mediated adenosine 3′,5′–cyclic monophosphate (cAMP) formation. These results indicate that in addition to the classic inhibitory DP1 receptor, eosinophils possess a second, novel DP2 receptor that is associated with PGD2-induced cell activation. These 2 receptors appear to interact to regulate eosinophil responses to PGD2, as blockade of DP1 receptor–mediated cAMP production by BWA868C resulted in enhanced DP2receptor–mediated stimulation of CD11b expression. The balance between DP1 and DP2 receptors could determine the degree to which prostaglandin D2 can activate eosinophils and may play a role in eosinophil recruitment in asthma.
Introduction
Prostaglandins (PGs), formed by the actions of cyclooxygenases 1 and 2 on arachidonic acid, have biological effects on many types of cells through the actions of 8 known receptors. PGE2 has 4 receptor subtypes (EP1-4 receptors), whereas PGs D2, F2α, I2, and thromboxane (TX) A2 each have a single receptor (DP, FP, IP, and TP receptors, respectively).1 The DP receptor is coupled positively to adenylyl cyclase through Gs,1 and this results in a strong inhibitory effect on platelet aggregation2 as well as bronchodilator3 and vasodilator4 effects in humans. The DP receptor and lipocalin-type PGD synthase are both abundant in the central nervous system, where PGD2 plays a role in regulating sleep5 and pain perception.6
Despite the DP receptor–mediated bronchodilator effect of PGD2, there is evidence that PGD2 may contribute to the pathogenesis of asthma. Hematopoietic-type PGD2 synthase is abundant in mast cells,7dendritic cells,8 and certain subpopulations of TH2 lymphocytes,8,9 all of which play critical roles in this disease.10 PGD2 is released from immunologically stimulated mast cells11 and TH2 cells,9 and its levels in bronchoalveolar lavage fluid increase dramatically following allergen challenge of asthmatic subjects.12 Recent evidence that DP receptor knockout mice are resistant to the pulmonary effects of antigen challenge provides further support for a role of PGD2 in asthma.13 Pulmonary infiltration of eosinophils and lymphocytes, levels of TH2 lymphocyte-derived cytokines, and hyperresponsiveness were all dramatically lower in the DP receptor–deficient mice compared with control wild-type mice.13 The reduction in eosinophil recruitment is interesting in view of the key role played by these cells in the pathophysiology of asthma owing to their release of proinflammatory cytokines, bronchoconstrictor cysteinyl leukotrienes (LTs), degradative enzymes, and other factors.14 Furthermore, in dogs, PGD2 caused a rapid reduction in circulating eosinophil levels,15 whereas tracheal superfusion of PGD2in vivo induced the recruitment of eosinophils but not neutrophils into the superfusate.16 It is unclear from the above studies whether the in vivo effects of PGD2 are due to direct effects on eosinophils or are caused by the release of mediators from other cells. However, PGD2 has been reported to directly stimulate calcium mobilization and LTC4 release in vitro in human eosinophils.17
The stimulatory effects of PGD2 on eosinophils are somewhat surprising, as this prostaglandin is thought to act via DP receptor–mediated stimulation of adenylyl cyclase.1 A number of studies have clearly shown that agents that raise adenosine 3′,5′–cyclic monophosphate (cAMP) levels in eosinophils inhibit various responses, including LTC4 release, chemotaxis, and degranulation.18 19 This raises the possibility that PGD2 can activate eosinophils by a mechanism unrelated to the DP receptor. The objectives of the current study were to determine whether PGD2 has a direct chemotactic effect on eosinophils and, if so, to investigate the hypothesis that this effect is mediated by a novel receptor for this compound.
Materials and methods
Materials
Prostaglandins and BW245C were purchased from Cayman Chemical (Ann Arbor, MI). BWA868C was kindly provided by Glaxo Wellcome (Stevenage, United Kingdom), and 5-oxo-6,8,11,14-eicosatetraenoic acid (5-oxo-ETE) was synthesized chemically.20N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)phallacidin (NBD-phallacidin) was obtained from Molecular Probes (Eugene, OR). Fluorescein isothiocyanate (FITC)–labeled mouse anti–human CD11b (Bear1) and the corresponding FITC-labeled isotype immunoglobulin G1 (IgG1) control antibody were purchased from Beckman-Coulter (Fullerton, CA). FITC-labeled mouse anti–human L-selectin (Leu-8), the corresponding control IgG2a antibody, and phycoerythrin (PE)–labeled mouse anti–human very late antigen 4 (VLA-4) were purchased from Becton Dickinson (San Jose, CA).
Preparation of blood cells
Unfractionated leukocytes were prepared by removal of red blood cells from whole blood, obtained from healthy human subjects, by treatment with Dextran T-500 (Amersham-Pharmacia Biotech, Piscataway, NJ) for 45 minutes at 4°C.21 This preparation was used for measurements of CD11b and L-selectin. Granulocytes were prepared by centrifugation of red cell–depleted leukocytes over Ficoll-Paque (Amersham-Pharmacia Biotech), followed by removal of remaining red cells by hypotonic lysis as described previously.21 This fraction was used for experiments on neutrophil chemotaxis. Purified eosinophils were obtained by treating granulocytes with anti-CD16–labeled paramagnetic microbeads (Miltenyi Biotec, Bergisch-Gladbach, Germany) and removing the labeled neutrophils on a column containing a steel matrix placed in a permanent magnet (MACS, Miltenyi Biotec). Eosinophils (95% ± 4% pure) were obtained in the pass-through fraction22 and were used for experiments evaluating chemotaxis, actin polymerization, and cAMP formation.
Measurement of chemotactic responses
Cell migration was measured as previously described23 by means of 48-well microchemotaxis chambers (Neuro Probe, Cabin John, MD) and Sartorius cellulose nitrate filters (8-μm pore size; 140-μm thickness) (Neuro Probe). Agonists were added to the bottom well in a volume of 30 μL phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, and 8.1 mM Na2HPO4 at a pH of 7.4) containing 1 mM CaCl2, 1 mM MgCl2, and 0.3% bovine serum albumin, whereas eosinophils or neutrophils (150 000 cells in 55 μL RPMI containing 0.4% ovalbumin) were added to each of the top wells. Following incubation for 2 hours at 37°C, the filters were fixed with mercuric chloride and stained with hematoxylin and chromotrope 2R.24 The numbers of cells on the bottom surfaces of the filters were counted in 5 different fields at a magnification of 400 × for each incubation, each of which was performed in duplicate.
Measurement of actin polymerization
Eosinophil F-actin was measured by means of NBD-phallicidin, which binds strongly to F-actin, the polymerized form of actin, but not to unpolymerized G-actin.25 Eosinophils (3 × 105 cells in 260 μL) were incubated with agonists for 20 seconds, followed by fixation with formaldehyde (30 μL of a 37% solution) at room temperature for 15 minutes. F-actin was then stained by incubation with lysophosphatidylcholine (30 μg in 15 μL) and NBD-phallacidin (49 pmol in 6.2 μL; final concentration, 0.15 μM) overnight in the dark at 4°C. The cells were then centrifuged at 700g for 5 minutes and resuspended in PBS (0.5 mL). The fluorescence intensity of the stained eosinophils was quantified by flow cytometry with a Becton Dickinson Facscalibur instrument.
Measurement of surface expression of CD11b and L-selectin
CD11b and L-selectin were measured as described previously.26 Unfractionated leukocytes (0.5 mL; 106/mL) in PBS containing Ca++ and Mg++ were incubated with agonists for either 10 minutes (L-selectin) or 15 minutes (CD11b). The incubations were terminated by the addition of ice-cold Facsflow (Becton Dickinson) and centrifugation. Following incubation of the pellets for 10 minutes at 4°C with mouse plasma (5 μL), the cells were incubated for 30 minutes at 4°C with PE-labeled anti–VLA-4 (5 μL) along with an FITC-labeled antibody (10 μL) to either CD11b or L-selectin or the appropriate isotype-matched control FITC-labeled antibody. After incubation with Optilyse C (0.25 mL) (Beckman-Coulter) for 15 minutes, the cells were centrifuged and fixed in 1% formaldehyde in PBS (0.4 mL). The distribution of fluorescence intensities among 60 000 cells was measured by flow cytometry. Eosinophils were gated out on the basis of their granularity (high side scatter) and labeling with anti–VLA-4 (PE fluorescence). CD11b or L-selectin was then measured in the eosinophil region on the basis of fluorescence due to FITC as previously described.26
Measurement of cAMP levels
Purified eosinophils (5 × 105) in 100 μL PBS were preincubated with the phosphodiesterase-4 inhibitor rolipram (10 μM) (Sigma Chemical, St Louis, MO) for 15 minutes at 37°C and incubated with prostanoids for a further 15 minutes. In certain experiments, BWA868C was added 2 minutes prior to the addition of prostanoids. Platelets were purified as described previously27 and resuspended in PBS (without rolipram). Aliquots (3 × 107 platelets in 100 μL) were incubated with prostanoids for 2 minutes at 37°C. In both cases, the reaction was terminated with cold ethanol (300 μL), and the precipitated proteins were removed by centrifugation (600g for 15 minutes). We measured cAMP in the supernatants using a competitive protein-binding radiometric assay (Diagnostic Products, Los Angeles, CA) according to the manufacturer's instructions.
Results
PGD2 is a potent and selective eosinophil chemoattractant
We first investigated the effects of PGD2 on the migration of human eosinophils, which were purified from a polymorphonuclear cell fraction by removal of neutrophils with iron-labeled anti-CD16.22 Chemotactic effects were evaluated by means of a modified Boyden chamber assay in which cells that had migrated through a nitrocellulose filter were stained and counted. As a positive control, we used 5-oxo-ETE, which we have shown to be the most efficacious eosinophil chemoattractant among lipid mediators, inducing a maximal response about 3 times greater than that of platelet-activating factor (PAF)23 and about 50% higher than the chemokine eotaxin.26 The present experiments indicate that PGD2 is a potent eosinophil chemoattractant with a median effective concentration (EC50) of 5 nM (Figure 1A). Although the maximal response of PGD2 is about one third that of 5-oxo-ETE, it is very similar to that of PAF.23 Furthermore, unlike 5-oxo-ETE28 and PAF,29 PGD2 does not have an appreciable effect on neutrophil migration (Figure 1B), which puts it in a unique position among lipid mediators as a selective eosinophil chemoattractant. This selective effect on eosinophil activation is reminiscent of the potent eosinophil chemoattractant eotaxin.30
The selectivity of PGD2 as an eosinophil chemoattractant.
The chemotactic effects of PGD2 and other eicosanoids on human eosinophils (A) and human neutrophils (B) were examined by means of a modified Boyden chamber assay followed by staining with hematoxylin and chromotrope 2R and counting cells that had migrated through to the bottom of the filter under a microscope. The results are expressed as percentages (± SE) of the maximal responses to 5-oxo-ETE (493 ± 68 cells per 5 high-power fields). Although it is not apparent from the Figure, a concentration of 1 μM 5-oxo-ETE induces close to the maximal response, with relatively little additional effect observed at higher concentrations. The number of cells migrating through the filter in control experiments with vehicle alone was 26 ± 4 cells per 5 high-power fields. Experiments were performed in duplicate on cells from the numbers of different individuals indicated for PGD2 (●; n = 8), 5-oxo-ETE (○, 5o-ETE; n = 8), PGF2α (▴; n = 4), PGE2 (▿; n = 3), and the TXA2 agonist U46619 (■; n = 3).
The selectivity of PGD2 as an eosinophil chemoattractant.
The chemotactic effects of PGD2 and other eicosanoids on human eosinophils (A) and human neutrophils (B) were examined by means of a modified Boyden chamber assay followed by staining with hematoxylin and chromotrope 2R and counting cells that had migrated through to the bottom of the filter under a microscope. The results are expressed as percentages (± SE) of the maximal responses to 5-oxo-ETE (493 ± 68 cells per 5 high-power fields). Although it is not apparent from the Figure, a concentration of 1 μM 5-oxo-ETE induces close to the maximal response, with relatively little additional effect observed at higher concentrations. The number of cells migrating through the filter in control experiments with vehicle alone was 26 ± 4 cells per 5 high-power fields. Experiments were performed in duplicate on cells from the numbers of different individuals indicated for PGD2 (●; n = 8), 5-oxo-ETE (○, 5o-ETE; n = 8), PGF2α (▴; n = 4), PGE2 (▿; n = 3), and the TXA2 agonist U46619 (■; n = 3).
The chemotactic effects of PGD2 on eosinophils are not mediated by receptors for other prostanoids
The stimulatory effect of PGD2 on eosinophils raised the question of whether it is acting via the classical DP receptor, which signals through Gs-mediated stimulation of adenylyl cyclase, as this would be expected to inhibit, rather than induce, eosinophil activation.18,19 As stimulatory effects of PGD2 have often been attributed to other prostanoid receptors, including FP31,32 and TP33 34receptors, we tested the effects of PGF2α, PGE2, and U46619, a TP receptor agonist, on eosinophil migration. None of these compounds exhibited significant chemoattractant effects on these cells, with the possible exception of PGF2α at the highest concentration tested (Figure 1A). These results clearly indicate that PGD2 does not induce eosinophil migration by interacting with receptors for other prostanoids.
PGD2 selectively stimulates CD11b expression on eosinophils
We also investigated the effects of PGD2 on other processes involved in the infiltration of eosinophils into tissues, including expression of the cellular adhesion molecule CD11b, which is important for the adherence of eosinophils to endothelial cells.35 Measurement of CD11b permitted more extensive experiments to be conducted because this does not require purification of eosinophils, which are present in blood in relatively small numbers. With the use of flow cytometry, it is possible to make simultaneous measurements on both eosinophils and neutrophils with the use of relatively small numbers of unfractionated leukocytes, while at the same time minimizing the artifactual activation of cells that occurs during purification.26
Eosinophils were gated out from other cells on the basis of high side scatter owing to their granularity and labeling with PE-labeled anti–VLA-4 as shown by the dot plot in the inset to Figure2A, and were readily distinguishable from neutrophils (Figure 2B, inset). PGD2 (1 μM) increased the expression of CD11b on eosinophils to about the same extent as an identical concentration of 5-oxo-ETE (Figure 2A). In contrast, PGD2 had only a very small effect on neutrophil expression of CD11b, whereas 5-oxo-ETE strongly stimulated its expression on these cells (Figure 2B).
Selective stimulation of CD11b expression on eosinophils by PGD2.
CD11b expression was measured by flow cytometry on eosinophils (A) and neutrophils (B), which were distinguished from one another and from other leukocytes on the basis of side scatter (SSC), a measure of granularity, and staining with PE-labeled anti–VLA-4. Eosinophils and neutrophils were gated out as shown by the dot plots in the insets. Eosinophils are shown by the black dots in the inset to panel A, whereas neutrophils are shown by the black dots in panel B. CD11b expression by the gated cells was measured by means of FITC-labeled anti-CD11b. Histograms are shown for cells stained with an FITC-labeled isotype control antibody (black) and with FITC-labeled anti-CD11b for unstimulated cells (control; light gray shading), PGD2(heavy line), and 5-oxo-ETE (5o-ETE, dark gray shading).
Selective stimulation of CD11b expression on eosinophils by PGD2.
CD11b expression was measured by flow cytometry on eosinophils (A) and neutrophils (B), which were distinguished from one another and from other leukocytes on the basis of side scatter (SSC), a measure of granularity, and staining with PE-labeled anti–VLA-4. Eosinophils and neutrophils were gated out as shown by the dot plots in the insets. Eosinophils are shown by the black dots in the inset to panel A, whereas neutrophils are shown by the black dots in panel B. CD11b expression by the gated cells was measured by means of FITC-labeled anti-CD11b. Histograms are shown for cells stained with an FITC-labeled isotype control antibody (black) and with FITC-labeled anti-CD11b for unstimulated cells (control; light gray shading), PGD2(heavy line), and 5-oxo-ETE (5o-ETE, dark gray shading).
Concentration-response curves for the effects of PGD2 and other eicosanoids on CD11b expression by eosinophils are shown in Figure 3A. PGD2(EC50, 5 nM) has a potency similar to that of 5-oxo-ETE (EC50, 7.5 nM) and a maximal response about 25% lower. PGE2 and the TP receptor agonist U46619 did not affect CD11b expression appreciably, whereas PGF2αhad detectable effects only at concentrations of 100 nM or above and appeared to be about 2 orders of magnitude less potent than PGD2 (Figure 3A). Similarly, the IP receptor agonist carbaprostacyclin was also without effect (data not shown). PGD2 had very little effect on CD11b expression by neutrophils (Figure 3B), unlike 5-oxo-ETE, which was a strong inducer of this response in both neutrophils and eosinophils.
Effects of PGD2 and other eicosanoids on CD11b expression by eosinophils and neutrophils.
CD11b expression was measured in eosinophils (A) and neutrophils (B) as shown in Figure 2. The results are expressed as percentages (± SE) of the maximal responses to 5-oxo-ETE for eosinophils (98% ± 9% above control) and neutrophils (114% ± 21% above control) after subtraction of control values. Experiments were performed on cells from the numbers of different individuals indicated for PGD2(●; n = 21) 5-oxo-ETE (○, 5o-ETE; n = 10), PGF2α(▴; n = 3), PGE2 (▿; n = 3), and U46619(■; n = 3).
Effects of PGD2 and other eicosanoids on CD11b expression by eosinophils and neutrophils.
CD11b expression was measured in eosinophils (A) and neutrophils (B) as shown in Figure 2. The results are expressed as percentages (± SE) of the maximal responses to 5-oxo-ETE for eosinophils (98% ± 9% above control) and neutrophils (114% ± 21% above control) after subtraction of control values. Experiments were performed on cells from the numbers of different individuals indicated for PGD2(●; n = 21) 5-oxo-ETE (○, 5o-ETE; n = 10), PGF2α(▴; n = 3), PGE2 (▿; n = 3), and U46619(■; n = 3).
PGD2 induces shedding of L-selectin from eosinophils but not neutrophils
Activation of granulocytes by chemoattractants often results in the shedding of L-selectin owing to the action of a metalloproteinase.36 Intact L-selectin was measured on eosinophils and neutrophils by gating out these cells by means of flow cytometry as discussed above for CD11b. PGD2 induced L-selectin shedding from eosinophils, although to a lesser extent than 5-oxo-ETE (Figure 4A). PGE2did not appreciably affect L-selectin on eosinophils. In contrast, PGD2, like PGE2, had no effect on L-selectin shedding in neutrophils, whereas 5-oxo-ETE strongly stimulated this response (Figure 4B).
Selective stimulation of L-selectin shedding on eosinophils by PGD2.
L-selectin expression was measured in eosinophils (A) and neutrophils (B) by a method analogous to that illustrated in Figure 2, except that FITC-labeled anti– L-selectin was used instead of FITC-labeled anti-CD11b. The results are expressed as the percentages of L-selectin that were shed following incubation with PGD2 (●), 5-oxo-ETE (○, 5o-ETE), and PGE2 (▿). The values obtained for unstimulated control cells were assumed to represent no shedding of L-selectin, whereas the values obtained following labeling with an FITC-labeled isotype control antibody were taken to be equivalent to complete shedding of L-selectin from the surface of the cell. All values are means ± SE of determinations on leukocytes from 4 individuals.
Selective stimulation of L-selectin shedding on eosinophils by PGD2.
L-selectin expression was measured in eosinophils (A) and neutrophils (B) by a method analogous to that illustrated in Figure 2, except that FITC-labeled anti– L-selectin was used instead of FITC-labeled anti-CD11b. The results are expressed as the percentages of L-selectin that were shed following incubation with PGD2 (●), 5-oxo-ETE (○, 5o-ETE), and PGE2 (▿). The values obtained for unstimulated control cells were assumed to represent no shedding of L-selectin, whereas the values obtained following labeling with an FITC-labeled isotype control antibody were taken to be equivalent to complete shedding of L-selectin from the surface of the cell. All values are means ± SE of determinations on leukocytes from 4 individuals.
PGD2 stimulates actin polymerization in eosinophils
Migration of leukocytes into tissues involves dramatic changes in cell shape that are dependent on actin polymerization. PGD2strongly induced the formation of polymerized F-actin in purified eosinophils with an EC50 (6.5 nM) and maximal response very similar to those of 5-oxo-ETE (EC50, 10 nM) (Figure5). This is quite interesting in view of our recent findings that 5-oxo-ETE induces a significantly stronger actin polymerization response in eosinophils than a variety of other chemoattractants, including PAF37 and eotaxin.26 In contrast to PGD2, PGE2 had no effect on actin polymerization, whereas PGF2α was active only at the highest concentration tested (1 μM).
PGD2 stimulation of actin polymerization in eosinophils.
Purified eosinophils were incubated for 20 seconds at 37°C with PGD2 (●; n = 5), 5-oxo-ETE (○, 5o-ETE; n = 5), PGF2α (▴; n = 4), and PGE2 (▿; n = 3). The cells were then permeabilized and stained with fluorescently labeled phallacidin. F-actin levels were measured by flow cytometry. The results are expressed as percentage increases (± SE) in F-actin content above basal levels.
PGD2 stimulation of actin polymerization in eosinophils.
Purified eosinophils were incubated for 20 seconds at 37°C with PGD2 (●; n = 5), 5-oxo-ETE (○, 5o-ETE; n = 5), PGF2α (▴; n = 4), and PGE2 (▿; n = 3). The cells were then permeabilized and stained with fluorescently labeled phallacidin. F-actin levels were measured by flow cytometry. The results are expressed as percentage increases (± SE) in F-actin content above basal levels.
Eosinophils possess DP receptors
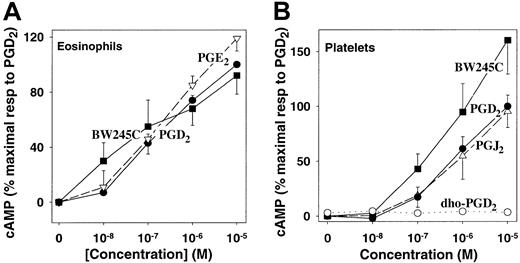
As it was not known whether eosinophils possess DP receptors, we investigated the effects of PGD2 and the highly selective DP receptor agonist BW245C on cAMP formation in these cells. BW245C has been reported to be slightly more potent than and to have over 300 times higher affinity for DP receptors compared with other prostanoid receptors.32 Both PGD2 and BW245C elevated cAMP levels in purified eosinophils, with the latter compound being slightly more potent (Figure 6A), clearly demonstrating the presence of classic Gs-coupled DP receptors on these cells. The effects of these compounds were comparable to that of PGE2, which stimulates adenylyl cyclase by interacting with EP2 and EP4receptors. The effects of PGD2 and BW245C on eosinophils were very similar to their effects on human platelets, which are known to possess DP receptors1 (Figure 6B). Consistent with previous reports,32 PGJ2 was equipotent with PGD2 in stimulating cAMP formation in platelets, whereas BW245C was more potent. In contrast, the PGD2 metabolite 13,14-dihydro-15-oxo-PGD2 had no effect on cAMP formation in platelets.
PGD2 stimulation of cAMP formation in eosinophils via DP receptors.
(A) Purified eosinophils were incubated with PGD2 (●), BW245C (▪), and PGE2 (▿) in the presence of rolipram (10 μM) for 15 minutes. Eosinophil cAMP levels were then measured by means of a protein binding assay. The values are means ± SE (n = 4) of determinations on from cells from 4 individuals, each performed in duplicate, and are expressed as percentages of the maximal response (2.25 ± 0.26 pmol cAMP per 106 cells) to PGD2. Basal levels of cAMP were 0.30 ± 0.15 pmol/106 cells. (B) Human platelets were incubated for 2 minutes at 37°C with PGD2 (●), BW245C (▪), PGJ2 (▵), and 13,14-dihydro-15-oxo-PGD2(○), followed by measurement of cAMP levels. The values are means ± SE of determinations on platelets from 4 individuals and are expressed as percentages of the maximal response to PGD2, which was 4.95 ± 1.06 pmol cAMP per 3 × 107 platelets, compared with a control value of 0.17 ± 0.05 pmol cAMP per 3 × 107 platelets.
PGD2 stimulation of cAMP formation in eosinophils via DP receptors.
(A) Purified eosinophils were incubated with PGD2 (●), BW245C (▪), and PGE2 (▿) in the presence of rolipram (10 μM) for 15 minutes. Eosinophil cAMP levels were then measured by means of a protein binding assay. The values are means ± SE (n = 4) of determinations on from cells from 4 individuals, each performed in duplicate, and are expressed as percentages of the maximal response (2.25 ± 0.26 pmol cAMP per 106 cells) to PGD2. Basal levels of cAMP were 0.30 ± 0.15 pmol/106 cells. (B) Human platelets were incubated for 2 minutes at 37°C with PGD2 (●), BW245C (▪), PGJ2 (▵), and 13,14-dihydro-15-oxo-PGD2(○), followed by measurement of cAMP levels. The values are means ± SE of determinations on platelets from 4 individuals and are expressed as percentages of the maximal response to PGD2, which was 4.95 ± 1.06 pmol cAMP per 3 × 107 platelets, compared with a control value of 0.17 ± 0.05 pmol cAMP per 3 × 107 platelets.
The effects of PGD2 on eosinophil migration and CD11b expression are not mediated by DP receptors
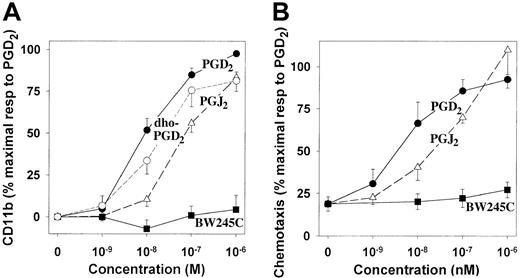
The relative potencies of the above compounds on CD11b expression in eosinophils were quite different from their potencies in stimulating platelet adenylyl cyclase. In contrast to its potent effects on platelet cAMP levels, BW245C has virtually no effect on CD11b expression (Figure 7A). PGJ2, which is equipotent to PGD2 in stimulating platelet adenylyl cyclase, is only about one tenth as potent in stimulating eosinophil CD11b expression. Furthermore, 13,14-dihydro-15-oxo-PGD2 was nearly as potent as PGD2 in stimulating CD11b expression, despite its lack of activity in platelets. The relative potencies of PGD2, PGJ2, and BW245C in stimulating eosinophil migration were nearly identical to those for simulation of CD11b expression (Figure7B). The striking differences in the structure-activity relationships for PGD2-induced stimulation of DP receptor–dependent cAMP formation and stimulation of CD11b and chemotactic responses provide compelling evidence that the latter effects are mediated by a novel stimulatory PGD2 receptor on eosinophils.
Structure-activity relationships for the effects of PGD2 and related compounds on CD11b expression and eosinophil migration.
(A) Human leukocytes were incubated with PGD2 (●; n = 12), BW245C (▪; n = 10), PGJ2 (▵; n = 6), and 13,14-dihydro-15-oxo-PGD2 (○; n = 7), followed by measurement of CD11b expression in eosinophils as illustrated in Figure2. The values are means ± SE and are expressed as percentages of the maximal responses to PGD2 after subtraction of the values obtained from unstimulated cells. (B) The chemotactic effects of PGD2 (●), BW245C (▪), and PGJ2 (▵) on purified human eosinophils were evaluated as described in the legend to Figure 1. The results are means ± SE of determinations on cells from 6 individuals and are expressed as percentages of the maximal responses to PGD2.
Structure-activity relationships for the effects of PGD2 and related compounds on CD11b expression and eosinophil migration.
(A) Human leukocytes were incubated with PGD2 (●; n = 12), BW245C (▪; n = 10), PGJ2 (▵; n = 6), and 13,14-dihydro-15-oxo-PGD2 (○; n = 7), followed by measurement of CD11b expression in eosinophils as illustrated in Figure2. The values are means ± SE and are expressed as percentages of the maximal responses to PGD2 after subtraction of the values obtained from unstimulated cells. (B) The chemotactic effects of PGD2 (●), BW245C (▪), and PGJ2 (▵) on purified human eosinophils were evaluated as described in the legend to Figure 1. The results are means ± SE of determinations on cells from 6 individuals and are expressed as percentages of the maximal responses to PGD2.
Interaction of PGD2 with classic DP receptors attenuates PGD2-stimulated eosinophil activation
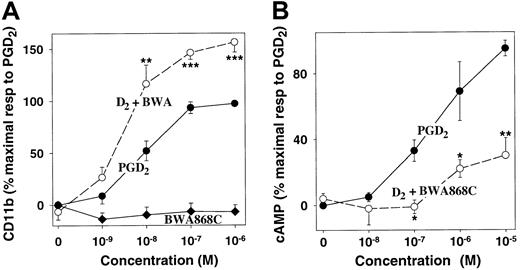
The existence of both stimulatory and inhibitory PGD2receptors on eosinophils raised the possibility that these receptors could interact to regulate eosinophil responses to PGD2, with classic DP receptors serving to limit PGD2-induced eosinophil activation. To test this hypothesis, we used the highly selective DP receptor antagonist BWA868C.32 39 Although BWA868C alone had no effect on CD11b expression at concentrations up to 1 μM, it strongly stimulated PGD2-induced CD11b expression, increasing the maximal response by about 60% (P < .001) (Figure 8A). The EC50 for PGD2 appeared to be reduced slightly, from 8 nM (PGD2 alone) to 4 nM, but this difference was not significant. In contrast, BWA868A (100 nM) strongly inhibited PGD2-induced cAMP formation in eosinophils (Figure 8B). The enhanced CD11b response to PGD2 in the presence of the DP receptor antagonist suggests that PGD2-induced stimulation of adenylyl cyclase can attenuate its effect on eosinophil activation, mediated by the novel PGD2 receptor. Furthermore, the failure of the DP receptor antagonist to inhibit PGD2-stimulated CD11b expression provides additional evidence for the existence of a second PGD2receptor.
Effects of the DP receptor antagonist BWA868C on PGD2-induced CD11b expression and cAMP formation in eosinophils.
(A) Leukocytes were incubated for 15 minutes with PGD2following preincubation for 2 minutes with either BWA868C (100 nM) (○) or vehicle (●). Alternatively, cells were incubated for 15 minutes with BWA868C alone (♦). CD11b expression on eosinophils was measured as shown in Figure 2. The values are means ± SE of determinations on eosinophils from 7 individuals and are expressed as percentages of the maximal responses to PGD2 alone, after subtraction of background values. (B) Purified eosinophils were preincubated with rolipram (15 minutes) and either BWA868C (100 nM) (○) or vehicle (●) for 2 minutes followed by incubation with PGD2 for a further 15 minutes. Then cAMP was measured by means of a protein-binding assay. The values are means ± SE of determinations on cells from 4 individuals and are expressed as percentages of the maximal responses to PGD2. Pvalues were determined by means of a paired t test on nonnormalized data. *, P < .05. **,P < .01. ***, P < .001.
Effects of the DP receptor antagonist BWA868C on PGD2-induced CD11b expression and cAMP formation in eosinophils.
(A) Leukocytes were incubated for 15 minutes with PGD2following preincubation for 2 minutes with either BWA868C (100 nM) (○) or vehicle (●). Alternatively, cells were incubated for 15 minutes with BWA868C alone (♦). CD11b expression on eosinophils was measured as shown in Figure 2. The values are means ± SE of determinations on eosinophils from 7 individuals and are expressed as percentages of the maximal responses to PGD2 alone, after subtraction of background values. (B) Purified eosinophils were preincubated with rolipram (15 minutes) and either BWA868C (100 nM) (○) or vehicle (●) for 2 minutes followed by incubation with PGD2 for a further 15 minutes. Then cAMP was measured by means of a protein-binding assay. The values are means ± SE of determinations on cells from 4 individuals and are expressed as percentages of the maximal responses to PGD2. Pvalues were determined by means of a paired t test on nonnormalized data. *, P < .05. **,P < .01. ***, P < .001.
Discussion
The present study clearly demonstrates the presence of a novel PGD2 receptor on human eosinophils that results in activation of these cells. This is supported by several lines of evidence: (1) PGD2 stimulates actin polymerization, CD11b expression, L-selectin shedding, and chemotaxis in eosinophils; (2) these effects are not mediated by receptors for other prostanoids, since they cannot be reproduced by agonists for these receptors, and (3) these effects are not mediated by the classic DP receptor, as they are neither induced by the potent DP receptor agonist BW245C nor inhibited by the potent DP receptor antagonist BWA868C. Furthermore, the PGD2 metabolite 13,14-dihydro-15-oxo-PGD2is only slightly less potent than PGD2 in stimulating CD11b expression on eosinophils but has no effect on DP receptor–mediated stimulation of cAMP formation in platelets. The specificity of the novel receptor for PGD2-related ligands (PGD2equals or exceeds 13,14-dihydro-15-oxo-PGD2, which equals or exceeds PGJ2, which is much greater than BW245C) is quite different from that of the classic DP receptor (BW245C equals or exceeds PGD2 = PGJ2, which is much greater than 13,14-dihydro-15-oxo-PGD2). It does not interact appreciably with ligands for EP (PGE2), IP (carbaprostacyclin), or TP (U46619) receptors. It would seem likely that PGF2α has weak activity at the novel PGD2 receptor, as it can activate eosinophils with about 1% of the potency of PGD2. It would seem unlikely that this is due to activation of FP receptors because of the low potency of PGF2α in activating eosinophils. Because of the specificity of this novel receptor for PGD2, we propose the designation DP2 receptor. The classic Gs-coupled DP receptor would then be designated as the DP1 receptor.
In addition to DP2 receptors, we have shown for the first time that eosinophils also possess classic DP1 receptors coupled to adenylyl cyclase. These receptors display the same specificity for PGD2-like compounds as the platelet DP1 receptor, as shown in the present study and various other studies.32,38 However, the magnitude of the cAMP response appeared to be less than that observed for platelets, possibly owing to limitations of cell numbers and rapid metabolism of cAMP by phosphodiesterase-4, which is known to be present in eosinophils.40 It was necessary to inhibit this enzyme with rolipram to observe the effect of PGD2 on cAMP levels in eosinophils, but this was not necessary with platelets. The presence of both DP1 and DP2 receptors on eosinophils could explain previous reports that PGD2 has both stimulatory and inhibitory effects on these cells.41
The ability of PGD2 to activate eosinophils while at the same time stimulating adenylyl cyclase is intriguing, as increased cAMP levels would be expected to attenuate eosinophil responses. The phosphodiesterase-4 inhibitor rolipram has been shown to inhibit eosinophil migration and CD11b expression in response to eotaxin,42 PAF,18,43,44 and C5a. Stimulation of adenylyl cyclase with forskolin44 or isoproterenol43 or addition of dibutyryl cAMP43had similar effects. This raised the possibility that PGD2could have opposing actions on eosinophils and that its stimulatory effect could be blunted by concomitant stimulation of adenylyl cyclase, especially at higher concentrations of PGD2. This hypothesis was confirmed by the finding that blocking DP1receptor–mediated cAMP formation in eosinophils with BWA868C resulted in a markedly enhanced DP2 receptor–mediated CD11b response to PGD2. Thus, it would appear that DP1 receptors could serve to negatively regulate DP2 receptor–mediated responses in eosinophils (Figure9). The balance between inhibitory DP1 and stimulatory DP2 receptors is likely to determine the final response of these cells to PGD2. A reduction in expression of DP1 receptors, for example, could enhance PGD2-mediated stimulation of eosinophils, which could be important in diseases associated with eosinophil infiltration, such as asthma.
Interaction of DP1 and DP2receptors in regulating eosinophil responses.
Stimulation of DP2 receptors by PGD2 leads to activation of eosinophils (CD11b expression), which is attenuated by simultaneous activation of DP1 receptors. Blockade of DP1 receptors with BWA868C removes this inhibitory effect and enhances PGD2-induced eosinophil activation.
Interaction of DP1 and DP2receptors in regulating eosinophil responses.
Stimulation of DP2 receptors by PGD2 leads to activation of eosinophils (CD11b expression), which is attenuated by simultaneous activation of DP1 receptors. Blockade of DP1 receptors with BWA868C removes this inhibitory effect and enhances PGD2-induced eosinophil activation.
In contrast to its stimulatory effects on eosinophils, PGD2appears to have an inhibitory effect on neutrophils. In the present study, we found that PGD2 induces little or no migration, CD11b expression, or L-selectin shedding in these cells. On the other hand, PGD2 has been reported to stimulate cAMP formation and to inhibit PAF responses in neutrophils.45 This suggests that neutrophils possess DP1 receptors, but lack appreciable numbers of DP2 receptors. It is possible that the opposite actions of PGD2 on neutrophils and eosinophils could further contribute to the selective accumulation of eosinophils in some conditions, such as asthma.
Although the effects of stimulation of DP1 receptors on neutrophils and eosinophils would be considered to be anti-inflammatory, it is clear that the overall role of DP1receptors in inflammation is much more complex, as it also appears to be involved in promoting inflammation. Deletion of this receptor in mice markedly reduced the pulmonary eosinophilia and hyperresponsiveness that occurred following antigen challenge of control animals. This was accompanied by reduced levels of the TH2 cytokines interleukin (IL)–4, IL-5, and IL-13 but no change in the TH1 cytokine interferon-γ or in IgE levels.13 These results suggest that in vivo the DP1 receptor contributes to eosinophil infiltration following antigen challenge. Because the DP1 receptor appears to attenuate eosinophil responses in vitro, it would appear likely that the stimulatory effect of this receptor in vivo is indirect, possibly mediated by increased production of the TH2 cytokines IL-4 and IL-5, which are known to be involved in eosinophil recruitment.30 Alternatively, the possibility that the lack of the DP1 receptor in these mice favored the development of a compensatory mechanism that could attenuate eosinophil responses cannot be ruled out.
In contrast to the DP1 receptor, the DP2receptor would appear to have a strictly proinflammatory effect, at least on the basis of its function in eosinophils. The proinflammatory and anti-inflammatory effects of PGD2 underline the complex roles of prostanoids in inflammation and the growing debate on their proinflammatory and anti-inflammatory effects.46 47
PGD2 is the second prostanoid to have multiple receptors that are clearly distinct from one another. The actions of PGE2 are mediated by 4 different receptors, 2 of which (EP2 and EP4) act by stimulation of adenylyl cyclase, whereas the others act by inhibition of adenylyl cyclase (EP3) and by increasing cytosolic calcium levels (EP1).1 The presence of these multiple receptors has explained the often-opposing actions of PGE2on various cells and tissues. Similarly, the existence of a second PGD2 receptor may clarify some of the discrepant reports in the literature documenting various effects of PGD2 that were not readily explained by its interaction with DP1receptors accompanied by activation of adenylyl cyclase.48Some of these effects may be due to interaction with receptors for other prostanoids, as PGD2 has a relatively high affinity for FP receptors32,49 and PGD2-mediated bronchoconstriction has been reported to be due to stimulation of TP receptors.33,34 In contrast, some other effects of PGD2, such as those on epithelial cell ion transport,50 are difficult to explain on the basis of classic prostanoid receptors and may be due to stimulation of DP2 receptors.
After completion of the present study, Hirai et al51reported that PGD2 is specifically bound by CRTH2 (chemoattractant receptor–homologous molecule expressed on TH2 cells), an orphan receptor previously identified as a marker for TH2 cells,52 which was also shown to be present on basophils and eosinophils.53PGD2 was found to induce migration of eosinophils, basophils, and TH2 cells that was blocked by an antibody to CRTH2.51 The binding affinities of various ligands for K562 cells transfected with CRTH251 are similar to their abilities to stimulate DP2 receptor–mediated responses in eosinophils, as found in the present study, suggesting that these 2 receptors are identical. Owing to the lack of sufficient numbers of human eosinophils, we were unable to conduct binding studies, but we have shown that, in addition to chemotaxis, PGD2 can induce a variety of other responses in these cells, including actin polymerization, CD11b expression, and L-selectin shedding. Moreover, the present study demonstrates an interaction between inhibitory DP1 receptors and stimulatory DP2 receptors, resulting in attenuated DP2-mediated responses to PGD2.
In conclusion, we have shown that eosinophils possess both a novel DP2 receptor, which is responsible for the chemoattractant effect of PGD2, and the classic DP1 receptor, which appears to play a regulatory role in these cells. The balance between these receptors is likely to regulate the response of these cells to PGD2 and may be important in asthma. The role of DP1 receptors is obviously complex, as they appear to contribute to eosinophil infiltration indirectly through effects on TH2 cell cytokine production.13 The direct and indirect effects of PGD2 on eosinophil migration as well as its strategic location in mast cells and antigen presenting cells suggest an important role for this prostaglandin in asthma.
Supported by Medical Research Council of Canada grant MT-6254 (W.S.P.), the J.T. Costello Memorial Research Fund, the National Institutes of Health grant DK44730 (J.R.), and the National Science Foundation grant CHE-90-13145 (J.R.) for an AMX-360 NMR instrument.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
William S. Powell, Meakins-Christie Laboratories, Department of Medicine, McGill University, 3626 St Urbain St, Montreal, QC, Canada H2X 2P2; e-mail: bill@meakins.lan.mcgill.ca.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal