Abstract
The clinical results in 107 patients receiving a peripheral blood stem cell (PBSC) graft mobilized by granulocyte colony-stimulating factor (G-CSF) from HLA-A, -B, and -DR–compatible unrelated donors were compared to 107 matched controls receiving unrelated bone marrow (BM) transplants. Engraftment was achieved in 94% of the patients in both groups. The PBSC graft contained significantly more nucleated cells, CD34+, CD3+, and CD56+ cells (P < .001), and resulted in a significantly shorter time-to-neutrophil (15 versus 19 days) and platelet engraftment (20 versus 27 days), compared to the BM control group (P < .001). Probabilities of acute graft-versus-host disease (GVHD) grades II to IV were 35% and 32% (not significant [NS]) and of chronic GVHD 61% and 76% (NS) in the PBSC and BM groups, respectively. There was no difference between the 2 groups in bacteremia, cytomegalovirus reactivation or disease, and fungal infection. The 3-year transplant-related mortality (TRM) rates were 42% in the PBSC group and 31% in the BM controls (P = .7) and the survival rates were 46% and 51%, respectively. The probability of relapse was 25% and 31% in both groups (NS), resulting in disease-free survival rates of 43% in the PBSC group and 46% in the BM controls (NS). In the multivariate analysis, early disease, acute GVHD grade 0 to I, and presence of chronic GVHD were independent factors associated with a better disease-free survival in this study. PBSC from HLA-compatible unrelated donors can be used safely as an alternative to BM for stem cell transplantation.
Introduction
The first report using peripheral blood as a source of stem cells for allogeneic hematopoietic stem cell transplantation (SCT) was published in 1989.1 Since then, the number of peripheral blood stem cell (PBSC) transplants has steadily increased.2-9 In 1995, about 500 PBSC transplants from HLA-identical siblings were reported to the European Group for Blood and Marrow Transplantation (EBMT); by 1996, the figure had increased to more than 1000.8 Until recent years most reports concerned PBSC grafts using HLA-A, -B, and -DR–identical sibling donors; data have been reported on only a few patients who received PBSC from unrelated donors.10-14 There have been several reasons for the reluctance to use PBSCs from unrelated donors. One concern has been the ethics of administering granulocyte colony-stimulating factor (G-CSF) to mobilize PBSCs from healthy volunteers. Another concern has been that the 10- to 15-fold higher donor T-cell content of PBSC would increase the risk of graft-versus-host disease (GVHD).13,15,16 Some reports have indicated a higher incidence of chronic GVHD after sibling PBSC transplantation.17-19 We report here the experience of PBSC grafts from unrelated donors at 3 European bone marrow transplant (BMT) units.
Patients and methods
Patients
The study group consisted of 107 consecutive patients who underwent PBSC transplantation with an HLA-identical unrelated donor at Essen (n = 44), Huddinge Hospital (n = 32), or Idar-Oberstein (n = 31) between February 1993 and September 1999. The patients were eligible for transplantation with PBSCs if they had a disease for which BMT was indicated and after approved informed consent was obtained from the donor and recipient. Most of the patients had a hematologic malignancy. Before 1998, relatively more patients underwent transplantation while not in remission, compared to those having the procedure later. The characteristics of the patients and donors are given in Table 1.
Patient and donor characteristics
| . | PBSC group . | BM group . |
|---|---|---|
| No. of patients | 107 | 107 |
| Recipient age | 35 (5-56) | 37 (1-55) |
| Donor age | 33 (20-53) | 36 (18-55) |
| Recipient sex (M/F) | 56/51 | 60/47 |
| Donor sex (M/F) | 72/35 | 62/44 |
| Diagnoses | ||
| AML | ||
| CR1 | 12 | 11 |
| CR2 | 8 | 11 |
| Not in remission | 16 | 13 |
| ALL | ||
| CR1 | 5 | 7 |
| CR2-3 | 6 | 4 |
| Not in remission | 5 | 6 |
| CML | ||
| CP1 | 39 | 42 |
| CP2 | 4 | 1 |
| Acc phase | 6 | 6 |
| Blast crisis | 2 | 3 |
| Lymphoma | 1 | 0 |
| Myelodysplastic syndrome | 2 | 2 |
| Aspartylglycosaminurea | 1 | 1 |
| Recipient CMV serology (−/+) | 43/64 | 47/59 |
| Donor CMV serology (−/+) | 61/46 | 58/48 |
| Follow-up, mo, median (range) | 11.1 (1.9-49)* | 20.8 (2.8-66) |
| . | PBSC group . | BM group . |
|---|---|---|
| No. of patients | 107 | 107 |
| Recipient age | 35 (5-56) | 37 (1-55) |
| Donor age | 33 (20-53) | 36 (18-55) |
| Recipient sex (M/F) | 56/51 | 60/47 |
| Donor sex (M/F) | 72/35 | 62/44 |
| Diagnoses | ||
| AML | ||
| CR1 | 12 | 11 |
| CR2 | 8 | 11 |
| Not in remission | 16 | 13 |
| ALL | ||
| CR1 | 5 | 7 |
| CR2-3 | 6 | 4 |
| Not in remission | 5 | 6 |
| CML | ||
| CP1 | 39 | 42 |
| CP2 | 4 | 1 |
| Acc phase | 6 | 6 |
| Blast crisis | 2 | 3 |
| Lymphoma | 1 | 0 |
| Myelodysplastic syndrome | 2 | 2 |
| Aspartylglycosaminurea | 1 | 1 |
| Recipient CMV serology (−/+) | 43/64 | 47/59 |
| Donor CMV serology (−/+) | 61/46 | 58/48 |
| Follow-up, mo, median (range) | 11.1 (1.9-49)* | 20.8 (2.8-66) |
AML indicates acute myeloid leukemia; ALL, acute lymphocytic leukemia; Acc phase, accelerated phase.
P < .001.
BMT control group
For each unrelated patient undergoing PBSC transplantation we selected a control patient who had received an HLA-A, -B, and -DR–compatible unrelated bone marrow (BM) graft at almost the same time. Controls were matched for diagnosis, stage of disease, age (< 20 years or > 20 years), and GVHD prophylaxis. All controls, except one, were matched for transplant center. Eighty of 107 (75%) controls were matched for all 4 criteria, and 101 of 107 (94%) were matched for at least 3 criteria. Among the controls, 43 came from Essen, 33 from Huddinge, and 31 from Idar-Oberstein. Patient and donor characteristics are given in Table 1.
Donors
All donors were HLA-A, -B, and -DR compatible with the patients. Before 1997, class I HLA typing was serologic. Since then, polymerase chain reaction-sequence-specific primer (PCR-SSP) low-resolution typing for class I was used. For HLA class II, genomic high-resolution DNA-based typing (PCR-SSP) was used.20 All patients undergoing transplantation at Huddinge University Hospital have recently been retrospectively retyped using PCR-SSP high-resolution typing for both HLA class I and II antigens (submitted). Before apheresis, all donors of PBSCs were treated with G-CSF (Rhône-Poulenc Rorer, Lyon, France or Amgen-Roche, Thousand Oaks, CA) for 4 to 6 days. The dose of G-CSF ranged from 9 to 12.5 μg/kg per day, administered subcutaneously once daily.
Conditioning
As preparative treatment before transplantation, most patients received cyclophosphamid (Cy) 60 mg/kg for 2 consecutive days, combined with total body irradiation (TBI) in a dose ranging from 10 to 13.5 Gy (Table 2). TBI doses were mainly 10 Gy in Huddinge (single dose) and Essen (fractionated) and 12 to 13.5 Gy (fractionated) in Idar-Oberstein. Thirty-eight patients were given busulfan (Bu) 16 mg/kg followed by 120 mg/kg Cy.21 Other combinations of conditioning regimens were also used and are given in Table 2. A priority was given to TBI, but if not possible, Bu was used.21 Detailed descriptions of the myeloablative therapy, immunosuppression, and patient care at the different centers have been reported elsewhere.10,22 23
Conditioning and immunosuppression
| . | PBSC group . | BM group . |
|---|---|---|
| No. | 107 | 107 |
| Conditioning | ||
| Cy/TLI | 0 | 1 |
| Cy/TBI | 67 | 80 |
| Cy/TBI + other drug | 8 | 7 |
| Holoxan/TBI | 0 | 2 |
| Bu/Cy | 22 | 16 |
| Bu/Cy + other drug | 5 | 1 |
| Bu/other | 4 | 0 |
| Cy/MEA | 1 | 0 |
| ATG/OKT-3 | 25/11 | 21/16 |
| GVHD prophylaxis | ||
| CsA or MTX | 2 | 3 |
| CsA + pred | 0 | 3 |
| CsA + MMF | 2 | 0 |
| CsA + MTX | 71 | 69 |
| CsA + MTX + pred | 25 | 31 |
| CsA + pred + MMF | 3 | 0 |
| CsA + MTX + pred + MMF | 3 | 1 |
| Patients receiving G-CSF | 53* | 32 |
| Median d with G-CSF | 10 (1-24) | 11 (5-30) |
| . | PBSC group . | BM group . |
|---|---|---|
| No. | 107 | 107 |
| Conditioning | ||
| Cy/TLI | 0 | 1 |
| Cy/TBI | 67 | 80 |
| Cy/TBI + other drug | 8 | 7 |
| Holoxan/TBI | 0 | 2 |
| Bu/Cy | 22 | 16 |
| Bu/Cy + other drug | 5 | 1 |
| Bu/other | 4 | 0 |
| Cy/MEA | 1 | 0 |
| ATG/OKT-3 | 25/11 | 21/16 |
| GVHD prophylaxis | ||
| CsA or MTX | 2 | 3 |
| CsA + pred | 0 | 3 |
| CsA + MMF | 2 | 0 |
| CsA + MTX | 71 | 69 |
| CsA + MTX + pred | 25 | 31 |
| CsA + pred + MMF | 3 | 0 |
| CsA + MTX + pred + MMF | 3 | 1 |
| Patients receiving G-CSF | 53* | 32 |
| Median d with G-CSF | 10 (1-24) | 11 (5-30) |
TLI indicates total lymphoid irradiation; MMF, mycomofetilphenolate; pred, prednisolone; MEA, mitoxantrone + etoposide + arabinoside-C.
P < .01.
As additional immunosuppressive treatment before transplant, one third of the patients (mainly at Huddinge) were given antithymocyte globulin (ATG-Fresenius, Fresenius AG, Bad Homburg, Germany, or Thymoglobulin, IMTIX-Sangstat, Lyon, France) or monoclonal anti–T-cell antibodies (Orthoclone OKT3, Ortho Biotech, Raritan, NJ) for 4 to 5 days.24
GVHD prophylaxis
The most common immunosuppression agent was cyclosporin A (CsA) combined with a short course (4 doses) of methotrexate (MTX; Table 2). The combination of CsA, MTX, and prednisolone was used in about one fourth of the patients.
Engraftment
Neutrophil engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count (ANC) of 0.5 × 109/L or above. Platelet engraftment was defined as the first of 7 days with platelet counts of 50 × 109/L or above without transfusion.
GVHD
Other definitions
Bacteremia was defined as the first positive blood culture related to a febrile episode during the first 30 days after transplantation. Cytomegalovirus (CMV) reactivation was determined weekly with PCR in leukocytes.27 Patients in Idar-Oberstein and Essen were followed weekly by the pp 65 antigen in blood leukocytes. CMV disease was defined as symptoms from the affected organ, combined with detection of CMV by histology, isolation, or immunohistochemistry, or typical retinitis changes.28Determinations of CD3+, CD34+, and CD56+ cells were performed on unseparated BM or PBSCs by flow cytometry, as previously described.12 G-CSF was given to 85 patients after transplantation for a median of 11 days (range, 1-30 days; Table 2).
Statistics
Analysis was performed on March 25, 2000, with a median follow-up time of 11.1 months (range, 1.9-49 months) in the PBSC group and 20.8 months (range, 2.8-66 months) in the BM group. Patients in the control group were matched according to (in ranking) center, diagnosis, disease stage, age (< 20 years or ≥ 20 years) and GVHD prophylaxis.
The probabilities of acute and chronic GVHD, transplant-related mortality (TRM), relapse, overall survival, and disease-free survival (DFS) were calculated with the Kaplan-Meier method,29comparing the groups with use of the log-rank test (Mantel-Haentszel).30 We used the Mann-WhitneyU test to compare the cell yield, time to engraftment, transfusions, and hospitalizations. In the univariate and multivariate analyses of risk factors for acute GVHD and prognostic factors for engraftment, the logistic regression model was used.31Patients dying before day 30 without GVHD were omitted from the GVHD analysis. When analyzing prognostic factors for chronic GVHD, TRM, survival, relapse, and DFS, the Cox regression model was used.32
In analyzing risk factors for relapse and chronic GVHD, only patients surviving more than 90 days after hematopoietic stem cell transplantation (HSCT) or having the event (relapse or chronic GVHD) before day 90 were included in the analysis. Factors significant at the 10% level in the univariate analysis were included in the multivariate analysis. The following factors were analyzed: MTX, nucleated cell dose, CD34 dose, CD3 dose, CD56 dose, ATG, G-CSF after transplant, stem cell source, disease, disease stage, pretransplant CMV serology, conditioning, patient age (< 30 years or ≥ 30 years), donor age (< 35 years or ≥ 35 years), and sex. For TRM, relapse, and survival, GVHD was also included.
Results
Graft content
The nucleated cell dose was 3 times higher in the PBSC group than in the BM control group (P < .001; Table3). The CD34+ cell dose was 2-fold and the CD3+ and the CD56+ cell doses more than 10-fold higher in the PBSC group (P < .001).
Cell yield in PBSC and BM grafts
| . | PBSC . | BM . | P . |
|---|---|---|---|
| Nucleated cell dose (× 108/kg) | 10.0 (2.2-51.1) | 3.1 (0.2-11.6) | < .001 |
| CD34+ cell dose (× 106/kg) | 6.1 (2.0-18.3) | 3.1 (0.5-10.7) | < .001 |
| CD3+ cell dose (× 106/kg) | 336 (75-1527) | 25.3 (3.5-57.1) | < .001 |
| CD56+ cell dose (× 106/kg) | 32.4 (8-379) | 2.4 (0.9-14.7) | < .001 |
| . | PBSC . | BM . | P . |
|---|---|---|---|
| Nucleated cell dose (× 108/kg) | 10.0 (2.2-51.1) | 3.1 (0.2-11.6) | < .001 |
| CD34+ cell dose (× 106/kg) | 6.1 (2.0-18.3) | 3.1 (0.5-10.7) | < .001 |
| CD3+ cell dose (× 106/kg) | 336 (75-1527) | 25.3 (3.5-57.1) | < .001 |
| CD56+ cell dose (× 106/kg) | 32.4 (8-379) | 2.4 (0.9-14.7) | < .001 |
Engraftment
Six patients in the PBSC group and 6 in the BM group died early, before engraftment (ANC > 0.5 × 109/L), giving an engraftment rate of 94% in both groups. Time-to-neutrophil engraftment was significantly shorter in the PBSC group (median, 15 days; range, 7-27 days) compared to the BM group (median, 19 days; range, 10-31 days) (P < .001). In the multivariate analysis, PBSC graft (odds ratio [OR] 3.74, CI 2.03-6.89, P < .001) and G-CSF after transplant (OR 2.87, CI 1.55-5.37,P = .001) were independent factors associated with faster neutrophil engraftment.
Platelet engraftment (> 50 × 109/L) was also significantly faster in the PBSC group (20 days; range, 8-395 days) but 27 days (18-210; P < .001) in the BM group. The CD34+ cell dose (> 5 × 106/L; OR 2.72, CI 1.38-5.37, P = .004) was the only factor associated with faster platelet engraftment in the multivariate analysis.
The median numbers of days from transplantation to discharge from hospital were similar in both groups, 37 days (range, 13-104 days) and 39 days (range, 14-150 days), respectively.
Transfusions
The numbers of platelet, erythrocyte, and granulocyte transfusions did not differ between the 2 groups, although there was a tendency to more platelet transfusions in the BM group, median 14 (range, 0-167) (P = .06) versus 10 (range, 0-144) in the PBSC group.
Infections
Bacteremia occurred in 45 (42%) of the patients in both groups. In the PBSC group, 33 patients had a coagulase-negative staphylococci (CNS) infection, 3 had a streptococcal infection, 5 had a pseudomonal infection, and 4 had other bacteria. In the BM group, 34 had a CNS infection, 4 a streptococcal infection, 3 a pseudomonal infection, and 4 other bacteria. CMV reactivation, as detected by PCR or pp 65 antigen, occurred in 33 and 34 patients in the PBSC and BM groups, respectively. CMV disease developed in 5 and 4 patients in the 2 groups, respectively. CMV disease consisted of interstitial pneumonitis in 4 patients and enteritis in one patient in the PBSC group. In the BM group, 2 patients had interstitial pneumonitis and one each had enteritis and retinitis. Invasive fungal infection was diagnosed in 8 patients in each group. Among the PBSC patients,Aspergillus was found in 3, Candida albicans in 3, and Candida glabrata in 2. In the BM group,Aspergillus was found in 5, C. albicans in 2, andPneumocystis carinii in 1.
GVHD
The cumulative probabilities of acute GVHD grades II to IV were similar in the 2 groups, 35% in the PBSC group and 32% in the BM group (NS). The probabilities of grades III to IV acute GVHD were 18% and 22% in the 2 groups, respectively. In the multivariate analysis, the use of OKT-3 was a significant risk factor for acute GVHD grades II to IV (OR 3.74, CI 1.62-8.67, P = .002).
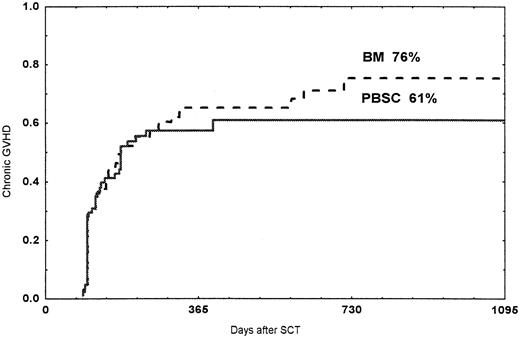
The cumulative probabilities of chronic GVHD were 61% among patients receiving a PBSC graft and 76% in patients receiving a BM graft (NS; Figure 1). Seven patients in the PBSC group and 10 in the BM group developed extensive chronic GVHD (NS). In the multivariate analysis, acute GVHD of any grade (relative risk [RR] 3.03, CI 1.72-5.37, P < .001), no ATG (RR 2.83, CI 1.51-5.31, P = .001), and chronic myeloid leukemia (CML; RR 1.84, CI 1.20-2.83, P = .005) were correlated to the development of chronic GVHD.
Probability of chronic GVHD.
Time and cumulative probabilities of chronic GVHD after SCT with PBSC and BM, using unrelated donors
Probability of chronic GVHD.
Time and cumulative probabilities of chronic GVHD after SCT with PBSC and BM, using unrelated donors
Survival and relapse
The TRM, all deaths except relapse, did not differ between the 2 groups (Figure 2). The TRM rate was 18% and 19% at 100 days and 32% and 31% at 12 months after transplantation in the 2 groups, respectively.
Probability of TRM.
Time and cumulative probabilities of TRM after SCT with PBSC and BM, using unrelated donors
Probability of TRM.
Time and cumulative probabilities of TRM after SCT with PBSC and BM, using unrelated donors
In the multivariate analysis, acute GVHD grades II to IV (relative hazard [RH] 8.94, CI 4.76-16.78, P < .001) was the only independent risk factor for TRM. Higher patient age (≥ 30 years; RH 2.01, 95% CI 1.19-3.42, P = .011) and late disease (RH 1.99, CI 1.17-3.39, P = .011) were independent risk factors for TRM if acute GVHD was not added in the multivariate analysis.
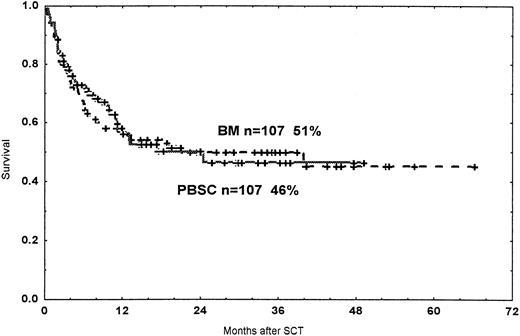
The 3-year probabilities of survival were 46% in the PBSC group and 51% in the BM group (NS; Figure 3). Among patients with a malignant disease in first remission (CR) or first chronic phase (CP; early disease), the probabilities of survival at 2 years were 61% and 68% in the PBSC group and the BM group, respectively (NS). In patients beyond the first CR/CP (late disease), the 2-year survival rates were 37% and 29%, respectively (NS). In the multivariate analysis, late disease (RH 2.25, CI 1.46-3.46,P < .001) and acute GVHD grades II to IV (RH 3.03, CI 1.97-4.66, P < .001) were independent risk factors for death after HSCT in this material. If GVHD was not introduced into the analysis, pretransplant CMV seropositivity (RH 1.54, CI 1.01-2.34,P = .046) was also associated with death.
Probability of survival.
Time and cumulative probability of survival after SCT with PBSC and BM, using unrelated donors.
Probability of survival.
Time and cumulative probability of survival after SCT with PBSC and BM, using unrelated donors.
Hematologic relapse was detected in 18 patients receiving PBSC and 20 receiving BM, giving a cumulative probability of 25% in both groups. Among patients having early disease, the 2-year probabilities of relapse were 2% in the PBSC group and 6% in the BM group (NS). Corresponding figures for patients in late disease were 49% and 52% (NS).
In the multivariate analysis, advanced disease (RH 10.91, CI 4.18-28.5,P < .001) and absence of chronic GVHD (RH 2.84, CI 1.42-5.64, P = .003) were independent risk factors for relapse. If GVHD was removed from the analysis, advanced disease and acute leukemia (AL; RH 2.51, CI 1.06-5.93, P = .04) were significantly associated with relapse.
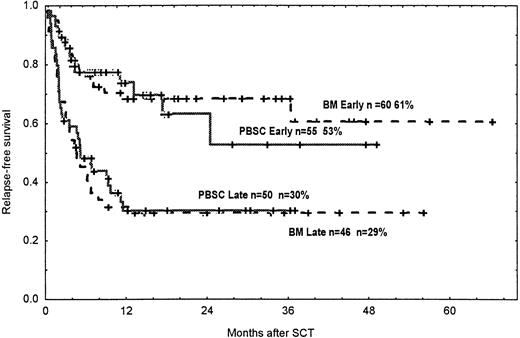
The rates for DFS at 3 years were similar in the 2 groups, 43% in patients in the PBSC group and 46% in the BM group (NS; Figure4). Among patients with CML, the DFS rates at 2 years were 62% and 64% in the PBSC and the BM groups, respectively.
Probability of DFS.
Time and cumulative probabilities of DFS after SCT among patients in early and late diseases given PBSC and BM from unrelated donors.
Probability of DFS.
Time and cumulative probabilities of DFS after SCT among patients in early and late diseases given PBSC and BM from unrelated donors.
Corresponding figures for patients with AL were 43% and 51% (NS). The probabilities of DFS and relapse in patients with early and late disease in the various diagnostic groups are given in Table4.
Probabilities of relapse and relapse-free survival among CML and AL patients, divided into early (CR1/CP1) and late (> CR1/CP1) disease
| . | No. . | Relapse (2 y), % . | DFS (2 y), % . |
|---|---|---|---|
| PBSC | |||
| CML early disease | 39 | 3 | 64 |
| CML late disease | 12 | 21 | 45 |
| AL early disease | 17 | 0 | 68 |
| AL late disease | 37 | 57 | 25 |
| Bone marrow | |||
| CML early disease | 42 | 0 | 70 |
| CML late disease | 10 | 38 | 40 |
| AL early disease | 18 | 21 | 64 |
| AL late disease | 36 | 57 | 26 |
| . | No. . | Relapse (2 y), % . | DFS (2 y), % . |
|---|---|---|---|
| PBSC | |||
| CML early disease | 39 | 3 | 64 |
| CML late disease | 12 | 21 | 45 |
| AL early disease | 17 | 0 | 68 |
| AL late disease | 37 | 57 | 25 |
| Bone marrow | |||
| CML early disease | 42 | 0 | 70 |
| CML late disease | 10 | 38 | 40 |
| AL early disease | 18 | 21 | 64 |
| AL late disease | 36 | 57 | 26 |
DFS indicates disease-free survival.
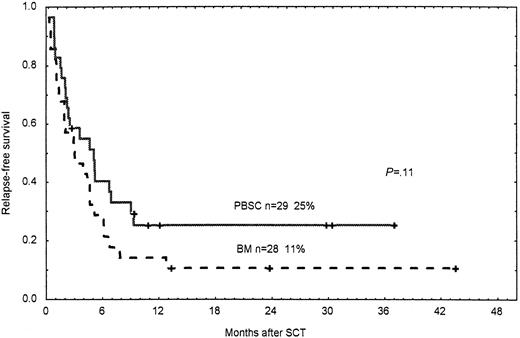
Among patients not in remission at transplant, DFS rates at 2 years were 25% for patients receiving a PBSC graft and 11% in the BM group (P = .11; Figure 5).
Probability of DFS.
Time and cumulative probabilities of DFS after SCT among patients not in remission/chronic phase given PBSC and BM from unrelated donors.
Probability of DFS.
Time and cumulative probabilities of DFS after SCT among patients not in remission/chronic phase given PBSC and BM from unrelated donors.
In the multivariate analysis, early disease (RH 2.97, CI 1.97-4.48,P < .001), acute GVHD grades 0 to I (RH 2.31, CI 1.54-3.49, P < .001), and the presence of chronic GVHD (RH 1.98, CI 1.28-3.04, P = .002) were independent prognostic factors for better DFS in this material. If GVHD was not added to the analysis, early disease was the only significant factor for a better DFS.
Discussion
The PBSC grafts contained a significantly higher cell dose than the BM grafts and this was correlated to faster neutrophil and platelet engraftment. This is in agreement with most other reports using sibling donors.5-8,12,14 The higher nucleated cell dose and CD34+ cell content seem to be the main factors for faster engraftment in the PBSC group.12,13,33 In the present study, more patients in the PBSC group received posttransplant G-CSF treatment, which promotes engraftment. In the multivariate analysis, G-CSF and PBSCs were independent prognostic factors for faster neutrophil engraftment. For platelet engraftment, the CD34+cell dose was the main factor for a faster engraftment. This agrees with other studies.12 33 Despite a faster engraftment being achieved, no difference in the number of days to discharge from hospital was seen. There may be several explanations for this. Even after engraftment, problems such as malnutrition, fever, GVHD, and infections remain before the patients can be discharged. The incidence of infections was the same as in the BM group despite the faster engraftment in the PBSC group.
In contrast, in a double-blinded randomized study, Powles and coworkers found that patients receiving PBSCs from HLA-identical siblings were discharged significantly earlier than those given BM.9This difference compared with our results could be explained by the fact that recipients of stem cells from siblings have fewer transplant-related complications than recipients of unrelated grafts.22
We found no difference in the incidence of moderate-to-severe acute GVHD between the 2 groups. This is important because it has been feared that the higher T-cell content in the PBSC grafts might cause more GVHD when using unrelated donors. This does not agree with the update of the EBMT randomized study in leukemic patients receiving grafts from HLA-identical siblings, where those receiving PBSC grafts had significantly more acute GVHD (N. Schmitz, oral communication, August 2000). One bias in the present study may have been that more patients in the PBSC group received G-CSF after transplant.
Some studies have indicated that G-CSF may affect the incidence and severity of acute GVHD, although most have not.34 But in both univariate and multivariate analysis, G-CSF after transplant did not affect acute GVHD in this study.
Roughly one third of the patients in both groups in our study developed moderate-to-severe acute GVHD, which is less than that reported by others using unrelated marrow.35,36 The use of ATG as part of pretransplant conditioning in some patients may have helped to reduce the incidence of acute GVHD.37 Polyclonal ATG was correlated to less acute GVHD in the univariate but not in the multivariate analysis. As we have previously reported, the use of OKT-3 increases the risk for acute GVHD.24 This is probably caused by a “cytokine release syndrome” on administration of OKT-3. However, the number of patients receiving ATG or OKT-3 were similar in the 2 groups.
No difference in the probability of chronic GVHD was seen in this study. Using HLA-identical sibling donors, some studies have reported a higher incidence of chronic GVHD when using PBSC.17-19The reason for absence of a difference in the present study may be the higher incidence of chronic GVHD using unrelated donors, compared to HLA-identical siblings.35,36,38,39 In this study, the incidences of chronic GVHD were 61% and 76% in the PBSC and BM groups, respectively. As previously reported, diagnosis of CML and acute GVHD of any grade were correlated to chronic GVHD.40
We found that patients not receiving polyclonal ATG had more chronic GVHD. The reason for this is that the absence of ATG is correlated to more acute GVHD and acute GVHD triggers chronic GVHD.40But, once again, the use of ATG was similar in the 2 groups. The incidences of bacteremia and CMV and fungal infections were almost identical in the 2 groups. Theoretically, the risk of CMV infection may be higher when using PBSC because the higher cell dose contains more viruses, but we found no such difference in this study. However, we analyzed only the number of patients with a CMV infection and not the occurrence of repeated infections.
With regard to survival, relapse, and DFS, the outcome was the same in patients receiving PBSC or BM. This agrees with most reports comparing PBSC with BM from HLA-identical sibling donors.7,8 Two studies, one from the International Bone Marrow Transplant Registry (IBMTR) and another from the M. D. Anderson Hospital, reported better DFS rates among patients with advanced leukemia using PBSCs rather than BM, using HLA-identical sibling donors.41,42 A randomized trial from Seattle also showed that leukemic patients with more advanced disease had fewer relapses and better DFS if they received PBSCs rather than BM.43 In our material, we found no difference in DFS among patients with CML or AL with early or late disease (Table 4 and Figure 4). However, when analyzing only patients not in remission at transplant, we found, in accordance with the studies from Seattle, IBMTR, and the M. D. Anderson Hospital, Houston, TX, in HLA-identical siblings, a better DFS for patients receiving PBSCs (Figure 5). But in our study the difference was not statistically significant (P = .11). This may be due to the low number of patients in this cohort and conclusions cannot be drawn based on this material. Further studies with PBSC using unrelated donors including more patients in advanced disease are needed.
There was a longer follow-up with BM in this study, because with more experience with PBSCs more patients and donors selected PBSCs compared to BM. In the PBSC group, 32% of the patients received transplants before 1998, compared to 53% in the BM group. However, indications for SCT using unrelated donors were the same during the period 1993-1999, when the patients were included for this study. Furthermore, any changes in treatment protocols that may have influenced the outcome variables have not been introduced in any of the 3 centers during this time period. Different GVHD prophylaxis and conditioning protocols were used in this study, but no statistical difference between the 2 groups existed. Furthermore, in the multivariate analysis significant and almost significant factors were included. Therefore, the finding of a similar survival using BM or PBSC seems sound and is not due to selection bias or differences in treatment or follow-up between the 2 groups.
When using unrelated donors, the incidence of chronic GVHD is higher than in HLA-identical siblings, which also carries a graft-versus-leukemia effect,44 45 thus reducing the risk of relapse. The shorter follow-up in the PBSC group may also have affected the results in the present study. However, this seems unlikely because the same protocol has been used throughout the study. HLA typing for class I has been genomic at one center during the last 3 years. This has not affected the outcome because genomic or serologic class I mismatched donor-recipient pairs were not included in this material.
Factors associated with a better DFS in our study (early disease, grades 0-I acute GVHD, and chronic GVHD) agree with other studies.46 47 Patients with chronic GVHD may run a slightly higher risk of late death not related to relapse, but this will probably be balanced by a lower risk of relapse. A long-term follow-up is warranted to find the answer.
To conclude, this study has shown that the use of PBSCs from unrelated donors is a safe and well-tolerated procedure, resulting in faster engraftment than BM, a good survival rate, and DFS.
We thank all nurses at the departments for excellent and skillful care and all volunteer donors for their generous gift to the patients.
Supported by grants from Swedish Cancer Society (0070-B97-11XBC), the Children's Cancer Foundation (1997/073, 1998/002), the Swedish Medical Research Council (K98-06X-05 971-18B), the FRF Foundation, and the Tobias Foundation (B11/98).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mats Remberger, Department of Clinical Immunology, F79, Huddinge University Hospital, SE-141 86 Huddinge, Sweden; e-mail:mats.remberger@impi.ki.se.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal