Abstract
Recent studies investigating thrombotic thrombocytopenic purpura (TTP) have implicated abnormal plasma von Willebrand factor (vWF)-cleaving metalloprotease activity in this disorder. It has been proposed that a metalloprotease cleaves unusually large (UL) multimers of vWF, which enter the circulation from the endothelium. Abnormal metalloprotease activity could result in ULvWF, which could participate in TTP. However, the diagnostic specificity of abnormalities in the plasma metalloprotease activity has not been established. A prospective study of vWF protease activity was performed using samples from 20 healthy controls, 20 patients with acute TTP, 20 patients with immune idiopathic thrombocytopenic purpura (ITP), 10 patients with disseminated intravascular thrombocytopenia (DIC), 10 patients with systemic lupus erythematosus (SLE,) and 5 thrombocytopenic patients with leukemia. Studies were performed blinded to the diagnosis. Samples from hospitalized patients with normal platelet counts were also tested. The vWF digests and multimer analysis were done using previously described methods. Six laboratory personnel independently scored each of the multimer gels. Reduced protease activity was observed in 9 of 20 patients with TTP. Reduced activity was also observed in 6 of 20 patients with ITP, 6 of 10 patients with DIC, 5 of 10 patients with SLE, 1 of 5 patients with leukemia, 2 of 20 healthy controls, and 3 of 25 hospitalized patients. This study indicates that abnormalities of vWF protease activity are not restricted to patients with the diagnosis of TTP.
Introduction
The factor known as von Willebrand factor (vWF) is a multimeric protein important for platelet adhesion. Physiologically, vWF is released from endothelial cells as unusually large (UL) multimers.1-4 Plasma vWF multimers are smaller, a finding that suggests that released ULvWF is cleaved by a combination of reductase and metalloprotease enzymes after it enters the plasma.3-6
Thrombotic thrombocytopenic purpura (TTP) is a rare disorder characterized by thrombocytopenia and schistocytic hemolytic anemia. TTP is often associated with fever and renal and neurologic dysfunction. Pathologically, individuals with TTP have evidence of intravascular, platelet-rich microthrombi that contain vWF. This has led many to postulate that abnormally reactive vWF contributes to the development of platelet-vWF–rich thrombi and tissue ischemia present in TTP.7-20
Many patients with TTP have abnormalities of plasma vWF during their acute illness.7-20 Recently, normal plasma was found to contain a metalloprotease activity that converts ULvWF multimers to the smaller forms in vitro. Several investigators subsequently reported that many patients with TTP have abnormally reduced vWF protease activity in their plasma.7,8,10,14,16,17,19,20 They implicated antibodies that inhibit the vWF metalloprotease in patients as the cause of sporadic but not familial TTP. These data have led to the hypothesis that patients with acute, sporadic TTP have antibody-mediated inhibition of the plasma metalloprotease, whereas patients with familial TTP may have a congenital defect of this enzyme.7,8,10,14,16 17
Although there are conflicting reports about the frequency of abnormal ULvWF multimer profiles in acute TTP and during remission, there has been general agreement that abnormalities of vWF are often observed in TTP.6-20 However, the sensitivity and specificity of the vWF metalloprotease assay has not been reported. This led us to prospectively examine the levels of plasma vWF-cleaving protease activity in patients with various thrombocytopenic disorders. Our results indicate that abnormalities in vWF protease activity occur in a significant proportion of patients with TTP. However, we observed identical decreases in vWF protease activity in association with other thrombocytopenic disorders and occasionally in nonthrombocytopenic controls.
Materials and methods
Materials
All reagents were obtained from Sigma Chemical (St Louis, MO) unless otherwise stated. Platelet counts were measured according to conventional laboratory methods. The vWF antigen levels were measured using an enzyme-linked immunoassay (Diagnostica Stago, Asniéres Cedex, France).
Methods
Study design.
A total of 95 samples from various patient groups were collected and coded to blind personnel to the patients' identities and diagnoses. Assays for vWF protease activity and antigen levels were performed. To rank the vWF protease activity, each multimer gel was analyzed independently by 6 laboratory personnel. Any disagreement was resolved by consensus prior to unblinding.
Patient and control samples.
This study was allowed by the university and hospital Ethics Review Committee, and all samples were drawn in compliance with ethics guidelines.
Test samples included sera from 20 healthy controls, 20 patients with acute TTP, 20 patients with idiopathic thrombocytopenic purpura (ITP), 10 patients with illnesses complicated by disseminated intravascular coagulation (DIC), 10 patients with systemic lupus erythematosus (SLE), and 5 thrombocytopenic patients with leukemia. This study included only adult subjects. Within the TTP group were 2 unrelated patients with familial TTP and 18 patients with acute, sporadic disease. The samples taken from the acute TTP patients were drawn before the patients received any treatment. Three TTP patients were tested both before and after plasmapheresis. All patients with TTP had thrombocytopenia (platelet counts < 100 × 109/L) and schistocytic hemolytic anemia (as indicated by red cell fragmentation on peripheral blood smear) with a negative direct Coombs test and no other identifiable cause for these abnormalities. Most had fluctuating neurologic signs that ranged from slight confusion to quadriparesis. Seven of the TTP patients had single, acute episodes of TTP followed by complete remission; 4 patients had chronic, relapsing TTP; 2 patients were unrelated females with familial TTP; 2 patients had pregnancy-related TTP and achieved remissions; and 5 patients had single, acute, fatal episodes of TTP. None had serum creatinine concentrations higher than 350 μM/L.
The diagnosis of ITP was based on the presence of thrombocytopenia, the absence of splenomegaly, no evidence of red cell fragmentation or hemolysis, and the absence of any secondary causes of thrombocytopenia. The patients diagnosed with DIC were thrombocytopenic and had an acquired coagulopathy with accelerated fibrinolysis. These patients had underlying conditions associated with DIC that included sepsis and malignancies. Patients were diagnosed with SLE based on the criteria of the American College of Rheumatology, were thrombocytopenic, and had documented positive tests for lupus anticoagulants in phospholipid-dependent coagulation tests. The patients with leukemia had the diagnosis confirmed by bone marrow aspirates, and all were thrombocytopenic at the time of testing. Their thrombocytopenia was related to chemotherapy-induced marrow suppression. All of the patients in this study were adults. Additionally, samples from 25 hospitalized patients with normal platelet counts and no evidence of schistocytic hemolytic anemia were tested in a nonblinded fashion. These patients were hospitalized on surgical and medical wards for procedures such as orthopedic surgery and colonoscopy and medical problems that included Crohn disease, myocardial infarction, and psychiatric disorders.
Serum samples were collected from patients and controls and processed within 2 hours of collection. The samples were aliquotted and frozen at −70°C until testing. Twelve patients had plasma samples (3.8% sodium citrate) drawn at the same time, and for these patients the plasma and sera were tested in parallel for vWF protease activity. To evaluate intraassay variability, 7 samples were submitted in duplicate with unique identifiers for each aliquot. Following unblinding, native vWF multimer analysis was done on samples with decreased vWF protease activity, including samples from 4 TTP patients, 2 ITP patients, 1 DIC patient, 2 SLE patients, and 5 control samples.
Purification of target vWF.
The vWF was purified according to previously described methods.5 Human cryoprecipitate, a gift of the Canadian Blood Services, Hamilton, ON, was gel-filtered using a Sepharose CL-2B column (Pharmacia, Uppsala, Sweden), equilibrated, and eluted using 0.13 M NaCl/0.01 M citrate/0.01 M Tris-HCl, pH 7.4. The vWF content of eluted fractions was analyzed by reduced 5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) as well as SDS-multimer gels containing 1.25% agarose (Seakem LGT, FMC Bioproducts, Rockland, ME) and 1% acrylamide (Bio-Rad Laboratories, Richmond, CA).1 The proteins were transblotted onto nitrocellulose (Pharmacia) before visualizing the vWF with peroxidase-conjugated rabbit antihuman vWF (Dako, Denmark) and chemiluminescent techniques (ECL, Amersham Pharmacia Biotech, England). The fractions containing the largest multimers of vWF were analyzed using SDS-PAGE and stained with Coomassie blue to confirm the absence of other contaminating proteins. These fractions were then tested in the vWF protease assay to confirm that there was no contaminating vWF protease activity. The purified fractions were pooled, adjusted to a concentration of 3 U/mL, aliquotted, and frozen at −70°C. Each aliquot was thawed only once.
Protease assay for vWF.
The vWF protease assay was performed using previously described methods.5,6,14 Test serum was diluted 1:3 in 0.15 M NaCL/10 mM Tris buffer, pH 7.4, and 100 μL was mixed with BaCl2 (10 mM) and serine protease inhibitors (1 mM Pefabloc, Boehringer Mannheim). Following a 5-minute incubation at 37°C, 50 μL purified protease-free vWF substrate (3 U/mL vWF antigen) was added.14 The substrate/test sample mixture was centered on a hydrophilic membrane (2 mm, VSWP, Millipore, Bedford, MA) and then dialyzed against 1.5 M urea/5 mM Tris hydrochloride, pH 8.0. Following a 24-hour incubation at 37°C, the digests were terminated by the addition of 10 mM ethylenediaminetetraacetic acid (EDTA). In addition, 3 samples from healthy controls and 3 TTP patients were tested using different dialysis buffers (1.0, 1.5, and 2.0 M urea/5 mM Tris, pH 8.0) to determine whether this altered the amount of protease activity detected. When varying concentrations of urea were tested, 1.0 and 1.5 M gave reproducible and comparable results, whereas 2.0 M urea decreased the proteolytic activity detected for all samples. Consequently, all results reported in this study were based on determinations using 1.5 M urea and 5 mM Tris, pH 8.0, as described by Furlan et al.5
The vWF multimers in the digests were analyzed using SDS-multimer gels containing 1.25% agarose and 1% acrylamide.1 Following electrophoresis (18 hours at 30 V), the proteins were transblotted onto nitrocellulose membranes and incubated with peroxidase-conjugated rabbit antihuman vWF antibodies. The vWF multimer patterns were developed using chemiluminescent techniques as described. Internal standards, on each gel, included target vWF, vWF digested by normal pooled serum, and vWF incubated with normal pooled serum containing 10 mM EDTA to block the metalloprotease activity.
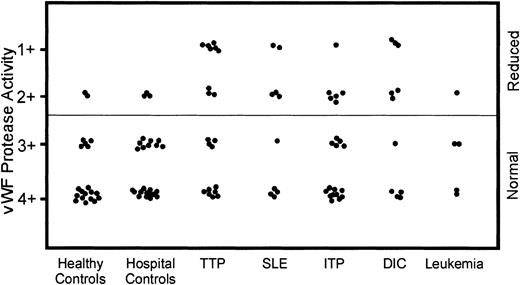
To ensure consistency in ranking, the degree of proteolysis of each sample was related to the migration of the target vWF run on the same gel. The total length of the target vWF multimer migration was divided into quadrants, and each quadrant was assigned a score from 4+ (only small vWF multimers remained) to 1+ (the complete array of vWF multimers remained intact). The score of vWF proteolysis of each sample was determined by matching its height of the vWF multimer migration to the corresponding quandrant established using the target vWF lane. Because the assay measured the ability of the test sample to proteolyze the target vWF, normal activity corresponded to the proteolysis of the large vWF multimers and scores of 3+ or 4+. Samples deficient in protease activity left large multimers intact and were ranked as 1+ or 2+ (Figure 1).
VWF multimer analysis of the metalloprotease proteolysis of target vWF induced by samples from patients with various thrombocytopenic disorders and control samples.
These 2 multimer gels represent the analysis of the vWF protease activity in some of the samples tested. After promoting digestion, the amount of vWF protease activity was determined by comparing the height of each individual multimer column to the target vWF (on the left of each gel), which represents 0 proteolysis. Following the metalloprotease activation assay, a decrease in the large-molecular-weight forms of the target vWF indicated normal vWF protease activity, and these samples were given scores of 3+ or 4+. The normal pooled serum (NP) demonstrated normal proteolysis. The addition of EDTA to the normal pool (NP + EDTA) prior to enzyme activation inhibited the protease activity and showed reduced protease activity with scores of 1+ or 2+. Target vWF, vWF plus normal pool, and vWF plus normal pool with added EDTA were run on each gel as standards. Some TTP samples (TTP 1, 2, 3, 5) had reduced vWF protease activity (1+ to 2+). Other samples from TTP patients (TTP 4 and 6) had normal vWF protease activity (3+ to 4+) and were indistinguishable from control or normal samples. Samples from patients with other thrombocytopenic disorders (ITP 1, 2, 3 and SLE 1) also had reduced vWF protease activity.
VWF multimer analysis of the metalloprotease proteolysis of target vWF induced by samples from patients with various thrombocytopenic disorders and control samples.
These 2 multimer gels represent the analysis of the vWF protease activity in some of the samples tested. After promoting digestion, the amount of vWF protease activity was determined by comparing the height of each individual multimer column to the target vWF (on the left of each gel), which represents 0 proteolysis. Following the metalloprotease activation assay, a decrease in the large-molecular-weight forms of the target vWF indicated normal vWF protease activity, and these samples were given scores of 3+ or 4+. The normal pooled serum (NP) demonstrated normal proteolysis. The addition of EDTA to the normal pool (NP + EDTA) prior to enzyme activation inhibited the protease activity and showed reduced protease activity with scores of 1+ or 2+. Target vWF, vWF plus normal pool, and vWF plus normal pool with added EDTA were run on each gel as standards. Some TTP samples (TTP 1, 2, 3, 5) had reduced vWF protease activity (1+ to 2+). Other samples from TTP patients (TTP 4 and 6) had normal vWF protease activity (3+ to 4+) and were indistinguishable from control or normal samples. Samples from patients with other thrombocytopenic disorders (ITP 1, 2, 3 and SLE 1) also had reduced vWF protease activity.
Statistical analysis.
Statistical analysis was done using Minitab (Minitab Release 13.1, Minitab, State College, PA) computer program.
Results
Validation of techniques
There was good consensus between each of the individual evaluators and complete agreement in distinguishing normal versus decreased vWF proteolysis. The agreement between scorers was strong (r > 0.9, Pearson correlation coefficient test). The 7 duplicate samples tested had reproducible levels of vWF proteolysis. As noted by other investigators, the 12 paired plasma and serum samples produced identical patterns of vWF proteolytic activity.5
Test results
The normal pool run on each gel consistently scored as having 4+ protease activity. When EDTA was added prior to incubation, the protease activity was reduced, resulting in scores of 1+. Nine (45%) of the 20 TTP samples had reduced protease activity scores in all assays. Seven of the TTP patients with reduced protease reactivity had acute TTP, whereas the other 2 were from unrelated patients with familial TTP. The 2 familial TTP patients had the most reduced activity of all the patient and control samples tested. Both of these patients have had lifelong TTP, initially responding to plasma infusion but now requiring regular plasmapheresis. Of the other 7 TTP patients with reduced protease activity, 2 had relapsing TTP and ultimately recovered; 3 achieved complete remissions following an acute, single TTP episode; and 2 died during their acute TTP. However, 11 other patients with acute TTP had normal vWF proteolysis and were indistinguishable from healthy controls and the normal pool (Figure 1). This group included 2 patients with pregnancy-related TTP who achieved complete remission; 2 patients with relapsing TTP; 4 TTP patients who achieved complete remissions following an acute, single TTP episode; and 3 TTP patients who died during their acute TTP. Of the 3 TTP patients tested before and after plasmapheresis, 2 patients had normal protease activity at both time points and the other patient had decreased proteolysis (2+) on admission and normal protease activity postplasmapheresis (3+).
Many patients with thrombocytopenic disorders other than TTP were noted to have reduced plasma vWF protease activity and were scored identically to the TTP patient samples. The proportion of patients with reduced vWF protease activity in each of the diagnostic groups was 6 of 20 ITP, 6 of 10 DIC, 5 of 10 SLE, and 1 of 5 leukemic patients (Figure2). Several of the control samples also had decreased vWF protease activity (3 of 25 hospital controls and 2 of 20 healthy controls) (Figure 2).
Protease activity of vWF in samples from patients with various thrombocytopenic disorders and control samples.
The vWF protease activity in samples from patients with various thrombocytopenic disorders, healthy controls, and hospitalized patients without thrombocytopenia was measured using previously described methods. Most of the samples from healthy controls and hospital controls with normal platelet counts had normal vWF protease activity (3+ to 4+); however, some samples had decreased vWF protease activity (1+ to 2+). Many of the TTP samples had decreased vWF protease activity, but there were also TTP samples with normal vWF protease activity. The other thrombocytopenic groups also had samples with decreased vWF protease activity.
Protease activity of vWF in samples from patients with various thrombocytopenic disorders and control samples.
The vWF protease activity in samples from patients with various thrombocytopenic disorders, healthy controls, and hospitalized patients without thrombocytopenia was measured using previously described methods. Most of the samples from healthy controls and hospital controls with normal platelet counts had normal vWF protease activity (3+ to 4+); however, some samples had decreased vWF protease activity (1+ to 2+). Many of the TTP samples had decreased vWF protease activity, but there were also TTP samples with normal vWF protease activity. The other thrombocytopenic groups also had samples with decreased vWF protease activity.
The in vitro measurement of decreased protease activity did not correlate with an in vivo abnormal pattern of native plasma vWF. The native vWF multimers from the 2 healthy controls with decreased protease activity, 3 hospital controls, as well as samples from patients with thrombocytopenic disorders (4 TTP, 2 ITP, 1 DIC, 2 SLE) were analyzed, and there was no evidence of increased ULvWF multimers associated with their decreased plasma vWF cleaving protease activity. Three of the 4 acute TTP patient samples had reduced plasma high-molecular-weight vWF multimers, which have been previously described in episodes of acute TTP.9,12 18
Relationship of vWF protease activity and platelet count and vWF antigen levels
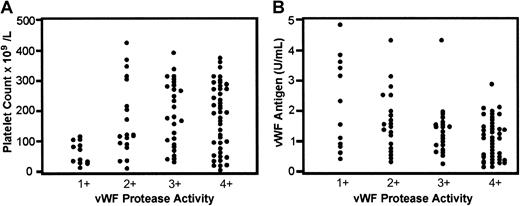
When vWF protease activity scores were compared with subject platelet counts, the most marked decreases in vWF proteolytic activity (1+) were noted in individuals with severe thrombocytopenia (P < .001, nonpaired Student t test). Also, the most marked decreases in vWF protease activity were seen in individuals with higher vWF antigen levels (P < .001, nonpaired Student t test) (Figure3).
The relationship between vWF protease activity to the platelet counts and vWF antigen levels.
The greatest reduction of vWF protease activity (1+) was evident in samples from patients with a variety of thrombocytopenic disorders, including 6 patients with TTP, 2 patients with SLE, 1 patient with ITP, and 3 patients with DIC. This group of patients also had lower platelet counts (P < .001) (A) and higher vWF antigen levels (P < .001) (B). Statistical analysis was done using a nonpaired Student t test (Minitab).
The relationship between vWF protease activity to the platelet counts and vWF antigen levels.
The greatest reduction of vWF protease activity (1+) was evident in samples from patients with a variety of thrombocytopenic disorders, including 6 patients with TTP, 2 patients with SLE, 1 patient with ITP, and 3 patients with DIC. This group of patients also had lower platelet counts (P < .001) (A) and higher vWF antigen levels (P < .001) (B). Statistical analysis was done using a nonpaired Student t test (Minitab).
Discussion
TTP is an uncommon but important clinical disorder. Without treatment the illness is usually fatal; however, plasma exchange with or without corticosteroids results in the survival of approximately 75% of patients with acute TTP. The illness is also important because it is mediated by the formation of intravascular platelet thrombi and a better understanding of the pathogenesis of this disorder could contribute to the understanding of other platelet-mediated thrombotic conditions. Finally, further defining TTP could lead to more effective and targeted therapies because the mainstay of therapy, plasma exchange, is an empiric treatment.12 21
A number of hypotheses have been put forward to explain the platelet thrombi that characterize TTP. These include an abnormal apoptotic factor,22,23 a variety of platelet aggregating factors,24-27 and abnormalities of vWF.7-20Our group has postulated that a platelet aggregating factor such as calpain, associated with platelet-derived microparticles, could participate in the disorder.11,25,28 Recently, other investigators have focused on factors that influence the cleaving of plasma vWF.7-10,14,16,17,19,20 Almost 20 years ago, Moake and associates noted abnormalities in vWF in patients with acute or convalescent familial TTP.13 Similar observations have been made by some but not all investigators.9,12,18,20 It is now agreed that many patients with TTP will have abnormalities of vWF, although the sensitivity and specificity of this observation is variable. Most recently, several groups of investigators have reported abnormally reduced cleavage of vWF by a plasma protease in patients with TTP.7-10,13,14,16,17,19 20 This is an attractive hypotheses because the abnormality could explain the abnormal vWF seen in the plasma of many patients with TTP.
In this report, we describe a study designed to evaluate the sensitivity and specificity of the finding of abnormal vWF protease activity in patients with TTP. The test samples were collected from a number of patients and controls in whom the cause of the thrombocytopenia was well defined. But these test samples were then coded and all further investigations performed in a blinded fashion. Our study was designed to minimize problems often associated with the performance of complex laboratory assays in patients with uncommon disorders. Each step was controlled and validated. As a first step, we prepared a consistent protease-free target sample of vWF that was used for all assay determinations. Second, we tested varying dilutions of urea to ensure optimal protease activity. We compared duplicate plasma and serum samples and, in agreement with others, found that identical results were obtained. We included a normal pool with and without EDTA on each gel to ensure consistency in ranking vWF protease activity. The samples were coded, and the degree of proteolysis was scored independently by 6 different laboratory personnel familiar with vWF gel analysis.
The technique we used to measure protease activity gave consistent and reproducible results, and there was excellent agreement among all observers. Additionally, the 7 blinded duplicate samples gave identical results. Consistent with the results of other investigators, we found that a number of our patients with acute TTP had detectable abnormalities of protease activity. However, because only 9 of the 20 TTP patient samples had reduced protease activity, the percentage that we found with abnormalities (45%) was lower than others have reported. Consistently, we observed that 2 unrelated patients with familial TTP had the most markedly reduced level of vWF protease activity. However, for the patients with acute TTP there was no relationship between protease activity and disease severity. Indeed, both groups of TTP patients—those with reduced and those with normal vWF protease activity—included patients with recurring TTP, patients who died, and patients who had complete recoveries. We also found unexpected results: An approximately similar proportion of patients with other thrombocytopenic disorders, including DIC, ITP, SLE, and leukemia, also had reduced protease activity similar to that of the TTP patients. As shown in Figures 1 and 2, the abnormalities in vWF proteolysis were indistinguishable for the TTP patients compared with patients with other thrombocytopenic disorders. Abnormalities of vWF protease activity were also noted in several well individuals who served as healthy laboratory controls, including one seemingly healthy investigator (J.G.K.) as well as several nonthrombocytopenic hospital controls. None of these controls had evidence of any hematologic disorder. Both healthy controls have unremarkable clinical histories. The 3 hospital controls with reduced vWF protease activity had diverse conditions that included amyotrophic lateral sclerosis, heart failure, and irritable bowel syndrome.
Taken together, our result suggests that abnormalities of vWF protease are seen in patients with TTP, but the observation has only moderate sensitivity (0.45) and a low specificity (0.30). Our studies indicate that within each of the control groups there is a spectrum of vWF protease activity. Given the study design (blinded and coded, with independent observers using a standard scoring system before breaking the code), it is unlikely that observer bias could explain our results. Similarly, differences in the laboratory technique are an unlikely explanation because we followed the protocols (including defined urea concentrations) described by other investigators.5,14Although other assays are available to measure abnormal vWF, the initial proteolysis of target vWF remains the same.7,19,20Because the protease has not been isolated, measurements of abnormalities of the protease activity depend on indirect measurements. Collagen binding assays and assays that use monoclonal anti-vWF antibodies are less time-consuming and are able to detect abnormal vWF structures.19 20 The benefit of using vWF multimer analysis as an end point to the metalloprotease activity is that the technology is a standard method of assessing vWF multimers in routine laboratories and can be easily understood and interpreted. By including internal standards on each multimer run, standardization of scoring was obtained.
The clinical significance of abnormalities of vWF protease activity remains uncertain. We observed a correlation between our results and the degree of thrombocytopenia as well as the relative concentration of vWF antigen level (Figure 3). However, it is important to note that a selection bias in our sample population could explain these observations. For example, our study focused on thrombocytopenic patients with a variety of disorders. Hence, this apparent association may be collateral rather than causal.
The studies described in this report indicate that abnormalities of vWF protease activity as measured in a standard assay are not correlated with the presence or absence of TTP.
Supported by grants from the Medical Research Council of Canada and the Heart and Stroke Foundation of Ontario.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
J. G. Kelton, Canada Research Chair in Transfusion Medicine, HSC 3W10, McMaster University Medical Centre, 1200 Main St W, Hamilton, ON, Canada, L8N3Z5.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal