Abstract
T-cell prolymphocytic leukemia (T-PLL) is a chemotherapy-resistant malignancy with a median survival of 7.5 months. Preliminary results indicated a high remission induction rate with the human CD52 antibody, CAMPATH-1H. This study reports results in 39 patients with T-PLL treated with CAMPATH-1H between March 1993 and May 2000. All but 2 patients had received prior therapy with a variety of agents, including 30 with pentostatin; none achieved complete remission (CR). CAMPATH-1H (30 mg) was administered intravenously 3 times weekly until maximal response. The overall response rate was 76% with 60% CR and 16% partial remission (PR). These responses were durable with a median disease-free interval of 7 months (range, 4-45 months). Survival was significantly prolonged in patients achieving CR compared to PR or no response (NR), including one patient who survived 54 months. Nine patients remain alive up to 29 months after completing therapy. Seven patients received high-dose therapy with autologous stem cell support, 3 of whom remain alive in CR 5, 7, and 15 months after autograft. Stem cell harvests in these patients were uncontaminated with T-PLL cells as demonstrated by dual-color flow cytometry and polymerase chain reaction. Four patients had allogeneic stem cell transplants, 3 from siblings and 1 from a matched unrelated donor. Two had nonmyeloablative conditioning. Three are alive in CR up to 24 months after allograft. The conclusion is that CAMPATH-1H is an effective therapy in T-PLL, producing remissions in more than two thirds of patients. The use of stem cell transplantation to consolidate responses merits further study.
Introduction
T-cell prolymphocytic leukemia (T-PLL) is a rare aggressive postthymic malignancy with distinctive clinical, morphologic, and cytogenetic features.1 T-PLL is often resistant to conventional chemotherapy and has a poor median survival (7.5 months).1 Although some patients may initially present with indolent disease, they eventually progress and the outcome is then similar.2 New therapeutic approaches are badly needed to improve outcome in this fatal disease.
CAMPATH-1H is a genetically reshaped human IgG1 anti-CD52 monoclonal antibody, consisting of the hypervariable regions of the parental rat antibody inserted into the framework regions of normal human immunoglobulin (IgG1) genes.3 The CD52 antigen is a nonmodulating glycosylphosphatidylinositol (GPI)-linked protein of unknown function abundantly expressed on normal and malignant lymphocytes, monocytes, macrophages, and eosinophils, but not on hemopoietic progenitor cells.4 In vitro, CAMPATH-1H is active in complement-mediated lysis and antibody-dependent cellular cytotoxicity (ADCC). Both these mechanisms may contribute to the therapeutic activity of this antibody in vivo. CD52+ cells vary in their sensitivity to lysis induced by CAMPATH-1H and this may be related to the antigen site density on different cell types.5
Promising results have been obtained using CAMPATH-1H antibodies in patients with a variety of lymphoproliferative disorders.6,7 These early studies included a patient with T-PLL who went into complete remission (CR) with the rat IgG2b antibody CAMPATH-1G.6 This prompted a larger phase 2 study to examine the role of CAMPATH-1H in this disease.8 We have now extended this study to include 39 patients and have also examined the use of high-dose treatment with autologous or allogeneic stem cell transplantation (SCT) in 11 patients.
Patients, materials, and methods
Patients
Thirty-nine patients with T-PLL received CAMPATH-1H treatment between March 1993 and May 2000 (Table1). Patients from a number of European centers have been entered into this study including 6 from Lille, France and 3 from Oslo, Norway. There were 28 men and 11 women, aged 34 to 78 years (median, 57 years). All but 2 of the patients had received prior therapy with a variety of agents, including 30 (77%) with pentostatin, 3 (8%) with other purine analogues (fludarabine, cladribine), 4 (10%) with chlorambucil, and 13 (33%) with combination chemotherapy (cyclophosphamide, doxorubicin, vincristine, prednisone [CHOP], cyclophosphamide, vincristine, prednisone [COP], vincristine, doxorubicin, prednisone, etoposide, cyclophosphamide, bleomycin [VAPEC-B]). Six patients (16%) had undergone splenectomy. No patient had achieved CR with prior therapy and 24 (61.5%) were resistant to treatment (either nonresponders or disease progression). Twelve patients had partial responses (PRs) to purine analogues (pentostatin 11, cladribine 1) followed by progressive disease in 10 and in the other 2 immediately by CAMPATH-1H treatment to eliminate residual disease.
Characteristics and responses of T-PLL patients treated with CAMPATH-1H
| Patient . | Age*/ sex . | Prior therapy . | Best response to prior therapy . | Site of disease* . | WCC* (× 109/L) . | Total dose CAMPATH . | Response to CAMPATH . | Duration of response . | Immuno-phenotype (CD)† . | Status, cause of death . | Survival from CAMPATH . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 49/F | VAPEC-B, Sx, DCF | PR | 1. B/BM | 1. 24 | 1. 530 mg | 1. CR | 1. 30 mo | 4+/8+ | Dead, infection | 54 mo |
| 2. B/BM | 2. 20.2 | 2. 670 mg | 2. CR | 2. 16 mo | |||||||
| 2 | 53/M | DCF | PR | 1. B/BM | 1. 8 | 1. 660 mg | 1. CR | 1. 21 mo | 4+/8+ | Dead, PD | 52 mo |
| 2. B/BM | 2. 14.3 | 2. 170 mg | 2. CR | 2. 6 mo | |||||||
| 3 | 61/M | DCF | NR | 1. B/BM | 1. 715 | 1. 890 mg | 1. CR | 1. 5 mo | 4+/8+ | Dead, PD | 17 mo |
| CHOP | 2. B/BM | 2. 105 | 2. 1325 mg | 2. CR | 2. 5 mo | ||||||
| 4 | 77/M | DCF | NR | 1. B/BM/Sp/H | 1. 148.8 | 1. 388 mg | 1. CR | 6 mo | 4+/8− | Dead, infection | 8 mo |
| 2. B/BM/Sp/H | 2. 16 | 2. 153 mg | 2. CR | 2. N/E | |||||||
| 5 | 43/M | DCF | PR | 1. Residual BM | Normal | 1. 180 mg | 1. CR | 23+ mo | 4+/8+ | Alive | 24+ mo |
| 2. Molecular relapse | 2. 180 mg | 2. CR | |||||||||
| 6 | 63/F | DCF | NR | 1. B/BM/Sp/H/S | 201 | 1. 803 mg | 1. CR | 10 mo | 4+/8− | Dead, PD in CNS | 16 mo |
| 2. C/B/S | 2. 263 mg | 2. PR | |||||||||
| 7 | 51/F | Prednisolone | NR | 1. B/BM/S | 1. 117 | 1. 436 mg | 1. CR | 7 mo | 4+/8− | Dead, PD | 9 mo |
| 2. B/BM/S | 2. 450 mg | 2. PD | |||||||||
| 8 | 63/M | DCF | PR | 1. B/BM/Sp/LN | 1. 94.0 | 1. 665 mg | 1. CR | 6 mo | 4+/8− | Dead, PD | 8 mo |
| 2. B/BM/Sp/LN | 2. 34.5 | 2. 40 mg | 2. PD | ||||||||
| 9 | 58/F | C + P, leukapheresis | NR | 1. B/BM/Sp/LN | 1. 456 | 1. 820 mg | 1. CR | 5 mo | 4+/8− | Dead, PD | 9 mo |
| 2. B/BM | 2. 38.4 | 2. 390 mg | 2. N/E | ||||||||
| 10 | 62/M | Chlor, DCF, Sx, CNOP | NR | B/BM | 95.6 | 1. 320 mg | 1. CR | 45 mo | 4−/8+ | Dead, PD | 46 mo |
| B/BM/H/LN | 35.0 | 2. 70 mg | 2. NR | ||||||||
| 11 | 47/F | NONE | B/BM | 1. 111 | 1. 900 mg | 1. CR | 25 mo | 4−/8+ | Dead, PD | 27 mo | |
| 2. 140 | 2. 800 mg | 2. NR | |||||||||
| 12 | 46/M | DCF, ALL-like CT | PR | 1. B/BM | 178 | 1. 883 mg | 1. CR | 4 mo | 4+/8+ | Dead, PD | 14 mo |
| 2. B/BM | 2. 300 mg | 2. NR | |||||||||
| 13 | 68/F | DCF | NR | B/BM/Sp | 76 | 800 mg | CR | 8 mo | 4+/8− | Dead, thrombosis | 9 mo |
| 14 | 59/M | DCF | PR | B/BM/LN | 4.8 | 333 mg | CR | 8 mo | 4+/8− | Dead, ALS | 15 mo |
| 15 | 74/M | DCF | NR | B/BM/Sp/H/LN | 323.4 | 388 mg | CR | 9 mo | 4+/8− | Alive | 23+ mo |
| 16 | 63/M | DCF | NR | B/BM/Sp | 318 | 540 mg | CR | 6+ mo | Alive | 16+ mo | |
| 17 | 57/M | DCF | PR | Residual BM | 6.4 | 466 mg | CR | N/E | 4+/8+ | Dead, TRM | 2 mo |
| 18 | 44/M | Steroids, methotrexate, Sx, 2CDA | PR | B/BM | 139 | 463 mg | CR | 19 mo | 4−/8+ | Alive | 22+ mo |
| 19 | 58/F | CHOP | NR | B/BM, LN, C | 115 | 1002 mg | CR | 7+ mo | 4+/8− | Alive | 10+ mo |
| 20 | 60/F | NONE | B/BM/LN/Sp | 315 | 493 mg | CR | 5+ mo | 4+/8− | Alive | 6+ mo | |
| 21 | 40/M | DCF, Sx | NR | B/BM | 36.5 | 423 mg | CR | 4 mo | 4−/8+ | Alive | 29+ mo |
| 22 | 38/M | DCF, CHOP | NR | B/BM/Lung | 34 | 540 mg | CR | 9 mo | 4+/8− | Dead, PD | 23 mo |
| 23 | 75/M | Chlorambucil | NR | B/BM | 484 | 483 mg | CR | 9 mo | 4−/8+ | Dead, Ca stomach | 9 mo |
| 24 | 78/M | DCF | PR | 1. B/BM/Sp | 1. 4.0 | 1. 230 mg | 1. PR | 5 mo | 4−/8+ | Dead, PD | 8 mo |
| 2. B/BM | 2. 219 | 2. 50 mg | 2. PD | ||||||||
| 25 | 60/M | CHOP | NR | B/BM/Sp/LN/H | 102.8 | 470 mg | PR‡ | N/E1-153 | 4+/8+ | Dead, infection | 2 mo |
| DCF | |||||||||||
| 26 | 67/M | DCF | PR | B/BM/Brain | 130 | 635 mg | PR | 1 mo | 4+/8+ | Dead, PD | 4 mo |
| 27 | 59/F | COP, DCF | NR | B/BM/Sp | 140 | 633 mg | PR | 7 mo | 4+/8+ | Dead, PD | 10 mo |
| 28 | 51/M | DCF | NR | B/BM/S | 88 | 1093 mg | PR | 4−/8+ | Alive | 12+ mo | |
| 29 | 56/M | CHOP, DCF | NR | B/BM/Sp/LN | 400 | 630 mg | PR | 4 mo | 4+/8+ | Dead, PD | 12 mo |
| 30 | 57/M | Steroids, DCF | PR | B/BM/C | 97.1 | 450 mg | NR1-155 | — | 4+/8+ | Dead, PD | 5 mo |
| 31 | 45/M | C + P, CHOP, DCF | NR | B/BM/Sp/LN/H | 660 | 630 mg | NR | — | 4+/8+ | Dead, PD | 5 wk |
| 32 | 38/M | DCF, Sx, various | PR | B/BM/S/C/H/A | 86 | 200 mg | NR | — | 4+/8+ | Dead, PD | 3 wk |
| 33 | 62/F | DCF | PR | B/BM/Sp/LN/H/P | 68.6 | 70 mg | NR | — | 4+/8− | Dead, PD | 1 wk |
| 34 | M | DCF | NR | BM/LN/Sp | 133 mg | NR | — | 4−/8+ | Dead, PD | 6 mo | |
| 35 | 67/F | 2CDA, FDR | MR | B/BM/S | 74.8 | 500 mg | NR | — | 4+/8+ | Dead, PD | 5 mo |
| 36 | 52/M | DCF | NR | B/BM/Sp/H/LN/S | 42.1 | 1163 mg | NR | — | 4+/8+ | Dead, PD | 8 mo |
| 37 | 50/M | DCF | NR | B/BM/Sp/H/LN | 14 | 180 mg | NR | — | 4−/8+ | Alive | 18+ mo |
| 38 | 34/M | COP, Sx, DCF | MR | BM/H/P/A | 230 | 283 mg | NR | — | 4+/8+ | Dead, PD | 3 wk |
| 39 | 45/M | CHOP, FDR, ESHAP | NR | B/BM/Sp/H | 50 | 213 mg | NE | — | 4+/8− | Dead, unknown | 2 wk |
| Patient . | Age*/ sex . | Prior therapy . | Best response to prior therapy . | Site of disease* . | WCC* (× 109/L) . | Total dose CAMPATH . | Response to CAMPATH . | Duration of response . | Immuno-phenotype (CD)† . | Status, cause of death . | Survival from CAMPATH . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 49/F | VAPEC-B, Sx, DCF | PR | 1. B/BM | 1. 24 | 1. 530 mg | 1. CR | 1. 30 mo | 4+/8+ | Dead, infection | 54 mo |
| 2. B/BM | 2. 20.2 | 2. 670 mg | 2. CR | 2. 16 mo | |||||||
| 2 | 53/M | DCF | PR | 1. B/BM | 1. 8 | 1. 660 mg | 1. CR | 1. 21 mo | 4+/8+ | Dead, PD | 52 mo |
| 2. B/BM | 2. 14.3 | 2. 170 mg | 2. CR | 2. 6 mo | |||||||
| 3 | 61/M | DCF | NR | 1. B/BM | 1. 715 | 1. 890 mg | 1. CR | 1. 5 mo | 4+/8+ | Dead, PD | 17 mo |
| CHOP | 2. B/BM | 2. 105 | 2. 1325 mg | 2. CR | 2. 5 mo | ||||||
| 4 | 77/M | DCF | NR | 1. B/BM/Sp/H | 1. 148.8 | 1. 388 mg | 1. CR | 6 mo | 4+/8− | Dead, infection | 8 mo |
| 2. B/BM/Sp/H | 2. 16 | 2. 153 mg | 2. CR | 2. N/E | |||||||
| 5 | 43/M | DCF | PR | 1. Residual BM | Normal | 1. 180 mg | 1. CR | 23+ mo | 4+/8+ | Alive | 24+ mo |
| 2. Molecular relapse | 2. 180 mg | 2. CR | |||||||||
| 6 | 63/F | DCF | NR | 1. B/BM/Sp/H/S | 201 | 1. 803 mg | 1. CR | 10 mo | 4+/8− | Dead, PD in CNS | 16 mo |
| 2. C/B/S | 2. 263 mg | 2. PR | |||||||||
| 7 | 51/F | Prednisolone | NR | 1. B/BM/S | 1. 117 | 1. 436 mg | 1. CR | 7 mo | 4+/8− | Dead, PD | 9 mo |
| 2. B/BM/S | 2. 450 mg | 2. PD | |||||||||
| 8 | 63/M | DCF | PR | 1. B/BM/Sp/LN | 1. 94.0 | 1. 665 mg | 1. CR | 6 mo | 4+/8− | Dead, PD | 8 mo |
| 2. B/BM/Sp/LN | 2. 34.5 | 2. 40 mg | 2. PD | ||||||||
| 9 | 58/F | C + P, leukapheresis | NR | 1. B/BM/Sp/LN | 1. 456 | 1. 820 mg | 1. CR | 5 mo | 4+/8− | Dead, PD | 9 mo |
| 2. B/BM | 2. 38.4 | 2. 390 mg | 2. N/E | ||||||||
| 10 | 62/M | Chlor, DCF, Sx, CNOP | NR | B/BM | 95.6 | 1. 320 mg | 1. CR | 45 mo | 4−/8+ | Dead, PD | 46 mo |
| B/BM/H/LN | 35.0 | 2. 70 mg | 2. NR | ||||||||
| 11 | 47/F | NONE | B/BM | 1. 111 | 1. 900 mg | 1. CR | 25 mo | 4−/8+ | Dead, PD | 27 mo | |
| 2. 140 | 2. 800 mg | 2. NR | |||||||||
| 12 | 46/M | DCF, ALL-like CT | PR | 1. B/BM | 178 | 1. 883 mg | 1. CR | 4 mo | 4+/8+ | Dead, PD | 14 mo |
| 2. B/BM | 2. 300 mg | 2. NR | |||||||||
| 13 | 68/F | DCF | NR | B/BM/Sp | 76 | 800 mg | CR | 8 mo | 4+/8− | Dead, thrombosis | 9 mo |
| 14 | 59/M | DCF | PR | B/BM/LN | 4.8 | 333 mg | CR | 8 mo | 4+/8− | Dead, ALS | 15 mo |
| 15 | 74/M | DCF | NR | B/BM/Sp/H/LN | 323.4 | 388 mg | CR | 9 mo | 4+/8− | Alive | 23+ mo |
| 16 | 63/M | DCF | NR | B/BM/Sp | 318 | 540 mg | CR | 6+ mo | Alive | 16+ mo | |
| 17 | 57/M | DCF | PR | Residual BM | 6.4 | 466 mg | CR | N/E | 4+/8+ | Dead, TRM | 2 mo |
| 18 | 44/M | Steroids, methotrexate, Sx, 2CDA | PR | B/BM | 139 | 463 mg | CR | 19 mo | 4−/8+ | Alive | 22+ mo |
| 19 | 58/F | CHOP | NR | B/BM, LN, C | 115 | 1002 mg | CR | 7+ mo | 4+/8− | Alive | 10+ mo |
| 20 | 60/F | NONE | B/BM/LN/Sp | 315 | 493 mg | CR | 5+ mo | 4+/8− | Alive | 6+ mo | |
| 21 | 40/M | DCF, Sx | NR | B/BM | 36.5 | 423 mg | CR | 4 mo | 4−/8+ | Alive | 29+ mo |
| 22 | 38/M | DCF, CHOP | NR | B/BM/Lung | 34 | 540 mg | CR | 9 mo | 4+/8− | Dead, PD | 23 mo |
| 23 | 75/M | Chlorambucil | NR | B/BM | 484 | 483 mg | CR | 9 mo | 4−/8+ | Dead, Ca stomach | 9 mo |
| 24 | 78/M | DCF | PR | 1. B/BM/Sp | 1. 4.0 | 1. 230 mg | 1. PR | 5 mo | 4−/8+ | Dead, PD | 8 mo |
| 2. B/BM | 2. 219 | 2. 50 mg | 2. PD | ||||||||
| 25 | 60/M | CHOP | NR | B/BM/Sp/LN/H | 102.8 | 470 mg | PR‡ | N/E1-153 | 4+/8+ | Dead, infection | 2 mo |
| DCF | |||||||||||
| 26 | 67/M | DCF | PR | B/BM/Brain | 130 | 635 mg | PR | 1 mo | 4+/8+ | Dead, PD | 4 mo |
| 27 | 59/F | COP, DCF | NR | B/BM/Sp | 140 | 633 mg | PR | 7 mo | 4+/8+ | Dead, PD | 10 mo |
| 28 | 51/M | DCF | NR | B/BM/S | 88 | 1093 mg | PR | 4−/8+ | Alive | 12+ mo | |
| 29 | 56/M | CHOP, DCF | NR | B/BM/Sp/LN | 400 | 630 mg | PR | 4 mo | 4+/8+ | Dead, PD | 12 mo |
| 30 | 57/M | Steroids, DCF | PR | B/BM/C | 97.1 | 450 mg | NR1-155 | — | 4+/8+ | Dead, PD | 5 mo |
| 31 | 45/M | C + P, CHOP, DCF | NR | B/BM/Sp/LN/H | 660 | 630 mg | NR | — | 4+/8+ | Dead, PD | 5 wk |
| 32 | 38/M | DCF, Sx, various | PR | B/BM/S/C/H/A | 86 | 200 mg | NR | — | 4+/8+ | Dead, PD | 3 wk |
| 33 | 62/F | DCF | PR | B/BM/Sp/LN/H/P | 68.6 | 70 mg | NR | — | 4+/8− | Dead, PD | 1 wk |
| 34 | M | DCF | NR | BM/LN/Sp | 133 mg | NR | — | 4−/8+ | Dead, PD | 6 mo | |
| 35 | 67/F | 2CDA, FDR | MR | B/BM/S | 74.8 | 500 mg | NR | — | 4+/8+ | Dead, PD | 5 mo |
| 36 | 52/M | DCF | NR | B/BM/Sp/H/LN/S | 42.1 | 1163 mg | NR | — | 4+/8+ | Dead, PD | 8 mo |
| 37 | 50/M | DCF | NR | B/BM/Sp/H/LN | 14 | 180 mg | NR | — | 4−/8+ | Alive | 18+ mo |
| 38 | 34/M | COP, Sx, DCF | MR | BM/H/P/A | 230 | 283 mg | NR | — | 4+/8+ | Dead, PD | 3 wk |
| 39 | 45/M | CHOP, FDR, ESHAP | NR | B/BM/Sp/H | 50 | 213 mg | NE | — | 4+/8− | Dead, unknown | 2 wk |
Patients 1-12 were treated twice; numbers 1 and 2 refer to first and second courses of CAMPATH-1H treatment.
For the duration of response, 3+ months denotes continuing CR at 3 months.
Sx indicates splenectomy; DCF, deoxycoformycin; B, blood; PD, progressive disease; Sp, spleen; H, liver; S, skin; C, central nervous system; LN, lymph nodes; N/E, nonevaluable; Chlor, chlorambucil; CNOP, cyclophosphamide, vincristine, prednisone, mitoxantrone; ALL-like CT, acute lymphoblastic leukemia–type chemotherapy regimen; ALS, amyotrophic lateral sclerosis; TRM, transplant-related mortality; 2CDA, cladrabine; C + P, chlorambucil and prednisolone; A, ascites; P, pleural effusion; FDR, fludarabine; ESHAP, methylprednisolone, etoposide, cytarabine, cisplatin.
At the time of CAMPATH-1H treatment.
All patients were CD2+, CD7+, CD3+, the table indicates phenotype with respect to CD4 and CD8 antigens.
Clearance of peripheral blood, Sp, S, and LN but BM hypoplasia and residual T-PLL.
Died of aplasia while in PR.
Peripheral blood and BM cleared but prolymphocytes reappeared here within 4 weeks.
Diagnosis was confirmed by morphology of peripheral blood and bone marrow (BM) smears, immunologic markers, and histology (BM and spleen).
Treatment
CAMPATH-1H (Wellcome Beckenham, [United Kingdom] or Ilex Oncology [Edinburgh, Scotland] compassionate use program) was administered as a 2-hour intravenous infusion. Informed consent was obtained prior to entry into the study. Patients received premedication with acetominophen and an antihistamine to ameliorate the first-dose reactions. An initial test dose of 3 or 10 mg was followed by dose escalation to 30 mg 3 times a week, as tolerated, until maximal response. Some patients received daily CAMPATH-1H and in 5 cases the dose was escalated up to 80 mg. Such dose increments were used empirically in patients who had failed to respond to a 30-mg schedule and in 2 cases was successful in inducing a response. In one patient CAMPATH-1H was administered subcutaneously. Patients received a median of 508 mg (range, 50-1325 mg) in any course of CAMPATH-1H therapy, given over 2 to 82 days (median, 35 days). Infection prophylaxis (oral cotrimoxazole and acyclovir or valacyclovir) was continued for up to 3 months following completion of treatment.
Response evaluation
A CR was defined as the absence of disease detectable by morphology of blood and BM (including trephine biopsy) and computed tomography (CT) scanning. PR was defined as 50% or more reduction in detectable disease, but short of a CR, maintained for 2 months or more. Detection of minimal residual disease by flow cytometry and polymerase chain reaction (PCR) was not included in the response criteria.
Disease-free interval (DFI) was measured from the end of CAMPATH-1H therapy to the time of relapse. Event-free survival (EFS) was measured from start of treatment to any event. Overall survival was determined from start of treatment to last follow-up or day of death. The analysis was conducted using the method of Kaplan and Meier.
Results
Responses
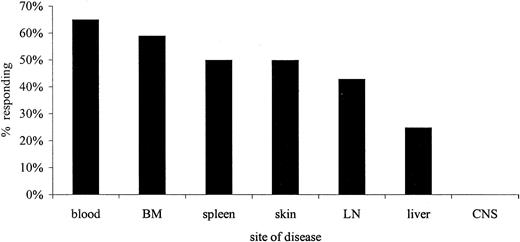
Thirty-eight patients were evaluable for response (Table 1). Patient 39 died suddenly, probably from intracranial hemorrhage, 2 weeks after commencing treatment. The overall response rate was 76%, with 60% CR and 16% PR (Table 2). These responses were durable, with a median DFI of 7 months (range, 4-45 months). Thirteen (34%) patients who had previously been resistant to chemotherapy, including 9 who had NR to pentostatin, achieved a CR following CAMPATH-1H. Figure 1 shows the responses according to site. Clearance of malignant cells from blood and BM was generally rapid although tumor lysis syndrome was not observed in any patient. Resolution of splenomegaly and skin infiltration occurred in half the affected patients and lymphadenopathy in 43%. However, patients with serous effusions and hepatic or central nervous system (CNS) involvement had a poor response to therapy. Patients with very bulky lymph node masses were also more resistant to treatment. In patient 19, CNS disease was effectively cleared by use of intrathecal triple therapy (methotrexate, cytosine arabinoside, and hydrocortisone) prior to commencing CAMPATH-1H treatment. There was no statistical difference in response according to patient age, sex, or presenting white blood cell count (WCC). Patients 5 and 17 received CAMPATH-1H after achieving a PR with prior therapy (pentostatin) and obtained a molecular remission followed by SCT (autograft, patient 5; allograft, patient 17).
Summary of response to CAMPATH-1H in T-PLL patients
| No. of patients | 38 |
| Median age (range) | 57 y (34-78) |
| M/F ratio | 2.5:1 |
| Overall response | 76% |
| CR | 60% |
| PR | 16% |
| NR | 24% |
| Median DFI (range) | 7 mo (4-45) |
| No. of patients | 38 |
| Median age (range) | 57 y (34-78) |
| M/F ratio | 2.5:1 |
| Overall response | 76% |
| CR | 60% |
| PR | 16% |
| NR | 24% |
| Median DFI (range) | 7 mo (4-45) |
Proportion of affected patients who responded to CAMPATH-1H according to the site of disease.
Proportion of affected patients who responded to CAMPATH-1H according to the site of disease.
Of 12 patients who were retreated with CAMPATH-1H following relapse, 5 (42%) achieved a second CR, and one a PR, with responses lasting 5 to 6 months. One of these patients (no. 5) had been treated for a molecular relapse, without any other evidence of disease. There was no statistical difference in the length of first CR or other disease characteristics, comparing the patients who did or did not have a second response.
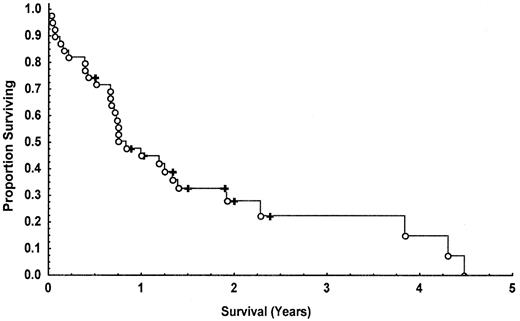
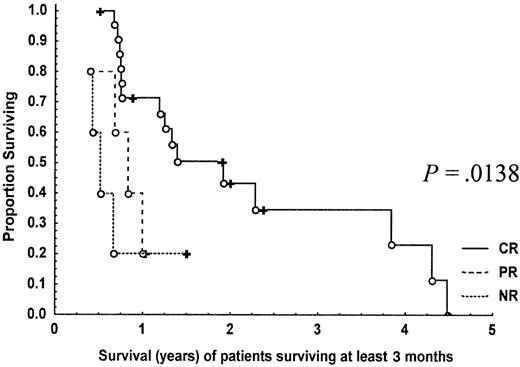
Nine (23%) of the 39 patients entered into the study remain alive. Of the patients who died, 4 died while still in CR, 1 from carcinoma of the stomach (patient 23), 1 from multiple arterial thromboses (patient 13), 1 from infection (patient 4), and 1 from treatment-related toxicity (allogeneic bone marrow transplant, patient 17); 1 died from amyotrophic lateral sclerosis but had also relapsed (patient 14); the remainder died from progressive disease. The median overall survival for the whole group was 10 months (Figure2). Survival was significantly prolonged in those patients achieving CR (median, 16 months) compared to partial (median, 9 months) and nonresponders (median, 4 months) (P = .00066; Figure 3). This observation holds true when the analysis is performed on patients surviving at least 3 months (P = .01380) adjusting for the confounding effect of early deaths (Figure4). Patients 1 and 2 survived for more than 4 years following treatment.
Kaplan-Meier curve showing the overall survival of T-PLL patients following treatment with CAMPATH-1H.
○ indicates dead; ✚, alive.
Kaplan-Meier curve showing the overall survival of T-PLL patients following treatment with CAMPATH-1H.
○ indicates dead; ✚, alive.
Kaplan-Meier curves showing the overall survival of T-PLL patients following treatment with CAMPATH-1H according to response (CR, PR, NR).
♦ indicates dead; ■, alive.
Kaplan-Meier curves showing the overall survival of T-PLL patients following treatment with CAMPATH-1H according to response (CR, PR, NR).
♦ indicates dead; ■, alive.
Kaplan-Meier curves showing the overall survival of T-PLL patients who were alive at 3 months following treatment with CAMPATH-1H according to response (CR, PR, NR).
○ indicates dead; ✚, alive.
Kaplan-Meier curves showing the overall survival of T-PLL patients who were alive at 3 months following treatment with CAMPATH-1H according to response (CR, PR, NR).
○ indicates dead; ✚, alive.
Immunology and cytogenetics
The malignant T cells from 13 patients (33%) were CD4+/CD8−, CD4+/CD8+in 16 (41%), and CD4−/CD8+ in 9 (23%). All cases tested were CD52+ at diagnosis, although cells from patients 10 and 11 were found to have lost CD52 expression at relapse and became resistant to further CAMPATH-1H therapy.
Twenty-eight patients (72%) had successful cytogenetic analysis; 2 had a normal karyotype, and the remainder had complex abnormalities. The most consistent abnormalities involved chromosome 14 including 15 patients with inv(14)(q11q32), 3 with t(14;14)(q11;q32), 2 with del(14)(q12) and 1 t(11;14)(q23;q11). Eleven patients had idic(8)(p11); in 8 patients this was found in association with inv(14)(q11q32), in 2 with t(14;14)(q11;q32) and in 1 with del(14)(q12). There was no correlation between overall or complete responses to CAMPATH-1H and immunophenotype or karyotype.
Autograft and allograft
Seven patients (18%) have proceeded to high-dose therapy (melphalan or cyclophosphamide and total body irradiation [TBI] or BCNU, etoposide, cytarabine, melphalan [BEAM]) with autologous SCT. Stem cell harvests (in 6 of 7 tested) were uncontaminated with T-PLL cells as demonstrated by dual-color flow cytometry and PCR for T-cell receptor (TCR) gene rearrangements. Patients 1 and 2 were autografted in their second remission. Patients 1, 2, 6, and 11 have subsequently relapsed after 5 to 14 months and died from progressive disease. Patients 5, 16, and 19 remain alive 5+, 7+, and 15+ months after autograft.
Four patients (10%) have had an allogeneic SCT (3 sibling [patients 17, 21, and 37], 1 matched unrelated donor [MUD; patient 28]). Two of the allografts (patients 28 and 37), including the MUD transplant, were performed using a nonmyeloablative conditioning regimen.9 Three patients are alive in CR, one of whom remains in molecular remission 2 years after transplant (patient 21). One died (patient 17), while still in remission, from treatment-related toxicity. Patient 37 had been resistant to initial induction therapy including CAMPATH-1H but achieved a good PR following BEAM chemotherapy and went on to receive a sibling allograft using a nonmyeloablative conditioning regimen. He remains well 11 months after transplant having received one donor lymphocyte infusion to maintain full donor chimerism. The mini-MUD transplant was performed on patient 28, who had only achieved PR to CAMPATH-1H and was showing signs of disease progression. These 2 cases illustrate the potential for allografting as salvage therapy in patients with refractory disease. Results are shown in Table 3.
Results of autologous and allogeneic SCT in T-PLL patients following CAMPATH-1H
| Patient no. . | Age/sex . | SCT . | Status at SCT . | Conditioning regime . | Current status . | Survival from SCT . |
|---|---|---|---|---|---|---|
| 1 | 49/F | Auto | Second CR | Melphalan/TBI | Died, PCP | 12 mo |
| 2 | 53/M | Auto | Second CR | Melphalan/TBI | Died, relapse | 17 mo |
| 5 | 62/M | Auto | First CR | BEAC | Alive, CR | 7+ mo |
| 6 | 63/F | Auto | First CR | BEAM | Died, CNS relapse | 8 mo |
| 11 | 47/F | Auto | First CR | Melphalan/TBI | Died, relapse | 19 mo |
| 16 | 63/M | Auto | First CR | Cyclo/TBI | Alive, CR | 15+ mo |
| 19 | 58/F | Auto | First CR | BEAM | Alive, CR | 5+ mo |
| 17 | 57/M | Sib Allo | First CR | Cyclo/TBI | Died, TRM | 3 wk |
| 21 | 40/M | Sib Allo | First CR | Cyclo/TBI | Alive, CR | 24+ mo |
| 37 | 50/M | Mini Sib Allo | NR to CAMPATH-1H, PR post mini-BEAM | FDR/melphalan/CAMPATH-1H | Alive, CR DLI | 11+ mo |
| 28 | 51/M | Mini MUD | PR | FDR/melphalan/CAMPATH-1H | Alive, CR | 2+ mo |
| Patient no. . | Age/sex . | SCT . | Status at SCT . | Conditioning regime . | Current status . | Survival from SCT . |
|---|---|---|---|---|---|---|
| 1 | 49/F | Auto | Second CR | Melphalan/TBI | Died, PCP | 12 mo |
| 2 | 53/M | Auto | Second CR | Melphalan/TBI | Died, relapse | 17 mo |
| 5 | 62/M | Auto | First CR | BEAC | Alive, CR | 7+ mo |
| 6 | 63/F | Auto | First CR | BEAM | Died, CNS relapse | 8 mo |
| 11 | 47/F | Auto | First CR | Melphalan/TBI | Died, relapse | 19 mo |
| 16 | 63/M | Auto | First CR | Cyclo/TBI | Alive, CR | 15+ mo |
| 19 | 58/F | Auto | First CR | BEAM | Alive, CR | 5+ mo |
| 17 | 57/M | Sib Allo | First CR | Cyclo/TBI | Died, TRM | 3 wk |
| 21 | 40/M | Sib Allo | First CR | Cyclo/TBI | Alive, CR | 24+ mo |
| 37 | 50/M | Mini Sib Allo | NR to CAMPATH-1H, PR post mini-BEAM | FDR/melphalan/CAMPATH-1H | Alive, CR DLI | 11+ mo |
| 28 | 51/M | Mini MUD | PR | FDR/melphalan/CAMPATH-1H | Alive, CR | 2+ mo |
Auto indicates autologous; PCP, Pneumocystis cariniipneumonia; BEAC: BCNU, etoposide, cytarabine, cyclophosphamide; cyclo, cyclophosphamide; sib, sibling; allo, allogeneic; mini, nonmyeloablative conditioning; DLI, donor lymphocyte infusion. Other abbreviations are defined in the text or in Table 1.
Toxicity
First-dose reactions characterized by fever, rigors, and nausea (grade 1/2) occurred in all patients, but no serious allergic reactions were observed. Rigors responded to treatment with Demerol. Urticaria was seen in 9 patients (23%) but was usually mild and transient. No patient experienced significant fluid retention. The major side effect of CAMPATH-1H therapy was prolonged lymphopenia (grade 4) lasting for up to 320 days after completion of CAMPATH-1H. Opportunistic infections included 1 case of cryptococcal meningitis, 1 cytomegalovirus (CMV) reactivation, 2 herpes zoster (1 case complicated by myelitis and retinitis), 2 Pneumocystis pneumonia (1 unconfirmed), and 1Legionella pneumonia. These patients all recovered following appropriate therapy. One patient died from an unidentified pulmonary infection during a second course of CAMPATH-1H given to treat disease relapse.
Transient hematologic toxicity (grades 3 and 4) was documented in 5 patients (13%). Four of these patients had an isolated thrombocytopenia and in one case there was a concomitant fall in hemoglobin and neutrophil count. The blood count recovered to normal within 14 days in all 5 patients. Prolonged pancytopenia with severe BM aplasia was seen in a further 2 patients, one of whom died from a fungal pneumonia.
Discussion
Our results confirm earlier observations that T-PLL is particularly responsive to treatment with the monoclonal antibody CAMPATH-1H.8 Overall (76%) and complete (60%) response rates are significantly higher than those reported previously for conventional therapies such as CHOP, with which a third of patients achieve short-lived responses (3 months) or pentostatin with a 40% overall response (12% CR).10 In those patients responding to CAMPATH-1H clearance of cells from the peripheral blood was rapid. However, in no case was this complicated by tumor lysis syndrome.
Although many of the CRs were durable (up to 45 months) and survival was prolonged, there is no evidence that CAMPATH-1H therapy alone alters the ultimate fatal outcome for patients with this aggressive malignancy. All but 2 patients followed up for more than a year have relapsed, including all but one of those who have received autografts with ostensibly uncontaminated stem cells. CAMPATH-1H is not therefore curative despite second responses. Consolidation therapy in first remission with autologous or allogeneic transplantation appears to prolong the DFI and improve survival. However, relapse after autograft seems to be inevitable. Because some stem cell harvests have been shown to be negative for T-PLL by PCR, these relapses may arise from other “sanctuary” sites where CAMPATH-1H is ineffective at clearing disease.
The graft-versus-leukemia (GVL) effect seen in allogeneic SCT is likely to be important and patients who are eligible for this treatment may be potentially curable.11 Because T-PLL patients are often over the age of 55 years the use of a nonmyeloablative conditioning may make allogeneic transplantation a possibility for a larger proportion of patients. This strategy may also be able to salvage patients who are resistant to first-line therapy.
Experience using CAMPATH-1H in the treatment of non-Hodgkin lymphoma and chronic lymphocytic leukemia (CLL) has shown that, although clearance of disease from blood and BM may be complete, bulky disease at other sites (lymph nodes, liver, CNS) may be resistant.12-16 This differential pattern of response may reflect poor accessibility of the antibody to tumor cells in different sites or to reduced or impaired effector cell function in these locations. This may explain similar observations in our study where patients with serous effusions or hepatic or CNS disease were more resistant to therapy. In the case of CNS disease the absence of local effector cells may be particularly important. It may be possible to augment host effector mechanisms by addition of cytokines such as granulocyte-macrophage colony-stimulating factor and in this way improve responses.17 The clinical observations suggest that CAMPATH-1H may be most effective in patients who present with blood and BM disease alone or in whom bulky disease has already been eradicated by conventional therapy.15
The reason for the particular sensitivity of T-prolymphocytes to CAMPATH-1H is unknown. It may be related to the high level of CD52 expression on the surface of the leukemic cells, which also has some predictive value for response.5 Specific genetic abnormalities, for example inactivation of the ATMgene,18 19 may also play a role in determining responsiveness to CAMPATH-1H therapy, although we found no correlation in our study.
The majority of patients who relapsed had CD52+ T cells and 6 of these patients went on to achieve second responses. However, 2 patients who had achieved durable CRs (25 and 45 months) following a first course of CAMPATH-1H relapsed with CD52− T cells and were thus resistant to further CAMPATH-1H therapy. The reason for this is unclear. The prolonged remissions seen in these 2 cases accompanied by relapse with CD52− cells suggests that CAMPATH-1H was successful at eliminating CD52+ cells. Studies in CLL have shown that, in some individuals, there is emergence of a paroxysmal nocturnal hemoglobinuria, CD52− clone following treatment with CAMPATH-1H.20 This clone was probably present at a very low level prior to treatment and is selected out after elimination of CD52+ cells. Another possibility is the acquisition of new somatic mutations in the PIGA gene.
CAMPATH-1H is a generally well-tolerated therapy. Acute infusion-related effects were only associated with initial therapy or with dose increments and were usually controlled by premedication. Antihistamines are probably less effective than steroids, but in this study prednisolone was not used to avoid confusion over response evaluation. Use of steroids would need to be restricted to the first 1 to 2 weeks of treatment to avoid increasing the immunosuppression. Systemic side effects may also be reduced by subcutaneous administration of CAMPATH-1H. Studies in CLL suggest that this does not reduce efficacy14 although we have no data to support this in T-PLL. The dose schedule of 30 mg 3 times a week was selected following results of a phase 1 study. However, the optimal dose and dose interval are not yet clearly established and need to be further evaluated.
The most significant side effect was prolonged lymphopenia associated with reactivation of viruses such as CMV and herpes and other opportunistic infections. Use of antibacterial and antiviral prophylaxis is important and should probably be continued for at least 3 months after completing therapy. Two patients developed prolonged and unexplained BM aplasia. In both cases this occurred as a relatively late complication following partial BM recovery and was not associated with a documented infection such as parvovirus. It is possible that an untested virus such as human herpesvirus 8, which has recently been implicated in posttransplant aplasia, was responsible.21CD52 is not expressed on hemopoietic progenitors and CAMPATH-1H would not therefore be expected to cause direct toxicity to stem cells.4 Comparable degrees of aplasia have not been observed in patients with B-cell malignancies and the cause for this event and its apparent association with T-PLL is not clear.
In summary, CAMPATH-1H is an effective and well-tolerated therapy in T-PLL, able to produce durable remissions in two thirds of patients. These results show a significant improvement over previous therapy and offer the hope of improved survival in the majority of patients. We would, therefore, consider CAMPATH-1H to be the first-line treatment in T-PLL. This agent is not, however, curative and other strategies such as SCT need to be further explored if the natural history of this aggressive malignancy is to be significantly altered.
We are grateful to the Ilex Corporation and Geoff Hale for supply of CAMPATH-1H, to John Swansbury for the statistical analysis, to Ricardo Morilla for flow cytometry, to Peter de Schuower for some of the PCR work, and to Stephen Devereux, Andrew Duncombe, Robin Ireland, M. Wetterwald, M. Ojeda, B. Mahe, and E. Carreras for providing patient data.
Supported by research funding from the Royal Marsden Hospital Trustees.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Claire Dearden, St George's Hospital Medical School, Cranmer Terrace, London SW17 OQT, United Kingdom; e-mail:claire.dearden@ccmail.stgh-tr.sthames.nhs.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal