Abstract
Early response to therapy is one of the most important prognostic factors in acute leukemia. It is hypothesized that early immunophenotypical evaluation may help identify patients at high risk for relapse from those who may remain in complete remission (CR). Using multiparametric flow cytometry, the level of minimal residual disease (MRD) was evaluated in the first bone marrow (BM) in morphologic CR obtained after induction treatment from 126 patients with acute myeloid leukemia (AML) who displayed aberrant phenotypes at diagnosis. Based on MRD level, 4 different risk categories were identified: 8 patients were at very low risk (fewer than 10−4 cells), and none have relapsed thus far; 37 were at low risk (10−4 to 10−3 cells); and 64 were at intermediate risk (fewer than 10−3 to 10−2 cells), with 3-year cumulative relapse rates of 14% and 50%, respectively. The remaining 17 patients were in the high-risk group (more than 10−2 residual aberrant cells) and had a 3-year relapse rate of 84% (P = .0001). MRD level not only influences relapse-free survival but also overall survival (P = .003). The adverse prognostic impact was also observed when M3 and non-M3 patients with AML were separately analyzed, and was associated with adverse cytogenetic subtypes, 2 or more cycles to achieve CR, and high white blood cell counts. Multivariate analysis showed that MRD level was the most powerful independent prognostic factor, followed by cytogenetics and number of cycles to achieve CR. In conclusion, immunophenotypical investigation of MRD in the first BM in mCR obtained after AML induction therapy provides important information for risk assessment in patients with AML.
Introduction
Investigation of minimal residual disease (MRD) has proven to be a valuable tool for predicting impending relapses before clinical and hematologic manifestations, and establishing different risk categories in patients with acute leukemia (AL).1-5The detection of residual leukemic cells is usually based on either molecular or immunophenotypical markers present in leukemic but not in normal cells, allowing for their specific discrimination. Most available information on large series of patients with AL correlates with childhood acute lymphoblastic leukemia (ALL). Thus, studies based on semiquantitative polymerase chain reaction (PCR)1,6 or immunophenotyping2,5 have demonstrated the clinical relevance of MRD investigation; both methods provide similar results. However, information on acute myeloid leukemia (AML) is scarce and is mostly restricted to the investigation of AML subgroups with molecular markers involved in chromosomal translocations, particularly t(15;17). In fact, monitoring of PML/RAR gene rearrangement is used for assessing molecular relapse and subsequent therapeutic intervention in acute promyelocytic leukemia (APL).4,7,8By contrast, few data have been reported on MRD analysis of AML by immunophenotyping.3,9,10 This is probably because of the technical difficulties related to the immunologic characterization of myeloid leukemias given that large panels of monoclonal antibodies are needed to cover all the different myeloid lineages and that several blast cell subpopulations frequently coexist at diagnosis.11-13 Altogether, these technical problems complicate the identification of phenotypic aberrancies in AML to be used as leukemia-associated markers for MRD detection.12-15 Nevertheless, in a preliminary study, our group has shown that immunophenotypical investigation of MRD predicts outcome in patients with AML and that the number of residual leukemic cells correlates with multidrug resistance.3
Most of the reported MRD studies have been based on sequential analysis of follow-up samples.1,2,5,6 However, it would also be of interest to exploit MRD techniques for the assessment of initial response to treatment, as a single time-point evaluation. Early response to therapy is one of the most, if not the most, important prognostic factor in AL. In childhood acute lymphocytic leukemia (ALL), it has been shown that morphologic evaluation of blast cells in peripheral blood (PB) after 1 week of steroid treatment16and molecular analysis of the bone marrow (BM) on day 1517help to identify different groups of patients at risk. In AML, the relevance of early response is also well established; thus, patients who do not enter into morphologic complete remission (mCR) after one cycle of induction therapy have poor prognoses.18Moreover, Estey et al19 have shown that patients requiring more than 51 days to achieve mCR also have lower survival rates. However, most of these latter data emerge from a negative indicator—the failure to achieve early mCR—which identifies patients at high risk as candidates for more intensive therapeutic approaches. By contrast, morphologic information provided about patients who enter into CR is not really relevant because, though some of these patients will remain in continuous CR, many others will relapse. Therefore, sensitive techniques are needed for a more precise assessment of the quality of the response to induction therapy. We have hypothesized that immunophenotypical evaluation of the first BM in mCR may identify patients at high risk for relapse from those who would remain in continuous CR. To test this hypothesis, we analyzed by multiparametric flow cytometry the BM in mCR from 126 patients with AML, and our results show that early immunophenotypical evaluation of MRD identifies different categories of patients at risk and may contribute to postinduction treatment stratification.
Patients, materials, and methods
Patients
Eligibility criteria for entering the study were unequivocal diagnosis of de novo AML based on morphologic, cytochemical, and immunophenotypical criteria20,21; phenotypically aberrant blast cells at diagnosis10,13; mCR with induction therapy22; and corresponding mCR BM sample for immunophenotypical investigation of MRD. Overall, 126 consecutive patients with AML fulfilled these prerequisites and were included in the present study. The initial series of patients consisted on 233 consecutive patients with de novo AML; of these, 175 patients (75%) displayed aberrant immunophenotypes at diagnosis. Among these patients, 126 (72%) achieved mCR and are the focus of the present study. Fifty-two of the 126 patients are described in a preliminary report.3 Sixty-five are men and 61 are women, and their mean age is 43 ± 18 years (median, 42 years). Distribution according to French–American–British (FAB) classification was as follows: M0 (11 patients), M1 (21 patients), M2 (19 patients), M3 (44 patients), M4 (14 patients), M5 (16 patients), and M6 (1 patient). Median ages of patients with and without APL were 39 ± 17 and 45 ± 18 years, respectively. All patients were uniformly treated according to the Spanish Pethema Cooperative group protocols of 1991 and 1996. Remission-induction therapy included 1 or 2 3/7 courses of anthracycline (60 mg/m2 daunorubicin or 12 mg/m2 idarubicin) and cytosine arabinoside (ARA-C) (200 mg/m2); 101 patients were administered one course, and the remaining 25 were administered 2 courses. Subsequently, all patients underwent one identical consolidation cycle, followed by intensification therapy with 2 courses of high-dose ARA-C (3 g/m2 every 12 hours for a total of 8 dosages) and either daunorubicin (45 mg/m2) or idarubicin (12 mg/m2) for 3 days. In 16 patients, intensification chemotherapy was replaced by an autologous transplantation, and in 12 patients it was replaced by allogeneic stem cell transplantation. Relapse-free survival and overall survival times were not significantly different in patients who underwent transplantation than in those receiving conventional chemotherapy (P = .8). Forty of the 44 M3 patients were uniformly treated with all-transretinoic acid plus chemotherapy.7,8 Overall, the mean follow-up time was 28 ± 23 months (median, 20 months), and 42 patients have already relapsed. Median overall and relapse-free survival times are 79 and 67 months, respectively. Morphologic CR was defined by the criteria proposed by Cheson at al22: (1) less than 5% blast cells without detectable Auer rods in a BM sample displaying more than 20% cellularity with maturation of all hemopoietic cell lines in the BM aspirate; (2) absence of extramedullary leukemia; (3) absence of leukemic blasts in PB; and (4) PB neutrophil count greater than 1.5 × 109/L and platelet count greater than 100 × 109/L.
The following variables collected at diagnosis were included in the database: age, white blood cell (WBC) count, platelet count, hemoglobin (Hb) level, percentage of blast cells in BM and absolute number in PB; number of cycles to achieve mCR, type of treatment, morphologic FAB classification, and cytogenetics. Karyotypic findings were grouped according to Grimwade et al28 with the following distribution: (1) favorable cytogenetic markers [t(15;17), t(8;21), inv 16)] in 49 patients; (2) intermediate (no abnormalities, +8, 11q23, del (9q), +22, and other abnormalities not included in the other groups) in 35 patients; and (3) adverse cytogenetics (complex, −7, abnormalities in 3q, del (5q) and −5) in 10 patients. In the remaining 32 patients, karyotypic information was unavailable (n = 25) or mitosis was not achieved (n = 7).
Immunophenotypical investigation of MRD
Erythrocyte-lysed whole BM samples obtained at diagnosis were analyzed, with a large panel of 3-color combinations of monoclonal antibodies, for the identification of phenotypic aberrancies that could be used later as so-called custom-built probes for the detection of residual blast cells displaying the same phenotypic profile in the BM in mCR obtained after induction therapy.11-15 Antigenic expression on blast cells at diagnosis was systematically analyzed by multiparametric flow cytometry (FACScan; Becton Dickinson, San José, CA) according to previously described methods13-15 using triple stainings with the following fluorochrome-conjugated (fluorescein isothiocyanate [FITC], phycoerythrin [PE], PerCP, or PE-Cy5) combinations of monoclonal antibodies (mAbs): CD15/CD117/CD34, CD15/CD33/CD34, CD15/CD34/HLA-DR, CD34/CD38/CD19, CD34/CD56/CD33, HLA-DR/CD33/CD34, CD7/CD13/CD19, CD65/CD11b/CD4, CD2/CD14/CD33, CD61/Glycophorin A/CD45, CD10/CD5/CD20, and CD71/CD11b. All mAbs were purchased from Becton Dickinson, except for CD117PE, CD34 PE-Cy5 (Immunotech, Marseilles, France) and CD19 PE-Cy5, CD33 PE-Cy5, CD13 PE-Cy5, CD4 PE-Cy5, CD65 FITC, CD45 PE-Cy5, and CD20 PE-Cy5 (Caltag Laboratories, San Francisco, CA).
Our aim was to define within leukemic cells those phenotypes that are absent or extremely infrequent in normal BM samples by using a 5-dimensional space formed by the 2 light scatter parameters (forward scatter [FSC] and side scatter [SSC]) and the 3 fluorescence-associated characteristics. The existence of 2 or more blast cell populations was established on the basis of a clearly differentiated antigen expression, as previously defined.23 Based on our previous experience,3,13,14,23 4 main types of aberrant phenotypes were considered: cross-lineage antigen expression, asynchronous antigen expression, antigen overexpression, and abnormal light scatter pattern. As a second step, once the immunophenotype of the leukemic cells for the above-mentioned panel of mAbs was established, the need for additional mAb combinations that could help identify the aberrant phenotypes was evaluated and, if appropriate, carried out to define a custom-built phenotypic pattern for follow-up studies. Table 1 presents the frequencies of the most common aberrant phenotypes detected in the present series. Although we have previously reported that phenotypic changes involving aberrant phenotypes may occur in 16% of patients with AML, these changes usually take place during long-term clonal evolution.14 Here, we have focused on MRD detection during an early phase of the disease (after induction therapy). Interestingly, at this stage, the only phenotypic change observed was the selection, in some patients, of a minor blast cell subpopulation, but these abnormalities had been identified at diagnosis. This represents an additional advantage of the early investigation of MRD.
Frequencies of aberrant phenotypes
| Aberrant phenotypes . | Number of cases . |
|---|---|
| Cross-lineage infidelity, 37 of 126 (29%) | |
| CD2 | 26 (21) |
| CD7 | 11 (9) |
| CD19 | 3 (2) |
| CD20 | 1 (0.8) |
| CD5 | 1 (0.8) |
| Antigen overexpression, 26 of 126 (21%) | |
| CD33+++ | 14 (11) |
| CD34 | 11 (9) |
| CD13 | 3 (2) |
| CD117 | 1 (0.8) |
| CD15* | 1 (0.8) |
| HLA DR | 1 (0.8) |
| Abnormal light scatter, 22 of 126 (17%) | |
| High FSC/SSC | |
| CD2 | 4 (2.6) |
| CD34 | 4 (2.6) |
| CD7 | 4 (2.6) |
| CD117 | 2 (1.5) |
| CD19 | 2 (1.5) |
| CD20 | 1 (0.8) |
| Low FSC/SSC | |
| CD13† | 3 (2) |
| CD33† | 1 (0.8) |
| CD15 | 1 (0.8) |
| Asynchronous antigen expression, 98 of 126 (78%) | |
| CD34+ HLA-DR−CD33+ | 11 (9) |
| CD34+CD56+ | 10 (8) |
| CD34+CD11b+ | 6 (5) |
| CD34+CD33++ | 4 (3) |
| CD34+CD14+ | 4 (3) |
| CD34+ CD117+HLA-DR− | 3 (2.3) |
| CD34+CD117− CD15+ | 3 (2.3) |
| CD34+ CD33− CD13+HLA-DR+ | 2 (1.5) |
| CD34+CD33− CD13+ HLA-DR− | 1 (0.8) |
| CD34+ CD33− CD117+HLA-DR+ | 1 (0.8) |
| CD117+CD33+ HLA-DR− | 14 (11) |
| CD117+ CD34−CD15−‡ | 8 (6) |
| CD117+CD11b+ | 7 (5.5) |
| CD117+CD33+ CD34− CD15+ | 5 (4) |
| CD117+ DR− CD15+ | 3 (2.3) |
| CD117+ DR−CD15−* | 2 (2.3) |
| CD117+DR+ CD33+ CD34− | 1 (0.8) |
| CD33++ HLA-DR− CD34−CD15− CD14− | 22 (17) |
| CD33− CD13+ | 18 (14) |
| CD33+ CD13− | 9 (7) |
| CD33+ HLA-DR+ CD4+CD45dim | 1 (0.8) |
| CD33++HLA-DR+ CD15−CD14−1-153 | 1 (0.8) |
| CD33+CD45d CD34− CD15−1-155 | 1 (0.8) |
| CD33+ HLA-DR+ CD56+CD13+ | 1 (0.8) |
| Aberrant phenotypes . | Number of cases . |
|---|---|
| Cross-lineage infidelity, 37 of 126 (29%) | |
| CD2 | 26 (21) |
| CD7 | 11 (9) |
| CD19 | 3 (2) |
| CD20 | 1 (0.8) |
| CD5 | 1 (0.8) |
| Antigen overexpression, 26 of 126 (21%) | |
| CD33+++ | 14 (11) |
| CD34 | 11 (9) |
| CD13 | 3 (2) |
| CD117 | 1 (0.8) |
| CD15* | 1 (0.8) |
| HLA DR | 1 (0.8) |
| Abnormal light scatter, 22 of 126 (17%) | |
| High FSC/SSC | |
| CD2 | 4 (2.6) |
| CD34 | 4 (2.6) |
| CD7 | 4 (2.6) |
| CD117 | 2 (1.5) |
| CD19 | 2 (1.5) |
| CD20 | 1 (0.8) |
| Low FSC/SSC | |
| CD13† | 3 (2) |
| CD33† | 1 (0.8) |
| CD15 | 1 (0.8) |
| Asynchronous antigen expression, 98 of 126 (78%) | |
| CD34+ HLA-DR−CD33+ | 11 (9) |
| CD34+CD56+ | 10 (8) |
| CD34+CD11b+ | 6 (5) |
| CD34+CD33++ | 4 (3) |
| CD34+CD14+ | 4 (3) |
| CD34+ CD117+HLA-DR− | 3 (2.3) |
| CD34+CD117− CD15+ | 3 (2.3) |
| CD34+ CD33− CD13+HLA-DR+ | 2 (1.5) |
| CD34+CD33− CD13+ HLA-DR− | 1 (0.8) |
| CD34+ CD33− CD117+HLA-DR+ | 1 (0.8) |
| CD117+CD33+ HLA-DR− | 14 (11) |
| CD117+ CD34−CD15−‡ | 8 (6) |
| CD117+CD11b+ | 7 (5.5) |
| CD117+CD33+ CD34− CD15+ | 5 (4) |
| CD117+ DR− CD15+ | 3 (2.3) |
| CD117+ DR−CD15−* | 2 (2.3) |
| CD117+DR+ CD33+ CD34− | 1 (0.8) |
| CD33++ HLA-DR− CD34−CD15− CD14− | 22 (17) |
| CD33− CD13+ | 18 (14) |
| CD33+ CD13− | 9 (7) |
| CD33+ HLA-DR+ CD4+CD45dim | 1 (0.8) |
| CD33++HLA-DR+ CD15−CD14−1-153 | 1 (0.8) |
| CD33+CD45d CD34− CD15−1-155 | 1 (0.8) |
| CD33+ HLA-DR+ CD56+CD13+ | 1 (0.8) |
Overexpression of CD15 in nongranulocytic lineage.
Basophilic lineage.
Mast cells express this phenotype with a higher expression of CD117.
Dendritic cells.
Basophilic lineage.
For the investigation of MRD in the mCR BM sample obtained after induction treatment, only those mAb combinations selected at diagnosis as informative for MRD studies were used. Additionally, to increase the sensitivity of the analysis, data acquisition in the flow cytometer was performed in 2 consecutive steps. Briefly, in the first step, all cells in the sample were acquired, and, at this point, at least 15 000 events per tube were measured. Subsequently, in a second step, a multiparametric live-gate was used to acquire more data on leukemic cells that might have been present in low numbers within the original BM sample. For that purpose, acquisition through an SSC/antigen live-gate was performed, and information was collected for at least 106 BM nucleated cells. Data analysis was based on the identification of cells with aberrant phenotypic features identical to those of leukemic cells at diagnosis.
For data acquisition, the Lysis II or Cell Quest software programs (Becton Dickinson) were used. The Paint-a-Gate Pro software program (Becton Dickinson) with the polynomial SSC transformation capability was used for further data analysis. Analysis was performed on gated blast cells according to previously defined methods.3,5,13-15 23
Statistical analysis
The χ2 and the Mann-Whitney U tests were used to estimate the statistical significance of differences between groups. Survival curves were plotted according to the method of Kaplan and Meier, and comparison between the curves was performed using the log-rank and Breslow tests.24 Relapse rates are reported as % ± 1 SE and overall survival rates as median ± 1 SE. In univariate analysis for relapse-free survival, the following variables were tested: age, WBC, platelet count, proportion of blast cells in BM, absolute number of blast cells in PB, number of cycles to achieve CR (1 or 2 courses), type of treatment, FAB classification, cytogenetics, and MRD level at the end of induction therapy.
Subsequently, multivariate analysis stepwise regression25was performed to explore the independent effect of variables that showed a significant influence on disease-free survival found by univariate analysis. Statistical analysis was performed using the SPSS software (version 8.0.1S; SPSS, Chicago, Illinois).
Results
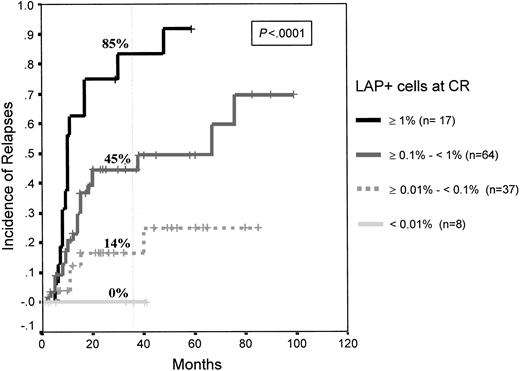
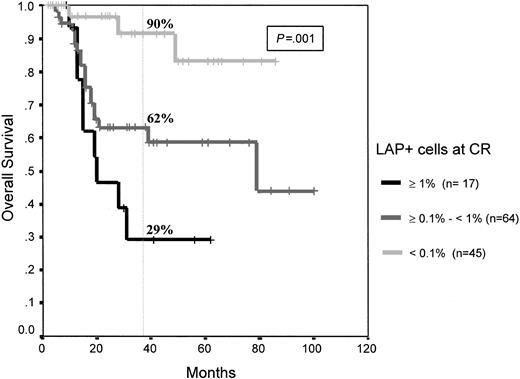
As mentioned, this study is based on 126 consecutive patients with de novo AML in whom blast cells displayed antigenic phenotypic aberrancies at diagnosis and who achieved mCR after induction therapy. Immunophenotypic analysis of the first BM in mCR demonstrated that, based on the level of residual phenotypically aberrant cells displaying a leukemia-associated phenotype (LAP+), it was possible to identify 3 different groups of patients at risk (P < .0001). Seventeen patients were in the high-risk category (MRD greater than 10−2 LAP+ cells) and had a 3-year cumulative relapse rate of 85% ± 9%; 64 patients were in the intermediate risk category (MRD: 10−3 to 10−2 LAP+ cells) and had a 3-year relapse rate of 45% ± 8%; and 45 patients were in a low-risk category (MRD fewer than 10−3 LAP+ cells) and had a relapse rate at 3 years of only 14% ± 6%. Moreover, within the low-risk group, 8 patients (18%) had fewer than 10−4LAP+ cells, and none of them have had relapses thus far. Thus, it is possible to grade 4 MRD levels (from less than 10−4 to greater than 10−2) that are associated with significantly different relapse rates (P < .0001) (Figure 1). MRD level not only influenced leukemia-free survival but also overall survival of patients with AML. Median overall survival has not been reached in the low-risk group, and it was 79 and 20 months for the intermediate and high-risk patient groups, respectively (P = 0.001) (Figure2).
Relapse rates in the 4 AML risk categories according to number of LAP cells in the first BM in mCR.
High risk (MRD: greater than 10−2 LAP+cells); intermediate risk (MRD: 10−3 to 10−2LAP+ cells); low risk (MRD: fewer than 10−3LAP+ cells); and very low risk (fewer than 10−4 LAP+ cells; none have had relapses).
Relapse rates in the 4 AML risk categories according to number of LAP cells in the first BM in mCR.
High risk (MRD: greater than 10−2 LAP+cells); intermediate risk (MRD: 10−3 to 10−2LAP+ cells); low risk (MRD: fewer than 10−3LAP+ cells); and very low risk (fewer than 10−4 LAP+ cells; none have had relapses).
Overall survival of patients with AML according to MRD levels.
High risk (MRD: greater than 10−2 LAP+ cells), intermediate risk (MRD: 10−3 to 10−2LAP+ cells), low risk (MRD: fewer than 10−3LAP+ cells); and very low risk (fewer than 10−4 LAP+ cells).
Overall survival of patients with AML according to MRD levels.
High risk (MRD: greater than 10−2 LAP+ cells), intermediate risk (MRD: 10−3 to 10−2LAP+ cells), low risk (MRD: fewer than 10−3LAP+ cells); and very low risk (fewer than 10−4 LAP+ cells).
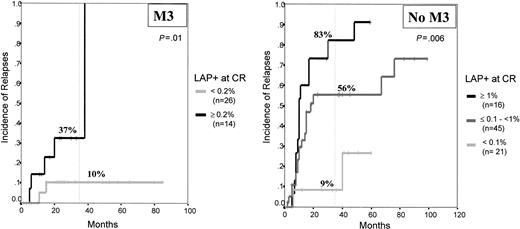
The prognostic impact of MRD levels was also observed once M3 and non-M3 leukemias were separately analyzed. Because of the reduced number of M3 patients (40 patients uniformly treated with all-trans retinoic acid), only 2 risk categories were established for M3, low risk (less than or equal to 2 × 10−3 LAP+ cells; n = 26) and high risk (greater than 2 × 10−3 LAP+ cells, n = 14), with cumulative relapse rates at 3 years of 10% ± 7% and 37% ± 13%, respectively (P = .01) (Figure3). Within the non-M3 leukemias (n = 82), once again 3 distinct risk-group categories could be established based on the level of MRD after induction therapy (fewer than 10−3, 10−3 to 10−2, and more than 10−2 residual LAP+ cells) with 3-year relapse rates of 9% ± 7%, 56% ± 9%, and 83% ± 10%, respectively (P = .006) (Figure 3).
Relapse rates of APL and non-APL patients with AML according to the MRD levels.
High risk (MRD: greater than 10−2 LAP+ cells), intermediate risk (MRD: 10−3 to 10−2LAP+ cells), low risk (MRD: fewer than 10−3LAP+ cells); and very low risk (fewer than 10−4 LAP+ cells).
Relapse rates of APL and non-APL patients with AML according to the MRD levels.
High risk (MRD: greater than 10−2 LAP+ cells), intermediate risk (MRD: 10−3 to 10−2LAP+ cells), low risk (MRD: fewer than 10−3LAP+ cells); and very low risk (fewer than 10−4 LAP+ cells).
Univariate analysis of prognostic factors revealed that, in addition to MRD levels, 4 other disease characteristics had significant impact on relapse-free survival: (1) cytogenetic characteristics (median survival times, 18 months, 40 months, and not reached for the adverse, intermediate, and favorable cytogenetic subgroups;P = .005); (2) number of chemotherapy cycles needed to achieve response (1 vs 2; median survival time, 77 vs 15 months, respectively; P = .003); (3) WBC (less than or greater than or equal to 50 × 109/L; median survival time, 67 vs 11 months, respectively; P = .003); and (4) absolute PB blast cells count (less than or equal to or greater than 50 × 109/L; median survival time, 67 vs 11 months, respectively; P = .009). By contrast, neither the type of consolidation treatment (chemotherapy vs autologous or allogeneic transplantation) nor the FAB classification or age showed significant influence on relapse-free survival. Subsequently, we analyzed the level of MRD in the patient subgroups according to the above-mentioned prognostic factors (Table2). Thus, on analyzing whether MRD level was different in patients who achieved mCR with one cycle of chemotherapy compared with those patients who needed 2 cycles, our results show that in the first group, MRD levels were lower than in the second (median, 1.9 × 10−3 vs 5.6 × 10−3 residual LAP+ cells;P = .02). Moreover, patients with low WBC (less than 50 × 109/L) counts also had lower levels of MRD than patients with higher absolute numbers of leukocytes (P = .01). Based on patient grouping according to the cytogenetic characteristics found at diagnosis, it was observed that patients with adverse karyotypic features had significantly higher levels of residual LAP+ cells than patients with favorable cytogenetics, whereas those with normal karyotype or another cytogenetic abnormality had intermediate MRD levels (Table 2). Regarding the influence of MRD on the outcome of different cytogenetic subgroups of patients, the results for patients with t(15;17) were identical to those reported above for M3 leukemias. The reduced number of patients in each of the other cytogenetic categories hampered appropriate statistical comparisons on the impact of MRD in these specific subsets of patients. Nevertheless, interesting trends emerged from these limited samples. After the exclusion of patients with t(15;17), within the 9 remaining t(15;17)-vepatients with favorable cytogenetics [t(8;21)+ and inv16+], only 2 patients had greater than 1% residual LAP+ cells after induction therapy, and both have relapsed. On the other hand, within the adverse cytogenetic group, 7 of the 8 patients with greater than 0.1% residual LAP+ cells have relapsed compared with only 1 of 3 patients with levels below this threshold. Reverse transcription (RT)–PCR studies were performed at the time of mCR in 22 patients with APL uniformly treated with all-trans retinoic acid plus chemotherapy. Most patients (n = 19; 87%) remained PCR positive, however, because quantitative PCR was not performed, it is not possible to compare these results with the level of MRD detected by immunophenotypic methods.
MRD levels in subsets of patients defined according to prognostic factors
| Patient categories . | MRD level (%)* . | P . |
|---|---|---|
| Cytogenetics | ||
| Favorable (n = 49) | 0.26 ± 0.33 (0.09) | |
| Intermediate (n = 28) | 0.8 ± 1.1 (0.30) | .05 |
| Adverse (n = 7) | 1.2 ± 1.6 (0.68) | |
| Number cycles to CR | ||
| One (n = 101) | 0.42 ± 0.75 (0.19) | .02 |
| Two (n = 25) | 1.05 ± 1.26 (0.56) | |
| WBC (× 109/L) | ||
| Less than 50 × 109 (n = 107) | 0.47 ± 0.74 (0.2) | .01 |
| Greater than 50 × 109 (n = 18) | 1.02 ± 1.49 (0.41) |
| Patient categories . | MRD level (%)* . | P . |
|---|---|---|
| Cytogenetics | ||
| Favorable (n = 49) | 0.26 ± 0.33 (0.09) | |
| Intermediate (n = 28) | 0.8 ± 1.1 (0.30) | .05 |
| Adverse (n = 7) | 1.2 ± 1.6 (0.68) | |
| Number cycles to CR | ||
| One (n = 101) | 0.42 ± 0.75 (0.19) | .02 |
| Two (n = 25) | 1.05 ± 1.26 (0.56) | |
| WBC (× 109/L) | ||
| Less than 50 × 109 (n = 107) | 0.47 ± 0.74 (0.2) | .01 |
| Greater than 50 × 109 (n = 18) | 1.02 ± 1.49 (0.41) |
Mean ± SD (median).
To explore whether MRD level was an independent prognostic factor for relapse-free survival of patients with AML, multivariate analysis was conducted. In 32 of 126 patients, either cytogenetic information or mitosis was not obtained. As a result, the number of patients for multivariate analysis was reduced to 94. Therefore, we decided to perform analyses with and without the cytogenetic information. In the latter, all 126 patients were included in the regression analysis. Only the MRD levels proved to have consistent, independent prognostic influence in the Cox model (P = .0003), whereas the number of cycles of chemotherapy (1 vs 2) needed to achieve morphologic response was on the limit of statistical significance (P = .058). Because M3 leukemia is sometimes considered a distinct AML subtype, we repeated the multivariate analysis for the non-M3 patients. Once again, the most significant variable for predicting relapse-free survival was MRD followed by response to one cycle of chemotherapy (P = .01). When cytogenetic information was included in the Cox model, the parameters selected as having independent prognostic influence on relapse-free survival were the MRD levels (P = .002) and cytogenetics (P = .03).
Discussion
Investigation of MRD has proven to be of relevant clinical value in the decision-making process for the most appropriate treatment of several hematologic malignancies, among them ALL, APL, and chronic myeloid leukemia.3,4,6-8,11 In AML, most MRD studies have been restricted to APL4,8 because the proportion of molecular targets available from non-M3 AML is still relatively small; in fact, only approximately 40% of patients with AML can be monitored by PCR.26 An alternative to molecular studies is the use of multiparametric flow cytometry immunophenotyping to monitor patients with AL who have aberrant phenotypic features. We have previously shown13 that on using multiparametric immunophenotyping, 70% to 80% of patients with AML have phenotypic aberrancies appropriate for MRD detection. However, in contrast to ALL, little information is available on the clinical value of MRD investigation by immunophenotyping of AML.3 9-11
In most therapeutic strategies for patients with AML, the treatment, including the possibility of transplantation, is administered within the first 6 months of diagnosis. Therefore, stratification of patients according to risk group should be based on parameters either already available at diagnosis or derived from response to initial treatment. The most relevant factors for risk stratification of AML, and probably the only ones used, are cytogenetics and morphologic evaluation of response to induction therapy.18,27-29 However, the latter parameter is of limited value because it identifies only a subgroup of patients with poor prognoses: those who do not achieve mCR. Among patients who do achieve CR, a high relapse rate persists that cannot be predicted using conventional morphology. Accordingly, more sensitive techniques, such as PCR or flow cytometry immunophenotyping, may be useful for the evaluation of MRD level in patients with AML in mCR. Although most MRD studies have focused on sequential follow-up samples, we hypothesize that early investigation of MRD in BM samples obtained immediately after induction treatment could help to subdivide AML patients who have achieved mCR into different risk categories. Our results show that 4 different patient risk groups can be clearly identified based on the number of residual cells displaying leukemia-associated phenotypes (LAP+ cells). Thus, none of the patients with fewer than 10−4 LAP+ cells have relapsed so far, whereas in the group at poor risk (MRD greater than 10−2) the median relapse-free survival time was only 10 months, with a cumulative relapse risk rate of 85% at 3 years. It should be noted that the assessment of MRD was also valuable when M3 and non-M3 AMLs were separately analyzed. RT-PCR studies were performed at the time of mCR in 22 patients with APL uniformly treated with all-trans retinoic acid plus chemotherapy. Most patients (n = 19; 87%) remained PCR positive, but because quantitative PCR was not performed, it was not possible to compare these results with the level of MRD detected by immunophenotypic methods. As mentioned above, cytogenetics is probably the most important prognostic criterion for patients with AML.27-29 Nevertheless, a shortcoming of this parameter is that informative karyotypes are only obtained in 60% to 70% of all patients with AML. Here, we have explored whether cytogenetic abnormalities influenced the level of MRD after induction therapy. Our results show that the level of MRD was significantly higher in patients with adverse karyotypes than in patients with favorable cytogenetics. Interestingly, even within each individual cytogenetic subgroup, MRD evaluation appears to be informative. Thus, patients with favorable cytogenetics will eventually have relapses only if they have high MRD levels (greater than 10−2) after induction therapy. By contrast, patients with adverse cytogenetics will have relapses unless MRD levels decrease to 10−4 residual LAP+ cells. These observations were confirmed in multivariate analysis showing that MRD levels after induction therapy, together with cytogenetics, are independent prognostic factors. Both parameters represent the best combination of variables to predict disease-free survival in AML. When cytogenetics was excluded from stepwise regression analysis (because information was unavailable for 32 patients), the most significant parameter was, once again, MRD level, together with the number of chemotherapy cycles (1 vs 2) needed to achieve morphologic response.
As previously mentioned, the strategy of monitoring MRD based on aberrant phenotypes can be used in 70% to 80% of patients with AML. For the remaining patients, new approaches must be developed. One possibility, recently explored in ALL,30 is based on the investigation of myeloid progenitors. In these precursor cells, the detection of deviations from the normal maturation pathways could reflect the presence of abnormal hemopoiesis that might signal impending relapse.
Different treatment strategies are available for consolidation therapy in AML patients, including autologous and allogeneic transplantation with the mini-allografting variants, and novel therapeutic weapons, such as monoclonal antibodies and immunotherapy. Some of these treatment modalities should still be considered experimental, and it would be desirable to test them in uniform groups of patients whose risk is defined by objective prognostic factors. Otherwise, identifying patients who could benefit from a particular treatment strategy would be extremely difficult, and progress in the therapeutic outcome of AML would be delayed. Our results show that early immunophenotypical evaluation of MRD in patients with AML identifies different risk groups and may contribute to postinduction treatment stratification.
We thank all members of the BIOMED-1 Concerted Action (BMH-CMT 94-1675), particularly Jacques van Dongen for fruitful discussion during the standardization of MRD studies, and M. Anderson and M. J. Rodrigo for their help with the English language.
Supported in part by national grants from Spain (FISS 95/1640,CICYT-SAF 94-038,AECC-95,FIS 00/0023-03) and integrated in the European BIOMED-1 Concerted Action (BMH-CMT 94-1675).
Accepted for oral presentation at the 2000 American Society of Hematology Meeting, San Francisco, CA, December 1-5, 2000.
J.F.S.M., M.B.V., M.G., and A.O. are members of the European BIOMED-1 Concerted Action (BMH-CMT 94-1675).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jesús F. San Miguel, Hematology Department, Hospital Universitario de Salamanca, Paseo de San Vicente 58-182, 37007 Salamanca. Spain; e-mail: sanmigiz@gugu.usal.es.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal