Glycoprotein V (GPV) is a subunit of the platelet GPIb-V-IX receptor for von Willebrand factor and thrombin. GPV is cleaved from the platelet surface during activation by thrombin, but its role in hemostasis is still unknown. It is reported that GPV knockout mice had a decreased tendency to form arterial occluding thrombi in an intravital thrombosis model and abnormal platelet interaction with the subendothelium. In vitro, GPV-deficient platelets exhibited defective adhesion to a collagen type I–coated surface under flow or static conditions. Aggregation studies demonstrated a decreased response of the GPV-deficient platelets to collagen, reflected by an increased lag phase and reduced amplitude of aggregation. Responses to adenosine diphosphate, arachidonic acid, and the thromboxane analog U46619 were normal but were enhanced to low thrombin concentrations. The defect of GPV null platelets made them more sensitive to inhibition by the anti-GPVI monoclonal antibody (mAb) JAQ1, and this was also the case in aspirin- or apyrase-treated platelets. Moreover, an mAb (V.3) against the extracellular domain of human GPV selectively inhibited collagen-induced aggregation in human or rat platelets. V.3 injected in rats as a bolus decreased the ex vivo collagen aggregation response without affecting the platelet count. Finally, surface plasmon resonance studies demonstrated binding of recombinant soluble GPV on a collagen-coupled matrix. In conclusion, GPV binds to collagen and appears to be required for normal platelet responses to this agonist.
Introduction
Platelets play a central role in hemostasis through their ability to adhere to a damaged vessel wall and to aggregate in response to agonists such as thrombin, collagen, or adenosine diphosphate (ADP),1 and these properties are known to be mediated by cell surface glycoproteins.2,3 However, the exact functions of numerous platelet glycoproteins that have been characterized biochemically remain unknown. Glycoprotein V (GPV, Mr 82 kd) is one of the most abundant glycoproteins at the surface of blood platelets and has long been identified,4-6 but its functional role is still subject to speculation. GPV is noncovalently linked to the GPIb-IX von Willebrand factor (vWF) receptor on the platelet surface.7,8 This type I transmembrane protein has a large extracellular domain comprising 15 Leu-rich motifs followed by a thrombin cleavage site.9,10 The specific release of a soluble 69-kd extracellular domain fragment (GPVf1) by thrombin has led to its proposal as a thrombin receptor,6 whereas cloning of the gene in rat and mouse revealed a well-conserved thrombin cleavage site.11 Studies in transfected cells have shown that GPV is required for efficient thrombin binding, possibly through a direct interaction with GPIbα.12,13 The release of a soluble fragment by thrombin has been used to develop a specific enzyme-linked immunosorbent assay for GPV that is being tested as a means of monitoring platelet activation under conditions of clinical thrombosis.14
The role of GPV as a thrombin receptor has been questioned on the basis of efficient platelet responses to high doses of thrombin when GPV had been enzymatically removed or cleavage was prevented by a blocking antibody.15,16 More recently, the functions of GPV have been reassessed in mouse strains lacking the GPVgene.17,18 These studies demonstrated that the absence of GPV did not provoke a bleeding syndrome and that they allowed normal GPIbα expression and vWF-mediated adhesion of the GPV-deficient platelets. The only defect reported by one group was an increased sensitivity of these platelets to activation by low concentrations of thrombin,18 suggesting that GPV may act as a negative modulator of the thrombin response.
The purpose of the present work was to evaluate in more detail possible functional defects of GPV null platelets, particularly regarding their interactions with components of the subendothelial matrix. Studies in an intravital thrombosis model indicated a decreased tendency of GPV null platelets to form occlusive thrombi, and in vitro adhesion tests indicated a defective initial attachment to collagen. These platelets had decreased reactivity to collagen, as demonstrated by a prolonged lag phase and reduced amplitude of aggregation especially at lower agonist concentrations. The collagen aggregation defect was also observed in normal human and rat platelets incubated with a monoclonal antibody (mAb) against the extracellular domain of GPV. Finally, recombinant soluble GPV inhibited collagen-induced platelet aggregation and bound to a collagen-coated matrix.
Materials and methods
Reagents
Mouse mAbs V.3 and V.1 against human GPV, the mouse mAb ALMA.16 against human GPIX, and rat mAbs RAM.1 against mouse GPIbβ and RAM.2 against mouse GPIIb-IIIa have been described previously.14,19 The hamster mAb 1C2 against mouse GPV was kindly provided by Dr Junichiro Fujimoto (Tokyo, Japan),20and the hamster mAb HMα2 against mouse α2β1 integrin (CD49b) was from Pharmingen-Becton Dickinson (Le Pont de Claix, France). Human vWF was purified from plasma cryoprecipitates by gel filtration chromatography according to a published procedure,21 and bovine vWF was kindly provided by Dr S. Jackson, Australian Centre for Blood Diseases (Box Hill, Australia). Soluble recombinant GPV was purified from culture media conditioned with GPV-transfected Chinese hamster ovary (CHO) cells using affinity chromatography on a V.1-Sepharose column as previously described.14
Insoluble bovine collagen type I, soluble human and rat collagen type I, soluble human collagen type III, ADP, the thromboxane analog U46619, PGI2, arachidonic acid, and mouse MOPC21 IgG1 were from Sigma Chemicals (St Louis, MO). Equine tendon collagen (a mixture of types I and III) were obtained from Nycomed (Münster, Germany), and human fibrinogen was obtained from Kabi (Stockholm, Sweden). Human serum albumin (HSA) and human α-thrombin were from the Etablissement Français du Sang-Alsace (Strasbourg, France). Recombinant hirudin was a gift of Dr A. Pavirani (Transgène SA, Strasbourg, France). Nutridoma-CS medium was purchased from Boehringer Mannheim (Mannheim, Germany), and heparin was from Hoffmann La Roche (Basel, Switzerland). Dichloro triazinyl amino fluorescein (DATF)–conjugated goat antirat F(ab)′2and fluorescein isothiocyanate (FITC)–conjugated anti–hamster immunoglobulin antibodies were from Jackson Immunoresearch (West Grove, PA).
Mouse strains
GPV−/− and GPV+/+ mouse colonies were established at the animal facilities of the Etablissement Français du Sang-Alsace by breeding heterozygotes provided by Dr Shaun Coughlin (University of California, San Francisco).17 These heterozygotes had been obtained by mating chimeric male mice (129/SvJae) with C57Bl/6 females. Genotyping was performed on mouse tail DNA by Southern blotting as described17 or by polymerase chain reaction amplification of a 444-nt GPV fragment (wild-type allele, 143-586 position11) or a 294-nt fragment in the Neo replacement cassette (KO allele).
In vivo murine thrombosis model
Thrombosis studies were performed according to a published method.22,23 Male mice of both genotypes (3-5 weeks old) were injected with genotype-matched calcein-labeled washed platelets (108 platelets/10 g body weight) and allowed to recover for at least 12 hours. The mice were then anesthetized, and the mesentery was exposed by abdominal incision. Arterioles (60–80-μm diameter) with shear flow rates estimated at 1400 second−1 were observed22 23 under an inverted fluorescence microscope (Leica DMIRB; Leica Microsystems SA, Wetzlar, Germany) coupled to a video camera (DAGE MTI, Michigan City, MI), and images were recorded on DVD-Ram tapes (WDR 200; Matsushita Electric Industrial, Osaka, Japan). After 30-μL application of a 250-mM FeCl3 solution, the arterioles were monitored for 20 minutes or until blood flow stopped. The operator was unaware of the mouse genotype while performing experiments.
Platelet preparation
Washed mouse and rat platelets were prepared from blood (9 vol) drawn from the abdominal aorta of anesthetized animals into a plastic syringe containing acid–citrate–dextrose (1 vol) and centrifuged at 1570g (10 seconds per milliliter blood). Platelet-rich plasma (PRP) was removed and centrifuged at 1570g for 10 minutes. The platelet pellet was washed twice at 1100g for 3-8 minutes in Tyrode buffer (137 mM NaCl, 2 mM KCl, 12 mM NaHCO3, 0.3 mM NaH2PO4, 1 mM MgCl2, 5.5 mM glucose, 5 mM HEPES, pH 7.3) containing 0.35% HSA and 7.5 nM PGI2 and resuspended at a density of 3 × 105 platelets per microliter in the same buffer in the presence of 0.02 U/mL ADP scavenger apyrase (adenosine 5′-triphosphate diphosphohydrolase, EC 3.6.1.5)—a concentration sufficient to prevent desensitization of platelet ADP receptors during storage. Washed human platelets were prepared from acid–citrate–dextrose anticoagulated blood obtained from aspirin-free healthy volunteers by sequential centrifugation as previously described.24 Platelets were kept at 37°C throughout all experiments. Citrated mouse and rat PRP was obtained by collecting blood into 3.15% sodium citrate followed by centrifugation at 1570g (10 seconds per milliliter blood) and adjusted to 3.105 platelets per microliter by the addition of plasma.
Platelet adhesion assays
Platelet adhesion under flow was studied using a modification of the method of Cranmer et al.25 Glass microcapillary tubes (microslides of 100-mm length and 0.2 × 2-mm cross-section; VitroCom, Mountain Lakes, NJ) were coated with 2.5 mg/mL insoluble bovine type I collagen for 2 hours at room temperature or with 50 μg/mL bovine vWF overnight at 4°C and then blocked with 0.1% HSA for 1 hour at room temperature. Citrate-anticoagulated whole blood was labeled with 5 μM DiOC6 for 10 minutes before perfusion with a syringe pump at 1500 seconds−1 (collagen studies) or 3000 seconds−1 (vWF studies) through the coated capillaries. The interaction of platelets with the matrix was viewed in real time under a fluorescence microscope and was saved for off-line analysis. The number of individual platelets adhering to the surface (6400-μm2 field) was analyzed frame by frame (24 frames/s) over the first 20 seconds.
Platelet adhesion under static conditions was studied on 8-well Super Teflon slides (HTC Super cured (R); Polylabo, Strasbourg, France) coated with 2.5 mg/mL collagen for 90 minutes or 20 μg/mL bovine vWF for 2 hours at room temperature and blocked with 0.1% HSA in phosphate-buffered saline (PBS) for 1 hour at room temperature. Washed platelets (30 000 platelets/well) in Tyrode-albumin buffer (collagen studies) or the same buffer containing 10 mM EDTA (vWF studies) were incubated in the wells at room temperature for 5 or 20 minutes. Slides were then washed 3 times with PBS, fixed with 4% 1-paraformaldehyde in PBS for 20 minutes, washed 3 times with PBS, and labeled with a 1/500 dilution of phalloidin–TRITC (Sigma) for 20 minutes in the dark. After 5 more washes in PBS, adherent platelets were covered with 5 μL Mowiol 4-88 solution (France Biochem, Meudon, France) and mounted for examination under a Leica DMR fluorescence microscope. Platelets were counted in 5 random fields (26 140 μm2/field).
Platelet aggregation
Aggregation was measured at 37°C by a turbidimetric method in a dual-channel Payton aggregometer (Payton Associates, Scarborough, Ontario, Canada). A 450-μL aliquot of platelet suspension was stirred at 1100 rpm and activated by the addition of different agonists, with or without antagonists, in the presence of human fibrinogen (0.05 mg/mL) and in a final volume of 500 μL. The extent of aggregation was estimated quantitatively by measuring the maximum curve height above the baseline. In studies with collagen, the lag phase was taken to be the time from agonist addition to the onset of aggregation.
Ex vivo aggregation of rat platelets
Anesthetized male Wistar rats (400 g) were given a bolus injection of V.1 or V.3 IgG1 (1 mg/kg) in the dorsal penile vein. After 20 minutes, 7 mL blood was withdrawn from the jugular vein into 3.15% sodium citrate. PRP was prepared, and aggregation studies were performed as described above.
Flow cytometry
Washed mouse platelets (3 × 105 platelets in 50 μL) were incubated with 20 μg/mL purified IgG or 50 μL hybridoma culture supernatant at 22°C for 30 minutes, washed in PBS, and incubated in the dark at 22°C for 30 minutes with 10 μg/mL DATF-conjugated goat anti–rat F(ab)′2 or FITC-conjugated goat anti–hamster immunoglobulin antibodies. Cells were then resuspended in PBS and analyzed on a FACScalibur fluorescence cytometer (Becton Dickinson, San Jose, CA) using Cell Quest software. The light scattering and fluorescence intensity from 10 000 platelets were collected using a logarithmic gain.
Surface plasmon resonance analysis
Experiments were performed using a Biacore 2000 apparatus (Biacore, Uppsala, Sweden). Activation of a CM5 sensor chip with the Biacore amine coupling kit was followed by the injection of 70 μL soluble or insoluble type I or type III collagen of variable origin diluted to 100 μg/mL in 10 mM sodium acetate, pH 4.8. Excess reactive amine groups were blocked with 1 M ethanolamine, pH 8.5. Parallel experiments were performed with sensor chips coupled with HSA or human vWF, and the total immobilized proteins corresponded to 8000 to 12 000 resonance units. Recombinant soluble GPV (59 μg/mL) in Hepes buffer saline containing EDTA and polysorbate (HBS-EP) buffer (0.01 M HEPES, 0.15 M NaCl, 3 mM EDTA, 0.005% [vol/vol], polysorbate 20, pH 7.4) was injected at 25°C at a flow rate of 5 μL/min using HBS as a running buffer. Responses (in resonance units) obtained on chips coupled with collagen or vWF were subtracted from those obtained with immobilized HSA using Biaevaluation 3.0 software. The surface was regenerated by 10-μL injection of 2 M NaCl.
Results
GPV null mice form less occlusive thrombi in an intravital arterial thrombosis model
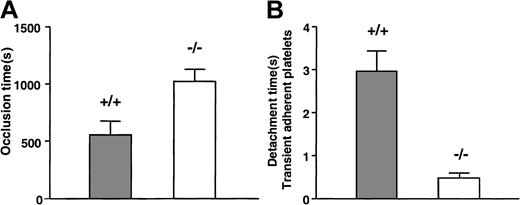
To evaluate the consequences of a lack of GPV in thrombotic situations, we used an established intravital microscopy model of thrombosis. Injury of the mesenteric arterioles of mice with FeCl3 leads to rapid platelet attachment to the vessel wall and progressive thrombus formation that eventually occludes the artery. The average time to occlusion was significantly longer in GPV null mice than in wild-type (WT) littermates (1027 ± 105 seconds [n = 10] vs 556 ± 123 seconds [n = 12]; P = .01) (Figure1A). Vessel lumen closure occurred in less than 20 minutes in 90% of WT mice, whereas half the arteries were still permeable at this time in GPV−/− mice. Analysis of the dynamics of platelet interaction at early times indicated that most subsequently detached from the matrix. The time interval between initial tethering and detachment of single attached platelets was significantly shorter in GPV null mice (0.47 ± 0.05 seconds, n = 24) than in GPV WT mice (2.97 ± 0.48 seconds, n = 34) (Figure 1B).
Effect of GPV deficiency in an intravital arterial thrombosis model.
GPV knockout (−/−, open bars) or WT (+/+, gray bars) mice were injected with calcein-labeled platelets of matching phenotype. After exposure of mesenteric arteries and FeCl3 injury, the arteries were observed for up to 20 minutes by fluorescence videomicroscopy. Time to vessel closure was determined (A), and vessels still permeable at the end of the experiment were scored as occluded in 20 minutes. Occlusion times in GPV-deficient mice (n = 10) were statistically prolonged (P = .01; 2-tailed t test) compared to those of littermate (+/+) controls (n = 12). During the first 2 minutes after injury, single platelets tethering to the vessel wall were randomly assigned and timed for detachment after frame-by-frame analysis (B) (n = 34 platelets from 5 arteries for WT mice and n = 24 platelets from 5 arteries for GPV null mice). Platelets detached significantly faster (P < .0001; 2-tailed ttest) in GPV null (0.47 ± 0.05 seconds) than in WT mice (2.97 ± 0.48 seconds). Results in panels A and B are expressed as mean values ± SEMs.
Effect of GPV deficiency in an intravital arterial thrombosis model.
GPV knockout (−/−, open bars) or WT (+/+, gray bars) mice were injected with calcein-labeled platelets of matching phenotype. After exposure of mesenteric arteries and FeCl3 injury, the arteries were observed for up to 20 minutes by fluorescence videomicroscopy. Time to vessel closure was determined (A), and vessels still permeable at the end of the experiment were scored as occluded in 20 minutes. Occlusion times in GPV-deficient mice (n = 10) were statistically prolonged (P = .01; 2-tailed t test) compared to those of littermate (+/+) controls (n = 12). During the first 2 minutes after injury, single platelets tethering to the vessel wall were randomly assigned and timed for detachment after frame-by-frame analysis (B) (n = 34 platelets from 5 arteries for WT mice and n = 24 platelets from 5 arteries for GPV null mice). Platelets detached significantly faster (P < .0001; 2-tailed ttest) in GPV null (0.47 ± 0.05 seconds) than in WT mice (2.97 ± 0.48 seconds). Results in panels A and B are expressed as mean values ± SEMs.
Defective adhesion of GPV null platelets to a collagen-coated surface
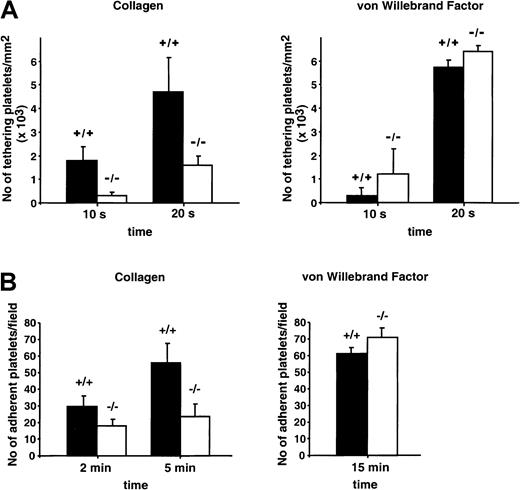
Defective interaction with components of the subendothelial matrix, including collagen, could contribute to the GPV knockout phenotype. Adhesion of GPV WT and GPV null platelets was, therefore, evaluated under flow conditions using microcapillaries coated with bovine type I collagen and citrated whole blood perfused at 1500 second−1. Initial attachment and adhesion of single platelets took place quickly after blood injection and was followed by the formation of stable aggregates progressively covering the adhesive surface. At early times (10-20 seconds), there was less attachment of individual platelets in blood from mice lacking GPV (Figure2A). This effect was also observed when hirudin was added to the citrated blood to completely inactivate thrombin (data not shown). In separate static adhesion tests using washed platelets, approximately 42% fewer GPV−/−platelets attached to collagen compared with WT cells (Figure 2B). A bovine vWF matrix showed comparable interaction with platelets of the 2 phenotypes under dynamic (3000 seconds−1, Figure 2A) and static (Figure 2B) conditions. Mouse platelets interacted weakly, if at all, with human vWF (data not shown).
Adhesion of WT and GPV-deficient platelets to a collagen- or vWF-coated surface.
(A) Fluorescence-labeled anticoagulated whole blood (3.15% trisodium citrate) from GPV null (−/−) or WT (+/+) mice was perfused at 1500 seconds-1 through glass microcapillaries coated with bovine type I collagen or at 3000 seconds-1 through glass microcapillaries coated with bovine vWF. Platelet attachment was continuously monitored by fluorescence videomicroscopy, and the number of platelets adhering to the surface during the first 10 or 20 seconds of flow was determined off-line. Results are the mean values ± SEMs from 3 to 5 separate experiments. (B) Washed platelets from GPV null (−/−) or WT (+/+) mice resuspended in Tyrode-albumin buffer containing apyrase were incubated with collagen- or bovine vWF-coated microslides in the absence of flow. The number of adherent platelets was determined at indicated time points after fixation and labeling with phalloidin-TRITC. Results are the mean values ± SEMs from 3 separate experiments.
Adhesion of WT and GPV-deficient platelets to a collagen- or vWF-coated surface.
(A) Fluorescence-labeled anticoagulated whole blood (3.15% trisodium citrate) from GPV null (−/−) or WT (+/+) mice was perfused at 1500 seconds-1 through glass microcapillaries coated with bovine type I collagen or at 3000 seconds-1 through glass microcapillaries coated with bovine vWF. Platelet attachment was continuously monitored by fluorescence videomicroscopy, and the number of platelets adhering to the surface during the first 10 or 20 seconds of flow was determined off-line. Results are the mean values ± SEMs from 3 to 5 separate experiments. (B) Washed platelets from GPV null (−/−) or WT (+/+) mice resuspended in Tyrode-albumin buffer containing apyrase were incubated with collagen- or bovine vWF-coated microslides in the absence of flow. The number of adherent platelets was determined at indicated time points after fixation and labeling with phalloidin-TRITC. Results are the mean values ± SEMs from 3 separate experiments.
Diminished collagen-induced aggregation of GPV null platelets
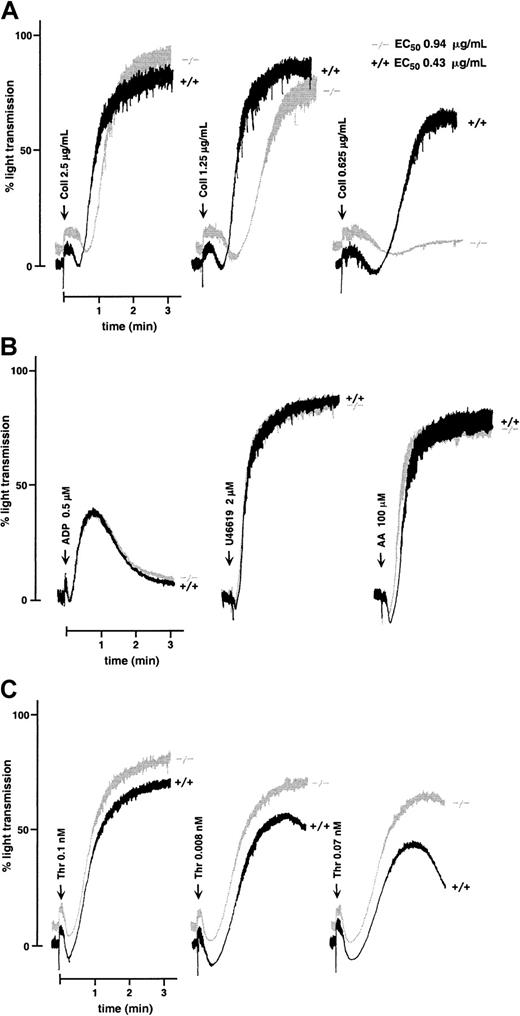
Washed WT mouse platelets activated with 2.5 μg/mL collagen in the presence of fibrinogen aggregated irreversibly after a typical 10- to 20-second lag phase before the onset of aggregation. At this agonist concentration, WT and GPV null platelets displayed no difference in the extent of aggregation at 3 minutes (Figure3A). However, close analysis of the tracings revealed a slight but reproducible increase in lag time in the GPV-deficient cells. At progressively lower doses of collagen, the diminished response of GPV null platelets became more evident, and at 0.625 μg/mL collagen—a concentration that induced 60% aggregation of WT platelets—no aggregation was detected using GPV-deficient platelets. The estimated concentration of collagen that induced half-maximal aggregation was 0.43 μg/mL for WT and 0.94 μg/mL for GPV null platelets. This decreased reactivity was limited to collagen because normal aggregation was observed in response to ADP, arachidonic acid, and the thromboxane analog U46619 (Figure 3B). Aggregation was also normal in response to nanomolar concentrations of thrombin (data not shown) but increased in response to lower (less than 0.1 nM) concentrations (Figure 3C). The collagen aggregation defect was further confirmed using citrated PRP instead of washed platelets (data not shown).
Aggregation of WT and GPV-deficient platelets.
Aggregation of washed platelets from GPV null (−/−) or WT (+/+) mice was initiated by the addition of collagen (Coll) (A), ADP, the thromboxane analog U46619, or arachidonic acid (AA) (B), or thrombin (Thr) (C), in the presence (A, B) or absence (C) of fibrinogen. Washed platelets were prepared from pools of 5 to 7 mice for each genotype. GPV null platelets exposed to decreasing doses of collagen gave a diminished response compared to WT platelets, reflected in an increased lag phase and a reduced amplitude of aggregation (A). Concentrations of collagen that induced half-maximal aggregation of WT and GPV null platelets are indicated as EC50 values. Aggregation responses to ADP, U46619, and arachidonic acid were normal in GPV-deficient platelets (B). GPV null platelets displayed enhanced aggregation in response to low doses of thrombin (C). Tracings are representative of at least 3 separate experiments.
Aggregation of WT and GPV-deficient platelets.
Aggregation of washed platelets from GPV null (−/−) or WT (+/+) mice was initiated by the addition of collagen (Coll) (A), ADP, the thromboxane analog U46619, or arachidonic acid (AA) (B), or thrombin (Thr) (C), in the presence (A, B) or absence (C) of fibrinogen. Washed platelets were prepared from pools of 5 to 7 mice for each genotype. GPV null platelets exposed to decreasing doses of collagen gave a diminished response compared to WT platelets, reflected in an increased lag phase and a reduced amplitude of aggregation (A). Concentrations of collagen that induced half-maximal aggregation of WT and GPV null platelets are indicated as EC50 values. Aggregation responses to ADP, U46619, and arachidonic acid were normal in GPV-deficient platelets (B). GPV null platelets displayed enhanced aggregation in response to low doses of thrombin (C). Tracings are representative of at least 3 separate experiments.
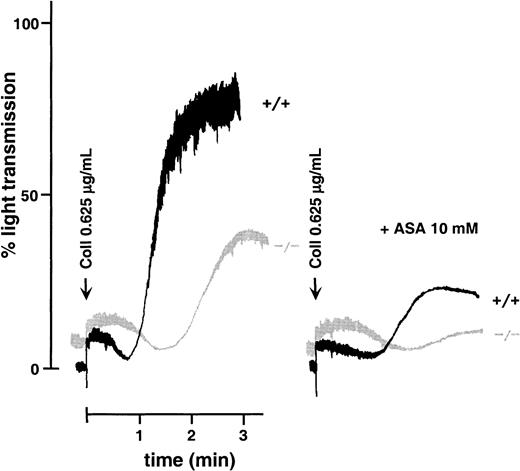
Platelet activation induced by collagen is typically accompanied by thromboxane A2 (TXA2) production, which in turn amplifies the aggregation response.26 After treatment with aspirin, both WT and GPV null platelets displayed a decreased response to collagen. Lower reactivity was nonetheless observed in GPV-deficient platelets (Figure 4), suggesting that the lower collagen reactivity of these cells was not due to a defect of the cyclo-oxygenase pathway. Moreover, responses to arachidonic acid and the TXA2 analog U46619 were normal in GPV null platelets (Figure 3B).
Effects of aspirin on collagen-induced aggregation of WT and GPV-deficient platelets.
Washed mouse platelets left untreated or preincubated with 10 mM aspirin (ASA) were activated with 0.625 μg/mL collagen (Coll) in the presence of fibrinogen. Aspirin inhibited the aggregation response of both GPV null (−/−) and WT (+/+) platelets, but a more prolonged lag phase was observed in GPV-deficient cells. This experiment was performed twice with comparable results.
Effects of aspirin on collagen-induced aggregation of WT and GPV-deficient platelets.
Washed mouse platelets left untreated or preincubated with 10 mM aspirin (ASA) were activated with 0.625 μg/mL collagen (Coll) in the presence of fibrinogen. Aspirin inhibited the aggregation response of both GPV null (−/−) and WT (+/+) platelets, but a more prolonged lag phase was observed in GPV-deficient cells. This experiment was performed twice with comparable results.
GPVI-dependent activation is affected in platelets lacking GPV
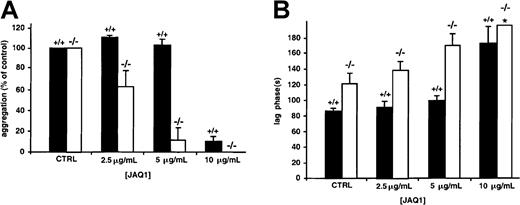
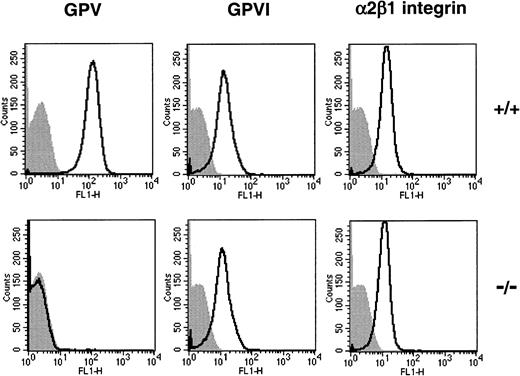
GPVI plays a major role in triggering the activation of platelets exposed to insoluble fibrillar collagen.27,28 At a 20-μg/mL concentration, JAQ1, a rat mAb specific for mouse GPVI,29 completely inhibits the aggregation induced by collagen concentrations below approximately 7 μg/mL.30When JAQ1 was used at lower doses (2.5 and 5 μg/mL), only partial inhibition, leading to a slightly increased lag phase, was observed in WT platelets, with no change in the amplitude of aggregation (Figure5). At these concentrations of JAQ1, GPV null platelets were more sensitive to inhibition than WT cells, displayed an extended lag phase, and decreased levels of aggregation. Contrary to WT platelets, the shape change induced by collagen was abolished in GPV null platelets treated with 10 μg/mL JAQ1. This increased sensitivity to JAQ1 was not attributed to lower expression of GPVI because identical levels were found on WT and GPV-deficient platelets by flow cytometry (Figure 6) and Western blot analysis (data not shown). Flow cytometry further revealed normal amounts of the α2β1 integrin (Figure 6), the GPIb-IX complex, and the αIIbβ3 integrin (data not shown) on GPV null platelets.
Effects of mAb against GPVI on collagen-induced aggregation of WT and GPV-deficient platelets.
Washed mouse platelets left untreated (CTRL) or preincubated with 2.5, 5, or 10 μg/mL JAQ1 against mouse GPVI were activated with 0.625 μg/mL collagen in the presence of fibrinogen. Amplitude of aggregation 3 minutes after the addition of collagen (percentage control) (A) and time to onset of aggregation (lag phase) (B) were determined in platelets from GPV null (−/−, open bars) and WT (+/+, black bars) mice. JAQ1 inhibited the collagen response of GPV null platelets to a greater extent than it did that of WT platelets, as shown by the smaller amplitude of aggregation and the increased lag time. Aggregation was significantly decreased (P ≤ .015, 2-tailed t test) in GPV null platelets than in WT platelets for 2.5 and 5 μg/mL JAQ1. The lag time was significantly increased (P ≤ .02, 2-tailedt test) in GPV null platelets than in WT platelets for control without JAQ1 and for 2.5 and 5 μg/mL JAQ1. No shape change was observed in −/− mice in the presence of 10 μg/mL JAQ1 (*). Results are expressed as mean ± SEM of 3 separate experiments.
Effects of mAb against GPVI on collagen-induced aggregation of WT and GPV-deficient platelets.
Washed mouse platelets left untreated (CTRL) or preincubated with 2.5, 5, or 10 μg/mL JAQ1 against mouse GPVI were activated with 0.625 μg/mL collagen in the presence of fibrinogen. Amplitude of aggregation 3 minutes after the addition of collagen (percentage control) (A) and time to onset of aggregation (lag phase) (B) were determined in platelets from GPV null (−/−, open bars) and WT (+/+, black bars) mice. JAQ1 inhibited the collagen response of GPV null platelets to a greater extent than it did that of WT platelets, as shown by the smaller amplitude of aggregation and the increased lag time. Aggregation was significantly decreased (P ≤ .015, 2-tailed t test) in GPV null platelets than in WT platelets for 2.5 and 5 μg/mL JAQ1. The lag time was significantly increased (P ≤ .02, 2-tailedt test) in GPV null platelets than in WT platelets for control without JAQ1 and for 2.5 and 5 μg/mL JAQ1. No shape change was observed in −/− mice in the presence of 10 μg/mL JAQ1 (*). Results are expressed as mean ± SEM of 3 separate experiments.
Flow cytometry analysis of the binding of mAbs to WT and GPV-deficient platelets.
Antibodies against mouse GPV (1C2), GPVI (JAQ1), and α2β1 integrin (HMα2) were incubated with washed platelets from GPV null (−/−) and WT (+/+) mice, and their binding was quantified by flow cytometry. Analysis of the FL1 histograms confirmed a lack of GPV on GPV null platelets, whereas GPVI and the α2β1 integrin were expressed at similar levels on WT and GPV-deficient platelets.
Flow cytometry analysis of the binding of mAbs to WT and GPV-deficient platelets.
Antibodies against mouse GPV (1C2), GPVI (JAQ1), and α2β1 integrin (HMα2) were incubated with washed platelets from GPV null (−/−) and WT (+/+) mice, and their binding was quantified by flow cytometry. Analysis of the FL1 histograms confirmed a lack of GPV on GPV null platelets, whereas GPVI and the α2β1 integrin were expressed at similar levels on WT and GPV-deficient platelets.
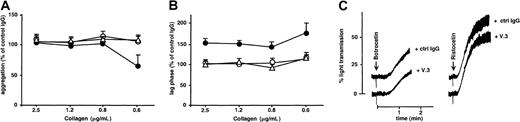
In vitro and ex vivo inhibition of collagen-induced platelet aggregation by an mAb against GPV
In view of the results with GPV null mice, we next tested the effects on normal human platelets of mAbs against the extracellular domain of GPV.14 One mAb (V.3) decreased the collagen responses of human platelets in washed platelet suspensions (Figure7) and citrated PRP (data not shown). The inhibition profile was similar to that observed in GPV null mice, with an increased lag phase at all agonist concentrations tested and with reduced amplitude of aggregation in response to low concentrations of collagen (Figure 7). V.3 had no influence on botrocetin- or ristocetin-induced agglutination or on aggregation induced by ADP,U46619, arachidonic acid, or high- or low-dose thrombin (data not shown). Other mAbs directed against distinct epitopes on GPVf1 (V.1) or against GPIX (ALMA.16) did not affect platelet aggregation in response to collagen or any other agonist (Figure 7).
Effects of mAbs against GPV on collagen- and vWF-induced aggregation of human platelets.
In panels A and B, washed human platelets incubated with MOPC21 IgG1 (control), mAb V.1 (●), or V.3 (○) against human GPV, or mAb ALMA.16 against human GPIX (▵) (all 20 μg/mL) were activated by the addition of collagen (2.5, 1.2, 0.8, or 0.6 μg/mL) in the presence of fibrinogen. The amplitude of aggregation at 3 minutes (A) and lag phase (B) were expressed as the percentages of the values for MOPC-treated platelets. Treatment with V.3 decreased the amplitude of aggregation for 0.6 μg/mL collagen (P = .02; 2-tailed ttest, A) and increased the lag phase for all collagen concentrations (P ≤ .01, 2-tailed t test, B), whereas V.1 and ALMA.16 had no effect on the collagen response. In panel C, washed human platelets incubated with MOPC21 (control) or mAb V.3 (both 20 μg/mL) were activated with 10 μg/mL human vWF and 5 μg/mL botrocetin or 0.7 μg/mL human vWF and 50 μg/mL ristocetin. Treatment with V.3 had no effect on vWF-dependent aggregation. Results are the mean ± SEM from 3 separate experiments (A, B), and tracings are representative of 3 experiments (C).
Effects of mAbs against GPV on collagen- and vWF-induced aggregation of human platelets.
In panels A and B, washed human platelets incubated with MOPC21 IgG1 (control), mAb V.1 (●), or V.3 (○) against human GPV, or mAb ALMA.16 against human GPIX (▵) (all 20 μg/mL) were activated by the addition of collagen (2.5, 1.2, 0.8, or 0.6 μg/mL) in the presence of fibrinogen. The amplitude of aggregation at 3 minutes (A) and lag phase (B) were expressed as the percentages of the values for MOPC-treated platelets. Treatment with V.3 decreased the amplitude of aggregation for 0.6 μg/mL collagen (P = .02; 2-tailed ttest, A) and increased the lag phase for all collagen concentrations (P ≤ .01, 2-tailed t test, B), whereas V.1 and ALMA.16 had no effect on the collagen response. In panel C, washed human platelets incubated with MOPC21 (control) or mAb V.3 (both 20 μg/mL) were activated with 10 μg/mL human vWF and 5 μg/mL botrocetin or 0.7 μg/mL human vWF and 50 μg/mL ristocetin. Treatment with V.3 had no effect on vWF-dependent aggregation. Results are the mean ± SEM from 3 separate experiments (A, B), and tracings are representative of 3 experiments (C).
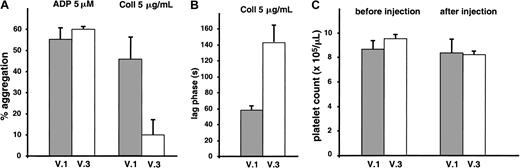
V.3 also reacted with rat GPV11 and demonstrated identical inhibition of collagen-induced aggregation of rat platelets (data not shown). In vivo effects of V.3 were tested by giving a bolus intravenous injection of the mAb (1 mg/kg) to Wistar rats and performing ex vivo aggregation studies on citrated PRP. Treatment with V.3 resulted in a decreased response to collagen but did not alter the ADP response (Figure 8). GPV occupancy was confirmed by flow cytometry with FITC-conjugated anti–mouse immunoglobulin F(ab)′2 (data not shown), and there was no significant change in platelet count after V.3 injection.
Inhibition of ex vivo collagen-induced aggregation of rat platelets following injection of V.3.
Wistar rats were given a bolus intravenous injection (1 mg/kg) of the mAb V.3, which cross-reacts with rat GPV, or the mAb V.1, which does not recognize rat GPV. Blood was drawn 20 minutes later for aggregation tests in citrated PRP (A, B) and platelet counts (C). Treatment with V.3 resulted in decreased amplitude of aggregation (A) and increased lag phase (B) in response to collagen compared to treatment with V.1, but it had no effect on the ADP response (A). There was no significant change in platelet count after V.3 or V.1 injection (C). Results are the mean values ± SEMs for 3 animals in each group.
Inhibition of ex vivo collagen-induced aggregation of rat platelets following injection of V.3.
Wistar rats were given a bolus intravenous injection (1 mg/kg) of the mAb V.3, which cross-reacts with rat GPV, or the mAb V.1, which does not recognize rat GPV. Blood was drawn 20 minutes later for aggregation tests in citrated PRP (A, B) and platelet counts (C). Treatment with V.3 resulted in decreased amplitude of aggregation (A) and increased lag phase (B) in response to collagen compared to treatment with V.1, but it had no effect on the ADP response (A). There was no significant change in platelet count after V.3 or V.1 injection (C). Results are the mean values ± SEMs for 3 animals in each group.
Soluble recombinant GPV binds to collagen and decreases collagen-induced platelet aggregation
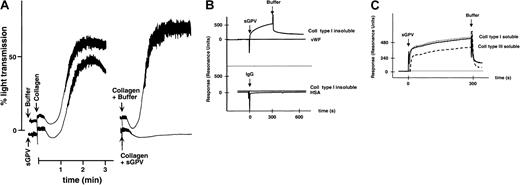
To test for a possible interaction of GPV with collagen, studies were performed using recombinant soluble forms of GPV consisting of the entire extracellular domain or the f1 fragment produced by thrombin. These proteins were purified from the culture supernatants of CHO cells transfected with the human GPV gene.14 Soluble GPV (4 μg/mL) inhibited the aggregation response induced by collagen (Figure 9A) but had no effect on the ADP response (data not shown). This inhibition was more effective when soluble GPV was preincubated with the collagen than when it was added to the platelet suspension before collagen. To look for a direct interaction of GPV with collagen, the binding of soluble GPV to collagen coupled to a dextran-coated chip was analyzed by surface plasmon resonance (Figure 9B-C). In the presence of bovine or equine fibrillar insoluble type I collagen, the sensor-grams displayed a time-dependent increase in resonance units indicating the binding of GPV. Binding was also observed on surfaces coupled to human- or rat-soluble type I or human-soluble type III collagen. No binding to albumin or vWF matrices and no binding of control IgG to the collagen matrix were detected.
Collagen-binding properties of soluble GPV.
Aggregation of washed human platelets (A) was initiated in the presence of fibrinogen by adding 1.25 μg/mL collagen 30 seconds after the addition of buffer or 4 μg/mL soluble GPV (sGPV) (left tracings) or by adding 1.25 μg/mL collagen that had been incubated for 3 minutes with buffer or with 4 μg/mL sGPV (right tracings). Soluble GPV inhibited aggregation in both cases but more efficiently when preincubated with collagen. Surface plasmon resonance sensorgrams were recorded on a Biacore 2000 apparatus (B, C). Kinetic studies of the interaction of sGPV or control IgG (IgG) with surfaces bearing covalently coupled bovine insoluble type I collagen, HSA, or human von Willebrand factor (vWF) were performed as described in “Materials and methods” (B). The interaction of soluble GPVf1 fragment (sGPV) with human (dotted line) or rat (solid line) soluble type I collagen or human soluble type III collagen (broken line) was studied in the same manner (C). Binding is indicated by an increase in resonance units, and the responses were corrected by subtraction of those obtained for control HSA. After 300 seconds, the surface was rinsed by the injection of buffer.
Collagen-binding properties of soluble GPV.
Aggregation of washed human platelets (A) was initiated in the presence of fibrinogen by adding 1.25 μg/mL collagen 30 seconds after the addition of buffer or 4 μg/mL soluble GPV (sGPV) (left tracings) or by adding 1.25 μg/mL collagen that had been incubated for 3 minutes with buffer or with 4 μg/mL sGPV (right tracings). Soluble GPV inhibited aggregation in both cases but more efficiently when preincubated with collagen. Surface plasmon resonance sensorgrams were recorded on a Biacore 2000 apparatus (B, C). Kinetic studies of the interaction of sGPV or control IgG (IgG) with surfaces bearing covalently coupled bovine insoluble type I collagen, HSA, or human von Willebrand factor (vWF) were performed as described in “Materials and methods” (B). The interaction of soluble GPVf1 fragment (sGPV) with human (dotted line) or rat (solid line) soluble type I collagen or human soluble type III collagen (broken line) was studied in the same manner (C). Binding is indicated by an increase in resonance units, and the responses were corrected by subtraction of those obtained for control HSA. After 300 seconds, the surface was rinsed by the injection of buffer.
Discussion
Questions regarding the exact function of platelet GPV have been a longstanding topic of research but have never been satisfactorily answered. The hypotheses raised to date mainly favored a role of GPV in platelet thrombin responses on the grounds of its selective cleavage by thrombin.6,9 11 Our present study establishes a novel role of GPV in platelet responses to collagen based on the following findings: (1) GPV knockout mice had a lower tendency to form occlusive thrombi in an intravital arterial thrombosis model, (2) GPV-deficient platelets displayed collagen adhesion and aggregation defects, (3) antibody against GPV blocked platelet aggregation induced by collagen in vitro and ex vivo, and (4) recombinant soluble human GPV bound to collagen.
The availability of a GPV knockout mouse strain provided a valuable tool to explore the functions of GPV. Initial reports indicated no major defect of primary hemostasis in GPV-deficient mice17,18 but gave no information on a possible effect in thrombotic situations. The present study provided indication of such a role in an intravital thrombosis model adapted to small animals such as rats and mice.22,23 In this model, ferric chloride injury of the mesenteric arteries provokes de-endothelialization and rapid platelet adhesion to the exposed subendothelium. Progressive platelet recruitment from the circulation generally leads to vessel closure. GPV deficiency did not prevent platelet interaction with the vessel wall; rather, it appeared to delay stable thrombus formation and, when a thrombus formed, to render it less stable and less prone to occlude the artery. There is now ample evidence that platelet GPIbα, the αIIbβ3 integrin, and their respective ligands, vWF and fibrinogen, are required to build stable thrombi after endothelial injury.31,32 However, recent studies have shown that vWF and fibrinogen double-knockout mice are still able to form occlusive thrombi, suggesting the involvement of alternative adhesive pathways such as collagen and its platelet receptors.23 Reports on patients with defects in the 2 main collagen receptors, α2β1 integrin and GPVI,33-35 indicate that they play a role in hemostasis, but the lack of platelet-specific knockout mice has precluded a study of their involvement in thrombosis. The GPV knockout strain is the first to demonstrate abnormal thrombus formation in vivo and a specific defect of collagen responses. The GPIb-IX complex and αIIbβ3 integrin pathways appear to be unaffected in GPV knockout platelets in view of the normal receptor levels, normal platelet interaction with vWF, and normal aggregation in the presence of fibrinogen.
Analysis of the dynamics of single platelet adhesion revealed an increased tendency to detachment, suggestive of weaker interaction with components of the subendothelium. This led us to explore the role of GPV in platelet adhesion in vitro by examining the interaction of GPV null platelets with a collagen-coated surface. Platelets lacking GPV displayed a decreased collagen interaction in flowing anticoagulated whole blood and static washed platelet suspensions, thus providing evidence for the involvement of GPV in platelet adhesive properties. This involvement of GPV in platelet adhesion to collagen was further confirmed by the defective collagen adhesion of human platelets treated with the V.3 GPV blocking mAb (data not shown). The adhesion defect appeared to be independent of vWF because it was observed with washed platelets, whereas GPV null platelets adhered normally to a vWF-coated surface. This agrees with results showing that the adhesion of CHO cells expressing optimal amounts of GPIb-IX to a vWF matrix under flow was not modified by the presence of GPV.25 Previous flow studies using whole blood at similar shear rates (1500 seconds−1) already indicated a vWF-GPIb–independent platelet interaction with collagen at early time points, which was attributed to the α2β1 integrin.36 GPVI and α2β1 integrin levels were normal in GPV null platelets and were, therefore, not likely to be responsible for the adhesion defect.
In accordance with the known importance of attachment to collagen fibers as a prerequisite for platelet activation and aggregation,27-29,36 GPV null or normal platelets incubated with the mAb V.3 displayed an increased lag phase at all collagen concentrations. At lower agonist concentrations, the defective adhesion led to reduced levels of aggregation. The current view of collagen-induced platelet activation is that the cells initially interact with the α2β1 integrin (GPIa-IIa) and GPVI and that the signal is then conveyed by GPVI.27,28 This protein is structurally related to immunoreceptors of the immunoglobulin superfamily37,38 and uses similar signaling pathways. A model has been put forward whereby GPVI couples collagen stimulation to phosphorylation of the FcR γ chain, leading to the activation ofsyk and PLCγ2.39 Platelets lacking GPVI or treated with GPVI-blocking mAbs nevertheless still respond to collagen, which indicates the existence of other collagen receptors on the platelet surface.28,34 Additional receptors proposed include GPIV (CD36)40 and unidentified proteins of 85 to 90 kd41 and 67 to 75 kd.42 The mechanism of inhibition of collagen-induced aggregation by V.3, as a consequence of its interaction with platelet GPV, is still unclear and will require additional experiments. Two observations make it seem unlikely that inhibition by V.3 is due to steric hindrance. First, other mAbs directed against the N-terminal (f1) domain of human GPV (V.1) or rat GPV (RPM.5 and RPM.9) do not inhibit collagen-induced platelet aggregation (Figure 7A,B and data not shown). Second, vWF-binding properties of GPIbα, which is in proximity to GPV, are not affected by V.3 (Figure 7C). It should, however, be mentioned that the mAb SZ2, an antibody against GPIbα, has been reported to inhibit collagen-induced platelet aggregation.43 Cooperation between distinct binding sites might ensure a complete response to collagen by providing multiple attachment points. Thus, one possibility is that GPV participates in a multimolecular signaling array by allowing correct presentation of collagen to a signaling receptor such as GPVI. An absence of GPV would, as observed in this study, reduce transduction efficiency and increase sensitivity to GPVI blocking mAbs. Another possibility that requires further investigation is that GPV directly participates in intracellular signaling. However, there is to date no precise indication that GPV has such properties, despite the detection in its intracellular domain of a putative binding site for the 14-3-3ζ signaling protein.44 The defective response of GPV knockout platelets did not seem to be caused by mechanisms unrelated to GPV. Global membrane perturbation resulting from the disappearance of a large number of integral membrane proteins was unlikely because CD9 null platelets (30 000-40 000 copies on WT cells) display normal responses to collagen (F.L., unpublished observations, July 2000). Similarly, the defect did not result from inefficient amplification of the collagen response by released ADP and thromboxane A2 generation given that GPV null platelets treated with apyrase (data not shown) or aspirin still exhibited a delayed shape change compared to WT cells.
Surface plasmon resonance binding studies, using a recombinant soluble form of GPV, provided evidence for a direct interaction of GPV with collagen, supporting the contention that GPV could serve as a collagen receptor. Lack of soluble GPV binding to other immobilized proteins, such as albumin, IgG, or vWF, and similar interaction with different types of collagen (soluble and insoluble, types I and III collagen) strongly suggest that binding was not caused by nonspecific capture. Analysis of the tracings indicates a high rate of dissociation of GPV from collagen and, hence, low-affinity interaction. To our knowledge, the collagen affinities of other purified receptors, such as α2β1 integrin or GPVI, have not been determined using Biacore and are unavailable. As mentioned earlier, platelet responses to collagen are thought to involve several receptors, including high-affinity adhesive receptors such as α2β1 integrin and low-affinity signaling receptors such as GPVI. In that context, the weak interaction of GPV may indicate that, like GPVI, it is a low-affinity receptor that may contribute independent signals or adhesion in response to collagen. The need to preincubate the GPV protein with collagen to get inhibition is what was found for GPVI, a recognized collagen receptor.37,38 Collagen receptors described to date essentially belong to the integrin or immunoglobulin superfamilies.2,3,27 GPV would, therefore, be the first example of a Leu-rich surface protein with collagen-binding properties, though fibromodulin and decorin, 2 Leu-rich proteoglycans, have been demonstrated to bind to collagen and to inhibit fiber formation. A collagen-binding site has been located in a Leu-rich element of decorin that includes a critical Glu residue.45 The epitope of the blocking mAb V.3, located within the f1 fragment of GPV containing 15 Leu-rich motifs, could represent a collagen-binding site. However, simple identification of the residues recognized by V.3 is hampered by its lack of recognition of the denatured protein. On the other hand, identification of the collagen region recognized by GPV will require binding studies using soluble GPV and collagen fragments, as were performed to identify binding sites of GPVI and α2β1 integrin.46 47
The only in vitro defect previously observed in GPV null platelets was an increased aggregation response in the presence of low concentrations of thrombin,18 confirmed in the present work. Early studies in human platelets using blocking antibodies or GPV removal suggested that GPV was not absolutely required to support thrombin-induced aggregation,15,16 and experiments in transfected cells have indicated that the association of GPV with GPIbα is necessary for efficient thrombin binding.12,13These results appear to disagree with those in GPV null platelets, which could be interpreted as evidence for a role of GPV as a negative modulator of the platelet thrombin response.18,48 An alternative explanation for the enhanced thrombin aggregation of GPV null platelets is that the lack of 12 000 copies of a thrombin substrate on the platelet surface leaves more enzyme available to bind to and activate its receptors, PAR3, PAR4,49 and GPIbα.50 This would result in an increased aggregation response, primarily at limiting concentrations of thrombin. Further studies will be required to establish the exact importance of GPV in normal platelet thrombin responses.
Interaction of platelets with collagen of the subendothelium plays an important role in normal hemostasis, as illustrated by the bleeding disorders encountered in patients with deficiencies of the 2 main collagen receptors, GPVI and the α2β1 integrin. Tail bleeding times were not significantly different between WT and GPV null mice (WT 87.6 ± 3.1 seconds [n = 60] vs GPV null 97.8 ± 4.7 seconds [n = 48]) confirming a lack of bleeding tendency. Therefore, GPV deficiency in humans would not be expected to produce significant bleeding. The shortened bleeding times observed in an independent knockout strain18 were not observed in the present study; this may be explained by differences in the bleeding time procedures.
The lower thrombotic tendency in mice lacking GPV and the diminished platelet collagen responses of rats treated with an anti-GPV antibody promote GPV as a new potential target for antithrombotics. Interestingly, the administration of GPV antibodies to rats or mice does not significantly modify the number of circulating platelets, contrary to the severe and prolonged thrombocytopenia with most antibodies directed against GPIbα.51 52 Platelet aggregation studies in the presence of recombinant soluble GPV suggest a role of released GPV as a negative modulator of platelet responses to collagen and thrombin. This might be important when soluble GPV is released into the circulation from activated platelets because it could limit thrombus growth on a collagen-rich subendothelial matrix.
In conclusion, this study has revealed an unsuspected role of GPV as a modulator of collagen-dependent platelet adhesion and activation. Evidence was provided that GPV is a binding site for collagen on the platelet surface and that the decreased collagen-binding properties of GPV null platelets is the likely explanation for their lower tendency, observed in vitro and in vivo, to adhere and to aggregate. The role of GPV in platelet collagen responses was further confirmed by the effect of a blocking antibody against GPV, pointing out new ways for pharmacologic inhibition of platelet function.
We thank Shaun Coughlin for providing the GPV null mouse strain, Shaun Jackson for pertinent comments, Sacha Dopheide for introducing us to the flow technique, Juliette Mulvihill for correcting the English of the manuscript, and Catherine Schwartz and Michèle Finck for animal care.
Supported by grants from ARMESA (P.M.) and GEHT (C.S.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
François Lanza, INSERM U.311, Etablissement Français du Sang-Alsace, 10 rue Spielmann, BP 36, 67065 Strasbourg Cédex, France; e-mail: francois.lanza@efs-alsace.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal