Abstract
Central venous catheters (CVCs) are a common adjunct to hemophilia therapy, but the risk of CVC-related deep venous thrombosis (DVT) in hemophiliacs is not well defined. In a previous study, 13 patients with CVCs had no radiographic evidence of DVT. However, recent abstracts and case studies demonstrate that DVT does occur. Therefore, this study sought to determine the frequency of DVT in children with hemophilia and long-term CVCs and to correlate venographic findings with clinical features. All hemophilia patients with tunneled subclavian CVCs in place for 12 months or more were candidates for evaluation. Patients were examined for physical signs of DVT and questioned about catheter dysfunction. Contrast venograms were obtained to identify DVT. Fifteen boys with severe hemophilia were evaluated, including 9 from the initially studied group of 13. Eight patients had evidence of DVT, 5 of whom previously had normal venograms. Five of 15 patients had clinical problems related to the CVC, all of whom had DVT. Four of 15 patients had suggestive physical signs; 3 had DVT. The mean duration of catheter placement for all patients was 57.5 months (range, 12-102 months). For patients with DVT, the mean duration was 66.6 ± 7.5 months, compared to 49.5 ± 7.2 months for patients without DVT (P = .06). No patient whose CVC was in place fewer than 48 months had an abnormal venogram. Many hemophilia patients with CVCs develop DVT of the upper venous system, and the risk increases with duration of catheter placement.
Introduction
Infusion of coagulation factor through peripheral veins is simple and effective, but frequent venipuncture is often painful and not possible for some patients with hemophilia. Central venous catheters (CVCs) facilitate the infusion of coagulation factors, especially in demanding regimens of primary prophylaxis and induction of immune tolerance in patients with inhibitors.1-3 Many families request CVCs for domiciliary, on-demand infusions as well. Thus, CVCs are an increasingly common adjunct to the therapy of hemophilia.
The CVCs can be problematic because of mechanical dysfunction and the risk of infection and deep venous thrombosis (DVT). Published series of hemophilia patients with CVCs have thoroughly described the risk of infection2,4-6; however, the frequency of thrombosis is not adequately documented. Indeed, a CVC is the greatest risk factor for DVT in childhood.7,8 As many as two thirds of children who receive total parenteral nutrition (TPN) or antineoplastic chemotherapy develop catheter-related DVT.9-13Nevertheless, investigation of CVC-related DVT in patients with hemophilia is lacking, perhaps because it seems paradoxical that individuals with bleeding disorders might develop DVT.
In 1998, we reported a favorable experience in 13 patients with hemophilia who had CVCs.14 None had definitive radiographic evidence of DVT in the upper venous system (the subclavian, brachiocephalic, or jugular veins, or the superior vena cava) despite having had catheters in place for 10 to 60 months (mean, 23 months). However, a recent abstract15 and several case studies16 17 indicate that thrombosis does occur in patients with hemophilia. Because we recently identified thrombi in several of our patients, we further investigated this issue.
Patients, materials, and methods
We reviewed the records of all patients with hemophilia at our center who had tunneled, internal CVCs (infusion ports, eg, Port-a-Cath), all of which were placed in a subclavian vein. Any patient whose CVC had been in place for more than 12 months was a candidate to be evaluated by contrast venography for DVT involving the subclavian, brachiocephalic, or jugular veins, or the superior vena cava. In particular, we attempted to re-evaluate each of the 13 patients whom we had previously studied.14 The potential risks of radiation exposure and contrast injection were discussed with the patients and their parents. No parent declined to have his or her child evaluated, and informed consent was obtained in each case.
Venograms were obtained by injection of radiocontrast medium into the ipsilateral antecubital vein (relative to the side of CVC insertion) by a method previously described.14 Thrombosis was defined as 2 or more of the following: (1) stenosis or occlusion (or both) of the superior vena cava or of the subclavian, brachiocephalic, or jugular vein; (2) poststenotic dilation; or, (3) prominent collateral veins proximal or distal to a probable stenosis or occlusion.
Patients who underwent venography were examined carefully for dilated veins on the chest wall and swelling, tenderness, or erythema of the arms. Patients' parents were questioned about difficulties using the CVC, such as resistance to infusion, absence of blood return, and pain with accession. We recorded any episode of documented pulmonary embolism and any history of respiratory distress or chest pain that might indicate pulmonary embolism.
We correlated the clinical features of the patients with the results of venography, and we compared the mean time of catheter insertion between the groups of boys with and without DVT using a one-tailed ttest. The probability of remaining free of DVT after insertion of a CVC was also calculated.
Results
Patients
Fifteen boys were evaluated, 9 from the previously studied group of 13 and 6 additional patients. The mean age of the patients at time of venography was 9.2 years (range, 4-14 years). Thirteen patients had severe factor VIII deficiency and 2 had severe factor IX deficiency. The reasons for CVC placement included primary prophylaxis (n = 12) and immune tolerance for an inhibitor (n = 3). The patients' characteristics are shown in Table 1.
Characteristics of patients
| Patient no.* . | Type of hemophilia . | Age at time of catheter insertion (y) . | Age at time of venogram (y) . | Reason catheter inserted . | Interval from catheter insertion to venogram (mo) . | Venogram result . |
|---|---|---|---|---|---|---|
| 1 | VIII | 6 | 9 | Prophylaxis | 42 | Normal |
| 9 | 12 | Replacement/prophylaxis | 46 | Normal | ||
| 2 | VIII | 5 | 6 | Prophylaxis | 10 | Normal |
| 9 | 48 | DVT | ||||
| 3 | VIII | 8 | 11 | Prophylaxis | 31 | Normal |
| 14 | 60 | Normal | ||||
| 4 | VII | 3 | 5 | Immune tolerance | 24 | Normal |
| 9 | 62 | DVT | ||||
| 5 | VIII | 4 | 6 | Prophylaxis | 17 | Normal |
| 9 | 65 | Normal | ||||
| 6 | VIII | 6 | 8 | Immune tolerance | 27 | Normal |
| 12 | 73 | Normal | ||||
| 7 | VIII | 4 | 6 | Prophylaxis | 28 | Normal |
| 10 | 75 | DVT | ||||
| 8 | IX | 1 | 3 | Prophylaxis | 30 | Normal |
| 7 | 79 | DVT | ||||
| 9 | IX | 3 | 8 | Prophylaxis | 59 | Normal |
| 12 | 102 | DVT | ||||
| 10 | VIII | 1.5 | — | Prophylaxis | — | Not done |
| 3 | 4 | Prophylaxis | 12 | Old DVT | ||
| 11 | VIII | 1 | 4 | Prophylaxis | 37 | Normal |
| 12 | VIII | 1 | 4 | Prophylaxis | 37 | Normal |
| 13 | VIII | 1 | 5 | Prophylaxis | 49 | DVT |
| 14 | VIII | 9 | 14 | Prophylaxis | 51 | DVT |
| 15 | VIII | 8 | 13 | Immune tolerance | 66 | Normal |
| Patient no.* . | Type of hemophilia . | Age at time of catheter insertion (y) . | Age at time of venogram (y) . | Reason catheter inserted . | Interval from catheter insertion to venogram (mo) . | Venogram result . |
|---|---|---|---|---|---|---|
| 1 | VIII | 6 | 9 | Prophylaxis | 42 | Normal |
| 9 | 12 | Replacement/prophylaxis | 46 | Normal | ||
| 2 | VIII | 5 | 6 | Prophylaxis | 10 | Normal |
| 9 | 48 | DVT | ||||
| 3 | VIII | 8 | 11 | Prophylaxis | 31 | Normal |
| 14 | 60 | Normal | ||||
| 4 | VII | 3 | 5 | Immune tolerance | 24 | Normal |
| 9 | 62 | DVT | ||||
| 5 | VIII | 4 | 6 | Prophylaxis | 17 | Normal |
| 9 | 65 | Normal | ||||
| 6 | VIII | 6 | 8 | Immune tolerance | 27 | Normal |
| 12 | 73 | Normal | ||||
| 7 | VIII | 4 | 6 | Prophylaxis | 28 | Normal |
| 10 | 75 | DVT | ||||
| 8 | IX | 1 | 3 | Prophylaxis | 30 | Normal |
| 7 | 79 | DVT | ||||
| 9 | IX | 3 | 8 | Prophylaxis | 59 | Normal |
| 12 | 102 | DVT | ||||
| 10 | VIII | 1.5 | — | Prophylaxis | — | Not done |
| 3 | 4 | Prophylaxis | 12 | Old DVT | ||
| 11 | VIII | 1 | 4 | Prophylaxis | 37 | Normal |
| 12 | VIII | 1 | 4 | Prophylaxis | 37 | Normal |
| 13 | VIII | 1 | 5 | Prophylaxis | 49 | DVT |
| 14 | VIII | 9 | 14 | Prophylaxis | 51 | DVT |
| 15 | VIII | 8 | 13 | Immune tolerance | 66 | Normal |
Patients 1 through 9 are both previously and newly studied; patients 10 through 15 are newly studied.
Three patients had more than one CVC. Patients 1 and 4 had their first CVCs replaced because of catheter tip migration. At the time of venography, their second CVCs had been in place for 46 and 62 months, respectively. Patient 10 had his CVC replaced after 41 months because of recurrent infection and mechanical dysfunction. His second CVC had been in place for 12 months when the venogram was obtained.
Three of the 4 previously studied patients who did not have a second venogram are no longer followed at our center. One had his CVC removed after 5 years because of catheter migration. The other 2 have had patent catheters for over 4 years; one of them had a normal contrast dye study of the line to test for patency. The remaining patient from the first study who was not re-evaluated outgrew his CVC, and it was replaced after 57 months. He is scheduled to have a venogram as part of his next comprehensive evaluation.
Contrast venography
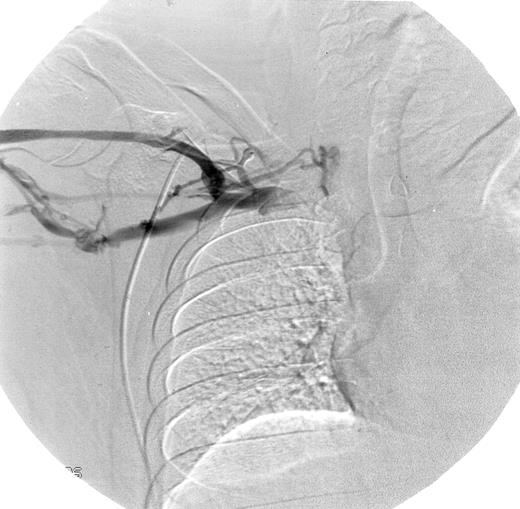
Eight of 15 patients (53%) had abnormal venograms consistent with DVT (Table 2). Five of the patients from the first study, all of whom had previously normal venograms, now had evidence of DVT. Three of the 6 newly evaluated patients also had abnormal venograms. Figure 1 shows a representative abnormal venogram (patient 14).
Patients with abnormal venogram
| Patient no. . | History of problems with CVC . | Physical examination . | Venogram findings . | Outcome . |
|---|---|---|---|---|
| 2 | No blood return and resistance to infusion | Normal | Venous stricture at catheter insertion and multiple collaterals around the left subclavian vein | Catheter removed |
| 4 | Discomfort in neck with CVC use and resistance to infusion | Few dilated veins on upper left chest wall and left shoulder; left arm 2 cm greater in diameter than the right | Occluded right subclavian vein with multiple collaterals | Catheter removed |
| 7 | None | Normal | Narrowing of the proximal right subclavian with mild poststenotic dilation | Scheduled catheter removal |
| 8 | None | Normal | Chronic thrombosis of left subclavian and brachiocephalic veins and superior vena cava; numerous mediastinal collaterals | Catheter removed |
| 9 | No blood return, resistance to infusion, discomfort with CVC use | Normal | Proximal subclavian vein narrowing with collateral formation; nonocclusive 4-5 cm thrombus | Catheter removed |
| 10 | None | Prominent chest wall veins and left arm 1 cm greater in diameter than the right | Thrombosis or stricture of left subclavian and brachiocephalic veins with multiple collaterals | Catheter removed |
| 13 | Resistance to infusion | Normal | Multiple collaterals around the right subclavian vein, suggesting a proximal stenosis | Catheter removed |
| 14 | No blood return | Right arm 2 cm greater in diameter than left | Stricture of right subclavian vein with multiple collaterals | Catheter removed |
| Patient no. . | History of problems with CVC . | Physical examination . | Venogram findings . | Outcome . |
|---|---|---|---|---|
| 2 | No blood return and resistance to infusion | Normal | Venous stricture at catheter insertion and multiple collaterals around the left subclavian vein | Catheter removed |
| 4 | Discomfort in neck with CVC use and resistance to infusion | Few dilated veins on upper left chest wall and left shoulder; left arm 2 cm greater in diameter than the right | Occluded right subclavian vein with multiple collaterals | Catheter removed |
| 7 | None | Normal | Narrowing of the proximal right subclavian with mild poststenotic dilation | Scheduled catheter removal |
| 8 | None | Normal | Chronic thrombosis of left subclavian and brachiocephalic veins and superior vena cava; numerous mediastinal collaterals | Catheter removed |
| 9 | No blood return, resistance to infusion, discomfort with CVC use | Normal | Proximal subclavian vein narrowing with collateral formation; nonocclusive 4-5 cm thrombus | Catheter removed |
| 10 | None | Prominent chest wall veins and left arm 1 cm greater in diameter than the right | Thrombosis or stricture of left subclavian and brachiocephalic veins with multiple collaterals | Catheter removed |
| 13 | Resistance to infusion | Normal | Multiple collaterals around the right subclavian vein, suggesting a proximal stenosis | Catheter removed |
| 14 | No blood return | Right arm 2 cm greater in diameter than left | Stricture of right subclavian vein with multiple collaterals | Catheter removed |
Abnormal venogram.
Patient 14 had multiple collateral vessels in the right mid-subclavian region providing antegrade flow around the obstruction of the proximal subclavian vein.
Abnormal venogram.
Patient 14 had multiple collateral vessels in the right mid-subclavian region providing antegrade flow around the obstruction of the proximal subclavian vein.
Patient 10 had transferred from another physician's care. His first CVC was in place for 41 months, but it was removed because of recurrent infection and mechanical dysfunction. This patient did not have prior radiographic studies. We evaluated him 12 months after insertion of a second CVC in the opposite subclavian vein. His venogram was consistent with DVT in his left superior venous system where his previous catheter had been inserted. There was no evidence of DVT in the right upper venous system, the location of the current CVC.
Clinical problems and physical examinations
Five of 15 patients had clinical problems related to their CVCs, such as discomfort with accession, resistance to infusion, and lack of blood return. All 5 had venographic evidence of DVT. The 3 other patients with DVT had no apparent clinical difficulties.
Four of the 15 patients had physical signs suggestive of DVT, such as slight arm swelling or prominent chest wall veins, but no patient had a functional deficit of the affected arm. Three of these 4 patients had abnormal venograms. The patient with the normal venogram had only mild prominence of superficial veins over the ipsilateral shoulder. In contrast, 5 patients with DVT had normal physical examinations.
Outcome and follow-up
The mean duration of catheter placement for all patients was 57.5 months (median, 60 months; range, 12-102 months). The mean duration of CVC placement in the patients with DVT was 66.6 ± 7.5 months (SEM) compared to 49.5 ± 7.2 months for the patients without DVT (P = .06). Figure 2 depicts the probability of remaining free of DVT after CVC insertion. No patient whose catheter was in place for fewer than 48 months had an abnormal venogram associated with his current CVC, whereas all patients whose catheter was in place longer than 73 months had venographic evidence of a DVT.
We removed the CVCs from 6 of the 8 patients with DVT because they no longer functioned or had migrated out of the subclavian vein. We discussed the risks of continued use of an apparently functional CVC associated with a DVT with the parents of the remaining 2 boys (patients 7 and 10). Patient 10 had his CVC removed. The family of patient 7 preferred to keep the CVC for frequent prophylactic infusions until they were comfortable with peripheral venous administration.
None of the 8 patients with DVT was treated with systemic fibrinolytic agents or anticoagulants. No patient presented with clinical signs suggestive of pulmonary thromboembolism. No patient had a catheter-related infection, except for patient 10 described previously.
We did not prospectively assay for inherited thrombophilic states in all patients. However, we did test patient 2 because his parents strongly desired placement of a second CVC. He had no demonstrable abnormalities of protein C, protein S, antithrombin, or prothrombin, but he was heterozygous for the factor V Leiden mutation. Because of this finding we discouraged replacement of the CVC and taught the family how to use peripheral veins instead.
Discussion
Regimens of primary prophylaxis beginning in the first year of life can prevent hemophilic arthropathy,1,18,19 and immune tolerance programs can eliminate inhibitors.1 Reliable venous access is needed for these treatments, but repeated peripheral venipuncture can be difficult or impossible in very young children. CVCs can make these intensive and effective treatment approaches more feasible and more convenient.20-22 However, the widespread use of CVCs is now being re-evaluated because of complications such as infection and thrombosis.
Five recent series describing more than 100 hemophilia patients with CVCs have documented the risk of catheter-related infection and mechanical dysfunction.2-6,19 Four of the studies mention catheter occlusion in a total of 8 patients, but only Blanchette and coworkers described venographic evidence of DVT in a single patient.19 We report herein a specific investigation for DVT using contrast venography in a cohort of unselected patients with hemophilia and long-term CVCs.
Although we initially reported that catheter-related thrombosis is rare in hemophilia patients,14 with longer follow-up we now realize that DVT is indeed common. Approximately half of our patients with CVCs in place for more than 1 year now have evidence of DVT. We found that the risk of DVT increased with time and was highest when CVCs had been in place 4 years or longer. Three of 8 patients with DVT in this series had suggestive physical signs, and 5 had evidence of catheter dysfunction. Yet, despite the common occurrence of thrombosis, no patient has had clinically apparent pulmonary embolism or symptomatic postphlebitic syndrome.
This study corroborates the findings of other observers reported in abstract form.15,16 Koerper and colleagues performed bilateral venograms on 11 asymptomatic children with hemophilia who had tunneled catheters in place for 1 to 6 years.16 Two patients (22%) had complete occlusion of the left brachiocephalic vein with development of collateral vessels. Blanchette and coworkers evaluated 16 patients with hemophilia who had indwelling catheters for 1 to 40 months using Doppler ultrasonography, dye studies of the central line testing for patency, and venograms.15 Ten of 16 patients (63%) had evidence of a DVT. The site of catheter insertion was not mentioned. Venography identified thrombi in the subclavian and brachiocephalic veins or involving the superior vena cava, but only ultrasonography identified thrombi in the internal jugular vein. These investigators concluded that both ultrasound and radiocontrast studies should be used to evaluate adequately the entire upper venous system. Our patients had only subclavian CVCs, and because it would be unlikely to have jugular venous thrombosis without DVT in contiguous veins, venography alone was deemed sufficient.
Thrombosis in patients with bleeding disorders is seemingly paradoxical. Young patients with hemophilia should not be prone to DVT. They have defective coagulation but are otherwise healthy, and they have no increased risk of concomitant inherited thrombophilia.23,24 Accordingly, Arbini and coworkers tested 21 patients with severe hemophilia but with mild bleeding symptoms for prothrombotic disorders. Only one was heterozygous for the factor V Leiden mutation, and none had an hereditary deficiency of antithrombin, protein C, or protein S.24 Patients with debilitating diseases such as cancer and short-gut syndrome also require tunneled catheters to facilitate the infusion of chemotherapy, blood products, or TPN. These conditions and their treatments can injure the endothelium and promote DVT within months of catheter insertion. As many as 50% of these patients develop CVC-related DVT.11,13 25 We have shown that this risk is ultimately the same for hemophilia patients, but we did not detect thrombosis until CVCs had been in place 4 years or longer. This suggests that thrombi form more slowly in patients with hemophilia, perhaps because hemostasis is only intermittently normalized by factor infusions.
Why so many patients develop DVT is not known. Vidler and colleagues reported 2 patients with severe hemophilia and an inhibitor who had CVC-related DVT.17 Both were treated with high-dose factor infusions and one with an activated prothrombin complex concentrate (PCC). The authors speculated that high-dose factor and procoagulant PCC therapy might have promoted thrombus formation. Three patients in our study received PCC; 2 did not develop DVT. The patient with DVT received PCC only through his first CVC, which was removed after 3 months because of migration of the catheter tip. His DVT occurred approximately 5 years after the insertion of a second CVC. Thus, exposure to a PCC is not a necessary antecedent of thrombosis in hemophilia patients. Perhaps the only requisites are chronic irritation of the vessel wall by a catheter tip and intermittent normalization of coagulation.
Although patients with hemophilia may develop catheter-related DVT, the benefits of CVCs, especially in young patients, likely outweigh the risks. However, caution is urged, and physicians should specifically monitor for DVT. Surveillance should include attention to difficulties with the infusion of factor and examination of the patient for physical signs of DVT. Patients should also be evaluated intermittently for the feasibility of peripheral venipuncture, and families should understand that a CVC is a temporary adjunct to therapy. Removal of catheters within 4 years might prevent thrombosis. Screening venography may be warranted for patients who require CVCs longer. If DVT occurs, one should strongly consider removal of the CVC and the use of peripheral veins instead. Further studies of CVC-related DVT are needed to define the adverse sequelae, the utility of routine screening, and the appropriateness of anticoagulant therapy.
Supported in part by National Institutes of Health Training Grant T32-CA09640, and grants from Maternal and Child Health Bureau (5 H30 MC00029-11), Centers for Disease Control and Prevention (U27/CCU613189-05), and the Children's Cancer Fund of Dallas.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
George R. Buchanan, Division of Hematology-Oncology, Department of Pediatrics, The University of Texas Southwestern Medical Center at Dallas, 5323 Harry Hines Blvd, Dallas, TX 75390-9063; e-mail: George.Buchanan@UTSouthwestern.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal