The hematopoietic stem cell underlying acute myeloid leukemia (AML) is controversial. Flow cytometry and the DNA-binding dye Hoechst 33342 were previously used to identify a distinct subset of murine hematopoietic stem cells, termed the side population (SP), which rapidly expels Hoechst dye and can reconstitute the bone marrow of lethally irradiated mice. Here, the prevalence and pathogenic role of SP cells in human AML were investigated. Such cells were found in the bone marrow of more than 80% of 61 patients and had a predominant CD34low/− immunophenotype. Importantly, they carried cytogenetic markers of AML in all 11 cases of active disease examined and in 2 out of 5 cases in complete hematological remission. Comparison of daunorubicin and mitoxantrone fluorescence emission profiles revealed significantly higher drug efflux from leukemic SP cells than from non-SP cells. Three of 28 SP cell transplants generated overt AML-like disease in nonobese diabetic–severe combined immunodeficient mice. Low but persistent numbers of leukemic SP cells were detected by molecular and immunological assays in half of the remaining mice. Taken together, these findings indicate that SP cells are frequently involved in human AML and may be a target for leukemic transformation. They also suggest a mechanism by which SP cells could escape the effects of cytostatic drugs and might eventually contribute to leukemia relapse.
Introduction
The demonstration that acute myeloid leukemia (AML) is a disease of hematopoietic stem cells (HSCs), rather than of committed myeloid progenitors, has raised fundamental questions concerning the true target of leukemic transformation in this disease.1-3 In most instances, the HSCs capable of initiating human AML in nonobese diabetic–severe combined immunodeficient (NOD/SCID) mice or other animal models have been exclusively CD34+CD38−,3-6although engraftment by CD34− cells has also been reported in isolated cases.7,8 Experimental evidence indicates that human bone marrow contains both CD34+ and CD34− HSCs and that both possess strong in vivo repopulating activity.9-11 Most intriguing, perhaps, was the emergence of CD34+ cells in animals transplanted with CD34− cells.9 These observations suggest that CD34− HSCs may be more primitive than cells expressing the CD34 antigen and, when transformed, could underlie some cases of AML.
We have selected murine bone marrow cells on the basis of their rapid efflux of fluorescent Hoechst dye, using flow cytometry to identify a small subset of these cells (0.05% of all marrow cells), which we have termed the side population (SP).10,12 Such cells have many of the properties of recognized HSCs, including long-term repopulating activity in mice as well as specific lineage–marker negativity. Importantly, SP cells were also found in a variety of mammalian species, including humans, where their frequency ranges from 0.01 to 0.09% (average, 0.03%).12 If leukemic SP cells are present in AML patients, their characteristics would be expected to greatly affect the response to therapy. For example, the ability of SP cells to expel the Hoechst dye might be associated with drug resistance through rapid efflux of cytotoxic drugs, and their repopulating activity could lead to disease recurrence from small numbers of leukemic cells. To test these hypotheses, we studied the bone marrow and peripheral blood mononuclear cells of children and adults with primary AML or AML secondary to myelodysplasia (MDS), both in relapse and in remission. The findings suggest that SP cells constitute a previously unrecognized population of human leukemic stem cells with potential clinical significance.
Patients, materials, and methods
Patients
The bone marrow and peripheral blood specimens analyzed in this study represented diagnostic material collected from children and adults with AML in accordance with the respective institutional guidelines of M. D. Anderson Cancer Center and Texas Children's Hospital. The clinical characteristics of the study population are reported in Table 1. Since the SP is a minute population within the samples, the specimens were analyzed in cohorts for the experimental questions addressed as indicated in the respective “Results” sections.
Characteristics of the patient population
| . | Secondary AML . | M1 . | M2 . | M3 . | M4 . | M5 . | M6 . | M7 . | Unclassified . | Total . |
|---|---|---|---|---|---|---|---|---|---|---|
| Total No. | 15 | 11 | 10 | 1 | 9 | 6 | 1 | 3 | 5 | 61 |
| Gender (M/F) | 12/3 | 7/4 | 6/4 | 1/0 | 3/6 | 6/0 | 1/0 | 1/2 | 4/1 | 40/21 |
| Age | ||||||||||
| Younger than 2 y | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 4 |
| 2-19 y | 1 | 3 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 8 |
| 20-49 y | 4 | 4 | 4 | 0 | 4 | 1 | 0 | 2 | 2 | 21 |
| Older than 49 y | 9 | 4 | 6 | 0 | 4 | 2 | 1 | 0 | 2 | 28 |
| Source of cell sample*,† | ||||||||||
| Bone marrow | 15‡ | 9 | 10 | 0 | 7 | 5‡ | 1 | 2 | 5‡ | 54‡ |
| Peripheral blood | 0 | 21-153 | 0 | 1 | 21-153 | 1 | 0 | 1 | 0 | 71-153 |
| Status of disease | ||||||||||
| Untreated | 5 | 51-153 | 4 | 1 | 6 | 1 | 0 | 2 | 1 | 251-153 |
| Remission | 6‡ | 3 | 3 | 0 | 0 | 3 | 1 | 0 | 2‡ | 18‡ |
| Relapsed/resistant | 4‡ | 3 | 3 | 0 | 31-153 | 2‡ | 0 | 1 | 2‡ | 181-153 |
| Prognostic implication of karyotype1-155 | ||||||||||
| Favorable | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Intermediate | 1 | 2 | 3 | 0 | 3 | 2 | 0 | 0 | 0 | 11 |
| Unfavorable | 14 | 9 | 7 | 0 | 6 | 4 | 1 | 2 | 5 | 48 |
| . | Secondary AML . | M1 . | M2 . | M3 . | M4 . | M5 . | M6 . | M7 . | Unclassified . | Total . |
|---|---|---|---|---|---|---|---|---|---|---|
| Total No. | 15 | 11 | 10 | 1 | 9 | 6 | 1 | 3 | 5 | 61 |
| Gender (M/F) | 12/3 | 7/4 | 6/4 | 1/0 | 3/6 | 6/0 | 1/0 | 1/2 | 4/1 | 40/21 |
| Age | ||||||||||
| Younger than 2 y | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 4 |
| 2-19 y | 1 | 3 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 8 |
| 20-49 y | 4 | 4 | 4 | 0 | 4 | 1 | 0 | 2 | 2 | 21 |
| Older than 49 y | 9 | 4 | 6 | 0 | 4 | 2 | 1 | 0 | 2 | 28 |
| Source of cell sample*,† | ||||||||||
| Bone marrow | 15‡ | 9 | 10 | 0 | 7 | 5‡ | 1 | 2 | 5‡ | 54‡ |
| Peripheral blood | 0 | 21-153 | 0 | 1 | 21-153 | 1 | 0 | 1 | 0 | 71-153 |
| Status of disease | ||||||||||
| Untreated | 5 | 51-153 | 4 | 1 | 6 | 1 | 0 | 2 | 1 | 251-153 |
| Remission | 6‡ | 3 | 3 | 0 | 0 | 3 | 1 | 0 | 2‡ | 18‡ |
| Relapsed/resistant | 4‡ | 3 | 3 | 0 | 31-153 | 2‡ | 0 | 1 | 2‡ | 181-153 |
| Prognostic implication of karyotype1-155 | ||||||||||
| Favorable | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Intermediate | 1 | 2 | 3 | 0 | 3 | 2 | 0 | 0 | 0 | 11 |
| Unfavorable | 14 | 9 | 7 | 0 | 6 | 4 | 1 | 2 | 5 | 48 |
The prognostic implication of the karyotype was classified according to Grimwade et al.17 The cases of acute myeloid leukemia (AML) that arose from pre-existing myelodysplasia are referred to as secondary AML.
Two 2 bone marrow samples were analyzed in 7 patients: 4 patients with secondary AML, 1 patient with AML M5, and 2 of the unclassified cases. In terms of disease status, the second samples were taken from 3 patients in complete hemotological remission and 4 patients with resistant or relapsed disease.
Both bone marrow and peripheral blood samples were available for 3 patients.
Includes patient or patients for whom 2 bone marrow samples were analyzed.
Includes patient or patients for whom both bone marrow and peripheral blood samples were available.
Cytogenetic findings were not available for one patient.
Cell separation, immunophenotyping, and fluorescence in situ hybridization analysis
Immediately after density centrifugation or transient liquid nitrogen cryopreservation of bone marrow or peripheral blood specimens, mononuclear cell (MNC) fractions were stained with the fluorescent dye Hoechst 33342 (Sigma, St Louis, MO) in a concentration of 5 μg/mL (37°C for 2 hours) as previously described.12 The SP population, characterized by the most rapid efflux Hoechst 33342, was identified and sorted by its fluorescence profile in dual wavelength analysis (405/30 and 670/40 nm) after excitation at 350 nm on a triple-laser instrument (MoFlow) (Cytomation, Fort Collins, CO), as previously described.10 12
SP cells were immunophenotyped simultaneously by means of fluorescein isothiocyanate (FITC), phycoerythrin (PE), and allophycocyanin (APC) as fluorescent agents excited from the second and third lasers at 488 nm (FITC and PE) and 633 nm (APC). Fluorochrome-conjugated antibodies (all from Becton Dickinson, San Jose, CA) were used to detect the antigens CD13, CD33, CD38, CD45, and CD71, thimine (Thy)–1, and glycophorin A, as well as for the appropriate isotype controls. For the sensitive detection of CD34, we employed a biotinylated first antibody against class II antigen (QBEnd10) (Coulter-Immunotech, Miami, FL) and a streptavidin-APC conjugate (Molecular Probes, Eugene, OR). Multidrug resistance (MDR)–1 was detected with the antibody MRK16 (Kamiya, Seattle, WA) in an indirect immunofluorescence procedure. All antibody incubations were performed at 4°C following the Hoechst 33342 staining at 37°C. Propidium iodide (2 μg/mL) (Sigma) was added to the samples following Hoechst and antibody staining to facilitate dead cell discrimination. All data acquired on the MoFlow were analyzed with the FlowJo analysis program (Tree Star, San Carlos, CA). The cells were sorted directly onto slides to facilitate fluorescence in situ hybridization (FISH) analysis, or into culture medium for all other puposes.
Immunostaining of whole bone marrow preparations from NOD/SCID mice to detect human leukemia engraftment was performed with directly conjugated antibodies against human CD45 (FITC) and human CD34 (PE) (Coulter-Immunotech). Analysis was performed on a FACScan flow cytometer (Becton Dickinson) and analyzed with either the CellQuest (Becton Dickinson) or the FlowJo program. Immunocytologic and immunohistochemical studies for human cells with the CD45-specific hybridoma clones 2B11 and PD7/26 (Dako, Carpinteria, CA) and diaminobenzidine were performed on sorted cell populations after cytocentrifugation and fixation in acetone (4°C, 10 minutes) and on formalin-fixed tissue specimens after conventional paraffin embedding and sectioning. FISH analysis followed standard protocols specifying SpectrumOrange-labeled alpha-satellite probes for chromosome 8 (D8Z2) (Vysis, Downers Grove, IL), chromosome 15 (D15Z3) (Vysis), and chromosome 7 (D7Z1) (Vysis) after pretreating the slides with pepsin (50 μg/mL, pH 2.0) (Sigma) for 5 minutes at 37°C, as previously described.13 14 Among the clinical samples, a frequency that exceeded 2 SDs from the mean healthy volunteer control value (D7Z1, 6% ± 0.79%; D8Z2, 2.4% ± 0.65%; D15Z3, 4.3% ± 0.76%) was considered evidence for a numerical aberration.
Efflux studies for daunorubicin and mitoxantrone
Freshly prepared or frozen patient MNC specimens were incubated with Hoechst 33342 and daunorubicin (Ben Venue Laboratories, Bedford, OH) at a final concentration of 0.5 μg/mL (37°C for 30 minutes followed by 15 minutes with Hoechst alone) or with mitoxantrone (Immunex, Seattle, WA) at 0.1 μg/mL (37°C for 30 minutes, 15 minutes with Hoechst alone). The drugs were added 60 minutes after the start of the Hoechst staining and removed by centrifugation in a centrifuge prewarmed to 37°C. The fluorescence emitted from daunorubicin after excitation at 488 nm was detected at 575/25 nm, while that from mitoxantrone was detected at 670/40 nm after excitation at 633 nm.
Transplantation of AML cells into NOD/SCID mice
Six- to 8-week-old NOD/SCID mice, maintained under defined flora conditions, were transplanted according to protocols approved by the Animal Protocol Review Committee of Baylor College of Medicine. At 12 to 24 hours before retro-orbital intraveneous injection of AML cell preparations, the animals were irradiated with 270 cGy from a 137Cs source. In 10 AML cases, the samples were transplanted without human cytokine supplementation or accessory cells. In 19 cases, 1 × 106 cryopreserved whole bone marrow cells from healthy volunteers were irradiated with 15 Gy and used as accessory cells. Human growth factors were also added in these cases. Beginning immediately after transplantation, mice received a total of 5 intraperitoneal injections at 2-day intervals that contained a mixture of recombinant methionyl human stem cell factor (rmetHuSCF, 10 μg per dose) (kindly provided by Amgen, Thousand Oaks, CA); interleukin-3 (rHuIL-3, 6 μg per dose) (generously provided by Immunex); granulocyte-macrophage colony stimulating factor (GM-CSF, sargramostim; 6 μg per dose) (Immunex); or HuIL-3/HuGM-CSF fusion protein (7 μg per dose) (provided by Immunex); granulocyte-colony stimulating factor (G-CSF, filgrastim; 6 μg per dose) (Amgen); and erythropoetin (epogen, 100 U per dose) (Amgen). Mice were killed and examined for engraftment of human cells 3 months after transplantation or earlier when a moribund state was observed. Single-cell preparations from hind-limb bone marrow, spleen, and liver were analyzed by fluorescence-activate cell sorter (FACS) for CD45+ and CD34+ cells. Tissues from spleen and liver were processed by formalin fixation and standard paraffin embedding for CD45 immunohistochemical studies, and DNA was extracted from bone marrow, spleen, and liver with a commercial DNA purification kit (Puregene) (Gentra, Minneapolis, MN) for Southern blot and polymerase chain reaction (PCR) analyses. Engraftment was based on FACS detection of human CD45+ cells (see above), Southern blot detection of human chromosome 17–specific alpha-satellite sequences, and PCR of human endogeneous retrovirus H (HER-H) sequences. Spleens and livers of the transplanted animals were analyzed by FACS, Southern blot, and immunohistochemistry against human CD45. For Southern blot analysis, 10 μg high-molecular–weight DNA per sample was digested with EcoR1 and hybridized to the chromosome 17–specific alpha-satellite probe (p17H8, kindly provided by J. Dick) with standard techniques,15 with the application of a nonradioactive chemoluminescent detection system (AlkPhos Direct) (Amersham, Frederick, MD). In nested PCR analysis, sequences of the type H human endogeneous retrovirus16 were amplified from 1 μg genomic DNA per sample with the outer primers us1 (5′-TCCTACAAGATCTAAATAATTCTTG-3′) and ds1 (5′-AGTGGCCAGATTTCTGGCAC-3′) at an annealing temperature of 55°C, with 20 cycles yielding a product of 869 base pairs (bp), and the inner primers us2 (5′-TTATACATTGTTCCCTCCCTAG-3′) and ds2 (5′-CCTGGCAGCTGCAGTT-3′) at an annealing temperature of 55°C, with 35 cycles yielding a product of 735 bp visualized in standard 1.2% ethidium bromide gels. This assay did not amplifiy a product from the bone marrow of 6 control mice irradiated and injected with irradiated human accessory cells 3 months before sacrifice. The sensitivities of the xenograft detection methods were compared by seeding bone marrow MNCs from NOD/SCID mice, which had been irradiated 3 months earlier, with decreasing numbers of the myeloid cell from HL60, KG1, and AML193 lines and analyzing the preparations as described for the experimental samples.
Results
Study population
Bone marrow and peripheral blood cells were derived from 71 diagnostic samples of AML from 61 children and adults (Table1) All French-American-British (FAB) subtypes of AML except M0 were represented in the study population. Five cases were unclassified; AML had developed from MDS in 15 cases. The original specimens were collected before treatment (n = 23), during remission (n = 18), or during relapse or resistance to chemotherapy (n = 18). The vast majority of patients (59 of 61) were classified as intermediate or poor risk by standard cytogenetic criteria.17 The male/female ratio was 2 to 1, and the median age was 48 years (range, 10 months–80 years).
Prevalence of SP cells in bone marrow and peripheral blood
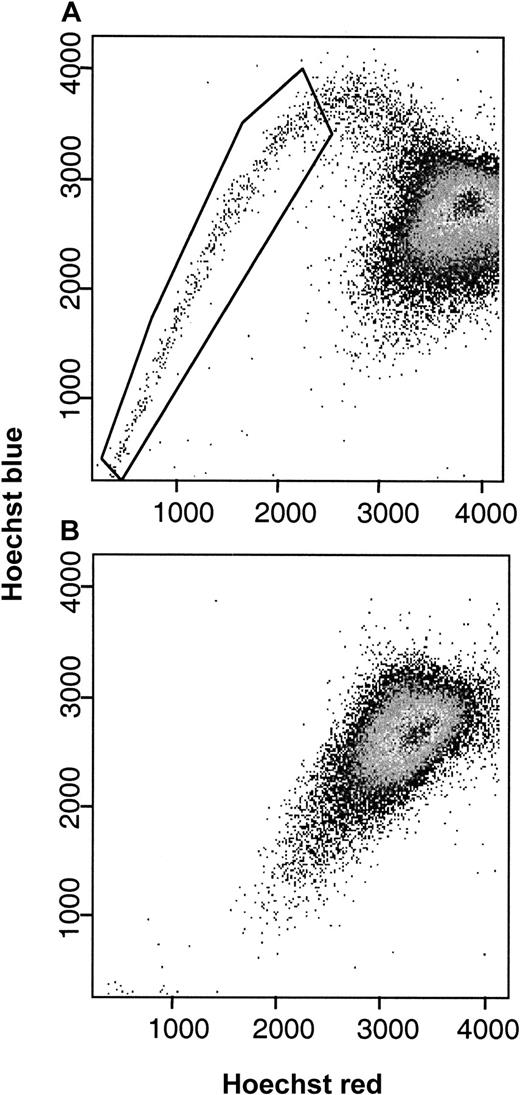
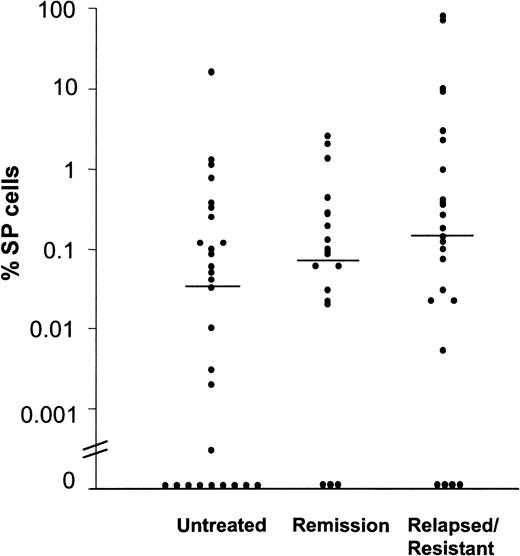
In normal human bone marrow, SP cells account for approximately 0.03% (range, 0.01%-0.09%) of all MNCs.10 To estimate the prevalence of SP cells in AML, we stained bone marrow and peripheral blood samples with Hoechst 33342 and then used dual-wavelength flow cytometry to identify cells with strong dye efflux activity (Figure 1). In 61 bone marrow and 10 peripheral blood specimens studied, an SP fraction was detected in 39 of the 52 patients with active disease (75%), including 19 of 28 (67%) samples from untreated patients and 20 of 24 (83%) samples from patients with drug-resistant or relapsed disease. Samples from 16 of the 19 specimens from patients (84%) in complete hematologic remission also contained SP cells (Figure 2). Only 4 of 10 leukemic blood samples, 6 from untreated patients and 4 from patients with relapsed or resistant disease, contained SP cells. The proportion of SP cells within the MNC fraction varied widely among patients, from 0.00% to 16% (median, 0.10%) in leukemic marrow and 0.00% to 80% (median, 0.00%) in leukemic blood. Although SP cell frequencies showed less variation in remission marrows, they exceeded the normal range in 10 of 21 samples. The highest SP cell fractions (16%, 71%, and 80%) were found in patients who were resistant to remission induction or salvage therapy. The prevalence of SP cells in bone marrow and peripheral blood samples was not correlated with FAB classification (data not shown) or with age at presentation.
Characteristic Hoechst 33342 staining profiles of MNC fractions from patients with AML.
SP cells (1.29% of MNCs, as boxed) were found in the bone marrow (panel A) but not the peripheral blood (panel B) of an AML patient with more than 90% blasts in both tissues.
Characteristic Hoechst 33342 staining profiles of MNC fractions from patients with AML.
SP cells (1.29% of MNCs, as boxed) were found in the bone marrow (panel A) but not the peripheral blood (panel B) of an AML patient with more than 90% blasts in both tissues.
Prevalence of SP cells in bone marrow and peripheral blood specimens of patients with AML.
The results are reported as percentages of viable MNCs per sample and were grouped according to the patients' disease stage (untreated, in complete hematological remission, or relapsed/resistant). Horizontal bars indicate median values.
Prevalence of SP cells in bone marrow and peripheral blood specimens of patients with AML.
The results are reported as percentages of viable MNCs per sample and were grouped according to the patients' disease stage (untreated, in complete hematological remission, or relapsed/resistant). Horizontal bars indicate median values.
Cell surface phenotype
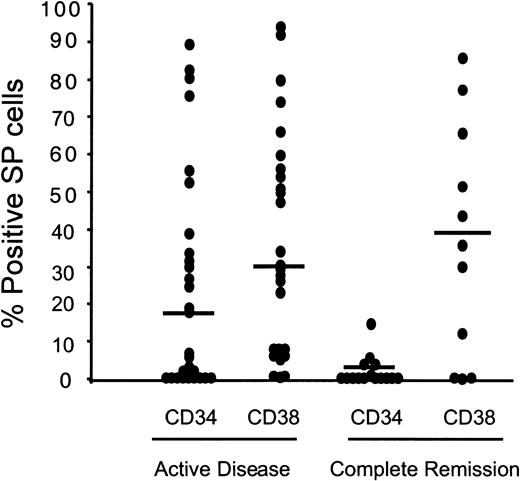
Normal human HSCs are classically defined as CD34+.18,19 Although the human SP fraction does contain some CD34+ cells, the surface phenotype is typically CD34low/−, with virtually no expression of the markers found on mature or differentiating hematopoietic cells.10 Because leukemic transformation and progression can alter the expression of surface antigens, we sought to determine the extent to which the immunophenotype of SP cells from AML patients differs from that of normal marrow SP cells. CD34 expression by SP cells was low or undetectable in more than half the cases of active AML, compared with the vast majority of the cases in complete hematologic remission (Figure 3). Six of the leukemic bone marrow samples had unexpectedly high CD34+ frequencies (53% to 97%). CD38 expression ranged from undetectable to greater than 85% of SP cells, in both leukemic and remission samples, in agreement with observations on normal cells.10 There was no correlation of CD34 and CD38 expression on the SP cells, regardless of the disease status (data not shown). Tests for Thy-1 (HSC marker), CD13/CD33 (myelomonocytic differentiation), CD71 (progenitor activation/proliferation), and MDR1, performed exclusively on samples of active disease, yielded positive results in 4 of 9 (2% to 63% positivity), 17 of 20 (9% to 100% positivity), 4 of 8 (3% to 17% positivity), and 8 of 14 (5% to 73% positivity) cases, respectively. Thus, SP cells in active AML are characterized by considerable case-to-case heterogeneity in expression of phenotypic markers, including CD34, while in cases in hematologic remission, they retain the CD34low/−CD38lowphenotype characteristic of normal SP cells.
Heterogeneous expression of CD34 and CD38 on the SP cells of patients with AML.
The patient samples were grouped into active disease (untreated and relapsed/resistant) or complete hematological remission. Median values are indicated by horizontal bars.
Heterogeneous expression of CD34 and CD38 on the SP cells of patients with AML.
The patient samples were grouped into active disease (untreated and relapsed/resistant) or complete hematological remission. Median values are indicated by horizontal bars.
Cytogenetic evidence of SP cell leukemic involvement
The alterations in prevalence and immunophenotype observed in the SP cells of patients with AML (Figures 1-2) compared with SP cells in healthy individuals suggests a dysregulation of SP associated with the disease, but does not establish their derivation from the original leukemic clone. We therefore used interphase FISH to test both leukemic and remission samples from 16 cases defined by trisomy 8 (n = 8), monosomy 7 (n = 7), or both (n = 1). In the 11 cases of active disease (blast cell count, 5% or greater), one or both of these cytogenetic markers were present in 9% to 90% (median, 41% ± 29%) of the SP cells in all of the cases, compared with 11% and 12.5% in 2 of the 5 cases in remission (Table2). The frequency of the defining cytogenetic abnormality among CD34low/− SP cells generally corresponded to that in the CD34+ non-SP fraction. These findings indicate a leukemic origin for a significant fraction of SP cells in most cases of active AML. The presence of marker-positive cells in some remission samples suggests a role of leukemic SP cells in disease persistence after chemotherapy.
Frequency of leukemia-associated aberrations of chromosomes 7 and 8 in the side population and non–side population stem cell compartments at initial diagnosis
| Patient* . | % Blasts in BM . | CD34low/−SP . | CD34+ non-SP . |
|---|---|---|---|
| Monosomy 7 | |||
| 1 | < 5 | 6 | 5 |
| 2 | < 5 | 12.5 | 0 |
| 3 | < 5 | 7.4 | 16 |
| 4 | 18 | 68 | 79 |
| 5 | 12 | 12 | 4.5 |
| 6 | 15 | 9 | 18.1 |
| 7a | < 5* | 5.5 | 6.7 |
| 8 | 90 | 84.5 | 93 |
| 9 | 80 (PB) | 90 | 89 |
| Trisomy 8 | |||
| 10 | 50 | 18 | 11.5 |
| 11 | 57 | 40 | 40 |
| 12 | 52 | 57.5 | 42.5 |
| 13 | 71 | 42.1 | 13.2 |
| 14 | 85 | — | 47.1 |
| 15 | 16 | 28.5 | 10 |
| 7b | < 5* | 11 | 19.5 |
| 16 | < 5 | 2 | 0.5 |
| Patient* . | % Blasts in BM . | CD34low/−SP . | CD34+ non-SP . |
|---|---|---|---|
| Monosomy 7 | |||
| 1 | < 5 | 6 | 5 |
| 2 | < 5 | 12.5 | 0 |
| 3 | < 5 | 7.4 | 16 |
| 4 | 18 | 68 | 79 |
| 5 | 12 | 12 | 4.5 |
| 6 | 15 | 9 | 18.1 |
| 7a | < 5* | 5.5 | 6.7 |
| 8 | 90 | 84.5 | 93 |
| 9 | 80 (PB) | 90 | 89 |
| Trisomy 8 | |||
| 10 | 50 | 18 | 11.5 |
| 11 | 57 | 40 | 40 |
| 12 | 52 | 57.5 | 42.5 |
| 13 | 71 | 42.1 | 13.2 |
| 14 | 85 | — | 47.1 |
| 15 | 16 | 28.5 | 10 |
| 7b | < 5* | 11 | 19.5 |
| 16 | < 5 | 2 | 0.5 |
The results of interphase fluorescence in situ hybridization are given as percentages of 200 sorted bone marrow cells.
BM indicates bone marrow; SP, side population; PB, peripheral blood.
Nine patients had fewer than 200 analyzable nuclei as follows: patient 1 non-SP (187 cells), patient 2 non-SP (12 cells), patient 3 SP (68 cells), patient 11 SP (30 cells), patient 12 SP (40 cells), patient 13 SP (138 cells)/non-SP (113 cells); patient 14 SP (136 cells); patient 7b SP (110 cells)/non-SP (173 cells) patient 15 non-SP (179 cells).
At initial diagnosis, the acute myeloid leukemia in case 7 was characterized by monosomy 7 and trisomy 8.
Efficient efflux of daunorubicin and mitoxantrone from SP cells
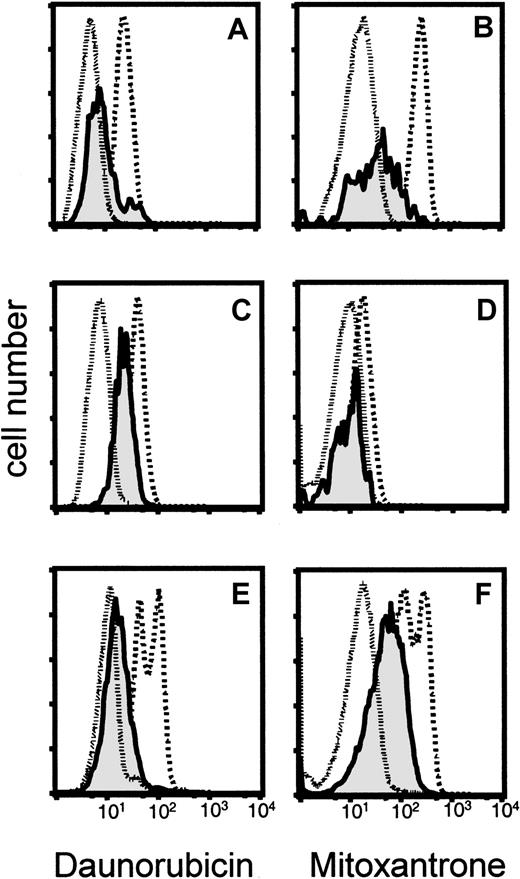
The ability of SP cells to efficiently expel Hoechst 33342 suggested a mechanism by which such cells could escape the lethal effects of antileukemic drugs. We therefore asked if daunorubicin and mitoxantrone, 2 commonly used agents in the treatment of AML, might be cotransported with this lipophilic dye. Mononuclear bone marrow cells from 2 childhood and 1 adult AML patient were simultaneously incubated with Hoechst 33342 and either daunorubicin or mitoxantrone, and the efflux of drug from the gated SP and non-SP cells was measured by its specific emission fluorescence at 575/25 nm (daunorubicin) or 670/40 nm (mitoxantrone). Comparison of the emission profiles of SP versus non-SP cells demonstrated increased efflux of daunorubicin and mitoxantrone together with Hoechst 33342 in each of the clinical samples of SP cells (Figure 4). Additional testing with leukemia/lymphoma cell lines and bone marrow samples from healthy volunteers confirmed the greater efficiency of cytostatic drug efflux from SP cells (data not shown).
Efflux of daunorubicin and mitoxantrone from leukemic SP cells in bone marrow samples from 3 patients with AML.
After incubation of the cells with Hoechst 33342 and daunorubicin (panels A, C, E) or mitoxantrone (panels B, D, F), the fluorescence emitted by the drugs was plotted for 3 gated cell populations: SP (solid line, shaded); non-SP (dashed line); and negative control, SP cells stained with Hoechst 33342 alone (dotted line).
Efflux of daunorubicin and mitoxantrone from leukemic SP cells in bone marrow samples from 3 patients with AML.
After incubation of the cells with Hoechst 33342 and daunorubicin (panels A, C, E) or mitoxantrone (panels B, D, F), the fluorescence emitted by the drugs was plotted for 3 gated cell populations: SP (solid line, shaded); non-SP (dashed line); and negative control, SP cells stained with Hoechst 33342 alone (dotted line).
Transplantable leukemic activity of SP cells
In competitive repopulation experiments, normal SP cells accounted for virtually all of the HSC activity of transplanted murine bone marrow.10,12 Thus, the SP cells we have identified in human AML should be able to regenerate the primary disease in immunodeficient mice. The limited number of SP cells available for transplantation, together with their likely quiescent state,12 led us to develop assays (see “Patients, materials, and methods”) that detect very low levels of human cell engraftment (maximum sensitivity, 0.001%, in a mixture of HL60 and AML193 leukemic blasts with NOD/SCID bone marrow). Subsequently, we injected variable numbers of either CD34low/− SP cells (range, 3.8 × 101 to 1 × 105; n = 29 cases) or the remainder of the Hoechst-positive cell population (range, 6.5 × 102 to 3.9 × 106; n = 29 cases) into 21 pairs of NOD/SCID mice (SP- vs non-SP cells).
Three SP cell transplants (from cases with initial SP fractions of 80%, 71%, and 16%) gave rise to AML-like disease in each recipient (Figure 5). Transplantation of non-SP cells led to overt leukemia in a single mouse injected with 1 × 105 cells from one of cases, in which SP cells had regenerated overt AML. Importantly, in mice transplanted with SP cells, low but persistent numbers of human cells were detected in murine bone marrows by immunocytology (8 of 14 mice) and PCR (6 of 18 mice), as well as in mice transplanted with non-SP cells (6 of 18 and 8 of 14 mice, respectively). In most of these instances, there was either engraftment or nonengraftment by each of the paired samples; engraftment by one but not the other cell type was unusual.
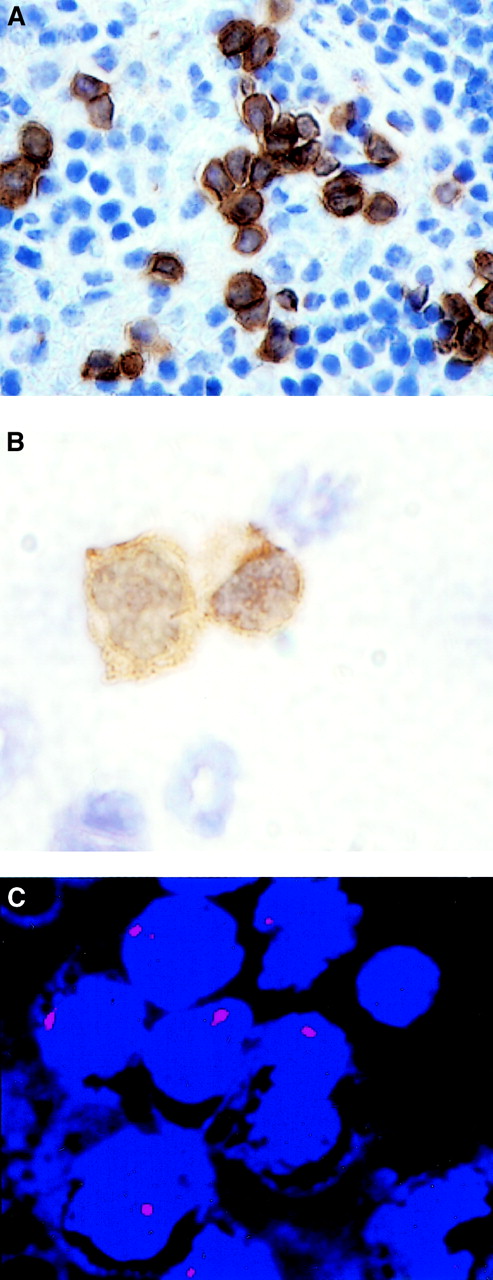
Generation of AML-like disease by human CD34low/− SP cells in the NOD/SCID mouse.
Leukemic blasts staining positively for human CD45 were found infiltrating the murine host spleen (panel A, 60 ×) and the bone marrow (panel B, 100 ×). FISH with a centromeric probe for chromosome 7 (D7Z1) showed monosomic blasts infiltrating the bone marrow of this mouse (panel C, 100 ×).
Generation of AML-like disease by human CD34low/− SP cells in the NOD/SCID mouse.
Leukemic blasts staining positively for human CD45 were found infiltrating the murine host spleen (panel A, 60 ×) and the bone marrow (panel B, 100 ×). FISH with a centromeric probe for chromosome 7 (D7Z1) showed monosomic blasts infiltrating the bone marrow of this mouse (panel C, 100 ×).
To determine the proportions of leukemic cells in the human hemopoietic xenografts, we applied FISH for monosomy 7 and trisomy 8 to the bone marrow MNCs of recipient mice. Table 3shows the proportions of SP and non-SP cells with marker-positive nuclei in 8 cases of AML with monosomy 7 or trisomy 8. In one case (No. 18), all of the human cells detected carried the relevant leukemic marker, while in the remaining cases, there was a mixture of diploid and cytogenetically aberrant cells. In the samples from a patient with trisomy 8 and monosomy 15 (No. 20), the engrafted SP cells were nearly all disomic for chromosome 8, in contrast to the repeated finding of trisomy 8 during the course of the disease. Finally, in a case of monosomy 7 (No. 17), the results of SP- and non–SP-cell engraftment showed a remarkable discrepancy. Whereas the progeny of transplanted non-SP cells were primarily marker positive (85%), those from SP cells were essentially disomic for chromosome 7. The morphologic features of the human diploid cell population infiltrating the bone marrow, spleen, and retrobulbar fat pad were unequivocally leukemic (data not shown).
Leukemic engraftment detected by fluorescence in situ hybridization from transplantation of CD34low/− side population versus non–side population cells into nonobese diabetic–evere combined immunodeficient mice
| Case . | Clonal marker . | Cell fraction . | Cell no. injected (× 10−3) . | % Engrafted human cells . | No. positive nuclei/total . | % Positive nuclei . |
|---|---|---|---|---|---|---|
| 17 | Monosomy 7 | SP | 10 | 32 | 18/200 | 9 |
| non-SP | 110 | 65 | 171/200 | 85 | ||
| 9 | Monosomy 7 | SP | 100 | 45 | 180/200 | 90 |
| non-SP | — | — | — | — | ||
| 6 | Monosomy 7 | SP | 0.45 | < 0.1 | 21/27 | 77 |
| non-SP | 160 | < 0.1 | 11/13 | 84 | ||
| 18 | Monosomy 7 | SP | 0.064 | < 0.1 | 5/5 | 100 |
| non-SP | 390 | < 0.1 | 5/5 | 100 | ||
| 19 | Monosomy 7 | SP | 1.40 | < 0.1 | 6/11 | 54 |
| non-SP | 545 | 0 | — | — | ||
| 20 | Trisomy 8 | SP | 100 | 90 | 1/200 | 0.5 |
| Monosomy 15 | SP | 100 | 90 | 191/200 | 95.5 | |
| non-SP | — | — | — | — | ||
| 21 | Trisomy 8 | SP | 0.33 | < 0.1 | 0/2 | 0 |
| non-SP | — | — | — | — | ||
| 22 | Trisomy 8 | SP | 0.90 | 0 | — | — |
| non-SP | 2500 | < 0.1 | 1/4 | 25 |
| Case . | Clonal marker . | Cell fraction . | Cell no. injected (× 10−3) . | % Engrafted human cells . | No. positive nuclei/total . | % Positive nuclei . |
|---|---|---|---|---|---|---|
| 17 | Monosomy 7 | SP | 10 | 32 | 18/200 | 9 |
| non-SP | 110 | 65 | 171/200 | 85 | ||
| 9 | Monosomy 7 | SP | 100 | 45 | 180/200 | 90 |
| non-SP | — | — | — | — | ||
| 6 | Monosomy 7 | SP | 0.45 | < 0.1 | 21/27 | 77 |
| non-SP | 160 | < 0.1 | 11/13 | 84 | ||
| 18 | Monosomy 7 | SP | 0.064 | < 0.1 | 5/5 | 100 |
| non-SP | 390 | < 0.1 | 5/5 | 100 | ||
| 19 | Monosomy 7 | SP | 1.40 | < 0.1 | 6/11 | 54 |
| non-SP | 545 | 0 | — | — | ||
| 20 | Trisomy 8 | SP | 100 | 90 | 1/200 | 0.5 |
| Monosomy 15 | SP | 100 | 90 | 191/200 | 95.5 | |
| non-SP | — | — | — | — | ||
| 21 | Trisomy 8 | SP | 0.33 | < 0.1 | 0/2 | 0 |
| non-SP | — | — | — | — | ||
| 22 | Trisomy 8 | SP | 0.90 | 0 | — | — |
| non-SP | 2500 | < 0.1 | 1/4 | 25 |
2 × 102 bone marrow MNC from NOD/SCID mice transplanted with sorted CD34low/− side population (SP) or non–SP cells were analyzed by fluorescence in situ hybridization with probes (D7Z1, D8Z2, D15Z3) for the respective clonal leukemia marker. The values are numbers (percentages) of all human cells detected by hybridization. Only the results from samples with positive fluorescence in situ hybridization signals are shown. Engraftment levels were estimated by staining 2 × 105 bone marrow mononuclear cells per slide, with the use of parallel samples, for human CD45+ cells.
SP indicates side population; NA, not available.
Discussion
Rapid efflux of fluorescent dyes, mainly rhodamine 123 and Hoechst 33342, has been widely used to identify HSC subpopulations in mammals.20-23 Analysis of Hoechst 33342 fluorescence at 2 wavelengths reveals a small subset of HSCs (SP cells) characterized by a CD34low/− phenotype and their high enrichment in bone marrow–repopulating activity in lethally irradiated mice.10,12 In human and rhesus monkey bone marrow, a similar CD34low/− SP population is present, which in the monkey has been shown to contain high long-term culture-initiating cell activity and the capacity to generate CD34+cells.10 Because of their probable biological significance in the human stem cell compartment, we sought to characterize SP cells in patients with AML. Such cells were identified in more than 80% of the cases analyzed, primarily in the bone marrow. In the 3 cases in which both peripheral blood and bone marrow were available for parallel analyses, SP cells were found only in the bone marrow, the classical niche for primitive HSCs. These findings establish SP cells as a discrete bone marrow HSC population in children and adults with AML and extend the results obtained with AML stem populations isolated primarily from peripheral blood.3,5,24 Whereas CD34+ HSCs are routinely used to achieve host engraftment in experimental models and in patients, cells lacking CD34 expression are a quiescent population with predominantly late engraftment potential.10,25 A series of reports based predominantly on the analysis of peripheral blood stem cell populations in patients with AML concluded that the leukemia-initiating cell is confined to the CD34+ cell population with only exceptional instances of leukemia-initiating activity reported for CD34low/−populations.3,24 By contrast, Terpstra et al,7,8 using resistance to 5-fluorouracil to select bone marrow AML stem cells, found the SCID-repopulating cells to be CD34− in one case and CD34+ with conversion to CD34− in a second case. Sato et al25 recently demonstrated dynamic reversibility of murine CD34 expression, depending on the activation status of the HSC compartment. Thus, in our study, the cases with strong CD34 expression on leukemic SP cells (Figure 3) might represent neoplastic dysregulation of an otherwise regulated association between CD34 negativity and the capacity for rapid efflux of lipophilic molecules.
Only low percentages of leukemic SP cells expressed CD71 or Thy-1, whereas approximately half expressed CD13 and/or CD33. Since the last 2 antigens were not expressed on the SP cells of healthy individuals, this observation raises the issue of phenotypic promiscuity in the AML stem cell compartment. In normal hematopoiesis, myeloid differentiation markers are generally not expressed on cells that retain the function of primitive HSCs, but this relationship may not be preserved once leukemic transformation has occurred. Most, if not all, of the previously described myeloid leukemia cell lines, capable of unlimited self-renewal in vitro, express myeloid differentiation antigens to some degree.
The repopulating activity of leukemic SP cells was tested by xenotransplantation into NOD/SCID mice. The minimal numbers of AML stem cells transplanted successfully into NOD/SCID mice in previous dilution experiments were 4 × 104 by Bonnet and Dick3 and 1 × 105 by Ailles et al,26 leading to an estimated frequency of leukemia-initiating cells of 0.2 to 100 per 106 and 0.7 to 45 per 107 AML peripheral blood cells, respectively. In the present study, we transplanted an average of 340 CD34− SP cells (range, 3.8 × 101 to 1 × 105 cells) per mouse. Engraftment resulted in AML-like disease in 3 of the recipient mice, each given high cell numbers (1 × 104 to 1 × 105). Notably, the occurrence of very high SP cell fractions in these 3 patients was associated with a rapidly progressive, refractory disease, and the frank engraftment in the NOD/SCID mice might in part reflect the aggressiveness of the disease observed on clinical grounds. Importantly, in most mice transplanted with low numbers of CD34− SP cells, persistent human cells could be detected with sensitive assays (eg, in 6 of 18 cases analyzed with nested HER-H PCR). The pathobiological significance of low numbers of xenografted leukemic cells persisting in the recipient bone marrow for at least 3 months after transplantation is uncertain. Considering that a sample of 2 × 105 murine bone marrow cells analyzed by immunocytology or PCR represents approximately 1 × 10−3of the whole bone marrow of an irradiated NOD/SCID mouse, 2 human cells would represent a total population of 2000 human cells. Since fewer than 2000 SP cells were transplanted in these animals, a positive finding by immunocytology or PCR would indicate low-level expansion of the cells in vivo. We speculate that these residual cells might be capable of re-establishing the leukemic clone, given the proper environmental cues, and could therefore be relevant to the problem of minimal residual disease in patients with AML.
The results of engraftment from non-SP controls were remarkably similar to those obtained with CD34low/− SP cells, even though appreciably higher numbers of the former were transplanted (mean, 8 × 105; range, 6.5 × 102 to 3.9 × 106). Indeed, the engraftment capacities of CD34low/− SP and non-SP cells from a given sample correlated well in most cases (Tables 1, 3). An explanation for this finding may reside in the toxicity associated with the minor groove DNA-binding dye Hoechst 33342.27 28 Assuming a reduced cytotoxic effect of Hoechst 33342 in the efficiently effluxing SP cells, one would predict a survival advantage for SP compared with non-SP cells. Thus, enrichment of the side population in cells capable of persisting in the NOD/SCID mice might be due to the cells' ability to resist the toxicity of Hoechst 33342, as well as the primitive nature of the cells themselves.
In contrast to most other definitions of HSCs, the distinguishing phenotype of SP cells is directly linked to a basic cell function, rapid efflux of lipophilic substances probably mediated by an MDR-related mechanism.10 We exploited this property to test a novel hypothesis: that antileukemic drugs might be expelled from SP cells by the same mechanism responsible for the rapid efflux of Hoechst 33342. Our results, obtained with daunorubicin and mitoxantrone in 3 AML samples and myeloid leukemia cell lines (data not shown), clearly support this prediction (Figure 4). In each comparison, cells within the SP population expelled either drug more efficiently than did their non-SP counterparts. This suggests that the increased drug efflux capacity of even small populations of leukemic SP cells could influence the outcome of therapy in patients with AML.
Increasing evidence that the AML leukemic clone is organized as a hierarchy of HSCs and committed progenitor cells with many similarities to the normal hematopoietic system has provided fresh insight into the mechanisms that initiate and maintain the myeloid leukemias. The original work of Bonnet and Dick,3 using AML cells from peripheral blood, suggested that CD34+ HSCs are the target for leukemic transformation and therefore the cells most worthy of exploiting in new therapeutic strategies. The findings we report demonstrate the involvement of CD34low/− SP cells in a substantial percentage of AML cases. Moreover, the ability of these cells to generate CD34+ HSCs as well as committed myeloid progenitors, together with their capacity for rapid efflux of antileukemic drugs, suggests a fundamental role in AML pathogenesis, perhaps as a major target for leukemic transformation. Increased understanding of the distinct transport capacity of SP cells, and the identification of immunologically accessible targets within this newly identified stem cell population, should facilitate studies of AML stem cell biology and the development of new therapeutic strategies.
We thank Brian Newsom and Mike Cubbage for their excellent work with the flow cytometer, and John Gilbert for helpful comments on the manuscript; the M. D. Anderson Leukemia Department physicians for clinical samples; and Amgen and Immunex for generously providing samples of human cytokines.
M.A.G. is a Fellow Scholar of the American Society of Hematology.
Supported in part by grants from the National Institutes of Health to M.A.G. (RO1 CA81179-01A1) and M.A. (PO1 CA55164, PO1 CA49639, and CA16672), and from the Deutsche Forschungsgemeinschaft (WU 310/1-1) to G.G.W.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Margaret A. Goodell, Center for Cell and Gene Therapy, Baylor College of Medicine, N1030, One Baylor Plaza, Houston, TX 77030; e-mail: goodell@bcm.tmc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal