Abstract
To maintain hemostasis under shear conditions, there must be an interaction between the platelet glycoprotein (GP) Ib-IX receptor and the plasma ligand von Willebrand factor (vWf). In platelet-type von Willebrand disease (Pt-vWD), hemostasis is compromised. Two mutations in the GPIbα polypeptide chain have been identified in these patients—a glycine-233 to valine change and a methionine-239 to valine change. For this investigation, these mutant proteins have been expressed in a Chinese hamster ovary cell model system. Ligand-binding studies were performed at various concentrations of ristocetin, and adhesion assays were performed under flow conditions. The Pt-vWD mutations resulted in a gain-of-function receptor. vWf binding was increased at all concentrations of ristocetin examined, and adhesion on a vWf matrix was enhanced in terms of cell tethering, slower rolling velocity, and decreased detachment with increasing shear rate. Two other mutations were also introduced into the GPIbα chain. One mutation, encompassing both the Pt-vWD mutations, created an increase in the hydrophobicity of this region. The second mutation, involving a valine-234 to glycine change, decreased the hydrophobicity of this region. Both mutations also resulted in a gain-of-function receptor, with the double mutation producing a hyperreactive receptor for vWf. These data further support the hypothesis that ligand binding is regulated by conformational changes in the amino-terminal region of GPIbα, thereby influencing the stability of the GPIbα–vWf interaction.
Introduction
Hemostasis under physiological flow conditions is dependent on the interaction between the platelet glycoprotein (GP)Ib receptor and the plasma ligand von Willebrand factor (vWf). GPIb is a heterodimer consisting of an α-chain disulfide linked to a β-chain.1 On the platelet surface, GPIb associates with 2 other glycoproteins to form the GPIb-V-IX complex.2-4 The amino-terminal domain of the GPIbα subunit contains the binding site for vWf,5-7 and numerous studies have examined specific residues within this domain to further understand the molecular basis of hemostasis.8-10
These studies have taken advantage of recombinant technology, which allows the examination of this platelet receptor in a heterologous cell line.11-13 Functional studies clearly show the competency of recombinant GPIb-IX expressed on the surfaces of Chinese hamster ovary (CHO) cells to bind human vWf.8,11,12,14-18 Binding to vWf can be measured in the presence of mediators such as ristocetin and botrocetin, and adhesion to immobilized vWf can be examined under flow conditions.14,17 18
The 2 mutations, associated with platelet-type von Willebrand disease (Pt-vWD), result in a receptor with an increased affinity for vWf. The first mutation identified was a nucleotide substitution from GGT to GTT (position 1281), resulting in a glycine-233 to valine change,19 and the second was an ATG to GTG (position 1299) substitution converting methionine-239 to valine.20,21Clinically, there is increased sensitivity to ristocetin leading to increased platelet aggregation in Pt-vWD, especially at low levels of ristocetin where aggregate formation in normal platelets is not induced.22 In addition, the shear stress at which Pt-vWD platelets will aggregate (14 dyne/cm2) is significantly reduced compared with that required to induce normal platelet aggregation (60 dyne/cm2).23 24
Two previous studies have examined vWf-binding to the Pt-vWD mutations using a recombinant soluble fragment of GPIbα encompassing histidine-1 to alanine-30225,26; binding studies were performed using immobilized GPIbα peptides. An additional study examined the G233V mutation; the recombinant fragment was coated onto latex beads, and binding to immobilized vWf was performed under flow conditions.27 Another study expressed a chimeric protein consisting of histidine-1 to valine-289 of GPIbα fused with the C-terminal domain of calmodulin.28 The data from these studies indicate that Pt-vWD mutations result in increased binding between GPIbα and vWf.
Recently, vWf binding to cell lines expressing GPIbα containing the Pt-vWD mutations as part of an intact receptor complex was examined.29 The focus of the study by Dong et al29 was the change to valine that occurs in Pt-vWD, and the use of scanning mutagenesis revealed other mutations to valine within this region of GPIbα that led to either gain-of-function or loss-of-function receptors.
In the current investigation, 4 phenotypic changes were introduced into GPIbα cDNA, and mutant GPIbα was expressed in CHO cells already expressing GPIbβ and GPIX. To determine the effect of the mutations associated with Pt-vWD, cell lines were created expressing the G233V and M239V mutations. Two other mutations were also examined. A double mutation encompassing both Pt-vWD substitutions was made (G233V/M239V), which would serve to further increase the hydrophobic nature of the receptor. A novel mutation in which valine-234 was replaced by a glycine was also introduced. This mutation is the converse of the Pt-vWD mutations and serves to decrease the hydrophobicity of this region. Valine-234 is the only valine residue positioned between glycine-233 and methionine-239 that does not form part of the hydrophobic face in the amphipathic helical structure proposed by Pincus et al.30,31 Valine-234 is also positioned opposite the 2 known Pt-vWD mutations in the proposed helical formation, as depicted by Dong et al.29
Binding of vWf to the 4 mutant proteins was examined in a membrane-bound and complexed form of GPIbα. Binding of soluble vWf demonstrated that the mutations resulted in a gain-of-function receptor. In addition, aggregation studies highlighted the uniqueness of the double mutant, which spontaneously bound vWf in the absence of ristocetin or shear. Examination of adhesion to immobilized vWf under flow conditions revealed that all the Pt-vWD mutants exhibited enhanced adhesion to vWf compared with wild type.
Materials and methods
Reagents
Monoclonal antibodies (mAbs) AK2 (anti-GPIbα) and AK3 (anti-GPIbα) and purified human von Willebrand factor were a kind gift from Dr Michael Berndt (Baker Medical Research Institute, Melbourne, Australia). The CHO cell line expressing the GPIbβ and GPIX subunits (CHO-βIX) and the cDNA for GPIbα were generously donated by Dr José López (Baylor College of Medicine, Houston, TX).
The plasmid pZeoSV and the antibiotic zeocin were from Invitrogen (Carlsbad, CA). The pAlter-1 vector was from Promega (Madison, WI), and the Qiafilter plasmid Maxi kit was from Qiagen (Hilden, Germany). Fluorescein isothiocyanate (FITC)-conjugated rabbit anti–mouse immunoglobulin (Ig)G antibody was from Silenus Laboratories (Melbourne, Australia), and the FITC-conjugated anti–human vWf was from Serotec (Oxford, United Kingdom). Fetal bovine serum and Dulbecco modified Eagle medium (DMEM) were from Trace Bioscientific (Melbourne, Australia). Sodium bicarbonate and ristocetin were from Sigma Chemical (St Louis, MO).
Site-directed mutagenesis
Full-length cDNA for GPIbα was subcloned into the mutagenesis vector pAlter-1. Site-directed mutagenesis was performed on single-stranded DNA as described by the manufacturer. Oligonucleotides G233V (PO4-TGG AAG CAA GTT GTG GAC G), M239V (PO4-TCA AGG CCG TGA CTT CTA ACG TG), and V234G (PO4-GCA AGG TGG TGA TGT CAA GG) were used to substitute glycine-233 for valine, methionine-239 for valine, and valine-234 for glycine, respectively. To achieve the double mutation glycine-233 and methionine-239 to valine, oligonucleotides G233V and M239V were used in combination. All oligonucleotides were made by Life Technologies (Gaithersburg, MD). DNA was sequenced to confirm the presence of the directed mutations and to identify any nonspecific mutations.
Transfection of Chinese hamster ovary cells
CHO-βIX cells were grown in DMEM containing L-glutamine and 4.5 g/L glucose, supplemented with 3.7 g/L sodium bicarbonate and 10% fetal bovine serum. Cells were incubated at 37°C in an atmosphere of 5% carbon dioxide and 90% humidity.
The cDNAs for wild-type GPIbα and mutated GPIbα were subcloned into the expression vector pZeoSV. Plasmid DNA was purified using the QIAfilter Plasmid Maxi kit, and 2 μg was introduced into the CHO-βIX cell line using Lipofectamine PLUS (Life Technologies). Two control cell lines were prepared. One cell line was transfected with wild-type GPIbα and designated wild type, and the other was transfected with vector alone and designated βIX-zeo. Cells were put under selection pressure after 72 hours using 350 μg/mL zeocin, and single-cell populations of cells expressing all 3 GPIb-IX subunits were prepared by single-cell deposition using a FACStar Plus flow cytometer (Becton Dickinson, San Jose, CA). Clones were analyzed by flow cytometry to confirm expression of the GPIb-IX complex.
Analysis of GPIb-IX surface expression by flow cytometry
Cells (1 × 105) were resuspended in DMEM–5% fetal bovine serum and incubated for 10 minutes on ice with mAb (2 μg/mL) against GPIbα (AK2 and AK3). Cells were washed twice with phosphate-buffered saline (PBS), incubated for 10 minutes on ice with FITC-conjugated rabbit anti–mouse IgG diluted 1:175, washed twice, and resuspended in 0.5% bovine serum albumin–PBS followed by flow cytometric analysis on a FACStar Plus (Becton Dickinson).
Ristocetin-mediated vWf-binding assay
Cells were harvested with 0.53 mM EDTA and washed twice with PBS. Cells (1 × 105) were incubated with either anti-GPIbα antibody AK3 or a mouse isotype control antibody (Becton Dickinson) and prepared for flow cytometric analysis. Cells (1 × 105) were also incubated for 20 minutes at room temperature with increasing concentrations of vWf (0-16 μg/mL) in the presence of 0, 0.25, 0.5, and 0.75 mg/mL ristocetin sulfate. Unbound vWf was removed with 4 washes in 0.1 M sodium acetate–0.5 × PBS. Cells were then incubated for 10 minutes on ice with FITC-conjugated anti-human vWf diluted 1:35 and washed twice with PBS. All cells were resuspended in 0.5% bovine serum albumin–PBS and analyzed by flow cytometry. Data are expressed as a ratio of the mean channel fluorescence for vWf binding over the mean channel fluorescence of AK3 binding to normalize for the level of GPIbα expressed by the various recombinant cells.
To determine whether the vWf binding observed was specific, cells were preincubated for 10 minutes on ice with the blocking anti-GPIbα antibody AK2 (50 μg/mL) before exposure to vWf.
Chinese hamster ovary cell aggregation assay
Wild-type, G233V, M239V, and G233V/M239V cells (1.6 × 106/mL) were resuspended in modified Tyrode buffer (10 mM HEPES, 12 mM NaHCO3, 137 mM NaCl, 2.7 mM KCl, 5 mM glucose, pH 7.4) containing 2 mM EDTA. Aggregation was initiated using 10 μg/mL human vWf in the absence of ristocetin with constant stirring. Aggregation was monitored using a Chronolog Dual-Channel Aggro-meter, and after 5 minutes cells were fixed using an equal volume of 2% paraformaldehyde. Cell samples were mounted onto glass microscope slides, and differential interference contrast images (× 5 objective; DMIRB microscope; Leica, Wetzlar, Germany) were captured using MCID image analysis software (Imaging Research, St Catharines, ON, Canada).
Flow-based adhesion assay
Flow assays were performed according to a modified method of Cooke et al.32 Microcapillary tubes (Microslides; Vitro Dynamics, Mountain Lakes, NJ) were coated overnight at 4°C with 100 μg/mL human vWf, washed 3 times with PBS, and blocked for 30 minutes with 25% heat-inactivated human serum. For these experiments, one representative clone for each mutation was examined. CHO cells were resuspended in Tyrode buffer (supplemented with 2 mM EDTA) at 1 × 106/mL and perfused through vWf-coated microcapillary tubes at a defined flow rate to generate a shear stress of 1 dyne/cm2 for 5 minutes. Cell tethering was assessed at 1, 3, and 5 minutes after cells first entered the microcapillary tube. After 5-minute perfusion, tethered cells were subjected to incremental increases in shear stress at 1-minute intervals from 1 to 5, 20, 40, and 60 dyne/cm2. Adherent cells were visualized using phase-contrast microscopy (IX70; Olympus, Tokyo, Japan), and images were video-recorded for off-line analysis. CHO cell tethering and rolling was quantitated as described previously.14Cell detachment was measured by the number of adherent cells/field over 5 fields at 5, 20, 40, and 60 dyne/cm2.
Statistical analysis
Student unpaired t test was used to test for differences between the cell lines. P values less than .05 were considered statistically significant.
Results
Generation of recombinant GPIbα-expressing cell lines
To examine the effects of the Pt-vWD and associated mutations, plasmids subcloned with either wild-type or mutant cDNA for GPIbα were constructed. They contained either of the 2 mutations associated with Pt-vWD (G233V and M239V), a change encompassing both Pt-vWD mutations or the V234G mutation. Cell lines were generated expressing wild-type GPIbα associated with GPIbβ and GPIX (wild type), a vector only control cell line (βIX-zeo), and the 4 GPIbα substitutions G233V, M239V, G233V/M239V, and V234G.
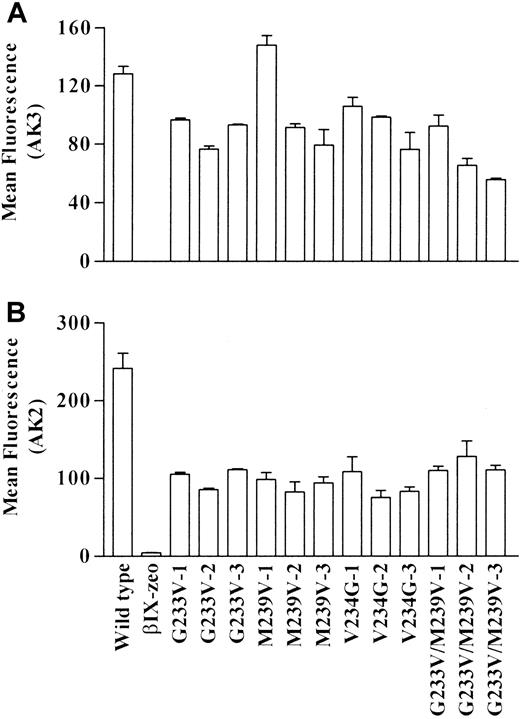
Surface expression of GPIbα was determined by incubating cells with mAb AK3; this was followed by flow cytometry analysis. The epitope for AK3 is within the macroglycopeptide region, and its binding should not be affected by the mutations. Data for 3 clones of each mutant cell line are presented in Figure 1A. Wild-type and all mutant cells bound AK3, indicating that these cells did express the GPIbα receptor on their surfaces. Binding was specific to GPIbα because βIX-zeo cells failed to bind AK3.
Surface expression of GPIb on recombinant cell lines.
Transfected cell lines were incubated with the anti-GPIbα mAbs (A) AK3 and (B) AK2. Cell autofluorescence was determined by use of a mouse isotype control antibody. Bound mAbs were detected using an FITC-labeled anti–mouse IgG antibody, followed by flow cytometric analysis. Mean fluorescence data are plotted ± SEM.
Surface expression of GPIb on recombinant cell lines.
Transfected cell lines were incubated with the anti-GPIbα mAbs (A) AK3 and (B) AK2. Cell autofluorescence was determined by use of a mouse isotype control antibody. Bound mAbs were detected using an FITC-labeled anti–mouse IgG antibody, followed by flow cytometric analysis. Mean fluorescence data are plotted ± SEM.
To determine whether the mutations caused conformational changes to the amino-terminal region of GPIbα, the cells were also incubated with mAb AK2. The epitope for AK2 has been localized to residues within the first leucine-rich repeat of GPIbα18 and is known to block vWf binding to platelets33 and recombinant GPIbα.18 Data presented in Figure 1B demonstrate that all the mutant clones bound significantly less AK2 than wild-type clones, indicating that the mutations did induce a conformational change to the amino-terminal region of GPIbα.
Binding of vWf to GPIbα on Chinese hamster ovary cells
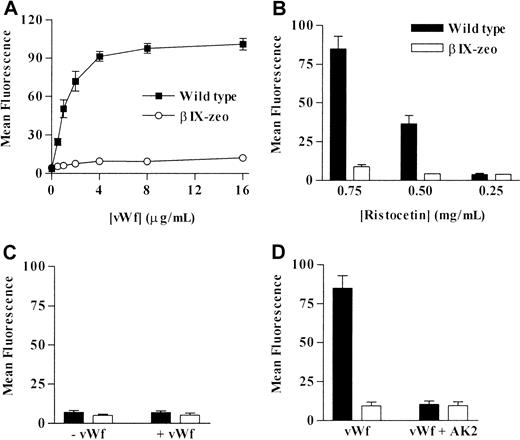
To examine the effect of the mutations on vWf binding, cell lines were treated with human vWf in the presence or absence of ristocetin. Wild-type cells incubated with 0.75 mg/mL ristocetin and vWf (0-16 μg/mL) bound vWf in a dose-dependent manner (Figure2A), whereas vWf was not detected bound to βIX-zeo cells. When the ristocetin concentration was reduced to 0.5 mg/mL, wild-type cells still bound increasing amounts of vWf as the ligand concentration increased, but the binding was 40% to 60% lower than that measured at 0.75 mg/mL (Figure 2B). Bound vWf was not detected on either wild-type or βIX-zeo cells at 0.25 mg/mL ristocetin (Figure 2B) or in the absence of ristocetin (Figure2C).
Effect of decreasing concentrations of ristocetin on vWf binding to control cell lines.
(A) βIX-zeo and wild-type control cell lines were incubated with increasing concentrations of vWf at 0.75 mg/mL ristocetin. Control cells were also incubated with 4 μg/mL vWf in (B) decreasing concentrations of ristocetin and in (C) the absence of ristocetin (mean ± SD, n = 5). (D) Finally, the cells were pre-incubated with 50 μg/mL AK2 or buffer alone before reacting with 4 μg/mL vWf in 0.75 mg/mL ristocetin to determine the specific nature of vWf binding to the recombinant cells (mean ± SD, n = 4). Bound vWf was detected using an FITC-conjugated anti–human vWf antibody, followed by flow cytometric analysis.
Effect of decreasing concentrations of ristocetin on vWf binding to control cell lines.
(A) βIX-zeo and wild-type control cell lines were incubated with increasing concentrations of vWf at 0.75 mg/mL ristocetin. Control cells were also incubated with 4 μg/mL vWf in (B) decreasing concentrations of ristocetin and in (C) the absence of ristocetin (mean ± SD, n = 5). (D) Finally, the cells were pre-incubated with 50 μg/mL AK2 or buffer alone before reacting with 4 μg/mL vWf in 0.75 mg/mL ristocetin to determine the specific nature of vWf binding to the recombinant cells (mean ± SD, n = 4). Bound vWf was detected using an FITC-conjugated anti–human vWf antibody, followed by flow cytometric analysis.
To determine the specificity of this binding, both wild-type and βIX-zeo cells were preincubated with the anti-GPIbα mAb AK2 to inhibit vWf-binding,18 32 followed by an incubation with 4 μg/mL vWf in the presence of 0.75 mg/mL ristocetin. vWf binding to wild-type cells was completely inhibited in the presence of AK2 (Figure2D).
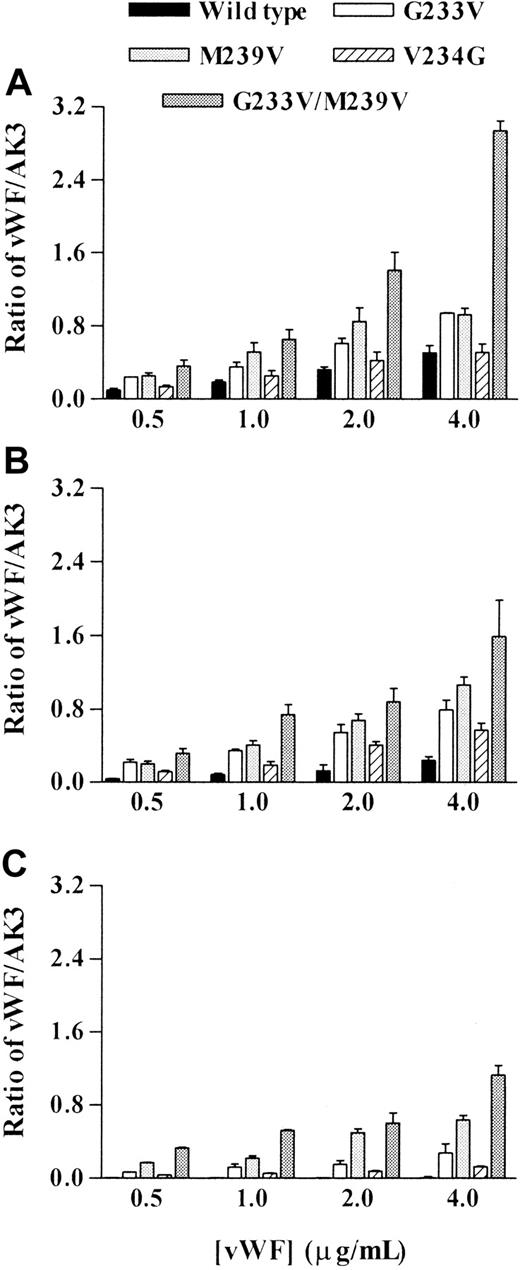
Examination of vWf binding to CHO cells expressing mutant GPIbα revealed significant differences when compared with cells expressing a wild-type receptor. Analysis was performed on at least 3 clones, and the data were combined for each cell line and normalized for GPIbα expression. At all concentrations of ristocetin examined (Figure3), the G233V and M239V cells bound significantly more vWf than wild type (n = 4; P < .05). V234G cells bound similar levels of vWf as did wild type at 0.75 mg/mL ristocetin. However, at lower vWf concentrations, V234G cells bound more vWf than wild type (n = 3; P < .05). G233V/M239V cells bound the most vWf at each concentration of ristocetin examined (n = 5; P < .05-.005). Of significance, this cell line was unique in its ability to bind vWf in the absence of ristocetin (Figure 4A), with the remaining mutants demonstrating no specific binding.
Effect of platelet-type vWD mutations on vWf-binding with decreasing concentrations of ristocetin.
Transfected cell lines were incubated with increasing concentrations of vWf (0-4 μg/mL) at decreasing concentrations of ristocetin: (A) 0.75, (B) 0.5, and (C) 0.25 mg/mL. Bound vWf was detected using an FITC-conjugated anti-human vWf antibody, followed by flow cytometric analysis. Data are expressed as a ratio of the mean fluorescence of vWf binding to that of AK3 binding. Mean ratio ± SEM from 3 experiments is shown.
Effect of platelet-type vWD mutations on vWf-binding with decreasing concentrations of ristocetin.
Transfected cell lines were incubated with increasing concentrations of vWf (0-4 μg/mL) at decreasing concentrations of ristocetin: (A) 0.75, (B) 0.5, and (C) 0.25 mg/mL. Bound vWf was detected using an FITC-conjugated anti-human vWf antibody, followed by flow cytometric analysis. Data are expressed as a ratio of the mean fluorescence of vWf binding to that of AK3 binding. Mean ratio ± SEM from 3 experiments is shown.
vWf interaction with the G233V/M239V cell line in the absence of ristocetin.
(A) Wild-type, G233V, M239V, V234G, and G233V/M239V cells were incubated with 0 or 4 μg/mL vWf in the absence of ristocetin. Bound vWf was detected using an FITC-conjugated anti–human vWf antibody, followed by flow cytometric analysis. (B) CHO cells (wild type, G233V, M239V, and G233V/M239V) were stirred for 5 minutes in the presence of 10 μg/mL human vWf without ristocetin. G233V/M239V was also pretreated for 10 minutes with 5 μg/mL AK2 before the addition of vWf (G233V/M239V + AK2). Cells were fixed and mounted onto glass microscope slides, and differential interference contrast images were captured as described in “Materials and methods.” (C) Aggregation traces for wild-type cells incubated with 10 μg/mL vWf in the presence (+/+) and absence (+/−) of 1 mg/mL ristocetin are presented. Traces for the G233V/M239V mutant incubated with vWf in the absence of ristocetin (+/−) and after pretreatment with AK2 (+/− and AK2) are also presented. Aggregation traces are representative of 3 experiments.
vWf interaction with the G233V/M239V cell line in the absence of ristocetin.
(A) Wild-type, G233V, M239V, V234G, and G233V/M239V cells were incubated with 0 or 4 μg/mL vWf in the absence of ristocetin. Bound vWf was detected using an FITC-conjugated anti–human vWf antibody, followed by flow cytometric analysis. (B) CHO cells (wild type, G233V, M239V, and G233V/M239V) were stirred for 5 minutes in the presence of 10 μg/mL human vWf without ristocetin. G233V/M239V was also pretreated for 10 minutes with 5 μg/mL AK2 before the addition of vWf (G233V/M239V + AK2). Cells were fixed and mounted onto glass microscope slides, and differential interference contrast images were captured as described in “Materials and methods.” (C) Aggregation traces for wild-type cells incubated with 10 μg/mL vWf in the presence (+/+) and absence (+/−) of 1 mg/mL ristocetin are presented. Traces for the G233V/M239V mutant incubated with vWf in the absence of ristocetin (+/−) and after pretreatment with AK2 (+/− and AK2) are also presented. Aggregation traces are representative of 3 experiments.
To verify these observations, CHO cell aggregation assays were performed using wild-type, G233V, M239V, and G233V/M239V cells. As demonstrated in Figure 4B-C and Table 1, wild-type, G233V, and M239V cells did not aggregate when stimulated with 10 μg/mL human vWf alone (in the absence of ristocetin). In contrast, G233V/M239V rapidly formed aggregates in the absence of ristocetin; maximal aggregation was observed within 1 minute. This aggregation was specific to the vWf-GPIb interaction because it was completely abolished by preincubating G233V/M239V cells with AK2.
Results of aggregation studies of CHO cell lines in the absence of ristocetin
| Cell line . | Aggregation (%) . |
|---|---|
| Wild type | 0 -6 |
| G233V | 0 -3 |
| M239V | 0 -6 |
| G233V/M239V | 72 -78 |
| Cell line . | Aggregation (%) . |
|---|---|
| Wild type | 0 -6 |
| G233V | 0 -3 |
| M239V | 0 -6 |
| G233V/M239V | 72 -78 |
One hundred percent aggregation is equivalent to wild-type cells incubated with 10 μg/mL vWf in the presence of 1 mg/mL ristocetin.
Adhesion of recombinant GPIbα expressing cells under flow conditions
To examine the effect of the Pt-vWD mutants and the 2 distinct mutations on the ability of cells to adhere to immobilized human vWf under physiologically relevant flow conditions, GPIbα-transfected CHO cells were perfused through vWf-coated microcapillary tubes. It has been demonstrated that under these conditions, GPIb-IX–transfected CHO cells are able to tether and roll on human vWf at a shear stress of up to 20 dyne/cm2.14 This adhesion is specific to GPIbα because CHO-βIX cells, or cells expressing wild-type GPIb-IX pretreated with AK2, fail to adhere. In the current study, 3 aspects of cell adhesion were compared; ability of cells to tether from flow, cell rolling velocity, and ability of cells to withstand the effects of increasing shear stress, providing an indication of the strength of adhesion. All flow experiments were performed in the presence of 2 mM EDTA to ensure that endogenous CHO cell integrins did not contribute to the adhesion process.
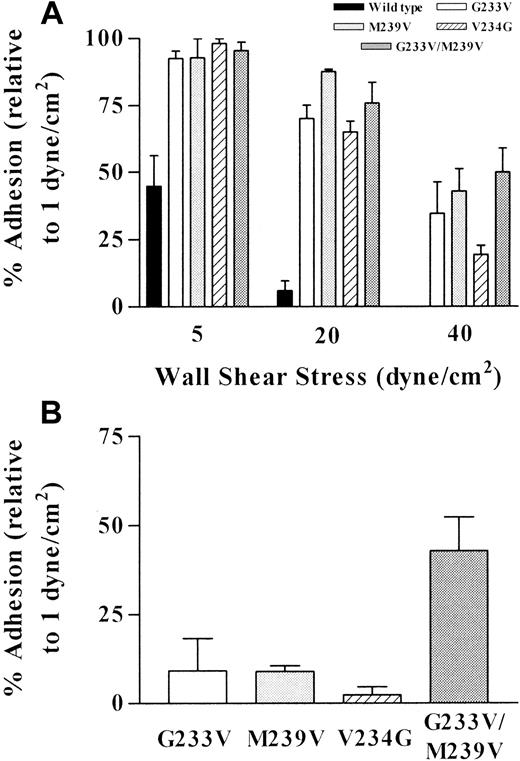
The data presented in Figure 5 indicate how the mutations in GPIbα affected the affinity of the vWf-GPIb interaction. Figure 5A demonstrates that all cell lines were able to tether and roll on surface-bound vWf at 1 dyne/cm2. However, the tethering ability of all 4 mutants was much greater than that of wild-type cells at all 3 time points analyzed. At 1 minute, tethering of mutant cells was between 145% and 179% of the tethering measured for wild-type cells, increasing to between 171% and 233% at 3 minutes. The greatest difference in tethering was observed at 5 minutes, when the number of adherent mutant cells was significantly higher than the number of wild-type cells for all 4 mutants (186%-293%, n = 3-4; P < .05-.01). These data suggest that all substituted forms of GPIbα expressed in CHO cells have increased affinity for vWf, as measured by an increased efficiency of cell tethering, compared with cells expressing the wild-type receptor.
Effect of platelet-type vWD mutations on adhesion to vWf under flow conditions.
Flow-based adhesion assays were performed as described in “Materials and methods.” (A) Cells were perfused at 1 dyne/cm2, and cell tethering was quantitated at 1, 3, and 5 minutes after cells first entered the capillary tube. Ten fields were analyzed at each time point for each cell line. Data are the mean ± SEM from 3 or 4 experiments. (B) Rolling velocities were compared for all cell lines at a shear stress of 5, 20, and 40 dynes/cm2. For each cell line, 5 randomly selected cells per field over 5 fields were analyzed for rolling velocity. Data are the mean ± SEM from 3 or 4 experiments. (Note: the rolling velocity of wild-type cells at 40 dynes/cm2 was only analyzed in one experiment given that in most experiments insufficient cells remained adherent for quantitation purposes).
Effect of platelet-type vWD mutations on adhesion to vWf under flow conditions.
Flow-based adhesion assays were performed as described in “Materials and methods.” (A) Cells were perfused at 1 dyne/cm2, and cell tethering was quantitated at 1, 3, and 5 minutes after cells first entered the capillary tube. Ten fields were analyzed at each time point for each cell line. Data are the mean ± SEM from 3 or 4 experiments. (B) Rolling velocities were compared for all cell lines at a shear stress of 5, 20, and 40 dynes/cm2. For each cell line, 5 randomly selected cells per field over 5 fields were analyzed for rolling velocity. Data are the mean ± SEM from 3 or 4 experiments. (Note: the rolling velocity of wild-type cells at 40 dynes/cm2 was only analyzed in one experiment given that in most experiments insufficient cells remained adherent for quantitation purposes).
Figure 5B demonstrates that when the shear stress was increased sequentially to 5, 20, and 40 dyne/cm2, the rolling velocity of all cell lines increased with increasing shear stress. More important, there were substantial differences in rolling velocities between wild-type and mutant cell lines, suggesting alterations in bond dissociation. Cells expressing wild-type receptor rolled the fastest at 5 and 20 dyne/cm2 and were unable to remain adherent above this shear stress. The rolling velocity at 5 dyne/cm2 was significantly faster than for all mutants (n = 3-4;P < .001-P < .05) except for the V234G mutation (n = 3; P > .05). Similar observations were made at 20 dyne/cm2. However, there were sufficient adherent wild-type cells to analyze rolling velocity in only 1 of 4 experiments. Statistical analysis was, therefore, not possible when comparing wild-type with mutant cell lines. Rolling velocities of the cells expressing the 2 known Pt-vWD mutations (G233V and M239V) were similar to each other (particularly at higher shear stress), indicating that a single substitution to valine either in position 233 or position 239 had a similar effect on ligand binding. Comparison of the 2 distinct mutations revealed a number of interesting observations. First, the rolling velocity of the double mutant was the slowest at all shear rates tested. This observation is indicative of a hyperreactive receptor, with dramatically reduced bond dissociation compared to the wild-type receptor. In contrast, the V234G mutation, though exhibiting enhanced tethering similar to the other mutants, rolled significantly faster than the double mutant at both 20 and 40 dyne/cm2(n = 3; P < .05). This observation raises the interesting possibility that the V-to-G substitution at this position primarily affects the association of the receptor–ligand interaction rather than the dissociation.
Increasing the shear stress also revealed a dramatic difference between wild-type cells and all mutant cell lines in terms of their ability to resist the detaching effects of increasing shear stress (Figure6A). In wild-type cells, increasing the shear stress to 5 dyne/cm2 resulted in only 45% of tethered cells remaining adherent, whereas at the same shear stress, more than 90% of all 4 mutants were still adherent. An additional increase to 20 dyne/cm2 virtually abolished the adhesion of wild-type cells (6% remained adherent) but had only a negligible affect on the adhesion of the mutants (65%-88% adherent). At 40 dyne/cm2, the few wild-type cells that remained adherent at 20 dyne/cm2 were detached from the matrix. The double mutant exhibited a slightly greater ability to remain adherent at 40 dyne/cm2 than the 2 known vWD mutations, but it was significantly more resistant to the detaching effects of high shear when compared with the V234G mutant (n = 3; P < .05).
Effect of platelet-type vWD mutations on shear-dependent cell detachment.
Cell detachment from the vWf matrix was determined at (A) 5, 20, and 40 dynes/cm2 and (B) 60 dynes/cm2, as described in “Materials and methods.” It is expressed as the percentage adhesion at each shear stress relative to the number of adherent cells at 1 dyne/cm2, arbitrarily defined as 100%.
Effect of platelet-type vWD mutations on shear-dependent cell detachment.
Cell detachment from the vWf matrix was determined at (A) 5, 20, and 40 dynes/cm2 and (B) 60 dynes/cm2, as described in “Materials and methods.” It is expressed as the percentage adhesion at each shear stress relative to the number of adherent cells at 1 dyne/cm2, arbitrarily defined as 100%.
Furthermore, when the shear stress was increased to 60 dyne/cm2, almost 50% of the cells expressing the double mutation retained the ability to adhere and roll on vWf (Figure 6B), whereas few of the other 3 mutants remained adherent. Combined with the rolling velocity data, this indicates that the overall adhesive strength of the GPIb-vWf interaction is increased with all substituted forms of GPIb, most significantly in cells expressing the double mutant.
Discussion
This study has demonstrated that the Pt-vWD mutations identified in GPIbα (G233V and M239V) result in a change in the interaction between GPIb and vWf, where there was an increase in the overall adhesive strength and stability of the mutant GPIb-vWf bond. The G233V and M239V mutants bound increased levels of soluble vWf at low concentrations of ristocetin, which has been observed with platelets obtained from patients with Pt-vWD.22 There was also increased cell tethering, decreased rolling velocity, and decreased detachment on immobilized vWf. These gain-of-function Pt-vWD mutations were also associated with decreased reactivity to the GPIbα mAb AK2, indicating a conformational change had occurred in the mutant receptors.
This study also examined the effect on vWf-binding associated with the change in hydrophobicity using the V234G and G233V/M239V mutants. Both these mutations also resulted in a gain-of-function receptor with decreased reactivity to AK2. Initially, these mutations were created to examine the effect of increasing or decreasing hydrophobicity on the GPIb-vWf interaction. The data indicated that a structural change seemed to have occurred with the change in hydrophobicity that resulted in the increased affinity for vWf. However, this does not answer why a structural change in this region would change the AK2 binding epitope. One possible suggestion is either that the binding region for AK2 is in proximity to the disulfide loop sequence encompassing the Pt-vWD mutations or that there is interaction between these 2 regions. The mutations described in this article might alter the conformation of the protein enough to influence both regions.
The change in the conformation of GPIbα has been proposed by Pincus et al.30,31 Computer modeling of the wild-type sequence encompassing glycine-233 has predicted that a beta-turn at the glutamine-232–glycine-233 junction had the lowest energy conformation. If glycine-233 were changed to valine, the lowest energy form would be an amphipathic alpha-helix. If methionine-239 were changed to valine, the lowest energy form would also be an amphipathic alpha-helix. This proposed change in the conformation of GPIbα might be important in regulating the GPIb-vWf interaction. Previous studies have suggested that the Pt-vWD mutations occur in a region involved in regulating vWf-binding.21,25,26 Similar to these studies,25,26 the G233V and M239V cells bound comparable levels of vWf at 0.75 and 0.5 mg/mL ristocetin (Figure 3). This indicates that there was a limit to the amount of vWf the mutant receptors could bind and that a change to the conformation of this region seems to affect how much ligand can be bound. This further substantiates the proposal that the conformation of the amino-terminal domain of GPIbα can influence vWf-binding, as proposed by Marchese et al27 and Pincus et al.31
The current study has also highlighted differences in the adhesive properties of the different recombinant forms of GPIbα. Results obtained from this study and from that of Marchese et al27 suggest that the G233V mutation led to a receptor with increased association and decreased dissociation for the A1 domain of vWf. We have made similar observations for the M239V and G233V/M239V mutations. Dong et al29 also reported decreased dissociation to immobilized vWf with CHO cells expressing GPIb-IX and containing the G233V and M239V mutations. Significantly, we found that the double mutant exhibited enhanced binding of soluble vWf and an increased ability to adhere to immobilized vWf compared with either the G233V or M239V single mutants. The double mutant was also unique in its ability to support vWf-mediated cell aggregation in the complete absence of ristocetin. To our knowledge this is the first report demonstrating that a mutant form of GPIb, expressed in CHO cells, can engage the A1 domain of vWf independent of changes in the conformation of vWf as a result of protein immobilization, shear stress, or the presence of artificial modulators. These findings appear to contrast those of Miura et al,28 who reported no difference in the dissociation constants between the M239V and G233V/M239V mutant fusion proteins (GPIbα-calmodulin) and suggested that the additional mutation had no additive effect on vWf binding. The 3 systems used to examine binding are distinct. Miura et al28 examined a chimeric protein consisting of amino acids 1-289 of GPIbα fused to the cytoplasmic domain of calmodulin, and Marchese et al27examined soluble GPIbα coated onto latex beads. Our study has examined the mature GPIbα protein associated with GPIbβ and GPIX and containing the cytoplasmic domain, including those regions involved in cell signaling.34 The double mutant possibly behaved differently in our model system because of possible effects on the receptor complex or on cell signaling not evident with the single mutants or with the purified extracellular fragment of GPIbα examined by Miura et al.28
Experiments comparing the ability of cells to form adhesion contacts with vWf (cell tethering) clearly demonstrate the increased association of recombinant GPIb-IX containing the Pt-vWD mutations with vWf. These observations indicate that the mutant receptors exhibit increased association to vWf, in agreement with the observations of Marchese et al27 but in contrast to the findings of Dong et al,29 who found no difference in the ability of mutant cells to adhere to immobilized vWf compared to wild-type cells. Furthermore, the experiments comparing the ability of cells to dissociate from vWf (cell detachment) also demonstrate the increased strength and decreased dissociation of recombinant GPIb-IX containing the Pt-vWD mutations with vWf. This is in agreement with the observations of Marchese et al27 and Dong et al29 but is in contrast to that observed by Miura et al,28 who reported no change in the dissociation constant between wild-type GPIbα and the G233V, M239V, and G233V/M239V mutants.
In conclusion, this investigation has clearly demonstrated that the glycine-233 to valine and the methionine-239 to valine mutations associated with Pt-vWD result in a GPIb-IX receptor with an increased affinity for vWf. These mutations lead to similar alterations in the kinetic properties of the vWf-GPIb interaction, implying that both mutations induce a similar conformational change in the GPIb receptor. In contrast, the V234G mutation and the G233V/M239V double mutation appear to induce functionally distinct receptors with unique biomechanical properties, with the double mutation resulting in a hyperreactive receptor for vWf.
We thank Dr José López for generously donating the cDNA for GPIbα and the CHO-βIX cell line. We also thank Dr Michael Berndt for supplying the various monoclonal antibodies and the purified human vWf used in the flow cytometry binding assay, Dr Rob Andrews for his technical advice, and Ms Leonie Gaudry for operation of the flow cytometer.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
A. Sasha Tait, Department of Haematology, Prince of Wales Hospital, PO Box 81, Randwick NSW 2031, Australia; e-mail:s.tait@unsw.edu.au.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal