Abstract
A study in 121 infants with severe combined immunodeficiency (SCID) was performed to determine the prevalence of an engraftment by transplacentally acquired maternal T cells and to explore clinical and immunological findings related to this abnormality. Each newly diagnosed patient with SCID presenting with circulating T cells was evaluated for chimerism by performing selective HLA typing of T cells and non–T cells. In patients with engraftment, maternal T cells were characterized phenotypically and functionally, and results were correlated with clinical findings in the patients. Maternal T cells were detected in the circulation in 48 patients; these cells ranged from fewer than 100/μL in 14 cases to more than 2000/μL in 4 cases (median, 415/μL). Clinical signs of graft-versus-host disease (GVHD) were absent in 29 patients. In the other cases, manifestations of GVHD were present, involving the skin and in 14 cases also the liver. Skin GVHD was mild in 8 patients. In these patients, as well as in patients with no signs of GVHD, maternal T cells were predominantly CD8+ and, with one exception, failed to respond to mitogen stimulation. In 9 patients, manifestations of skin GVHD were prominent. T cells in these cases were predominantly CD4+ and responded, with one exception, to mitogen stimulation. In 8 of the cases with prominent skin GVHD, the underlying SCID variant was characterized by the absence of B cells. In this study, further understanding is provided of a phenomenon that is responsible for the significant heterogeneity of clinical and immunological findings in SCID.
Introduction
Severe combined immunodeficiency (SCID) represents a genetically heterogeneous disorder characterized by profound disturbances in lymphocyte development, usually resulting in complete failure of T-cell maturation and the absence of T- and B-cell functions.1 A repeatedly described abnormality in SCID patients is the presence of maternal T lymphocytes in the circulation.2-6 This complication results from a prenatal or perinatal placental passage of maternal blood (maternal-fetal transfusion) and the failure in SCID patients to recognize and to reject foreign cells, allowing maternal T cells to persistently engraft. This unusual phenomenon in SCID has a number of perplexing aspects. One is the inconsistency of HLA-nonidentical maternal T cells to induce graft-versus-host disease (GVHD). In a number of patients, this complication was found to be completely absent, in spite of the presence of substantial numbers of circulating maternal T cells, while in other patients, mild or severe GVHD manifestations were present.2,3,5,6,8,10 This inconsistency and variability of GVHD in patients engrafted with maternal T cells is in sharp contrast to the experience in severely immunocompromised patients who receive transfusions of nonirradiated blood products containing viable T cells. Under those circumstances, GVHD has been noted to be a rapidly fatal complication, owing mainly to fulminant liver and marrow failure.7 In a number of studies, engrafted maternal T cells were noted to be functionally defective, with complete failure to proliferate upon in vitro stimulation with specific antigens, including the absence of alloreactivity against patient-derived cells, and variable, usually profoundly depressed, responses to mitogen-induced polyclonal stimulation.3,4 8-10 The basis of this unusual functional status of engrafted maternal T cells remains largely undefined. Since it was observed in patients both without and with GVHD, the variability of GVHD manifestations induced by maternal T cells remains an enigma.
In the study reported on here, we analyze, in a large cohort of patients with SCID, the prevalence and clinical and immunological findings of maternal T-cell engraftment and explore the basis of a markedly variable presentation of this complication.
Patients and methods
Patients
Between 1982 and 1999, a total of 121 infants were admitted to the Pediatric Hospital of the University of Ulm with clinical and laboratory findings consistent with the diagnosis of SCID; these findings included severely depressed or absent T-cell immunity, hypogammaglobulinemia or agammaglobulinemia, and a small or absent thymus by radiography or ultrasound. Thirty-one patients were diagnosed shortly after birth because of positive family histories; in the remaining 90 patients, the mean age at diagnosis was 5 months. Sixty percent of the patients were of German origin; the others were predominantly of Turkish background. In the latter group, 90% of parents were consanguinous. Variants of SCID were determined on the basis of family history, patient's sex, and immunological and enzymatic findings, as well as molecular analysis identifying the underlying genetic defects.
Identification of maternal lymphocytes
Laboratory evaluations in each patient included immunophenotyping of blood mononuclear cells (MNCs). In patients with detectable T cells, we examined whether the cells originated from the patient or the mother. For this purpose, blood MNCs were separated to obtain cell fractions enriched for T cells and for non–T cells, by means of either rosette formation with sheep red blood cells or, during the more recent period of the study, anti-CD8 beads and anti–HLA-DR (Dynal, Hamburg, Germany) as previously described.9-11 The resulting cell fractions were separately HLA-typed by means of standard, complement-mediated cytotoxicity assays. Chimerism was further analyzed by flow cytometry by means of anti–human major histocompatibility complex (MHC) class I–specific mouse monoclonal antibodies (One Lambda, Krefeld, Germany), allowing maternal and patient cells to be directly distinguished. These antibodies were combined with monoclonal antibodies directed against T cells, B cells, natural killer (NK) cells, and monocytes for 2-color immunofluorescence as described.10 This method also provided a fast and sensitive tool to detect engrafted T cells at levels as low as 1%. Absolute T-cell numbers were calculated on the basis of differential white blood cell counts.
Characterization of maternal T cells
T-cell functions were determined in vitro by measuring3H-TdR incorporation after stimulation of blood MNCs with phytohemagglutinin (PHA) at 20 μg/mL (PHA-P) (Difco Laboratories, Detroit, MI), anti-CD3 monoclonal antibody (mAb) (OKT3) (Ortho Diagnostic System, Raritan, NY), irradiated allogeneic cells, and recall antigens as described.10 To determine cytokine synthesis, blood MNCs were incubated for 6 hours in the presence of phorbolmyristate acetate (PMA) (10 ng/mL), ionomycin (750 ng/mL) (Sigma-Aldrich, Deisenhofen, Germany), and brefeldin A (1 μL/mL) (Golgi-PlugR) (Pharmingen, Hamburg, Germany). Subsequently, cells were stained with an anti-αCD3 mAb, fixed with Cytofix/Cytopermr (Pharmingen), and incubated with antibodies directed against the following cytokines: interferon γ (IFN-γ), tumor necrosis factor-α (TNF-α), interleukin-2 (IL-2) and IL-4, and the corresponding isotype control antibodies (Pharmingen), according to the instructions of the supplier. Analysis was performed by flow cytometry.
Results
Detection of maternal lymphocyte chimerism in 48 patients
Within the total group of 121 SCID patients, 48 patients (40%) were found to have maternal T lymphocytes in the circulation. Maternal cells were noted only within the T-cell–enriched blood MNCs. Restriction of engraftment to the T-cell compartment was confirmed by directly investigating chimerism by means of 2-color immunofluorescence. In these studies, which were performed in 30 of the 48 cases, maternally derived B cells, NK cells, or monocytes were not detected except in one case, in which NK cells were also of maternal origin. The simultaneous presence of both maternal and autologous T cells was never observed, with the sensitivity of this assay allowing an engraftment level as low as 1% to be detected.
Clinical presentation of patients with maternal T cells
In 29 (60%) of the 48 patients, clinical manifestations of GVHD were absent, while the other 19 patients presented with findings consistent with GVHD. Manifestations most commonly involved the skin and occurred in 2 main variants: a chronic eczematous skin rash that developed insidiously during the second or third month (8 cases) and a severe dermatitis characterized by generalized exfoliative erythrodermia developing 2 to 6 weeks after birth and persisting as a generalized, desquamatous dermatitis (9 patients). Most patients with this latter variant also presented or developed markedly increased palpable lymph nodes, hepatosplenomegaly, and total alopecia. Clinical manifestations in these patients were indistinguishable from patients with Omenn syndrome without maternal T cells. One patient presented at birth with a bullous erythrodermia, without other signs of GVHD.
Another manifestation of GVHD, observed in 14 cases, was hepatic disease. With the exception of one case, liver GVHD was always associated with skin GVHD and consisted of mild to moderate elevation of liver enzymes without jaundice, except for 2 patients who had severe cholestatic disease; in one patient, this was the only GVHD manifestation. One patient with skin GVHD also presented with a nephritis characterized histologically by extensive peritubular infiltrations of T lymphocytes. In all patients, histomorphological studies of skin and liver revealed findings of cell-mediated inflammatory reactions consistent with GVHD. Notably, histomorphological evidence of infiltrations correlated with the prominence of clinical manifestations.
Other abnormalities related to GVHD involved the hematological compartment, with eosinophilia in 16 patients and agranulocytosis in 12 patients. In 6 of the latter patients, agranulocytosis was present at birth, consistent with the diagnosis of reticular dysgenesis. The clinical presentation and immunological findings in these latter 6 patients will be presented in detail in a separate report (in preparation).
Prevalence of engrafted maternal T cells
An analysis of the prevalence of maternal T-cell engraftment in different variants of SCID, based on the total population of 121 patients, is presented in Table 1. In patients with SCID characterized by a complete absence of autologous T cells and either the absence or presence of variable numbers of B cells (B− SCID and B+ SCID), 16 of 26 patients (62%), and 26 of 52 patients (50%), respectively, were found to be engrafted. The highest prevalence rate was observed in patients with reticular dysgenesis, as all 6 patients with reticular dysgenesis demonstrated maternal T-cell engraftment. Maternal T cells were not observed in patients with SCID caused by enzymatic deficiencies, which included 8 patients with adenosine deaminase deficiency and 2 patients with purine nucleoside phosphorylase deficiency. In addition to MHC class II deficiency (10 patients), other variants of SCID in which maternal cells were not observed were Omenn syndrome (9 patients), SCID similar to ZAP-70 deficiency with an absence of CD8+ cells but the presence of CD4+ T cells (5 patients), and undefined SCID with low numbers of host T cells (3 patients).
Prevalence of engraftment of maternal T cells in different severe combined immunodeficiency variants
| . | Patients (no.) . | Maternal T-cell engraftment . | |
|---|---|---|---|
| (no.) . | (%) . | ||
| Reticular dysgenesis | 6 | 6 | 100 |
| B− SCID | 26 | 16 | 62 |
| B+ SCID | 52 | 26 | 50 |
| ADA/PNP deficiency | 10 | 0 | 0 |
| MHC class II deficiency | 10 | 0 | 0 |
| Omenn syndrome | 9 | 0 | 0 |
| Absent CD8+ T cells | 5 | 0 | 0 |
| Other T+ SCID | 3 | 0 | 0 |
| All patients | 121 | 48 | 40 |
| . | Patients (no.) . | Maternal T-cell engraftment . | |
|---|---|---|---|
| (no.) . | (%) . | ||
| Reticular dysgenesis | 6 | 6 | 100 |
| B− SCID | 26 | 16 | 62 |
| B+ SCID | 52 | 26 | 50 |
| ADA/PNP deficiency | 10 | 0 | 0 |
| MHC class II deficiency | 10 | 0 | 0 |
| Omenn syndrome | 9 | 0 | 0 |
| Absent CD8+ T cells | 5 | 0 | 0 |
| Other T+ SCID | 3 | 0 | 0 |
| All patients | 121 | 48 | 40 |
SCID indicates severe combined immunodeficiency; ADA, adenosine deaminase; PNP, purine nucleoside phosphorylase; MHC, major histocompatibility complex.
We also analyzed whether the rate and severity of GVHD induced by maternal T cells differed in patients with distinct SCID variants. Interestingly, the severe variant of skin GVHD was observed almost exclusively in patients with B− SCID (8 of 9 cases) (Table2). In contrast, the milder form, as characterized by chronic eczema, was observed only in patients with B+ SCID (8 of 8 cases) but never in patients with B− SCID. Thirteen of the 16 patients with B−SCID were analyzed for mutations of RAG1 or RAG2, and mutations were detected in 5 and excluded in the other 8 cases. The proportion of patients with severe GVHD was 40% (2 of 5) in the former and 63% (5 of 8) in the latter group. In 10 patients with B+ SCID, the disorder was caused by mutations in the gene encoding the common γ-chain, which was excluded in 9 other B+ SCID patients. The incidence of GVHD was similar in both groups (3 of 10 versus 3 of 9).
Graft-versus-host disease in B− and B+ severe combined immunodeficient patients with maternal T cells
| . | Patients . | GVHD . | ||
|---|---|---|---|---|
| None . | Mild . | Severe . | ||
| B− SCID | 16 | 8 | — | 8 |
| RAG 1/2 mutation | ||||
| Yes | 5 | 3 | — | 2 |
| No | 8 | 3 | — | 5 |
| Not tested | 3 | 2 | — | 1 |
| B+SCID | 26 | 17 | 8 | 1 |
| Yc mutation | ||||
| Yes | 10 | 7 | 3 | — |
| No | 9 | 6 | 2 | 1 |
| Not tested | 7 | 4 | 3 | — |
| . | Patients . | GVHD . | ||
|---|---|---|---|---|
| None . | Mild . | Severe . | ||
| B− SCID | 16 | 8 | — | 8 |
| RAG 1/2 mutation | ||||
| Yes | 5 | 3 | — | 2 |
| No | 8 | 3 | — | 5 |
| Not tested | 3 | 2 | — | 1 |
| B+SCID | 26 | 17 | 8 | 1 |
| Yc mutation | ||||
| Yes | 10 | 7 | 3 | — |
| No | 9 | 6 | 2 | 1 |
| Not tested | 7 | 4 | 3 | — |
Data are given as number of patients.
GVHD indicates graft-versus-host disease; SCID, severe combined immunodeficiency.
We further analyzed a number of other variables and determined whether correlations with GVHD existed. These variables included an analysis of HLA incompatibilities between patients and mothers, who as expected were in most instances (35 cases) fully haplo-mismatched for MHC class I and MHC class II antigens, while in 13 patients partial matching or homozygosity of MHC class I and/or class II antigens of the nonshared haplotype were observed. We also analyzed the number of previous pregnancies in the mothers, assuming the possibility of sensitization of maternal T cells to paternally derived histocompatibility antigens expressed by patients. Furthermore, we analyzed infectious complications, including cytomegalovirus and bacille Calmette-Guérin, representing the most common systemic infections in the patients. We obtained no evidence that any of these variables were related to differences in GVHD (data not shown).
Characterization of maternal T cells and correlation with GVHD
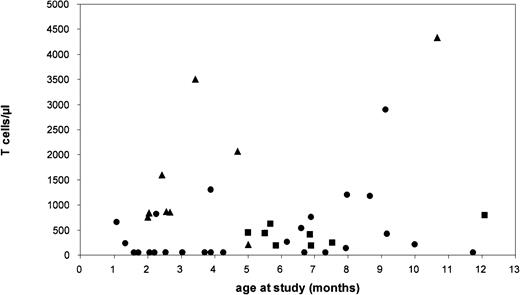
We next assessed whether GVHD in the patients was reflected by differences in the numbers and the characteristics of maternal T cells. From this analysis, we excluded 2 patients with exceptional manifestations of GVHD not observed in the other patients (one presenting as newborn with acute dermatitis, the other with isolated cholestatic liver disease at birth). Only data obtained prior to the institution of immunosuppression were used in patients requiring such treatment. Patients were classified into 3 main groups according to skin manifestations: group 1 (n = 29) had no GVHD; group 2 (n = 8) had GVHD manifesting as chronic eczema; and group 3 (n = 9) had GVHD characterized by severe dermatitis. Maternal T cells were detectable at highly variable numbers. As demonstrated in Figure1, there was no correlation with the age of patients at the time of the study. The largest variability in T-cell numbers was observed in group 1 patients without GVHD: in 14 of these patients, T cells were extremely low (fewer than 100/μL; fewer than 5% of blood MNCs); in the other 15 patients in group 1, T-cell numbers were higher, ranging from 130/μL to 2900/μL (median, 650/μL). In group 2 patients with mild skin GVHD, the median T-cell number was 418/μL (range, 180/μL to 800/μL). In group 3 patients, the median T-cell number was 1738/μL (range, 670/μL to 4300/μL). These findings indicated a trend toward higher T-cell numbers in patients with more prominent GVHD, but also showed that in approximately half of the patients without GVHD, T cells were in a similar range as in group 2 and group 3 patients.
Number of circulating maternal T cells and age at study in 48 patients with SCID.
Patients were classified into 3 groups differing in their manifestation of skin GVHD: group 1 had no manifestation; group 2 presented with chronic eczema; and group 3 presented with severe exfoliative erythrodermia. ● indicates group 1; ▪, group 2; and ▴, group 3.
Number of circulating maternal T cells and age at study in 48 patients with SCID.
Patients were classified into 3 groups differing in their manifestation of skin GVHD: group 1 had no manifestation; group 2 presented with chronic eczema; and group 3 presented with severe exfoliative erythrodermia. ● indicates group 1; ▪, group 2; and ▴, group 3.
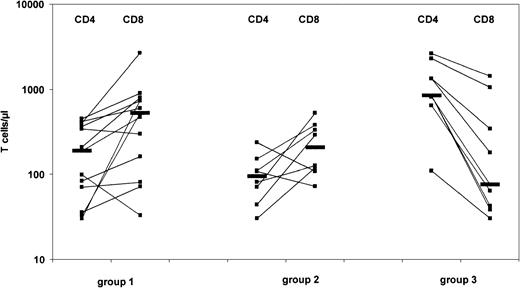
We next assessed whether the maternal T cells in these patient groups differed with respect to phenotypical and functional characteristics. These studies could not be performed in 16 group 1 patients owing to their low T-cell numbers; this reduced the number of analyzed patients in group 1 to 13 cases. A comparison of the number of CD4+and CD8+ T cells revealed striking differences. In patients with prominent GVHD (group 3), we observed a predominance of CD4+ cells. In contrast, both group 1 and group 2 patients showed, with the exception of 4 cases, a predominance of CD8+ cells (Figure 2). A comparison of the functional properties of the maternal T cells, as determined by proliferation to stimulation with PHA, is shown in Figure3. In group 1 and group 2 patients, these mitogenic responses were severely depressed, with stimulatory indices (SIs) less than 20 in 18 of 19 patients. These findings were different in 8 analyzed patients in group 3: T cells showed significant proliferation to PHA (median SI, 86; range, 40-133), although still lower as compared with responses observed in healthy adult controls (median SI, 170; range, 120-210). Interestingly, addition of exogenous IL-2 resulted in augmented PHA-induced T-cell proliferation in group 1 (median SI, 129; range, 37-280) and to a lesser degree in group 2 patients (median SI, 17,8; range, 1-61). In contrast, PHA responses were, with one exception, not augmented by exogenous IL-2 in group 3 patients or in controls (Figure 3). Similar data were observed following stimulation of T cells with an anti-CD3 mAb (OKT3) in the absence or presence of IL-2 (data not shown). Proliferative responses to recall antigens and to allogeneic cells were absent in all cases except one, where maternal T cells responded normally to allogeneic cells in a mixed lymphocyte reaction (data not shown). All together, these data show that a significant mitogen-induced proliferation of maternal T cells correlates with a high level of skin GVHD (group 3). Moreover, the inverse correlation holds, with an association between a low level of GVHD and low proliferation.
CD4+ and CD8+ maternal T cells in 30 SCID patients with T cells at levels exceeding 100/μL blood.
Patients were classified into 3 groups as described in Figure 1. Horizontal bars indicate median numbers in each group of patients.
CD4+ and CD8+ maternal T cells in 30 SCID patients with T cells at levels exceeding 100/μL blood.
Patients were classified into 3 groups as described in Figure 1. Horizontal bars indicate median numbers in each group of patients.
In vitro proliferative responsiveness of engrafted maternal T cells.
T cells were stimulated with PHA alone or in the presence of exogenous IL-2. Patients were classified into 3 groups as described in Figure 1. Controls consisted of 10 healthy adults. Horizontal bars indicate median values.
In vitro proliferative responsiveness of engrafted maternal T cells.
T cells were stimulated with PHA alone or in the presence of exogenous IL-2. Patients were classified into 3 groups as described in Figure 1. Controls consisted of 10 healthy adults. Horizontal bars indicate median values.
Intracellular cytokines were determined in PMA/ionomycin–activated T cells in 15 patients (6 patients in group 1; 4 patients in group 2; and 5 patients in group 3). Production of IL-2, IFN-γ, and TNF-α was found to be highly variable, but these cytokines were detected in T cells isolated from all patients in the 3 groups. Significant IL-4 cytokine synthesis was not observed in any of the patients. The cytokine profile observed in all studied patients thus was representative of TH1/Tc 1 (Tc1) but not TH2/Tc2 cells.
Discussion
The human placenta forms an incomplete barrier for blood cells, allowing bidirectional passage of nucleated blood cells.13By means of sensitive methods, microchimerism by maternal cells was observed in up to 42% of cord blood samples from healthy newborns.14 15 While the survival of maternal cells is usually limited owing to effective rejection by an immunocompetent organism, in patients with SCID a well-known phenomenon, characterized by long-term engraftment of maternal T cells, results. In the present study, 40% of 121 SCID patients were found to have this abnormality. It is likely that maternal-fetal transfusions occur even more frequently than these numbers suggest. This is implied by our findings in subgroups of patients with different variants of SCID, where we observed engraftment rates as high as 100%. The failure, on the other hand, to detect circulating maternal cells in SCID variants characterized by the presence of functionally impaired autologous T cells and by enzymatic deficiencies also indicates that, in SCID, effective mechanisms may exist to prevent engraftment of maternal cells.
An unusual and previously undescribed outcome of a maternal-fetal transfusion in SCID, as observed in one quarter of our patients, was the presence of maternal T cells in the circulation at very low levels: less than 100/μL. These patients were always asymptomatic with respect to manifestations of GVHD. The maternal T cells were increasingly recognized when very sensitive methods were applied to evaluate chimerism, with the use of an HLA-specific mAb for 2-color immunofluorescence in fluorescence-activated cell sorter analysis. Of note, 7 patients with very low T-cell numbers were older than 3 months, indicating that T cells in these cases actually persisted at very low levels and were not, as one might speculate, in the process of expansion or disappearance. It should also be mentioned that the proportion of patients presenting microchimerism with maternal lymphocytes may in fact be higher, as our findings suggest, since our method of detecting maternal T cells in the blood was at the 1% level. It will be of interest to prospectively analyze SCID patients with the use of even more sensitive methods based on the detection of maternal cells by molecular techniques.
We observed marked heterogeneity in the clinical presentation of patients engrafted with maternal T cells. Frequently, this finding was not associated with manifestations of GVHD. In fact, asymptomatic patients constituted the majority in our series (60%). At present, the basis of this variable capacity of maternal T cells to induce GVHD is poorly understood. The large database provided by our series of patients allowed us to assess whether the observed variability in clinical presentation correlated with specific characteristics of engrafted T cells, and indeed our analysis of T-cell subpopulations as well as of their functional characteristics revealed such correlations.
The mechanisms accounting for the selection of distinct subgroups of maternal T cells differing in phenotype and function and in their ability to induce clinical GVHD manifestations remain to be determined. T lymphocytes, particularly CD4+ cells, become activated and proliferate in response to alloantigen exposure. Since the fetal host, with very few exceptions, represents an HLA–haplo-identical environment for maternal T cells, this does not explain why the engrafted T cells induce such variable symptoms in the patients. We also found no evidence indicating that the presence of systemic infections, which may trigger additional T-cell activation pathways, correlated with the development or severity of GVHD. Alternatively, maternal T-cell responses may be restricted owing to a limited number of transplacentally acquired cells, exhibiting a narrowed receptor repertoire. Indeed, we and others previously observed restricted receptor repertoires of maternal T cells in several patients.10 12 While these findings may be interpreted as indicating an extensive and selective expansion of a small subset of transplacentally acquired T cells, the mechanism triggering these cells to expand remains to be elucidated.
In patients without GVHD or with mild GVHD, regulatory mechanisms may modify and suppress the proliferative properties of transfused maternal T cells, whereas in the absence of these suppressive mechanisms or in the presence of additional stimulatory mechanisms, maternal T cells, predominantly CD4+ cells, proliferate and induce inflammatory reactions. It is of significant interest that in our study the group of patients with prominent GVHD was composed almost exclusively of patients with B− variants of SCID. In patients with B− SCID, NK-cell functions, as determined by their cytotoxic activities against MHC class I–negative cell lines, are usually normal. Thus, not only do functional NK cells in B− SCID fail to protect against engraftment of maternal T cells, but their presence was even associated with development of severe inflammatory skin manifestations. This observation suggests that components of the innate immune system in SCID patients may affect the proliferative and functional activities of engrafted T cells, a postulate that we are currently investigating.
Our study discloses a surprisingly broad range of clinical and immunological findings in SCID engrafted with maternal T cells. Clinically, it will be important to more closely evaluate the significance of this abnormality for the treatment of SCID, in particular for the outcome of a stem cell transplantation. From the immunological standpoint, it seems most interesting to understand the signaling pathway for survival, activation, and proliferation of maternal T cells, which also may be rendered silent in an alloantigenic host.
The authors thank Naomi Taylor for critical comments and valuable discussions during preparation of the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Wilhelm Friedrich, Department of Pediatrics, University Hospital of Ulm, Prittwitzstr 43, 89075 Ulm, Germany; e-mail: wilhelm.friedrich@medizin.uni-ulm.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal