Abstract
Various pathologic conditions, such as hemorrhage, hemolysis and cell injury, are characterized by the release of large amounts of heme. Recently, it was demonstrated that heme oxygenase (HO), the heme-degrading enzyme, and heme are able to modulate adhesion molecule expression in vitro. In the present study, the effects of heme and HO on inflammation in mice were analyzed by monitoring the biodistribution of radiolabeled liposomes and leukocytes in conjunction with immunohistochemistry. Small liposomes accumulate in inflamed tissues by diffusion because of locally enhanced vascular permeability, whereas leukocytes actively migrate into inflammatory areas through specific adhesive interactions with the endothelium and chemotaxis. Exposure to heme resulted in a dramatic increase in liposome accumulation in the pancreas, but also intestines, liver, and spleen exhibited significantly increased vascular permeability. Similarly, intravenously administered heme caused an enhanced influx of radiolabeled leukocytes into these organs. Immunohistochemical analysis showed differential up-regulation of the adhesion molecules ICAM-1, P-selectin, and fibronectin in liver and pancreas in heme-treated animals. Heme-induced adhesive properties were accompanied by a massive influx of granulocytes into these inflamed tissues, suggesting an important contribution to the pathogenesis of inflammatory processes. Moreover, inhibition of HO activity exacerbated heme-induced granulocyte infiltration. Here it is demonstrated for the first time that heme induces increased vascular permeability, adhesion molecule expression, and leukocyte recruitment in vivo, whereas HO antagonizes heme-induced inflammation possibly through the down-modulation of adhesion molecules.
Introduction
The inflammatory response consists of a complex cascade of orchestrated signals resulting in increased permeability of blood vessels, changes in blood flow, and migration of leukocytes from blood to affected tissues.1 Vascular permeability results from the partial retraction of endothelial cells of small venules in the vicinity of inflammation, leaving small intercellular gaps (approximately 0.1-0.4 μm). This so-called vascular leakage results in slower blood flow by allowing the passage of water, salts, and small proteins from the plasma into the damaged area, whereas blood cells are retained within the vessels.1 In normal circumstances the endothelial layer is nonadhesive for leukocytes. However, during inflammation, activated endothelial cells increase the surface expression of specific adhesion molecules, such as intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), endothelial leukocyte adhesion molecule (E-selectin), and P-selectin.2 This increased cell surface adhesion enables circulating activated leukocytes to specifically interact with their ligands on the endothelium.2 Although the inflammatory response of the host is considered essential in the protection against pathogens, activated leukocytes and endothelial cells may also cause cellular and organ damage by excessive release of proteases and reactive oxygen species (ROS).3,4 Therefore, the inflammatory process must be tightly regulated by specific mediators, such as anti-inflammatory cytokines and acute-phase proteins.1
Heme and inflammation
Heme (iron protoporphyrin IX) serves as the functional group of various proteins, including hemoglobin, myoglobin, nitric oxide synthase, and cytochromes.5 Heme is therefore essential for diverse biologic processes.5 The vascular endothelium is continuously exposed to circulating erythrocytes and to exogenous hemoglobin and heme released by damaged cells. Excess of free heme may constitute a major threat because heme catalyzes the formation of ROS, resulting in oxidative stress and, subsequently, cell injury.6,7 Large amounts of free hemoproteins are released during hemolysis or rhabdomyolysis under various pathologic conditions, such as hemorrhage, hematoma, hemoglobinopathies, excessive blood transfusion, and muscle injury.8-12 The heme moieties are readily oxidized and subsequently dissociate from methemoglobin.13,14 In addition, during ischemia-reperfusion, heme may be released from mitochondrial P450 cytochromes and other denatured heme-proteins and contribute to inflammatory changes and cellular injury associated with oxidative stress. Free heme is highly lipophilic and will rapidly intercalate into the lipid membranes of adjacent cells.5 We and others15,16 observed that exposure to heme stimulates the expression of adhesion molecules ICAM-1, VCAM-1, and E-selectin on endothelial cells in vitro, probably through heme-mediated generation of intracellular ROS. These activated endothelial adhesion molecules may subsequently recruit leukocytes, forming one of the main characteristics of inflammation.1
Heme oxygenase and inflammation
Heme oxygenase (HO) is the rate-limiting enzyme in the degradation of heme. It breaks down the pro-oxidant heme into the vasodilator carbon monoxide, iron, and the antioxidant biliverdin.17Biliverdin is rapidly converted by biliverdin reductase to bilirubin.18 The heme-derived oxidant, iron, is directly sequestered and inactivated by coinduced ferritin.19 Among the 3 separate isoforms of the HO protein known to date, HO-1 is highly inducible by a great variety of stimuli, including oxidative stress, heat shock, UV radiation, ischemia-reperfusion, heavy metals, cytokines, and nitric oxide and its substrate heme.19-21HO-2 and, to a lesser extent, the recently discovered HO-3 iso-enzyme are predominantly constitutively expressed and function probably in normal heme capturing and metabolism.21,22 The strong adaptive response of HO-1 expression on the diverse array of stress stimuli suggests an important role for HO distinct from heme degradation.23 In fact, the overexpression of HO-1 is associated with the resolution of inflammation23 and with protection against heme- and hemoglobin-mediated toxicity.24,25 The importance of HO in the degradation of heme and the possible involvement in inflammation is further illustrated by recent observations in mice and humans deficient in HO-1 gene expression.26,27 The absence of HO-1 results phenotypically in the presence of consistently high serum heme concentrations (ca. 0.5 mM) and various oxidative and inflammatory complications.26,27 Despite the growing evidence of a protective role for HO, the mechanism by which HO down-regulates inflammation is only scarcely understood. Interestingly, recent observations suggest that the mechanism of HO-1–mediated resolution of inflammation may originate from the modulation of adhesion molecule expression. HO-1 overexpression was shown to attenuate adhesion molecule expression in vitro and in vivo, whereas selective inhibition of HO-1 activity aggravated adhesion molecule expression.15,28-30 Intense interest has recently focused on the biologic effects of carbon monoxide, biliverdin, and bilirubin. Until recently, these heme-derived metabolites were considered toxic waste products, but accumulating data suggest they have anti-oxidative, anti-inflammatory, antiapoptotic, and signaling properties and possibly immune modulatory functions.31-35 Therefore, we postulate that, in vivo, large amounts of free heme exert inflammatory actions resulting in the expression of adhesion molecules and the recruitment of leukocytes, whereas HO down-regulates inflammation through a mechanism involving the down-modulation of adhesive properties. To examine this hypothesis, the roles of heme and HO as inflammatory modulators were investigated in a mouse model.
Materials and methods
Antibodies
The following monoclonal rat antimouse antibodies were used: YN1/1 (anti–ICAM-1), B220 (anti-CD45R [B-cell marker]), MK2/7 (anti-VCAM-1), GR-1 (granulocyte marker), F4/80 (macrophage marker; Caltag Laboratories, Burlingame, CA), Lyt-2 (anti-CD8), MT-4 (anti-CD4), and kT3 (anti-CD3) (Serotec, DPC, Breda, The Netherlands). In addition, the polyclonal rabbit antibodies CD62P (anti–human–P selectin) (Pharmingen, San Diego, CA), A0245 (anti–human fibronectin) (DAKO, Glostrup, Denmark), SPA895 (anti–rat HO-1; cross-reacts with mouse HO-1) (Stressgen Biotechnologies, Victoria BC, Canada), and OSA200 (anti–rat HO-2; Stressgen) were used. As secondary antibodies, fluorescein isothiocyanate fluorochrome conjugated to mouse anti–rat immunoglobulin (Ig) G (Zymed Laboratories, San Francisco, CA), biotin-labeled goat anti–rat IgG (Vector Laboratories, Burlingame, CA), or biotin-labeled anti–rabbit IgG antibodies (Pharmingen) were used.
Animals
Male BALB/c mice or male C57Bl/6 (H-2b) mice, 8 to 12 weeks of age, were obtained from Charles River Wiga (Sulzfeld, Germany). The mice were kept under specified pathogen-free conditions in the Central Animal Laboratory (University Medical Center Nijmegen, The Netherlands). They had free access to water and were fed standard laboratory chow (Hope Farms, Woerden, The Netherlands). All experiments were performed in accordance with the guidelines of the Animal Experiments Committee of UMC Nijmegen.
Porphyrin solutions
The following porphyrins were used: hemin (Sigma, St Louis, MO) and tin (stannic) mesoporphyrin (SnMP) (Porphyrin Products, Logan, UT). At low concentrations SnMP acts as a potent and selective competitive inhibitor of HO-activity in vitro and in vivo.17,36 All porphyrin solutions were freshly prepared as previously described.37 In short, hemin was dissolved together with Trizma base in a 0.1-M NaOH solution and diluted in saline. Next, pH 11 to 12 was adjusted to pH 8 with HCl. The solution was then filter-sterilized, protected from light and directly used.
Heme administration protocol
Tail veins of mice were injected with heme, lysed erythrocytes, or saline. The final heme concentration in the serum was calculated by estimating the total blood volume as 71 mL/kg. Mice were anesthetized with ether. Blood was drawn by cardiac puncture or through the retro-orbital vein, and mice were killed by cervical dislocation. Erythrocyte lysis was performed as follows: erythrocytes were collected after density gradient centrifugation of peripheral blood, lysed by subjection to repeated freeze-thaw cycles, and filtered (0.45 μm).
Spectral measurement of heme
Heme concentration in the serum was determined spectrophotometrically by the pyridine hemochromogen assay.38 Briefly, 900 μL solution A (3 parts pyridine, 1 part 1 M NaOH) was added to 450 μL heme solution, vortexed, and used as a baseline for the spectrum between 500 and 600 nm. Next, fresh sodium dithionite was added for reduction. The sample was vortexed, and the spectrum between 500 and 600 nm was recorded. Dilution of the heme standard and the serum samples was, respectively, 40 × and 18 ×. Heme concentration was measured using the specific micromolar extinction coefficient of heme for the delta optical density between the peak at 557 nm and the minimum at 540 nm as 0.0207.
Preparation of HYNIC-PEG-liposomes
Polyethyleneglycol-2000 (PEG)–liposomes containing hydrazinonicotinamide-conjugated-distearoylphosphatidyl-ethanolamine (HYNIC-PEG-liposomes) were prepared as previously described.39 Liposomes were sized by multiple extrusion through pairs of stacked polycarbonate membranes using a medium pressure extruder (Lipex Biomembranes, Vancouver, BC, Canada). Phospholipid recovery after liposome preparation was 80% on average. Particle size distribution was determined by dynamic light scattering with a Malvern 2000 system equipped with a 25-mW neon laser (Malvern Instruments, Malvern, United Kingdom). Mean size of the small HYNIC-PEG-liposomes was 80 to 85 nm with a polydispersity index less than 0.1. In the experiments, the liposomes were administered intravenously at a dose of 5 μM/kg phospholipids in a volume of 0.1 mL.
Radiolabeling of liposomes
Preformed HYNIC-PEG-liposomes were labeled with technetium99mTc as described previously.39 Briefly, to 0.1 mL liposomes a mixture of 10 mg N-[Tris(hydroxymethyl)-methyl]glycine] (Tricine, Fluka, Zwijndrecht, The Netherlands), 10 μg stannous sulfate in 0.5 mL saline, and 500 mEq 99mTcO4− in saline (10 MBq/μM phospholipid) was added. The mixture was incubated for 15 minutes at room temperature. Labeling efficiency was always greater than 95%, and liposomes were used without any further purification.99mTc-labeled HYNIC liposomes have been shown to be highly stable: no significant release of the radiolabel was observed after incubation with DTPA, cysteine, or glutathione or after 48 hours of incubation in serum at 37°C.39
Administration of 99mTc-labeled liposomes, gamma camera imaging, and biodistribution studies
The role of heme on vascular permeability was examined using99mTc-labeled liposomes.40 41 Mice (C57Bl/6) intravascularly received either 150 μL phosphate-buffered saline (PBS) (n = 5) or 150 μL heme (750 μM, intravascular concentration; n = 5) followed 15 minutes later by the administration of radiolabeled liposomes (100 μL) through the lateral tail vein. Animals were anesthetized with a mixture of Ethrane (Abbott BV, Amstelveen, The Netherlands), nitrous oxide (N2O), and oxygen and were placed prone on a single-head gamma camera equipped with a parallel-hole, low-energy collimator. Mice were imaged at 5 minutes and at 1, 4, and 23 hours after injection (at least 100 000 counts/image). After 24 hours, the mice were anesthetized with ether, blood was drawn, and the mice were killed by cervical dislocation. Tissues were dissected to determine the biodistribution of99mTc. Blood samples, lungs, liver, heart, kidneys, spleen, thymus, pancreas, intestines, brain, and femur were collected and weighed, and their radio activity was measured in a shielded well-type gamma counter (Wizard, Pharmacia-LKB, Uppsala, Sweden). To correct for physical decay and to calculate the uptake of the radiopharmaceuticals in each tissue sample as a fraction of the injected dose, aliquots of the injected dose were counted simultaneously.
Immunofluorescence analysis
To examine the inflammatory properties of heme, the effect of heme on leukocyte migration was analyzed using radiolabeled leukocytes. Heparinized peripheral blood of 12 male C57Bl/6 mice was obtained through cardiac puncture. Leukocytes were isolated using sedimentation of erythrocytes with 2% dextran T500/PBS.42 Isolated leukocytes were analyzed for the presence of different subsets using FACS analysis. Cells (2 × 105) were incubated (30 minutes, 4°C) in PBS containing 0.5% wt/vol bovine serum albumin (Roche Molecular Biochemicals, Mannheim, Germany) and 0.01% sodium azide (Merck, Hohenbrunn, Germany), with appropriate dilutions of either mAb against a specific subset. Subsequently, cells were incubated with FITC-labeled goat (Fab′)2 anti–rat IgG mAb for 30 minutes at 4°C. Relative fluorescence intensity was measured by FACScan analysis (Becton Dickinson).
Indium-111 labeling of leukocytes and administration, gamma camera imaging, and biodistribution studies
Leukocytes were labeled with indium-111 (111In)–oxinate (Amersham, Hertogenbosch, The Netherlands) at room temperature as described previously.43 After washing, the labeling efficiency was determined by expressing the activity in the cell pellet as a fraction of the total amount of radioactivity added. Leukocyte viability before and after radiolabeling was greater than 95% as measured by trypan blue exclusion. Five mice per experimental group were injected in the lateral tail vein with either 150 μL saline or heme (750 μM; intravascular concentration) followed 15 minutes later by 1.5 × 106 syngeneic white blood cells in 100 μL saline with 15 μCi/mouse. In vivo distribution of the radiolabeled leukocytes was visualized scintigraphically using a gamma camera (Siemens Orbiter; Siemens, Hoffmann Estate, IL) equipped with a parallel-hole, medium-energy collimator. For tissue biodistribution, groups of 5 mice were killed and dissected 24 hours after injection of the 111In-labeled leukocytes. Blood samples, lungs, liver, heart, kidneys, spleen, thymus, pancreas, intestines, brain, and femur were dissected, weighed, and counted in the gamma counter. To correct for radioactivity decay, injection standards were counted simultaneously.
Histochemistry
Tissues were fixed in Unifix (Klinipath, Duiven, The Netherlands), dehydrated, and embedded in paraffin. Sections 4 μm thick were stained with hematoxylin and eosin using standard protocols. The presence of heme in the liver was analyzed using a benzidine staining technique.44
Immunohistochemistry
Dissected tissues were frozen with Tissue-Tek (Sakura Finetek Europe B.B., Zoeterwoude, The Netherlands) and stored at −80°C. Cryostat sections (4 μm) were collected on Super frost slides (Menzel Gläser, Freiburg, Germany). Immunohistochemical analysis was performed using protocols of the provider (Vector Laboratories). After counterstaining with hematoxylin, the slides were analyzed.
Statistical analysis
Statistical significance was defined by Student ttests. P < .05 was considered significant.
Results
Distribution of heme in situ
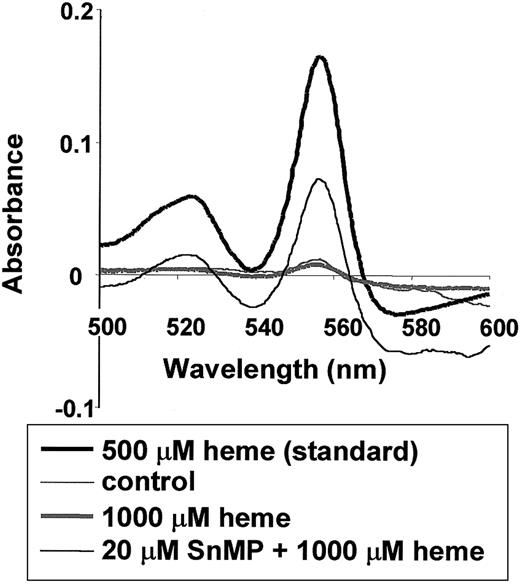
To determine the distribution of intravenously administered heme, heme concentrations in the serum of mice were measured by pyridine hemochromogen analysis. After intravenous administration of heme, a sharp decrease in serum heme concentration was observed during the first few hours (1-4 hours) (data not shown). After 24 hours, mice had heme levels similar to those in control mice (1 × 10−5M) (Figure 1, gray thin and thick spectra, respectively). However, in mice pretreated for 24 hours with a competitive inhibitor of HO activity, SnMP, followed by heme administration, elevated levels of heme were still apparent in the serum, even after 24 hours (1.3 × 10−4 M) (Figure 1, gray spectrum). These results indicate that HO is essential in the fast clearance of heme from the circulation. Furthermore, aggregation of heme molecules probably did not play a major role in our experiments because there was no apparent shift in wavelength (β band, 524 nm; α band, 557 nm) in the heme spectra of the serum samples compared to a freshly prepared heme solution (500 μM).45
Effect of HO activity on heme levels in the serum of BALB/c mice.
Twenty-four hours after mice were injected with saline or 1000 μM heme, the levels of heme in the serum were measured using the pyridine hemochromogen assay (thin and thick gray lines, respectively). Delta absorbance between 540 and 557 nm of the spectra correlates with the amount of heme (see “Materials and methods”). The thin black line represents mice pretreated for 24 hours with 20 μM SnMP, an inhibitor of HO activity, followed by treatment with 1000 μM heme for 24 hours. Thus, the inhibition of HO activity results in prolonged presence of heme in the vascular system. Further, the wavelengths of the serum heme spectra do not differ from those of a fresh heme standard (thick black line).
Effect of HO activity on heme levels in the serum of BALB/c mice.
Twenty-four hours after mice were injected with saline or 1000 μM heme, the levels of heme in the serum were measured using the pyridine hemochromogen assay (thin and thick gray lines, respectively). Delta absorbance between 540 and 557 nm of the spectra correlates with the amount of heme (see “Materials and methods”). The thin black line represents mice pretreated for 24 hours with 20 μM SnMP, an inhibitor of HO activity, followed by treatment with 1000 μM heme for 24 hours. Thus, the inhibition of HO activity results in prolonged presence of heme in the vascular system. Further, the wavelengths of the serum heme spectra do not differ from those of a fresh heme standard (thick black line).
To analyze whether heme is taken up from the circulation into the organs, the livers of mice receiving heme or saline were examined for the presence of heme using benzidine staining. Massive increases in heme levels could be observed in parts of the liver of heme-treated animals compared to mice receiving saline (Figure2).
Presence of heme in the liver.
Heme was assayed by benzidine staining of liver sections of BALB/c mice treated for 4 hours with saline or 1000 μM heme. In control mice (A), heme was confined to the vicinity of vessels, whereas in heme-treated mice (B) most liver cells stained positive (dark staining).
Presence of heme in the liver.
Heme was assayed by benzidine staining of liver sections of BALB/c mice treated for 4 hours with saline or 1000 μM heme. In control mice (A), heme was confined to the vicinity of vessels, whereas in heme-treated mice (B) most liver cells stained positive (dark staining).
In vivo distribution of liposomes: vascular permeability
To analyze the effect of free heme on vascular permeability, we determined the in vivo distribution of intravenously injected radiolabeled liposomes in C57Bl/6 mice. Mice that received99mTc-labeled liposomes in combination with either PBS or heme were monitored by gamma camera imaging. Radiolabeled liposomes were present in the well-perfused heart and liver directly after injection and during the time course of the experiment. However, within 4 hours of intravascular administration of liposomes, a clear distinction could be observed between the 2 experimental groups (Figure3A). Mice exposed to heme showed significant shifts in liposome accumulation from the heart region toward the organs in the abdominal region.
Effect of heme on vascular permeability.
Permeability was analyzed by gamma camera imaging and biodistribution of 99mTc-labeled liposomes in C57Bl/6 mice. (A) Scintigraphic image of mice 4 hours after injection with99mTc-labeled liposomes in the presence of either PBS or heme. A clear shift of radiolabeled liposomes from the heart in control mice to the abdominal region in heme-treated mice can be observed. (B) Effect of heme on the biodistribution of 99mTc-labeled liposomes in mice 24 hours after injection. Animals treated with PBS and heme are represented by white bars and black bars, respectively. Results are expressed as percentage of injected dose per organ (% ID). All values are indicated as mean ± SD of 5 mice. There is a significant increase in the accumulation of liposomes in the pancreas, liver (P < .00005), spleen, kidneys, intestines, brain (P < .01), and femur (P < .05) of the heme-treated animals compared to the PBS-treated animals. (C) Biodistribution of 99mTc-labeled liposomes in mice 24 hours after injection, corrected for weight. Animals treated with PBS and heme are represented by white bars and black bars, respectively. Results are expressed as a percentage of injected dose per 0.1 g tissue (% ID/0.1 g). All values are indicated as mean ± SD of 5 mice. There is a significant increase in the accumulation of liposomes in the pancreas (P < .0005), spleen (P < .01), liver, and intestines (P < .05) of the heme-treated animals compared to the PBS-treated animals.
Effect of heme on vascular permeability.
Permeability was analyzed by gamma camera imaging and biodistribution of 99mTc-labeled liposomes in C57Bl/6 mice. (A) Scintigraphic image of mice 4 hours after injection with99mTc-labeled liposomes in the presence of either PBS or heme. A clear shift of radiolabeled liposomes from the heart in control mice to the abdominal region in heme-treated mice can be observed. (B) Effect of heme on the biodistribution of 99mTc-labeled liposomes in mice 24 hours after injection. Animals treated with PBS and heme are represented by white bars and black bars, respectively. Results are expressed as percentage of injected dose per organ (% ID). All values are indicated as mean ± SD of 5 mice. There is a significant increase in the accumulation of liposomes in the pancreas, liver (P < .00005), spleen, kidneys, intestines, brain (P < .01), and femur (P < .05) of the heme-treated animals compared to the PBS-treated animals. (C) Biodistribution of 99mTc-labeled liposomes in mice 24 hours after injection, corrected for weight. Animals treated with PBS and heme are represented by white bars and black bars, respectively. Results are expressed as a percentage of injected dose per 0.1 g tissue (% ID/0.1 g). All values are indicated as mean ± SD of 5 mice. There is a significant increase in the accumulation of liposomes in the pancreas (P < .0005), spleen (P < .01), liver, and intestines (P < .05) of the heme-treated animals compared to the PBS-treated animals.
To study this shift in more detail, both experimental groups were killed after 24 hours, and the biodistribution of the liposomes was determined quantitatively in various organs and blood (Figure 3B). Liposome levels in the blood of the heme-treated mice were significantly lower. In contrast, in the pancreases of heme-treated mice, the influx of radiolabeled liposomes was 20 times higher than in PBS-treated animals. Furthermore, a significant uptake of liposomes was detected in the liver, spleen, intestines, femur, brain, and kidneys of heme-treated animals compared to animals receiving PBS. No significant change in liposome accumulation was found in the heart, lungs, and thymus. The amount of radiolabeled liposomes expressed per 0.1 g tissue is depicted in Figure 3C. These data confirm the scintigraphic imaging data and show a significant increase in liposome sequestration, corresponding to an increase in vascular permeability in liver, spleen, intestines, and even a 25-fold increase in the pancreas of heme-treated animals. Thus, tissue biodistribution clearly shows that heme provokes a differential increase in vascular permeability, as reflected by liposome accumulation in diverse organs.
Effect of heme on leukocyte migration
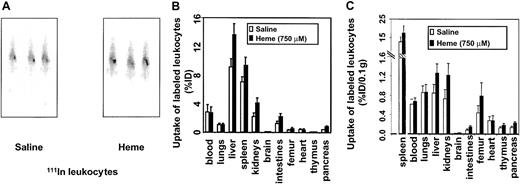
In view of our findings on heme-induced vascular permeability, the inflammatory effects of heme were further examined by analyzing the possible role of heme in leukocyte migration. Leukocytes were labeled ex vivo with 111In and injected into syngeneic mice. The migratory activity of 111In leukocytes was evaluated by comparing radiolabeled cell trafficking from the circulation into the tissues of the mice receiving heme relative to the control mice. C57Bl/6 mice receiving intravenously 111In-labeled leukocytes showed uptake of the radiolabeled cells in the liver and spleen directly after injection. However, 21 hours after the administration of heme or saline, a distinction was observed (Figure4A). Clearly, a larger fraction of the leukocytes had accumulated in the spleens of the heme-treated animals. After 24 hours, biodistribution was performed. Significant leukocyte accumulation was observed in spleen, liver, kidneys, intestines, femur, brain, and pancreas of heme-treated animals than in animals receiving saline (Figure 4B). No significant differences in leukocyte uptake were observed in the heart, thymus, or lung tissues.
Effects of heme on leukocyte influx.
Leukocyte accumulation was measured by gamma camera imaging and biodistribution of 111In-labeled leukocytes in C57Bl/6 mice. (A) Scintigraphic images of mice 21 hours after injection with either saline or heme, followed by administration of111In-labeled leukocytes. Note that there is an increased uptake of radiolabeled leukocytes in the spleen of this heme-treated animal. (B) Effects of heme on the biodistribution of111In-labeled leukocytes in C57Bl/6 mice 24 hours after injection. Animals treated with saline and heme are represented by white bars and black bars, respectively. Results are expressed as percentage injected dose per organ (% ID). All values are indicated as mean ± SD of 5 mice. There is a significant increase in the accumulation of radiolabeled leukocytes in the pancreas, kidneys (P < .0001), intestines, liver, brain, spleen (P < .01), and femur (P < .05) of the heme-treated animals compared to the saline-treated animals. (C) Biodistribution of 111In-labeled leukocytes in mice 24 hours after injection corrected for weight. Animals treated with saline and heme are represented by white bars and black bars, respectively. Results are expressed as percentage of injected dose per 0.1 g tissue (% ID/0.1 g). All values are indicated as mean ± SD of 5 mice. Accumulation of radiolabeled leukocytes in the pancreas, intestines, kidneys, brain, liver (P < .01), thymus, femur, and spleen (P < .05) of the heme-treated animals were significantly increased compared to levels in the saline-treated animals.
Effects of heme on leukocyte influx.
Leukocyte accumulation was measured by gamma camera imaging and biodistribution of 111In-labeled leukocytes in C57Bl/6 mice. (A) Scintigraphic images of mice 21 hours after injection with either saline or heme, followed by administration of111In-labeled leukocytes. Note that there is an increased uptake of radiolabeled leukocytes in the spleen of this heme-treated animal. (B) Effects of heme on the biodistribution of111In-labeled leukocytes in C57Bl/6 mice 24 hours after injection. Animals treated with saline and heme are represented by white bars and black bars, respectively. Results are expressed as percentage injected dose per organ (% ID). All values are indicated as mean ± SD of 5 mice. There is a significant increase in the accumulation of radiolabeled leukocytes in the pancreas, kidneys (P < .0001), intestines, liver, brain, spleen (P < .01), and femur (P < .05) of the heme-treated animals compared to the saline-treated animals. (C) Biodistribution of 111In-labeled leukocytes in mice 24 hours after injection corrected for weight. Animals treated with saline and heme are represented by white bars and black bars, respectively. Results are expressed as percentage of injected dose per 0.1 g tissue (% ID/0.1 g). All values are indicated as mean ± SD of 5 mice. Accumulation of radiolabeled leukocytes in the pancreas, intestines, kidneys, brain, liver (P < .01), thymus, femur, and spleen (P < .05) of the heme-treated animals were significantly increased compared to levels in the saline-treated animals.
After correcting for weight differences between organs, marked uptake of leukocytes in the spleen, liver, kidneys, thymus, intestines, femur, brain, and pancreas was assessed (Figure 4C). The effect of heme-induced radiolabeled leukocyte migration was subsequently corroborated by microscopic autoradiography (data not shown). These autoradiography studies showed heme-mediated leukocyte infiltration, further supporting a role for heme as an inflammatory mediator.
Effects of heme and heme oxygenase on leukocyte infiltration and adhesion molecule expression
Leukocyte infiltration and expression of several adhesion molecules was examined (immuno)histochemically to determine which inflammatory changes occur after heme administration. First, hematoxylin and eosin–stained sections of liver and pancreas from BALB/c mice were analyzed 24 hours after intravenous heme administration for the presence of infiltrates of leukocytes. Livers of heme-treated animals revealed several foci with pronounced leukocyte infiltration. These inflammatory infiltrates were frequently accompanied by necrotic areas (Figure5A-D). Serum alanine aminotransferase levels, which are increased under conditions involving necrosis of hepatocytes, had clearly increased after the administration of heme to mice (data not shown), supporting our observations on heme-induced liver injury. Interestingly, granulocyte influx levels into the livers of mice, as seen after treatment with heme alone, were similar to those of mice receiving lysed erythrocytes with the same concentration of heme (data not shown).
Effects of heme and HO on leukocyte infiltration.
Light microscopy pictures of liver sections of BALB/c mice stained with hematoxylin and eosin (H&E) (original magnification, × 40 [A,C,E,G], or × 400 [B,D,F,H]). Mice were treated for 24 hours with saline (A, B) or 750 μM heme (C, D). The lower 2 panels represent, respectively, mice pretreated for 24 hours with SnMP, followed by treatment with saline (E,F) or 750 μM heme (G,H) for 24 hours. Heme-treated mice show inflammatory lesions accompanied by leukocyte influx and liver cell injury. Animals in which HO-activity was inhibited by pharmacologic means (SnMP) show more severe inflammatory changes after heme exposure, as exemplified by larger lesions and more aggravated inflammatory cell infiltrates.
Effects of heme and HO on leukocyte infiltration.
Light microscopy pictures of liver sections of BALB/c mice stained with hematoxylin and eosin (H&E) (original magnification, × 40 [A,C,E,G], or × 400 [B,D,F,H]). Mice were treated for 24 hours with saline (A, B) or 750 μM heme (C, D). The lower 2 panels represent, respectively, mice pretreated for 24 hours with SnMP, followed by treatment with saline (E,F) or 750 μM heme (G,H) for 24 hours. Heme-treated mice show inflammatory lesions accompanied by leukocyte influx and liver cell injury. Animals in which HO-activity was inhibited by pharmacologic means (SnMP) show more severe inflammatory changes after heme exposure, as exemplified by larger lesions and more aggravated inflammatory cell infiltrates.
Next, the effect of HO activity on heme-induced leukocyte influx was investigated. Heme administration in mice lacking HO activity resulted in a significantly greater influx of leukocytes into the liver (Figure 5G-H) in comparison with that in mice given heme alone (Figure 5C-D) or SnMP alone (Figure 5E-F). Thus, HO activity protects against heme-mediated leukocyte infiltration.
Sections of pancreas from mice exposed to heme also showed severe alterations when compared to control mice treated with saline (Figure6). In the heme-treated animals, the presence of inflammatory hallmarks such as interstitial edema and cellular infiltration could easily be observed, corroborating our findings obtained in the biodistribution studies with the radiolabeled liposomes and leukocytes.
Effect of heme on inflammatory changes in the pancreas.
Sections of the pancreas of mice treated for 24 hours with saline (A,B) or heme (750 μM) (C,D) were stained with H&E (original magnification, × 40 [A,C] or × 400 [B, D]). Exposure to heme resulted in a variety of inflammatory changes in the pancreas, as illustrated by leukocyte influx and interstitial edema.
Effect of heme on inflammatory changes in the pancreas.
Sections of the pancreas of mice treated for 24 hours with saline (A,B) or heme (750 μM) (C,D) were stained with H&E (original magnification, × 40 [A,C] or × 400 [B, D]). Exposure to heme resulted in a variety of inflammatory changes in the pancreas, as illustrated by leukocyte influx and interstitial edema.
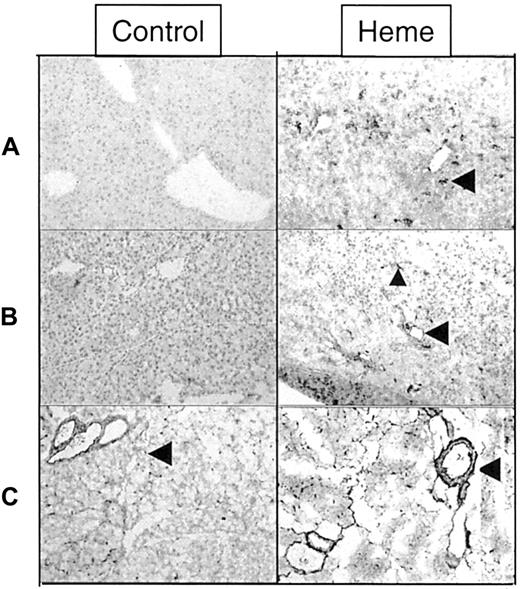
A panel of specific monoclonal antibodies against macrophages, granulocytes, T lymphocytes, and B lymphocytes was used to distinguish leukocyte subsets involved in heme-mediated migration. From these immunohistochemical stainings, it was evident that granulocytes formed the main leukocyte component of cellular infiltrate (Figure7A), whereas some macrophages were also observed (data not shown). T and B lymphocytes could not be detected within this time frame (1-24 hours; data not shown). In contrast, granulocyte infiltration was already evident in the liver as early as 1 hour after exposure. To investigate which adhesion molecules could be involved in mediating this migration, sections of liver and pancreas were examined for the expression of ICAM-1, VCAM-1, P selectin, and fibronectin. Heme strongly induced the expression of ICAM-1 in pancreas and liver on the luminal surfaces of vascular endothelial cells. Infiltrated and vascular leukocytes also became activated by heme, as illustrated by their enhanced ICAM-1 expression (Figure 7B). Vascular fibronectin expression was increased in sections of the pancreas of heme-treated animals. Fibronectin also seemed to stain other types of cells in the liver (Figure 7C). P selectin was expressed in mice 1 hour after heme administration, but P selectin could not be detected in mice treated with heme for 24 hours (data not shown).
Effect of heme on leukocyte infiltration and adhesion molecule expression.
Immunohistochemical analysis of liver and pancreas tissues of BALB/c mice after 24 hours of intravenous injection with either saline (left panel) or heme (750 μM) (right panel). (A) Detection of granulocytes (dark staining, arrowhead) in the liver using immunohistochemical analysis. Note the pronounced influx of granulocytes into the liver of heme-treated mice (GR-1 antibody). (B) ICAM-1 immunoreactive proteins (dark staining) in liver sections of mice treated with saline or heme (YN1/1 antibody). ICAM-1 can be identified on the endothelial lining (arrowhead) and on infiltrating leukocytes of heme-treated animals (small arrowhead). (C) Localization of fibronectin in pancreas sections (dark staining, arrowhead) (A0245 antibody). The expression of fibronectin proteins was clearly enhanced in the heme-treated animals (dark staining, arrowhead).
Effect of heme on leukocyte infiltration and adhesion molecule expression.
Immunohistochemical analysis of liver and pancreas tissues of BALB/c mice after 24 hours of intravenous injection with either saline (left panel) or heme (750 μM) (right panel). (A) Detection of granulocytes (dark staining, arrowhead) in the liver using immunohistochemical analysis. Note the pronounced influx of granulocytes into the liver of heme-treated mice (GR-1 antibody). (B) ICAM-1 immunoreactive proteins (dark staining) in liver sections of mice treated with saline or heme (YN1/1 antibody). ICAM-1 can be identified on the endothelial lining (arrowhead) and on infiltrating leukocytes of heme-treated animals (small arrowhead). (C) Localization of fibronectin in pancreas sections (dark staining, arrowhead) (A0245 antibody). The expression of fibronectin proteins was clearly enhanced in the heme-treated animals (dark staining, arrowhead).
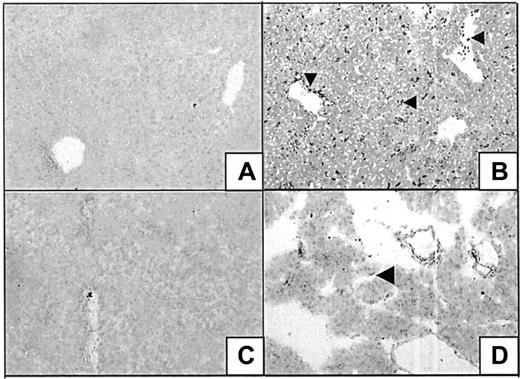
Immunohistochemical localization of HO expression
Heme oxygenase-1 and -2 expression levels were determined in liver and pancreas after the administration of saline or heme. HO-2 levels in hepatocytes were low and did not change in response to heme treatment in liver and pancreas (data not shown). However, HO-1 expression levels, not detectable in saline-treated animals, were strongly induced in distinct cell populations in the liver after exposure to heme. The irregular shapes and dendritic extensions suggest the presence of Kupffer cells, though no HO-1 expression has been found in the liver of saline-treated animals (Figure 8A-B). However, other cells such as sinusoidal endothelial cells and leukocytes may be involved, as demonstrated by the HO-1–positive staining of leukocytes present within the peripheral blood and of cells lining the vessels. Hepatocytes exhibited little or no staining in control or heme-treated animals. In the pancreas, heme-induced HO-1 expression was also restricted to single cells, most likely infiltrating leukocytes (Figure 8C-D).
Heme oxygenase expression in liver and pancreas.
Liver (A-B) and pancreas (C-D) of BALB/c mice after 24-hour exposure to either saline (A, C) or heme (B,D) were assayed for the expression of HO-1 immunoreactive proteins (SPA895 antibody). HO-1 is hardly detectable in control animals but is highly induced after heme exposure in residing Kupffer cells (arrowhead) and (infiltrating) leukocytes (arrowhead) or lining cells (arrowhead).
Heme oxygenase expression in liver and pancreas.
Liver (A-B) and pancreas (C-D) of BALB/c mice after 24-hour exposure to either saline (A, C) or heme (B,D) were assayed for the expression of HO-1 immunoreactive proteins (SPA895 antibody). HO-1 is hardly detectable in control animals but is highly induced after heme exposure in residing Kupffer cells (arrowhead) and (infiltrating) leukocytes (arrowhead) or lining cells (arrowhead).
Discussion
In this study we extended our earlier in vitro observations of heme-induced expression of endothelial adhesion molecules and the antiadhesive properties of HO activity to an in vivo model. Our results show that there are important roles for heme and HO in modulating inflammation in vivo (Figure 9). These are the first data that provide evidence of a proinflammatory role for heme in vivo. Heme administration resulted in increased vasopermeability, adhesion molecule expression, and tissue infiltration of leukocytes, which are hallmarks of inflammation. In contrast, HO has anti-inflammatory properties; the inhibition of HO activity exacerbated heme-induced inflammation. Furthermore, HO is crucial for the fast clearance of vascular heme. Our finding that heme and HO modulate inflammatory processes in an antagonistic manner offers an exciting new insight into the pathogenesis of diverse inflammatory processes, such as wound healing, ischemia-reperfusion injury, and vasculitis.
Model for the role of heme and heme oxygenase in inflammation.
Free heme interacts with the endothelial cell membrane (thick black line), resulting in oxidative stress, vasopermeability, adhesion molecule induction, leukocyte binding–migration, and HO-1 expression. In contrast, HO-1, the heme-degrading enzyme, acts as a feedback modulator by antagonizing the oxidative and inflammatory actions of heme through the formation of vasodilator carbon monoxide and antioxidants biliverdin/bilirubin.
Model for the role of heme and heme oxygenase in inflammation.
Free heme interacts with the endothelial cell membrane (thick black line), resulting in oxidative stress, vasopermeability, adhesion molecule induction, leukocyte binding–migration, and HO-1 expression. In contrast, HO-1, the heme-degrading enzyme, acts as a feedback modulator by antagonizing the oxidative and inflammatory actions of heme through the formation of vasodilator carbon monoxide and antioxidants biliverdin/bilirubin.
Several studies have reported that the administration of autologous whole blood increases vascular permeability and inflammation.46,47 In addition, Baldwin48showed recently that possible oxygen-carrying blood substitutes, the modified hemoglobin molecules αα-Hb and PEG-Hb, cause venular leakage in the rat mesentery. These effects resulted from changes in endothelial actin cytoskeleton and an increased number of endothelial gaps. Based on our findings, this effect is most likely mediated by the heme component of blood cells and hemoglobin. Our experiments with99mTc-labeled liposomes demonstrated a dramatic increase in vascular permeability in the pancreas after exposure to vascular heme. Heme also induced liposome accumulation in several other organs, such as the liver, spleen, and intestines.
However, despite our finding that heme administration caused a local increase in vascular permeability, we cannot rule out that heme-derived metabolites are responsible for these observations. Interestingly, recent data suggest that nitric oxide is capable of blocking inflammatory permeabilization.49 It is, therefore, tempting to speculate that carbon monoxide, which shares many functions with nitric oxide, also modulates vascular permeability.
There is a major distinction between the mechanism of liposome trafficking and leukocyte migration. Liposomes accumulate in inflamed tissues because of locally enhanced vascular permeability, whereas leukocytes actively migrate to inflammatory areas through specific adhesive interactions with the endothelium and chemotaxis.2,39-41111In-labeled leukocytes are an important clinical tool to image inflammatory foci in vivo.43 Our data clearly demonstrate that the presence of large amounts of vascular heme result in inflammatory infiltrates into various organs. Heme-induced leukocyte influx in our mouse model was present in spleen, liver, and intestines but not in lungs or heart. Although the pancreas showed substantially enhanced leukocyte accumulation, this increase was not as dramatic as the changes in permeability. Heme-induced enhancement in vasopermeability and leukocyte infiltration in mice was corroborated with (immuno)histochemical data, supporting our hypothesis that heme is a proinflammatory mediator. In the time frame studied (1-24 hours), heme-induced leukocyte infiltrates in liver and pancreas mainly consisted of granulocytes, whereas macrophages and lymphocytes were hardly present.
The present data demonstrate that the excess of free vascular heme forms a severe risk for inflammation in several organs, such as the liver and the pancreas. The liver is a major organ that helps in the detoxification of free heme molecules and biliary excretion of their metabolites, such as bilirubin, and requires self-protective mechanisms for tolerance against heme toxicity. Within the liver, Kupffer cells and liver sinusoidal cells are thought to play prominent roles in maintaining this tolerance.50 Accumulating evidence supports the concept that HO-1 induction is necessary to protect liver and pancreas homeostasis from stress conditions.26,27,50-52 The mechanism of heme-induced inflammation and organ damage might relate to our observation that heme activates proinflammatory genes and leukocyte recruitment whereas, in contrast, HO-1 attenuates proinflammatory signals. Previously, we showed that heme activates the endothelium in vitro, resulting in an up-regulation of proinflammatory genes ICAM-1, VCAM-1, and E-selectin.15 This effect is likely to be mediated by ROS and a compromised redox status, because glutathione attenuates heme-induced adhesion.28 Adhesion molecule up-regulation on the endothelium of inflamed tissues strongly suggests that the observed leukocyte influx is mediated through heme-induced cell surface expression of adhesion molecules such as selectins, fibronectin, and ICAM-1. The heme-induced inflammatory response caused a dose-dependent, highly reproducible leukocyte influx in inflamed tissues as monitored (immuno)histochemically. Our finding that besides endothelial activation, leukocytes were also activated after exposure to heme underscores the proinflammatory potential of heme. The present study contributes to insight into the mechanisms through which heme-hemoglobin overloading causes the deterioration of organ homeostasis under disease conditions.
The importance of heme in inflammatory processes in vivo is further emphasized by the rapid increased expression of hemoglobin and heme scavengers, haptoglobin and hemopexin, respectively, in response to inflammation.53-56 Hemopexin selectively delivers heme to cells expressing hemopexin receptors as present on cells in the liver and spleen. Observed differential effects of heme on the various organs may be partly related to a differential heme uptake by the various organs.
HO-1 is also induced during the resolution of inflammatory processes and may act as a feedback mechanism. Augmentation of HO-1 expression by gene transfer provides cellular resistance against hemoglobin–heme toxicity.24,25 We have previously shown that HO activity diminishes adhesion molecule expression.28 Antisense strategies demonstrated that mainly the HO-1 isoform is responsible for the down-regulation of these proinflammatory genes.28Thus, down-modulation of adhesion molecule expression may be part of the mechanism by which HO-1 functions in the resolution of inflammatory processes. Recent evidence suggests the involvement of the HO-1 downstream mediators carbon monoxide and biliverdin/bilirubin in signal transduction pathways, such as p38 mitogen-activated protein kinase.34 We are investigating whether HO-1 overexpression down-modulates proinflammatory gene expression by interfering with NF-κB translocation, analogous with NO and antioxidants.57 58
It was also demonstrated that HO activity is crucial for the fast clearance of heme from the circulation. In addition, the inhibition of HO activity dramatically increased heme-induced leukocyte infiltration into the liver and pancreas, suggesting that HO antagonizes the inflammatory actions of heme. Interestingly, heme breakdown products appear to mediate a down-regulation of low-density, lipoprotein-mediated monocyte chemotaxis.59 Our data suggest that HO activity acts in a similar fashion in inhibiting heme-mediated granulocyte chemotaxis.
Hancock et al16 elegantly demonstrated that HO activity is crucial for successful organ transplantation; organs not expressing HO-1 are rejected and develop microvascular dysfunction and arteriosclerosis. Ischemia-reperfusion injury is thought to play a major role in the pathogenesis of transplant rejection.60Based on our data, the elevated release of denatured hemoproteins, derived from injured cells, may form a major factor in the initiation or progression of inflammation during these processes and increase immune cell influx or activity after cellular damage, whereas HO activity prevents or ameliorates heme-induced inflammatory actions through the generation of its downstream anti-inflammatory effector molecules carbon monoxide and biliverdin/bilirubin.
Because lysed erythrocytes with similar concentrations of heme induced a granulocyte influx in the liver resembling the levels observed after heme alone, our observations after heme administration may be compared to the pathophysiological setting of severe hemolysis or rhabdomyolysis. Diseases characterized by vascular abnormalities, such as acute renal failure, hemorrhagic shock, hemolytic uremic syndrome, and thrombotic thrombocytic purpura, are associated with increased hemolysis,61-64 and one could speculate that heme causes the inflammatory onset in these diseases. Moreover, it was recently postulated that excessive heme release plays a major role in vaso-occlusive events in sickle cell disease.65,66 During hemolytic events, a sudden local increase in heme might overwhelm heme-hemoglobin scavengers and HO, leaving them unable to neutralize the oxidative and inflammatory effects of hemoglobin-heme and resulting in locally enhanced adhesion molecule expression, recruitment of inflammatory cells, and vascular dysfunction. This is exemplified by hemophilic hemarthrosis, in which blood cells entering the synovial space cause inflammatory complications and damage to the joints.67,68 A proinflammatory role for heme is further supported by clinical observations of thrombophlebitis69 after the administration of heme in healthy volunteers, demonstrating that heme can cause vascular inflammation followed by vascular obstruction in vivo. In addition, strenuous exercise can elicit muscle or soft tissue injury accompanied by myoglobinuria and inflammatory responses70,71 and activation of granulocytes.72
Although large amounts of heme act as pro-oxidative and proinflammatory modulators, other studies suggest that low concentrations of heme may be protective through the fast up-regulation of HO-1.29 30Future studies are warranted to determine this dual character of heme and whether preinduction and priming of the antioxidative–anti-inflammatory effects of the HO system can protect the body from severe insults, such as inflicted by hemoglobin-heme.
The novel model we propose (Figure 9) explains the clinically observed inflammatory manifestations accompanied by increased free heme levels. The observation that heme combines oxidative and inflammatory actions in vivo suggests that vascular heme causes endothelial cell injury, leading to inflammatory lesions and the formation of vascular inflammatory disorders. Our findings suggest an important contribution of heme to inflammation. On the other hand, HO activity is crucial in antagonizing heme-induced effects and protecting tissues from oxidative and inflammatory insults. The previously unrecognized inflammatory properties of heme and the attenuating role of HO may form the basis for the development of alternative approaches in controlling inflammation.
We thank Gerrie Grutters, Bianca Lemmers, Debbie Smits, and Geert Poelen (Central Animal Laboratory, University Nijmegen, The Netherlands) for their skilled assistance in the animal experiments. We also thank Peter Laverman and Emile Koenders for their expert help with the preparation and labeling of liposomes.
Supported by the Vanderes Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Frank A. D. T. G. Wagener, Department of Tumor Immunology, University Medical Center Nijmegen, PO Box g101, 6500 HB Nijmegen, The Netherlands; e-mail:f.wagener@dent.kun.nl.



![Fig. 5. Effects of heme and HO on leukocyte infiltration. / Light microscopy pictures of liver sections of BALB/c mice stained with hematoxylin and eosin (H&E) (original magnification, × 40 [A,C,E,G], or × 400 [B,D,F,H]). Mice were treated for 24 hours with saline (A, B) or 750 μM heme (C, D). The lower 2 panels represent, respectively, mice pretreated for 24 hours with SnMP, followed by treatment with saline (E,F) or 750 μM heme (G,H) for 24 hours. Heme-treated mice show inflammatory lesions accompanied by leukocyte influx and liver cell injury. Animals in which HO-activity was inhibited by pharmacologic means (SnMP) show more severe inflammatory changes after heme exposure, as exemplified by larger lesions and more aggravated inflammatory cell infiltrates.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/6/10.1182_blood.v98.6.1802/7/m_h81811529005.jpeg?Expires=1765883687&Signature=Rxdzf0wz8IOLe-686Sxia1tjH~10s~RfWHqD0mPj8vGo10Qptfvst7Ra9b4GnJ-prSyVivlOLnIsShlL6ihS1KArDGQ1h3bGptttafqcLeeQfXw6x9TebMg1RJ9rh9v-QTZExvtQLvxeMi0E4ZW0bDMZkUQLhimgVmeNnZ6SHMq8mHJdMgfBlpQtD-jyGR~4Xktn40~FUYfDiHCNRKPs8xUKyZF6uJpfqF~BECke~vf3s6mvl4aLTDludmJhbp9pRE5h6iT~WC481UTp5O3N8YEYmArHObRE4tA54OyJr0rgwHNvswhWgudQMUhyEbuTEeH16qJUjkvtw~63eP77sQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Effect of heme on inflammatory changes in the pancreas. / Sections of the pancreas of mice treated for 24 hours with saline (A,B) or heme (750 μM) (C,D) were stained with H&E (original magnification, × 40 [A,C] or × 400 [B, D]). Exposure to heme resulted in a variety of inflammatory changes in the pancreas, as illustrated by leukocyte influx and interstitial edema.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/6/10.1182_blood.v98.6.1802/7/m_h81811529006.jpeg?Expires=1765883687&Signature=RRq6r8GV7y56aOmhqcadVl1hg1jk4EMqjxDIkzheo0ht2NA8JO4NNNdxn4bdQNRBPDFMVasLkinp2RMC7pgqYmfWEdYbGtL39ozy2wczvVVPEV63Lqk1UB5Ol8kQB6o2AYb6jZERZBggJoqgJ0yqJByTpc0HJd3SYFDG3KxkGA3phrot7qY8vQ21YrWNCXeq5cdJ18sZKUQXG4qVwvAG3LMOB0URgP8XS7S33JWNkYWhA7EPtrb8RQ3gl2782ZMK~QvNd6A~B0kMPMyllekMzT8F5n6SH3DrRMJr9CMD7DcARRwdZpdkVxCsDnjS-KsODNAcPClVi8VHCa2DpyExCw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal