Recently, we have demonstrated that human platelets carry preformed CD40 ligand (CD154) molecules, which rapidly appear on the platelet surface following stimulation by thrombin. Once on the surface, platelet CD154 induces an inflammatory reaction of CD40-bearing endothelial cells. This study shows that strong platelet agonists other than thrombin also lead to the expression of CD154 on the platelet surface. At the same time, several lines of evidence are presented that together indicate that thrombotic events in the vasculature are generally accompanied by activation of the inflammatory potential of platelet CD154. This study also reports the constitutive expression of CD40, the receptor for CD154, on platelets. The binding of CD154 to coexpressed CD40 in the platelet aggregate leads within minutes to hours to the cleavage of membrane-bound surface CD154 and the release of an 18-kd soluble form of the molecule. Soluble CD154 (sCD154), in contrast to transmembrane CD154, can no longer induce an inflammatory reaction of endothelial cells. These findings indicate that the interaction of platelet CD154 with CD40 on neighboring cells is temporally limited to prevent an uncontrolled inflammation at the site of thrombus formation. Thus, similar to the very tight regulation of the CD154-CD40 interaction in the immune system, an effective mechanism controls the inflammatory potential of platelet CD154 in the vascular system.
Introduction
CD40, a 48-kd transmembrane protein homologous to the tumor necrosis factor (TNF) receptor, was first described as a constitutive cell-surface antigen on B cells.1,2 The CD40 ligand (CD40L, TRAP, gp39, CD154) is a 33-kd transmembrane homologue of TNF-α and was originally identified as a surface molecule expressed on activated T cells.3-5 Subsequent analysis of “immunodeficiency with hyper-IgM” demonstrated that interaction of CD40L on activated T cells with CD40 on B cells is required for the IgM to IgG isotype switch and thus revealed the pivotal role of the CD40L/CD40 pair for humoral immunity.6 Later, CD40 was also found to be constitutively expressed on monocytes, macrophages, and dendritic cells.7 In these cells, CD154, through interaction with CD40, initiates inflammatory responses such as synthesis of interleukin (IL)-1, IL-6, or TNF-α8 and thus functionally resembles its structural homologue TNF-α.
More recently, CD40 was identified on vascular endothelium and was shown to mediate signals leading to de novo expression of adhesion molecules and release of proinflammatory cytokines and chemokines by endothelial cells.9-11 In these original experiments, it was assumed that only activated T cells bearing CD154 can activate endothelial cells via CD40.9-11 This paradigm changed with our recent finding that platelets carry preformed CD154 molecules and rapidly express them on the cell surface after activation by thrombin.12 Furthermore, we could demonstrate that the interaction of CD154 on activated platelets with CD40 on endothelial cells elicits an inflammatory reaction of the endothelium, which is characterized by expression of the adhesion molecules E-selectin, vascular cell adhesion molecule (VCAM)-1, and intercellular adhesion molecule (ICAM)-1 and the secretion of the chemokines IL-8 and MCP-1.12 These findings allowed the conclusion that also in the vascular system, the action of platelet CD154 resembles the biologic effects of TNF-α.
Although our work demonstrated the role of thrombin in the induction of an “inflammatory platelet surface,” the presented data did not answer the question whether the expression of CD154 on the cell surface is a general phenomenon accompanying platelet activation. In the experiments presented here, we demonstrate that other strong platelet agonists also induce the expression of CD154 on the platelet surface.
Given the abundance of platelets, the identification of CD154 on activated platelets revealed a formidable inflammatory potential in the vascular system. It remained unclear, however, which mechanisms restrict the action of CD154 in the circulation and thus prevent an uncontrolled inflammation of the vasculature. Pertinent to this question, we also report here the constitutive expression of CD40, the receptor for CD154, on the surface of resting platelets. We describe how the interaction of CD154 on activated platelets with coexpressed CD40 temporally limits the duration of CD154 expression and thus effectively curtails the inflammatory action of platelet CD154.
Materials and methods
Isolation and activation of platelets
Platelet-rich plasma (PRP) was generated by centrifuging citrated human blood from healthy donors at 150g for 10 minutes. Purified platelets were isolated essentially as described.13 In brief, PRP was supplemented with 2 mM EDTA and centrifuged over a discontinuous albumin density gradient (25% over 34% endotoxin-free bovine serum albumin [BSA]; Sigma Chemical, St Louis, MO) in Tyrodes/HEPES buffer (138 mM NaCl, 2.9 mM KCl, 1 mM MgCl2, 1 mM glucose, 0.5 mM NaH2PO4, 20 mM HEPES, pH 7.4) at 750g for 30 minutes. The platelets in the interphase were layered onto a Sepharose 2B column (Pharmacia, Freiburg, Germany), eluted with Tyrodes/HEPES buffer, 0.3% BSA, and adjusted to 2 × 108/mL in Tyrodes/HEPES buffer. Both purified platelets and PRP were incubated at 37°C for 30 minutes before activation. For flow cytometry and time-course experiments on soluble CD154 (sCD154) release, platelets were stimulated with 0.2 U/mL human thrombin (Boehringer Mannheim, Germany) at 37°C without stirring. For immunoprecipitation, platelets were stimulated as above, except that aggregation was induced by stirring at 37°C.
Flow cytometry
After activation, platelets were fixed with 1% paraformaldehyde, washed, and reacted with platelet-specific gpIb monoclonal antibody (mAb) 142.11 (IgG114), CD40 mAb G28-5 (IgG1; American Type Culture Collection, Rockville, MD), or mAb 89 (IgG115), CD154 mAb TRAP1 (IgG116), or isotype control mAb MOPC-21 (IgG1; Sigma). Platelets were washed and stained with fluorescein isothiocyanate–conjugated goat anti–mouse polyclonal antibodies (Jackson, West Grove, PA). Platelets were identified by forward- and side-scatter and the expression of gpIb. For each determination, at least 10 000 platelets were analyzed on a FACScalibur (Becton Dickinson, Heidelberg, Germany). Human umbilical vein endothelial cells (HUVECs) were stained with ICAM-1 mAb BBIG-I1 (R&D, Minneapolis, MN), E-selectin mAb H18/7 (American Type Culture Collection), and VCAM-1 mAb VIII-6G10 (American Type Culture Collection).
Immunoprecipitation and Western blotting
Peripheral blood mononuclear cells (PBMCs) were isolated from human blood of healthy donors by centrifugation over a Ficoll density gradient (Biochrom, Berlin, Germany). Platelets (1 × 109) and PBMCs (2 × 107) were lysed in NP-40 buffer (150 mM NaCl, 1 mM EDTA, 1% [vol/vol] Nonidet P-40, 50 mM Tris/HCl, pH 8.0) containing the protease inhibitors phenylmethylsulfonyl fluoride, leupeptin A, pepstatin, and aprotinin (all from Boehringer Mannheim). Samples were precleared using Sepharose-4B beads (Pharmacia), reacted with glycine, and immunoprecipitated using mAb TRAP216 or mAb G28-5 directly coupled to Sepharose-4B beads. To analyze the binding of sCD154 to CD40, supernatants of unstimulated and activated platelets (0.2 U/mL thrombin for 1 to 24 hours) were precleared 2 times with Protein A beads (Pharmacia), and sCD154 was immunoprecipitated using a chimeric CD40-Ig fusion protein17 (generously provided by Dr Kurrle, Behring-Werke, Marburg, Germany) and Protein A beads. Immunoprecipitates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 12% gel) under reducing conditions, blotted onto a polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA), and stained with specific antisera using the Western Light Plus-Kit and CDP-Star as substrate (Tropix, Bedford, MA). Generation of the anti-CD154 serum has been described before.18 The anti-CD40 serum was obtained by immunizing rabbits with the recombinant extracellular domain of human CD40.
Determination of sCD154 release
For the determination of sCD154 levels, samples of activated platelets were routinely centrifuged at 2000g for 5 minutes, after experiments with additional ultracentrifugation (4 hours at 250 000g) excluded any influence on the values obtained. sCD154 levels in the supernatants were determined using a specific enzyme-linked immunosorbent assay (ELISA).16 Briefly, 96-well plates (Maxisorb; Nunc, Roskilde, Denmark) were preincubated with mAb LL2 (kindly provided by Dr Brière, Schering-Plough, Dardilly, France), the wells were washed with phosphate-buffered saline (PBS) containing 0.05% Tween 20, and the samples were applied after dilution in Tyrodes/HEPES buffer (washed platelets) or PBS/1% BSA (PRP). Following incubation with peroxidase (POD)–labeled mAb TRAP1, the assay was developed with 3,3′,5,5′ tetramethylbenzidine. The signal obtained with 1 ng/mL of a soluble chimeric recombinant mIL-4/hCD154 reagent (kindly provided by Dr Kurrle, Behring-Werke) was arbitrarily defined as 1 U/mL of sCD154.
Analysis of platelet phosphoproteins
Aggregation assay
Aggregation assays were performed in a Kontron Uvikon 930 photometer (Kontron, Watford, United Kingdom) equipped with a stirring device, a heated cuvette holder, and time-driven recording of transmission values. Platelets were adjusted to 2 × 108in plasma or Tyrodes/HEPES buffer, 0.3% BSA, and 2 mM CaCl2; both preparations gave identical results. Collagen (Nycomed, Munich, Germany), epinephrine, platelet-activating factor (PAF), and adenosine 5′-diphosphate (ADP) (all from Sigma) were used as agonists. Stimulation of platelets in buffered solution with ADP was performed in the presence of 0.5 mg/mL fibrinogen and 0.2 U/mL apyrase (both from Sigma). After the measurement of aggregation (10 minutes), the samples were further incubated for 50 minutes at 37°C without stirring to achieve optimal release of sCD154, then centrifuged at 2000g for 5 minutes, and the concentration of sCD154 was determined in the platelet-free supernatant. In some experiments, anti-CD40 mAb G28-5 or control mAb MOPC-21 was added to the platelet suspension at various concentrations (1 to 10 μg/mL) 20 minutes before or together with the above-mentioned agonists. In some experiments, goat anti–mouse Ig serum (Nordic Immunological Lab, Netherlands) was added at 1:150 dilution to induce cross-linking of mAb G28-5 on the platelet surface. Finally, all types of experiments were performed with platelets isolated by a different procedure19 and analyzed in an aggregometer (Platelet Aggregation Profiler, Model PAP-4; Bio/Data, Horsham, PA).
Separation of blood components for sCD154 release
Whole blood from healthy donors was collected into 4 mM EDTA, and cell counts were determined with a hemocytometer. Centrifugation at 150g for 20 minutes yielded PRP. Platelet-poor plasma was generated by centrifugation of PRP at 1300g, followed by centrifugation at 4000g, for 15 minutes each. Nucleated cells were separated from whole blood by Ficoll-Hypaque centrifugation. The interphase containing lymphocytes and monocytes and the pellet containing granulocytes and erythrocytes were collected separately and washed 3 times at 150g to remove contaminating platelets. Cells were resuspended in the original concentrations in platelet-poor plasma. Samples of each cell preparation were incubated at 37°C, and clotting was induced by the addition of 20 mM calcium or 0.2 U/mL human thrombin. After 1 hour, cell-free supernatants were collected and analyzed for sCD154 by ELISA.
Isolation and activation of endothelial cells
HUVECs were isolated by collagenase treatment.21Cells were cultured in endothelial cell growth medium with endothelial cell growth supplement (PromoCell, Heidelberg, Germany). HUVECs were used after the second and third passage and were cocultured with supernatants of activated platelets (0.2 U/mL thrombin for 1 hour). Furthermore, HUVECs were incubated with CD154 myeloma transfectant P3xTB.A74 at a ratio of 1:5, or reacted with sCD154 present in serum-free culture supernatant of the myeloma transfectant P3xTB.A7,4 or a combination of both CD154 transfectant and sCD154. In the sCD154-containing supernatants, any residual membrane fragments were removed by centrifugation at 1000g for 30 minutes and at 250 000g for 4 hours. After coculture, HUVECs were dislodged with PBS containing 5 mM EDTA and analyzed by flow cytometry.
Immunohistology
Frozen sections (8 μm) of fresh postmortem human venous thrombi were fixed with acetone for 10 minutes, incubated with the mAb TRAP1, G28-5, 142.11, WAPS 12.2 (anti–P-selectin; American Type Culture Collection), or isotype control mAb MOPC-21, and stained using the alkaline phosphatase antialkaline phosphatase (APAAP) technique (Dako, Glostrup, Denmark) with new fuchsin as chromogen. Counterstaining was performed with Mayer hematoxylin (Sigma). Artificial thrombi were generated in parallel by activating PRP with 0.2 U/mL human thrombin, immediately followed by vortexing. After incubation for up to 24 hours at 37°C, the artificial thrombi were snap-frozen, and cryosections were stained for CD154, gpIb, P-selectin, or CD40 using the tyramide signal amplification system (NEN Life Science Products, Boston, MA). AEC (3-amino-9-ethylcarbazole) was used as chromogen.
Results
CD40 is constitutively expressed on the surface of platelets
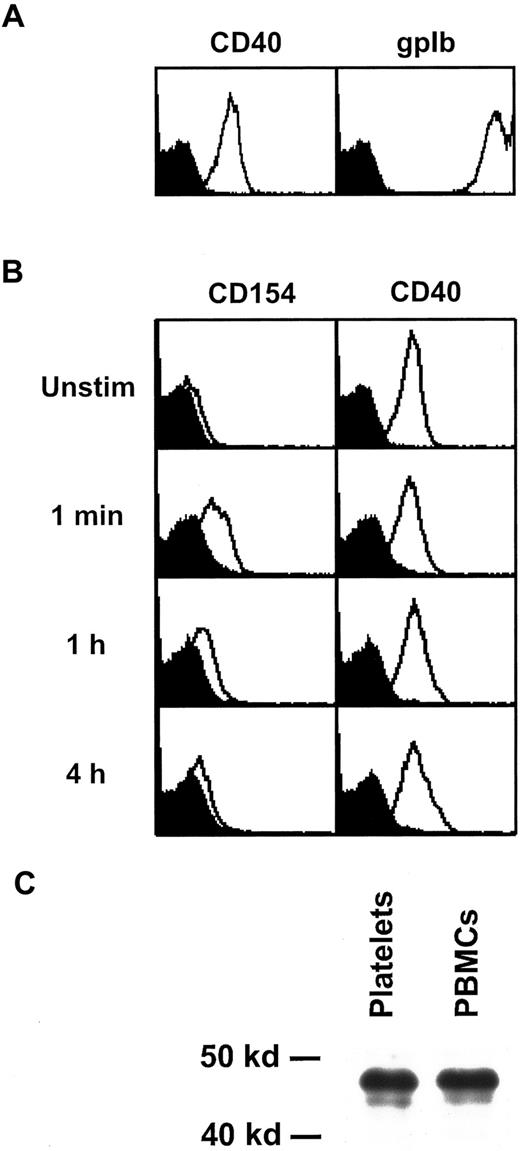
In the course of our studies on platelet activation, we noticed that platelets express substantial amounts of CD40 on their cell surface. Figure 1A shows flow cytometry profiles of unstimulated platelets stained with the CD40-specific mAb G28-5 and, for comparison, with mAb 142.11, which recognizes the platelet marker gpIb. To assess the levels of CD40 expression on activated platelets, we stimulated platelets with thrombin for up to 4 hours and analyzed them by flow cytometry using expression of CD154 as a positive control. As demonstrated earlier,12 CD154 is expressed on the platelet surface within seconds of activation, after which its levels gradually decline (Figure 1B). In contrast to this activation-dependent expression of CD154, no significant changes in CD40 levels were noticed in a series of experiments (Figure 1B). Similar results for CD40 expression on resting or activated platelets were obtained using mAb 89, another CD40-specific reagent (results not shown). To compare the CD40 molecule expressed on platelets with CD40 on B cells and monocytes, we immunoprecipitated CD40 from lysates of platelets or PBMCs and subjected them to Western blotting using a CD40-specific antiserum. An identical protein band of the expected size of 48 kd was detected in both samples (Figure 1C). Taken together, these data demonstrate that human platelets constitutively express CD40.
Expression and structure of platelet CD40.
(A) Unstimulated platelets were analyzed by flow cytometry for expression of CD40 and gpIb. (B) Washed platelets were stimulated for the indicated time periods with 0.2 U/mL thrombin and analyzed for expression of CD154 and CD40. The background signal obtained with the isotype control antibody is shown in black. (C) CD40 was immunoprecipitated from lysates of platelets and PBMCs with mAb G28-5 and detected by Western blotting with a CD40-specific antiserum. Unstim indicates unstimulated.
Expression and structure of platelet CD40.
(A) Unstimulated platelets were analyzed by flow cytometry for expression of CD40 and gpIb. (B) Washed platelets were stimulated for the indicated time periods with 0.2 U/mL thrombin and analyzed for expression of CD154 and CD40. The background signal obtained with the isotype control antibody is shown in black. (C) CD40 was immunoprecipitated from lysates of platelets and PBMCs with mAb G28-5 and detected by Western blotting with a CD40-specific antiserum. Unstim indicates unstimulated.
Interaction of CD154 with CD40 on the cell surface of activated platelets leads to cleavage of CD154
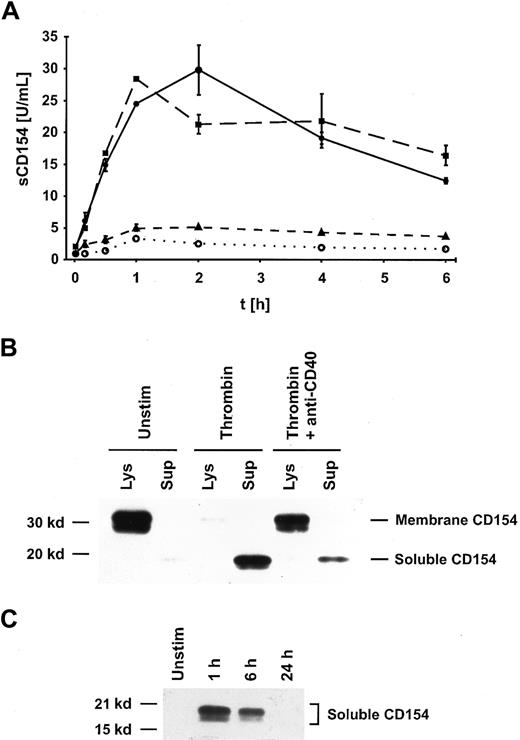
On T cells, the interaction of CD154 with CD40 on B cells or dendritic cells induces a rapid down-regulation of membrane-bound CD154,22,23 and this process is accompanied by the release of a soluble 18-kd trimeric form of CD154 (sCD15423), which can be measured using a specific ELISA.16 To determine the mechanism of the observed down-regulation of CD154 following its initial expression on platelets (Figure 1B), we measured whether sCD154 is released into the supernatant. Platelets were incubated with 0.2 U/mL thrombin for up to 6 hours, and the amount of sCD154 detectable in the supernatants was determined. A substantial release of sCD154 (up to 50 U/mL of sCD154/2 × 108 platelets in 1 mL) could be measured within 1 to 2 hours of platelet activation, after which the levels of detectable sCD154 gradually declined (Figure2A).
Interaction of CD154 with CD40 on the cell surface of activated platelets leads to cleavage of CD154.
(A) Platelets were either left unstimulated (○) or were activated with 0.2 U/mL thrombin for the indicated times, and the concentration of sCD154 in the supernatants was determined using a specific ELISA. The data are shown as mean values ± SD of duplicates and are representative of 4 independent experiments. The low background levels of sCD154 observed in unstimulated platelets probably reflect a minimal activation of platelets through the isolation and incubation procedures. ●, activated; ▪, activated plus mAb MOPC-21; ▴, activated plus mAb G28-5. (B) CD154 was immunoprecipitated from lysates and supernatants of unstimulated or activated (0.2 U/mL thrombin for 1 hour) platelets with mAb TRAP2 and detected by Western blotting with a CD154-specific antiserum. In parallel, platelet activation was performed in the presence of anti-CD40 mAb G28-5 (10 μg/mL). Unstim indicates unstimulated; Lys, lysate; Sup, supernatant. (C) Platelets were either left unstimulated or activated for the indicated time periods, and sCD154 was immunoprecipitated with a chimeric CD40-Ig reagent. The immunoprecipitate was subjected to SDS-PAGE and detected by Western blotting with a CD154-specific antiserum. Unstim indicates unstimulated.
Interaction of CD154 with CD40 on the cell surface of activated platelets leads to cleavage of CD154.
(A) Platelets were either left unstimulated (○) or were activated with 0.2 U/mL thrombin for the indicated times, and the concentration of sCD154 in the supernatants was determined using a specific ELISA. The data are shown as mean values ± SD of duplicates and are representative of 4 independent experiments. The low background levels of sCD154 observed in unstimulated platelets probably reflect a minimal activation of platelets through the isolation and incubation procedures. ●, activated; ▪, activated plus mAb MOPC-21; ▴, activated plus mAb G28-5. (B) CD154 was immunoprecipitated from lysates and supernatants of unstimulated or activated (0.2 U/mL thrombin for 1 hour) platelets with mAb TRAP2 and detected by Western blotting with a CD154-specific antiserum. In parallel, platelet activation was performed in the presence of anti-CD40 mAb G28-5 (10 μg/mL). Unstim indicates unstimulated; Lys, lysate; Sup, supernatant. (C) Platelets were either left unstimulated or activated for the indicated time periods, and sCD154 was immunoprecipitated with a chimeric CD40-Ig reagent. The immunoprecipitate was subjected to SDS-PAGE and detected by Western blotting with a CD154-specific antiserum. Unstim indicates unstimulated.
When the same experiment was performed in the presence of anti-CD40 mAb G28-5, the release of sCD154 was essentially abrogated, whereas the presence of a control antibody was without effect (Figure 2A). These experiments indicate that the interaction of CD154 with CD40 triggers the release of CD154 from the cell surface, probably by the action of a membrane-bound protease.
The release of CD154 from the platelet surface is the sole mechanism for the down-regulation of CD154
The presence of membrane-bound CD154 and sCD154 in platelets and supernatants was determined in the same experimental setup as used for the measurement of sCD154 release, except that platelet aggregation was induced by stirring. With unstimulated platelets, immunoprecipitation followed by Western blotting revealed the presence of transmembrane CD154 (33 kd and 28 kd) in the platelet lysate and gave no signal for the 18-kd sCD154 in the supernatant (Figure 2B). Upon activation of platelets by 0.2 U thrombin for 1 hour, the signal for the transmembrane form of CD154 almost fully disappeared and sCD154 became detectable in the supernatant (Figure 2B). The presence of blocking antibodies against CD40 (mAb G28-5) or against CD154 (mAb TRAP1) largely prevented the transformation of the membrane-form of CD154 to sCD154 after thrombin activation (Figure 2B and not shown). These results confirm the conclusions reached from the experiments measuring sCD154 in the supernatants. The complete disappearance of the transmembrane form of CD154 in activated platelets indicates that the CD154 molecule is not partly internalized, as observed in T cells, but is fully released as sCD154 into the extracellular milieu. The released sCD154 can bind to CD40, as determined by immunoprecipitation with a recombinant soluble CD40-Ig reagent (Figure 2C). The amount of immunoprecipitable sCD154 in the platelet supernatants decreased over time with an approximate half-life of 6 hours (Figure 2C), probably because of the inherent instability of the trimeric sCD154 (see “Discussion”).
Agonists inducing sCD154 release
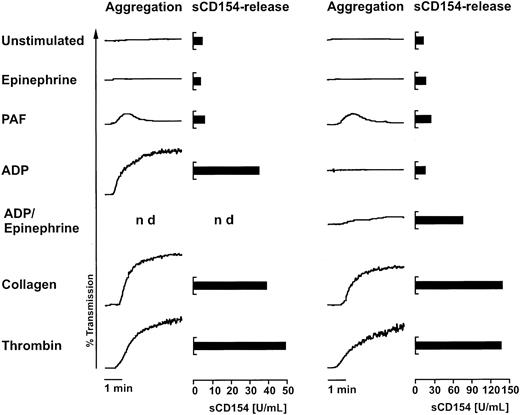
In the next series of experiments, we asked whether platelet agonists other than thrombin lead to cell-surface CD154 expression and sCD154 release. With the exception of thrombin, these agonists require stirring of platelets to exert their activation potential, and some of them irreversibly aggregate platelets. The aggregated platelets cannot be analyzed by flow cytometry, and therefore we previously could not determine whether CD154 is expressed on the platelet surface following activation by these agents. The discovery of the sCD154 release phenomenon allowed us to examine this issue. To this end, platelets were activated in the standard aggregometry assay, and the levels of sCD154 in the sample supernatants were determined 1 hour later. Addition of epinephrine, which did not induce aggregation, or addition of PAF, which induced only a slight and reversible aggregation, did not lead to release of sCD154 above the background levels observed with unstimulated platelets (Figure 3). In clear contrast, the addition of collagen or thrombin to stirred platelets triggered strong aggregation and resulted in a substantial release of sCD154 (Figure 3). The response to ADP and ADP plus epinephrine showed substantial donor variability; in responding platelet preparations, a release of sCD154 could be observed (Figure3). Although strong platelet aggregation in the experiments was usually accompanied by the release of sCD154, the 2 events appear not to be causally linked because thrombin activation of platelets without stirring gave no overt aggregation but nevertheless resulted in CD154 expression and substantial sCD154 release (Figures 1 and 2A). In summary, the aggregation experiments indirectly determined that not only thrombin, but also other strong platelet agonists, lead to the expression of CD154 on the platelet surface.
Agonists inducing sCD154 release.
Purified platelets in buffered solution were activated with 10 μM epinephrine, 1 μM PAF, 10 μM ADP, a combination of epinephrine and ADP (10 μM each), 10 μg/mL collagen, or 0.2 U/mL human thrombin. After the aggregation assay, the samples were incubated without stirring for an additional 50 minutes before the concentration of sCD154 was determined in the respective supernatants with an ELISA. Results are shown for aggregation experiments with 2 different donors to demonstrate the observed variability in the response to ADP. The data are representative of 6 independent experiments.
Agonists inducing sCD154 release.
Purified platelets in buffered solution were activated with 10 μM epinephrine, 1 μM PAF, 10 μM ADP, a combination of epinephrine and ADP (10 μM each), 10 μg/mL collagen, or 0.2 U/mL human thrombin. After the aggregation assay, the samples were incubated without stirring for an additional 50 minutes before the concentration of sCD154 was determined in the respective supernatants with an ELISA. Results are shown for aggregation experiments with 2 different donors to demonstrate the observed variability in the response to ADP. The data are representative of 6 independent experiments.
The central function of CD40 on the surface of platelets is the down-regulation of expressed CD154
Our experiments demonstrated that interaction of CD154 with CD40 is required for cleavage of CD154 (Figure 2). Thus, CD40 mediates a signal leading to the proteolytic cleavage of CD154. In a series of experiments, we sought to determine whether CD40 has additional effects on the function of platelets. Experiments designed to detect any blocking effects of mAb G28-5 on platelet aggregation triggered by various platelet agonists (for the setup of these experiments, see Figure 3) failed to reveal any effects (data not shown). In addition, we examined the phosphorylation pattern of 32P-labeled platelet proteins after incubation with mAb G28-5, which is known to induce protein phosphorylation in B cells,24 either in the presence or absence of optimal amounts of thrombin. Again, we could not observe any significant effects. These experiments led us to the conclusion that the interaction between CD154 and CD40, apart from inducing CD154 cleavage, does not participate in platelet activation and is not required for platelet aggregation.
Platelets express CD154 in the physiologic blood-clotting reaction
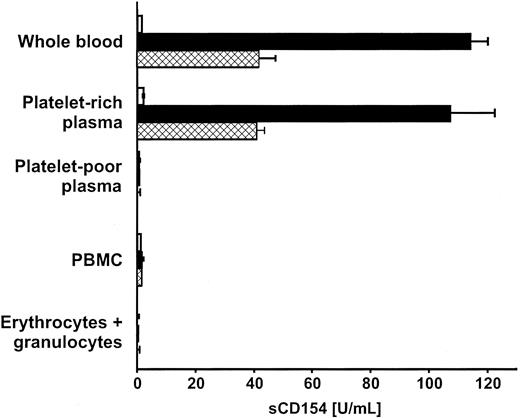
Our experiments predicted that aggregating platelets in clotting blood would release sCD154. To test this prediction, blood samples collected in EDTA were coagulated by the addition of calcium or thrombin, and sCD154 was measured in aggregate-free serum. Up to 110 U/mL sCD154 was found within 1 hour after induction of blood coagulation, whereas virtually no sCD154 was detected without addition of the agonists (Figure 4). Essentially the same amounts of sCD154 were released when PRP was prepared from the identical whole-blood samples and assayed in parallel (Figure 4). Only background sCD154 signals were obtained with platelet-poor plasma or with physiologic concentrations of PBMCs, erythrocytes, or granulocytes suspended in platelet-poor plasma (Figure 4). In addition to the clotting experiments, we immunostained cytocentrifuged PBMCs of healthy donors for the presence of intracellular or extracellular CD154 and obtained no signal (data not shown). Taken together, these data allow us to conclude that CD154 is expressed and later released by platelets in clotting blood and that platelets are the only source of sCD154 in this reaction. Inherently, these results also reveal that platelets are the predominant source of CD154 in the vascular system.
Expression of CD154 and subsequent sCD154 release occur in the physiologic blood-clotting reaction.
Whole blood collected in EDTA, or cellular blood components obtained from the same blood samples and adjusted in autologous plasma to physiologic concentrations, were subjected to the clotting reaction by addition of 20 mM calcium (■) or 0.2 U/mL human thrombin (▩) for 1 hour at 37°C. sCD154 concentrations were determined in cell-free supernatants by ELISA. ■, unstimulated. Experiments were performed in triplicate; data are shown as mean ± SD and are representative of 3 independent experiments.
Expression of CD154 and subsequent sCD154 release occur in the physiologic blood-clotting reaction.
Whole blood collected in EDTA, or cellular blood components obtained from the same blood samples and adjusted in autologous plasma to physiologic concentrations, were subjected to the clotting reaction by addition of 20 mM calcium (■) or 0.2 U/mL human thrombin (▩) for 1 hour at 37°C. sCD154 concentrations were determined in cell-free supernatants by ELISA. ■, unstimulated. Experiments were performed in triplicate; data are shown as mean ± SD and are representative of 3 independent experiments.
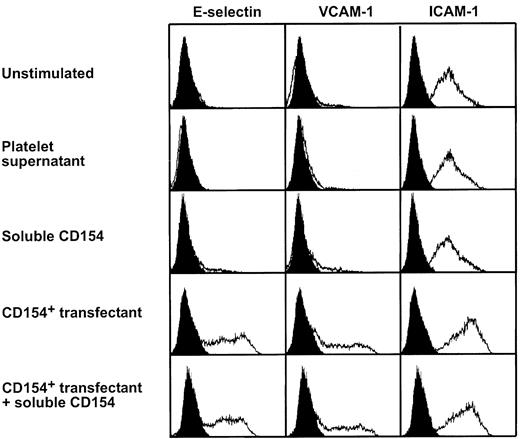
Soluble CD154 does not elicit an inflammatory response in endothelial cells
We have previously demonstrated that the membrane-bound form of CD154 on platelets induces a significant inflammatory reaction of endothelial cells expressing CD40.12 Because sCD154 is capable of binding to CD40 (Figure 2C), we sought to determine whether sCD154 has similar biologic properties. To this end, HUVECs were cultured for 4 hours in medium alone, in supernatants from thrombin-activated platelets, or in supernatants from a CD154-expressing transfectant containing high amounts of sCD154 (1000 U/mL). In parallel, HUVECs were cocultured with a CD154 transfectant (Figure 5). As reported previously,12 the transmembrane form of CD154 potently induced the expression of E-selectin and VCAM-1 in HUVECs and up-regulated ICAM-1 (Figure 5). In contrast, supernatants containing sCD154, either alone (Figure 5) or in combination with suboptimal amounts of TNF-α (not shown), did not exert any effects on the expression of the adhesion molecules (Figure 5), and also failed to induce the synthesis of IL-8 (not shown). Furthermore, coculture of the CD154 transfectant with HUVECs in the presence of 1000 U/mL sCD154 did not modulate the biologic effects of membrane-bound CD154 (Figure 5), suggesting a substantially lower affinity of sCD154 to CD40. Taken together, these experiments demonstrate that the soluble form of CD154, in contrast to the membrane-bound form, does not elicit an inflammatory response of endothelial cells.
Soluble CD154 does not induce an inflammatory response of endothelial cells.
HUVECs were cultured for 4 hours in medium alone, in a supernatant from thrombin-activated platelets (corresponding to 2 × 108platelets/mL, containing 40 U/mL sCD154), or in a supernatant containing 1000 U/mL sCD154. In parallel, HUVECs were cocultured with the CD154 transfectant P3xTB.A7 alone, or cocultured with the CD154 transfectant P3xTB.A7 in the presence of 1000 U/mL sCD154. Thereafter, the HUVECs were dislodged from the culture dishes and analyzed for expression of the adhesion molecules E-selectin, VCAM-1, and ICAM-1 by flow cytometry. The effects observed with the CD154 transfectant could be fully blocked with CD154-specific mAb TRAP1 (not shown and Henn et al12).
Soluble CD154 does not induce an inflammatory response of endothelial cells.
HUVECs were cultured for 4 hours in medium alone, in a supernatant from thrombin-activated platelets (corresponding to 2 × 108platelets/mL, containing 40 U/mL sCD154), or in a supernatant containing 1000 U/mL sCD154. In parallel, HUVECs were cocultured with the CD154 transfectant P3xTB.A7 alone, or cocultured with the CD154 transfectant P3xTB.A7 in the presence of 1000 U/mL sCD154. Thereafter, the HUVECs were dislodged from the culture dishes and analyzed for expression of the adhesion molecules E-selectin, VCAM-1, and ICAM-1 by flow cytometry. The effects observed with the CD154 transfectant could be fully blocked with CD154-specific mAb TRAP1 (not shown and Henn et al12).
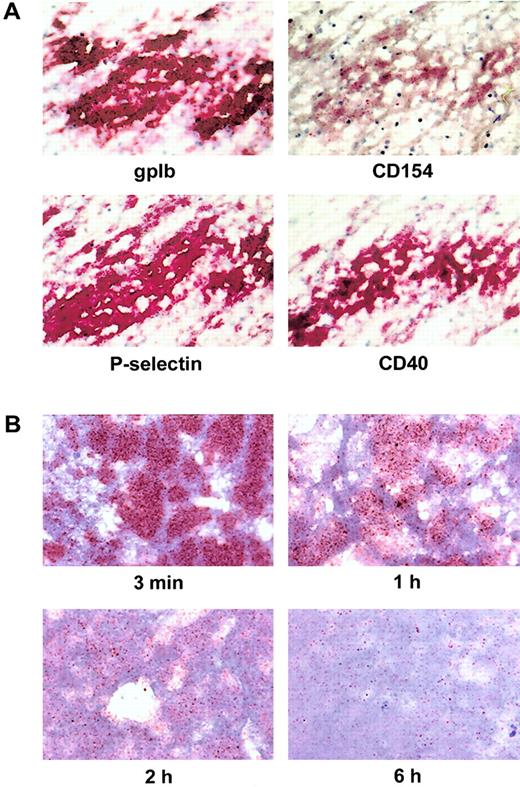
Intravital expression of CD154 and CD40 in human thrombi
To assess the intravital expression of CD154 and CD40 in thrombi, we stained serial sections of human venous thrombi for CD154, CD40, platelet marker gpIb, and platelet activation marker P-selectin (Figure6A). Nuclear staining revealed numerous leukocytes in the thrombus. The staining for gpIb clearly marked the area of platelet accumulation in the thrombus and coincided with the expression of P-selectin and CD40 (Figure 6A). In contrast, the number of CD154+ platelets was substantially lower. In many areas of the thrombus, CD154 expression was in fact restricted to single platelets (Figure 6A). To assess the expression of CD154 in a thrombus under controlled conditions, we generated artificial thrombi in vitro and analyzed sections from these thrombi at various time points. Sections obtained after 3 minutes gave significant signals when staining for CD154 (Figure 6B). Over the next few hours, the signals decreased substantially, and only relatively few CD154+platelets were observed after 6 hours (Figure 6B). Thus, the histology results obtained with intravitally formed thrombi and also with in vitro generated thrombi support the concept of a quick initial expression and function of platelet CD154, followed by gradual inactivation through coexpressed CD40 in the later phases of thrombus formation.
Expression levels of gpIb, P-selectin, CD154, and CD40 on platelets in human thrombi.
(A) Expression of CD154 and other platelet markers in intravitally formed thrombi. Consecutive serial cryostat sections of fresh venous thrombi were immunostained for the platelet-specific marker gpIb, the activation marker P-selectin, CD154, and CD40 by the APAAP technique. Shown is a characteristic result. (B) Time course of CD154 expression in thrombi generated in vitro. Thrombi were generated by addition of 0.2 U/mL thrombin to PRP and vortexing. After the indicated time periods, the thrombi were snap-frozen, and consecutive sections were immunostained for the expression of CD154 and other platelet markers. Signal obtained for CD154 is shown at 3 minutes, 1 hour, 2 hours, and 6 hours using the tyramide amplification system. The immunostaining patterns of gpIb, P-selectin, and CD40 did not change significantly over the time examined (not shown). In all experiments, counterstaining was performed with hematoxylin; original magnification was 600 ×.
Expression levels of gpIb, P-selectin, CD154, and CD40 on platelets in human thrombi.
(A) Expression of CD154 and other platelet markers in intravitally formed thrombi. Consecutive serial cryostat sections of fresh venous thrombi were immunostained for the platelet-specific marker gpIb, the activation marker P-selectin, CD154, and CD40 by the APAAP technique. Shown is a characteristic result. (B) Time course of CD154 expression in thrombi generated in vitro. Thrombi were generated by addition of 0.2 U/mL thrombin to PRP and vortexing. After the indicated time periods, the thrombi were snap-frozen, and consecutive sections were immunostained for the expression of CD154 and other platelet markers. Signal obtained for CD154 is shown at 3 minutes, 1 hour, 2 hours, and 6 hours using the tyramide amplification system. The immunostaining patterns of gpIb, P-selectin, and CD40 did not change significantly over the time examined (not shown). In all experiments, counterstaining was performed with hematoxylin; original magnification was 600 ×.
Discussion
We have shown earlier that human platelets express preformed CD154 on the cell surface within a very short period of time after activation by thrombin.12 Furthermore, we have demonstrated that platelet-bound CD154 induces an inflammatory reaction of endothelial cells via CD40.12 Our present work indicates that not only thrombin, but also other strong platelet agonists, can trigger platelets to express CD154 on the cell surface. Together with observations on CD154 expression in intravital thrombi and in vitro generated thrombi, our data indicate a link between the formation of a thrombotic plug and the immediate generation of a local inflammatory signal in the affected vessel in vivo.
Another essential finding of our present study was the detection of CD40, the receptor for the CD154 molecule, on the surface of resting platelets. Quite conspicuously, platelets are thus capable of coexpressing CD154 and its receptor CD40. CD40 has been identified on several cell types in the immune and vascular systems, and its ligation by CD154 usually induces a marked activation of these cells.8 Although CD40 on platelets is indistinguishable from CD40 on other cells by a number of criteria, we found no evidence for a direct involvement of CD40 in the activation or aggregation of platelets. Apparently, binding of CD40 by its ligand is not essential for the hemostatic function of platelets, and this conclusion is indirectly supported by the absence of an overt bleeding disorder in “hyper-IgM” patients lacking functional CD154 molecules.
We observed that the interaction of CD154 on stimulated platelets with the coexpressed CD40 receptor leads within minutes to hours to a proteolytic cleavage of CD154 and the release of sCD154, the soluble form of this molecule. The generated 18-kd sCD154 molecule is similar to sCD154 released from activated T cells16 and can also bind CD40 (Figure 2C). Interestingly, even high levels of sCD154 were not capable of inducing any inflammatory reaction of endothelial cells. These results fundamentally differ from the data with recombinant forms of soluble CD154 obtained by various investigators using endothelial cells in vitro9,10 or performing experiments in vivo.25 These stabilized forms of recombinant sCD154 clearly do not reflect the biology of natural sCD154. Natural sCD154, although capable of binding to CD40-Ig reagents, is relatively unstable. This conclusion reached from experiments using physical methods26 is supported by our finding of a relatively rapid decline of detectable sCD154 in our ELISA measurements, which is paralleled by the decrease of immunoprecipitable sCD154 (Figure 2). Taken together, our data indicate that cleavage of CD154 from the surface of activated platelets is a central mechanism limiting the inflammatory action of CD154 in the vascular system.
We have attempted to use the generation of sCD154 as a diagnostic indicator of platelet activation in vivo. To date, plasma samples from patients undergoing balloon angioplasty and patients with active systemic lupus erythematosus (SLE) were examined without positive results, and only patients receiving extracorporeal circulation exhibited increased sCD154 levels in the plasma (Henn et al, unpublished data). Our findings contradict a recent report on the presence of substantial amounts of sCD154 in the plasma of SLE patients,27 but the discrepancy can easily be explained by the failure of the authors to remove platelets from the plasma samples. The difficulty in measuring sCD154 levels in platelet-free plasma in various pathologic conditions may result from a high dilution of generated sCD154 in the circulation, which potentially could be compensated for by a very sensitive detection system. In vitro, however, the measurement of sCD154 allows easy monitoring of platelet activation, and this could be exploited in transfusion-medicine research.
Given our findings on the coexpression of CD154 and CD40 on platelets, the following scenario in vivo can be assumed. Once platelets are strongly activated in the vascular system, CD154 is rapidly expressed on the cell surface of aggregating platelets. As a result, platelet CD154 interacts with CD40 on the neighboring endothelial cells and at the same time with CD40 on monocytes trapped in the thrombus (Figure6). In both endothelial cells and monocytes, this interaction probably immediately triggers an inflammatory response. These CD154 molecules, which are not engaged by CD40 on other cell types, bind to CD40 coexpressed in the platelet aggregate. All of these interactions result over time in the transformation of the proinflammatory form of transmembrane CD154 into the biologically inactive, soluble form of the molecule. The generation of sCD154 takes minutes to hours in vitro, and a similar time scale can be assumed in vivo. The scant expression of CD154 observed in intravitally formed thrombi as well as the time course of the decline of CD154 detection in experimentally formed thrombi (Figure 6) seem to support the conclusions reached.
The tightly restricted action of platelet CD154 remarkably resembles the regulation of the molecule in activated T cells. There, CD154 is located intracellularly most of the time and, when translocated to the cell surface following a major histocompatibility complex–restricted stimulus, only briefly interacts with CD40 on partner cells (eg, B cells). On ligation to CD40, T-cell CD154 is either internalized22 or shed in a soluble form, with a major sCD154 product resulting from the cleavage between E112 and M113 (single-letter amino acid code).16 Despite the short duration of CD154 interaction with CD40 on cells of the immune system, the biologic effects of this interaction are impressive, one example being its pivotal role in the switch from IgM to IgG production by B cells.6 In fact, a very tight regulation of CD154 in the immune system is required because unduly prolonged exposure to a biologically effective form of CD154 leads to a massive inflammation or even triggers the development of lymphomas.28-30 The basic rules observed with T-cell CD154 are apparently also valid for platelet CD154. Thus, it can be assumed that the limited duration of CD154 expression on platelets prevents an excessive inflammation of the endothelium and of CD40+ cells in the vicinity of a hemostatic plug.
When compared with its structural and functional homologue TNF-α, platelet CD154 differs in several respects. One major difference is the rapidity of action. The preformed CD154 molecules exert their inflammatory action in the instant of the formation of a thrombus, whereas the de novo synthesis of TNF-α and other proinflammatory mediators requires several hours. Another important difference is the range of action. The proinflammatory effects of transmembrane CD154 are restricted to the area of platelet aggregation, whereas TNF-α is active in both its transmembrane and soluble forms.31 The inflammatory signal exerted by platelet CD154 is thus quick, powerful, uniform, local, and of limited duration. Only when the neighboring endothelial cells and possibly also monocytes start to secrete an array of inflammatory mediators in response to CD40 ligation by platelet CD1548 can the local inflammation be further (up-) regulated and sustained for a prolonged period of time.
The mechanism limiting CD154 function on platelets may not suffice in chronic vascular diseases such as atherosclerosis. There, aggregation of platelets on altered endothelium and plaque rupture are ever-recurring events in the formation of progressive lesions. Atherosclerotic plaques contain aggregated platelets expressing CD15412 in the vicinity of CD40-bearing macrophages and fibroblasts, cell types capable of synthesizing proinflammatory mediators and matrix proteases when triggered via CD40.32-34 It thus seems likely that platelet CD154 contributes to the inflammatory component of atherosclerosis, in which CD40 was shown to play a prominent role.35 Apart from the postulated involvement in chronic vascular disease, it can be assumed that platelet CD154 also participates in the generation of local inflammatory signals in acute thrombotic events such as heart and brain infarction. The involvement of platelet CD154 in predominantly inflammatory conditions of the vascular system cannot be assessed as yet. Given the high incidence of thrombosis in inflamed vessels, it seems likely that platelet CD154 interacts with CD40 on endothelial cells also under these conditions.
We thank Dr Michael Gräfe for the culture of endothelial cells and Dr Ioannis Anagnostopoulos for providing us with specimens of venous thrombi.
Supported by Deutsche Forschungsgemeinschaft grant Kr827/10-2 (R.A.K.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Richard A. Kroczek, Molecular Immunology, Robert Koch-Institute, Nordufer 20, 13353 Berlin, Germany; e-mail:kroczek@rki.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal