Abstract
Exposure of hematopoietic cells to DNA-damaging agents induces cell-cycle arrest at G1 and G2/M checkpoints. Previously, it was shown that DNA damage–induced growth arrest of hematopoietic cells can be overridden by treatment with cytokine growth factors, such as erythropoietin (EPO) or interleukin-3 (IL-3). Here, the cytokine-activated signaling pathways required to override G1 and G2/M checkpoints induced by γ-irradiation (γ-IR) are characterized. Using factor-dependent myeloid cells stably expressing EPO receptor (EPO-R) mutants, it is shown that removal of a minimal domain required for PI-3K signaling abrogated the ability of EPO to override γ-IR–induced cell-cycle arrest. Similarly, the ability of cytokines to override γ-IR–induced arrest was abolished by an inhibitor of PI-3K (LY294002) or by overexpression of dominant-negative Akt. Moreover, the ability of EPO to override these checkpoints in cells expressing defective EPO-R mutants could be restored by overexpression of a constitutively active Akt. Thus, activation of a PI-3K/Akt signaling pathway is required for cytokine-dependent suppression of DNA-damage induced checkpoints. Together, these findings suggest a novel role for PI-3K/Akt pathways in the modulation of growth arrest responses to DNA damage in hematopoietic cells.
Introduction
Exposure of mammalian cells to DNA-damaging agents triggers a variety of responses that include apoptotic cell death and cell-cycle arrest.1 Failure of DNA-damaged cells to commit to either of these pathways, before the restoration of DNA integrity, may contribute to tumorigenic development by allowing the accumulation of new mutations.2,3 Cellular determinants that predict whether cells will arrest or undergo apoptosis in the presence of genotoxic stress are not completely defined. Protection from DNA damage–induced apoptosis may be conferred by the expression of antiapoptotic proteins, such as Bcl-2 and Bcl-XL.4 Various hematopoietic cytokines induce the expression of antiapoptotic proteins, and this coincides with their ability to protect against DNA damage–induced cell death.5-7 In addition to the inhibition of apoptosis, growth factors acting through the hematopoietic cytokine receptor superfamily have also been shown to override the cell-cycle arrest response to DNA damage in hematopoietic cell lines.7However, the signaling pathways responsible for this ability to override DNA damage–induced cell-cycle checkpoints have not been defined.
The classical pathway coupling DNA damage with cell-cycle arrest involves up-regulation of p53 and its transcriptional targets. In response to DNA damage, p53 induces the expression of growth inhibitory genes, such as p21Cip1 and GADD45.8 However, the existence of p53-independent mechanisms resulting in cell-cycle arrest has been demonstrated in lymphoid cells derived from p53−/− mice.9 Similarly, hematopoietic cell lines lacking p53 protein arrest in the G1 and G2/M phases after treatment with ionizing irradiation.7 Thus, it remains unclear what mechanisms regulate the cell-cycle arrest response to DNA damage in hematopoietic cells. Further, it is not obvious how signaling pathways regulated by growth-promoting cytokines influence these G1 and G2/M cell-cycle checkpoints.
Members of the hematopoietic cytokine receptor superfamily include the receptors for erythropoietin (EPO) and interleukin-3 (IL-3).10 These receptors regulate a number of cellular functions in hematopoietic cell lineages, including growth and development. Receptors of this family function by activating one or more members of the Jak family of tyrosine kinases. For example, the receptor for EPO (EPO-R) associates with and activates Jak-2 through a cytoplasmic domain proximal to the membrane.11,12 This membrane-proximal domain of EPO-R is sufficient to mediate EPO-dependent proliferation and up-regulation of antiapoptotic genes in factor-dependent myeloid cell lines. The precise signaling pathways that mediate proliferative and antiapoptotic responses to EPO are undefined. However, it has been clearly demonstrated that EPO-R truncation mutants, lacking as many as 106 amino acids of the C-terminal domain (eg, EPO-R[H]), retain all activities required to inhibit cell death and to promote growth of factor-dependent myeloid cell lines under normal culture conditions.13-16 Notably, the EPO-R(H) truncation mutant lacks the domain responsible for EPO-dependent activation of Ras/Erk- and PI-3 kinase (PI-3K)–dependent signaling pathways. This domain contains a recruitment site for the p85 subunit of PI-3K at tyrosine 479 of EPO-R.17,18 Activated PI-3K phosphorylates inositol lipids resulting in the recruitment of pleckstrin homology (PH) domain-containing proteins to the membrane.19 In response to cytokines such as EPO and IL-3, the activation of PI-3K leads to activation of the PH domain-containing serine–threonine kinase, Akt.20-22 Alternatively, EPO activation of the Ras/Erk pathway occurs through the recruitment of adaptor proteins, including Shc, CrkL, and Ship1, to unidentified sites in the C-terminal tail of EPO-R.23-25 The ability of EPO-R(H) to support proliferation suggests that EPO activation of Ras/Erk and PI-3K/Akt pathways is dispensable to EPO-dependent cell growth under normal culture conditions.
We have previously shown that murine myeloid cell lines stably expressing wild-type EPO-R are resistant to both γ-IR–induced apoptosis and cell-cycle arrest when cultured in EPO or IL-3.7 In these cells, the apoptotic response to DNA damage could also be bypassed by the ablation of p53 protein or by the forced expression of Bcl-2 or Bcl-XL. However, all cells lacking this apoptotic response still arrested in both G1 and G2/M after γ-IR in the absence of cytokine treatment. Similarly, cells expressing EPO-R(H) remained viable but arrested in G1 and G2/M after treatment with γ-IR in the presence of EPO. Thus, activities associated with the C-terminal domain of EPO-R are dispensable to cell growth under normal culture conditions, but they are required for EPO to override DNA damage–induced cell-cycle checkpoints in G1 and G2/M. In the present study, we have shown that the ability of EPO and IL-3 to override DNA damage–induced growth arrest is dependent on their ability to activate PI-3K/Akt signaling pathways. These studies demonstrate that PI-3K/Akt signaling, which is normally dispensable to cytokine-dependent proliferation, is required for promoting cell-cycle progression in the face of DNA damage.
Materials and methods
Complemetary DNA constructs
The truncated EPO-R complementary DNA (cDNA), EPO-R(d465), was prepared by polymerase chain reaction (PCR) mutagenesis in which a stop codon was introduced after the codon for tyrosine 463 of the murine EPO-R cDNA.26 The truncated receptor, EPO-R(H), has been previously described.15 A point mutation converting Y479 of EPO-R to phenylalanine (EPO-R[Y479F]) was prepared by PCR mutagenesis.27 A cDNA encoding Bcl-XL28 was obtained by reverse transcription–PCR amplification from RNA obtained from murine myeloid DA3 cells. cDNAs encoding HA-tagged wild-type Akt, constitutively active Akt (myrAkt-HA), and dominant-negative Akt (HA-Akt[K179M]) have been described.29 All cDNAs were cloned into the pXM expression vector for stable expression in 32D cells.
Cell lines and culture conditions
Cells expressing wild-type EPO-R or various mutant EPO-Rs were generated by electroporation of 10 μg pXM expression vector containing EPO-R cDNA or mutant EPO-R cDNAs into 32D cells. Stable clones of 32D cells expressing either EPO-R(wt), EPO-R(H), EPO-R(d465), or EPO-R(Y479F) were selected for their ability to grow in 5 U/mL EPO. Cells stably expressing Bcl-XL, wild-type HA-Akt, myrAkt-HA, or HA-Akt(K179M) were obtained by cotransfecting 10 μg pXM expression vector plus 1 μg pSV2-Neo, followed by selection with 1 mg/mL G418. G418-resistant clones were screened by Western blot analysis for the expression of the appropriate constructs. All cell lines were maintained in RPMI 1640 plus 10% fetal bovine serum (FBS) and recombinant murine IL-3 (70 pg/mL). For stimulation experiments, cells were washed free of cytokine and were cultured in RPMI plus 10% FBS for 6 hours. In experiments involving inhibition of PI-3K, 10 μM LY294002 (Calbiochem, San Diego, CA) or dimethyl sulfoxide (DMSO; 0.2%) was added at the beginning of the 6-hour incubation. Subsequently, cells were treated with EPO or IL-3 or were left unstimulated for 8 minutes before lysis.
Western blot analysis
Cells were lysed in 0.5% NP-40, 10% glycerol, 50 mM Tris (pH 8.0), 0.1 mM EDTA, 150 mM NaCl, 0.1 mM Na3VO4, 50 mM NaF, 1 mM dithiothreitol (DTT), 0.1 mM phenylmethylsulfonyl fluoride, and 1 μM microcystin. Lysates were cleared of insoluble material by centrifugation at 4°C. Whole cell lysates (5 × 105 cell equivalents per lane) were resolved by SDS-PAGE and transferred to a polyvinylidene difluoride (PVDF) membrane, and membranes were probed with appropriate antisera or antibodies and were visualized by ECL (Amersham, Piscataway, NJ). Expression of HA-tagged Akt was detected using an anti-HA antibody (Roche Molecular Biochemicals, Indianapolis, IN) or an anti–human Akt1/PKB PH domain antiserum (Upstate Biotechnology, Lake Placid, NY). Erk phosphorylation was determined using an anti–phospho-Erk (P-Erk) antibody (Santa Cruz, Santa Cruz, CA). Stat-5 phosphorylation was determined using an anti–phospho-Stat-5A/B antibody (Upstate Biotechnology). Additionally, membranes were probed with an anti-Erk2 antiserum (Santa Cruz) to control for equal loading in each lane.
Cell-cycle analysis
Cells were collected, washed in phosphate-buffered saline, and resuspended (5 × 105 cells/mL) in RPMI 1640 medium containing 10% FBS and either IL-3 (1.4 ng/mL or 0.07 ng/mL), EPO (5 U/mL), or no cytokine. For experiments involving the inhibition of PI-3K, cells were cultured in 10 μM LY294002 or 0.2% DMSO immediately after resuspension. All cultures were incubated for 2 hours at 37°C, 5% CO2. Subsequently, parallel cultures were either exposed to 4 Gy γ-IR from a cesium (Cs) 137 source or left untreated. Cells were returned to a 37°C, 5% CO2incubator for 24 hours and were then assayed for viability and DNA content. Cell viability was determined by trypan blue exclusion. For cell-cycle analysis, cells were collected by centrifugation and resuspended at 1 × 106 cells/mL in propidium iodide (PI) staining buffer (0.1% sodium citrate, 0.1% Triton-X 100, and 50 μg/mL PI) and were treated with 1 μg/mL RNase at room temperature for 30 minutes. Cell-cycle histograms were generated after analysis of PI-stained cells by fluorescence-activated cell sorting (FACS) with a Becton Dickinson FACScan. For each culture, at least 1 × 104 events were recorded. Histograms generated by FACS were analyzed by ModFit Cell Cycle Analysis Software (Verity, Topsham, ME) to determine the percentage of cells, in each phase (G1, S, and G2/M).
Akt kinase assay
Akt kinase assays were performed in vitro using an Akt Kinase Assay Kit (catalog no. 9840; New England Biolabs, Beverly, MA). Akt was immunoprecipitated from whole cell lysates of stimulated 32D cells (2 × 106 cell equivalents) with immobilized anti-Akt antibodies. The immunoprecipitated Akt was subjected to a kinase reaction containing 25 mM Tris (pH 7.5), 5 mM β–glycerol-phosphate, 2 mM DTT, 0.1 mM Na3VO4, 10 mM MgCl2, 200 μM adenosine triphosphate, and 1 μg GSK-3α fusion protein. Subsequently, the GSK-3α fusion protein was resolved by SDS-PAGE and transferred to PVDF membranes, and the membranes were probed with an anti-phospho–GSK antibody and visualized using enhanced chemiluminescence (ECL; Amersham).
Results
Cytokine-treatment of 32D cells overrides a DNA damage–induced cell-cycle arrest
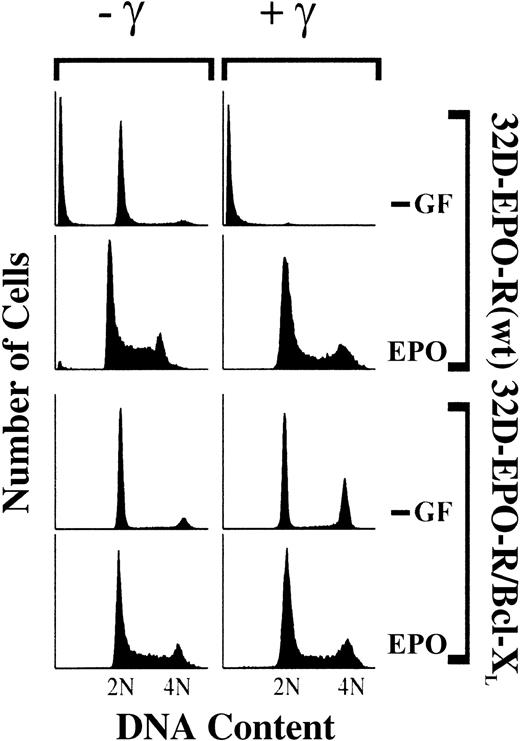
Factor-dependent 32D cells expressing wild-type EPO-R (32D–EPO-R[wt]) exhibit an asynchronous cell-cycle profile when cultured in EPO (Figure 1). When withdrawn from cytokine growth factors (−GF), these cells arrested largely in the G1 phase (77%) and much less in G2/M (5%). γ-IR of 32D–EPO-R(wt) cells resulted in complete cell death in the absence of cytokine, whereas irradiated cells treated with EPO remained viable and asynchronously growing. To confirm that cytokine treatment specifically overrides a DNA damage–induced growth arrest, 32D–EPO-R(wt) cells were stably transfected with a Bcl-XL expression construct to prevent DNA damage–induced cell death in the absence of cytokine treatment. Similar to parental cells, 32D–EPO-R/Bcl-XLcells proliferate asynchronously when cultured in EPO and arrest predominantly in G1 phase (77%) rather than in G2/M (13%) in the absence of cytokine (Figure 1). By contrast, irradiated 32D–EPO-R/Bcl-XL cells arrested in both G1 (54%) and G2/M (34%) phases in the absence of cytokine. Therefore, 32D cells arrest in G2/M specifically in response to DNA damage, not simply as a result of cytokine withdrawal. Moreover, this G2/M arrest is overridden in response to signals activated by EPO-R.
DNA damage specifically induces a G2/M-phase arrest in the absence of cytokine treatment.
32D cells expressing EPO-R(wt) or cells expressing both EPO-R(wt) and Bcl-XL (32D–EPO-R/BclXL) were cultured in medium lacking cytokine growth factors (−GF) or with 5 U/mL EPO. Subsequently, cultures were exposed to 4 Gy γ-IR (+γ) or were left untreated (−γ). Thirty-three hours after irradiation, cells were stained with PI and analyzed by FACS.
DNA damage specifically induces a G2/M-phase arrest in the absence of cytokine treatment.
32D cells expressing EPO-R(wt) or cells expressing both EPO-R(wt) and Bcl-XL (32D–EPO-R/BclXL) were cultured in medium lacking cytokine growth factors (−GF) or with 5 U/mL EPO. Subsequently, cultures were exposed to 4 Gy γ-IR (+γ) or were left untreated (−γ). Thirty-three hours after irradiation, cells were stained with PI and analyzed by FACS.
p85 recruitment site of EPO-R is required for EPO to override γ-IR–induced cell-cycle arrest
Previous studies have shown that activated receptors for EPO and IL-3 are coupled to a variety of signaling pathways, including the activation of Ras/Erk, PI-3K/Akt, and Stat-5. Truncation of the 106 C-terminal amino acids from EPO-R (EPO-R[H]) removes domains required for PI-3K/Akt and Ras/Erk activation and also abrogates the ability of EPO to override DNA damage–induced cell-cycle checkpoints.7 To further characterize the region of EPO-R that is necessary for preventing the γ-IR–induced cell-cycle arrest, a series of C-terminal truncated receptors was constructed. EPO-R(d465) lacks the 17 C-terminal amino acids of EPO-R, which includes a single tyrosine at position 479 (Figure 2A). In addition, a point mutant was prepared in which the reported PI-3K recruitment site at tyrosine 47917 was converted to phenylalanine (EPO-R[Y479F]). Each receptor construct was stably expressed in 32D cells.
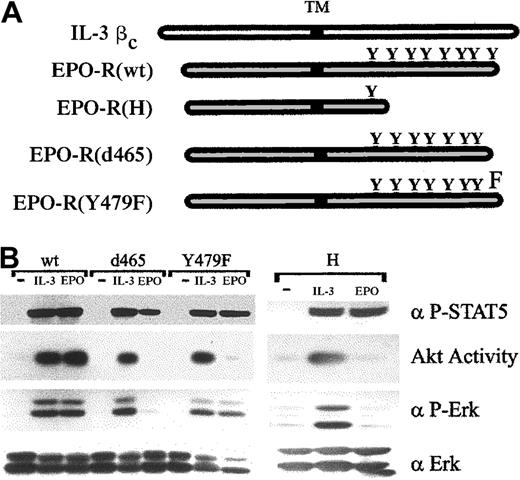
Signaling activities of wild-type and mutant cytokine receptors.
(A) Structures of wild-type and mutant receptors are diagrammed. Positions of the transmembrane domain (TM) and phosphorylatable tyrosine (Y) are indicated. (B) 32D cells stably expressing wild-type (wt) or mutated EPO-R were left unstimulated (−) or were stimulated with 1.4 ng/mL IL-3 or 5 U/mL EPO for 8 minutes. Akt catalytic activity was assayed from cell lysates in an in vitro kinase assay (see “Materials and methods”). Alternatively, whole cell lysates were Western blotted with anti–phospho-Erk (α P-Erk) or anti–phospho-Stat-5A/B (α P-STAT5) antibodies. As a control for loading, lysates were probed with anti-Erk2 (α Erk) antiserum.
Signaling activities of wild-type and mutant cytokine receptors.
(A) Structures of wild-type and mutant receptors are diagrammed. Positions of the transmembrane domain (TM) and phosphorylatable tyrosine (Y) are indicated. (B) 32D cells stably expressing wild-type (wt) or mutated EPO-R were left unstimulated (−) or were stimulated with 1.4 ng/mL IL-3 or 5 U/mL EPO for 8 minutes. Akt catalytic activity was assayed from cell lysates in an in vitro kinase assay (see “Materials and methods”). Alternatively, whole cell lysates were Western blotted with anti–phospho-Erk (α P-Erk) or anti–phospho-Stat-5A/B (α P-STAT5) antibodies. As a control for loading, lysates were probed with anti-Erk2 (α Erk) antiserum.
Before assessing their roles in overriding cell-cycle checkpoints, the signaling activities of each form of EPO-R were assessed through their ability to activate Akt and to phosphorylate Erk and Stat-5 in response to EPO (Figure 2B). In cells expressing EPO-R(wt), treatment with EPO resulted in activation of Akt, Erk, and Stat-5, as expected. By comparison, 32D–EPO-R(H), 32D–EPO-R(d465), and 32D–EPO-R(Y479F) contained little or no EPO-stimulated Akt activity, yet the ability to induce Stat-5 phosphorylation was retained. Interestingly, no EPO-induced Erk phosphorylation could be detected in 32D–EPO-R(d465) cells, whereas 32D–EPO-R(Y479F) cells were fully competent for this activity. The lack of EPO-induced activation of both Erk and Akt in 32D–EPO-R(d465) cells was similar to that observed in cells expressing the more severely truncated EPO-R(H) receptor. As controls, IL-3 induced the activation of Akt, Erk, and Stat-5 in all cell lines assayed, whereas none of these activities were observed in cells cultured in the absence of cytokine. These data indicate a dual role for the 17 C-terminal amino acids of EPO-R in the regulation of PI-3K and Ras/Erk pathways, and they confirm a specific requirement for tyrosine 479 in the regulation of PI-3K.
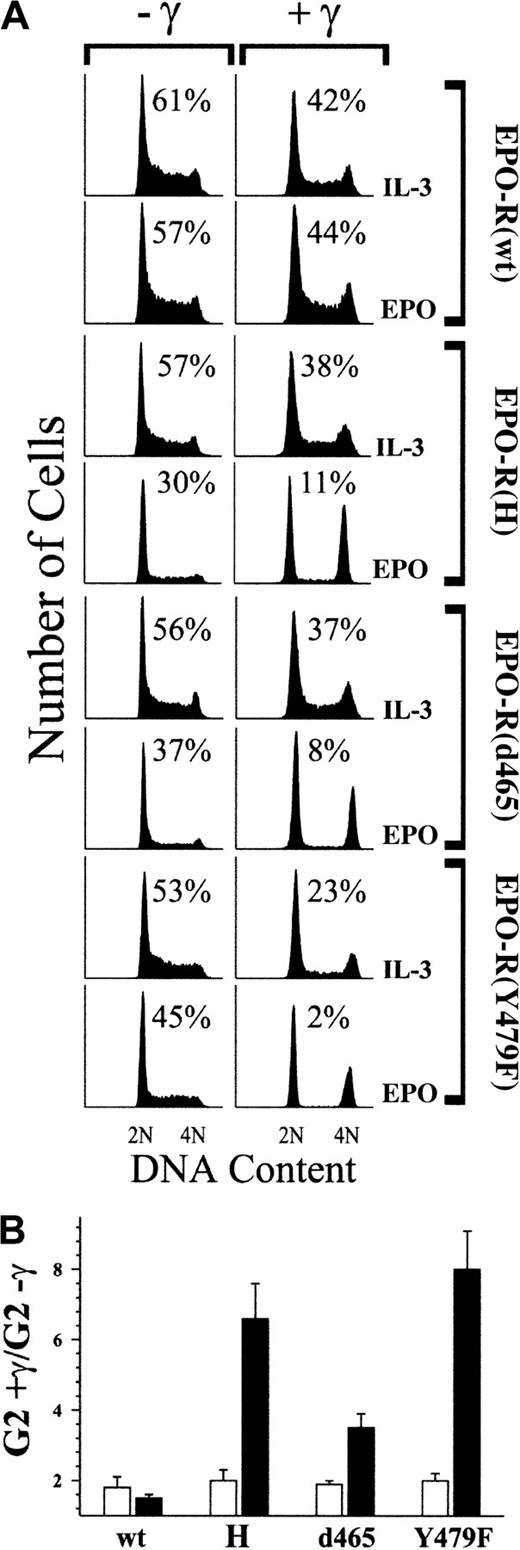
32D cell expressing the various EPO-R mutants were then tested for their ability to override DNA damage–induced cell-cycle arrest in response to EPO treatment. Each line was cultured in the presence of EPO and was exposed to 4 Gy γ-IR or was left unirradiated. Twenty-four hours after irradiation, the cell-cycle status of each culture was determined by flow cytometry. As a control for the activity of a wild-type cytokine receptor in each cell line, cells cultured in IL-3 were also examined. Similar to the experiment shown in Figure 1, all cell lines cultured in the absence of cytokine were nonviable after γ-IR, whereas unirradiated cultures in the absence of cytokine were largely viable yet arrested in G1 (data not shown). 32D–EPO-R(wt) cells maintained an asynchronous cell-cycle profile after exposure to γ-IR when cultured in EPO or IL-3 (Figure3A). A slight decrease in S phase and an increase in the G2/M population (less than 2-fold; Figure 3B) after γ-IR are indicative of a partial effect on cell-cycle progression, even in the presence of cytokine. In contrast, 32D–EPO-R(H), 32D–EPO-R(d465), and 32D–EPO-R(Y479F) cells were largely arrested in G1 and G2/M phases after exposure to γ-IR in the presence of EPO, as indicated by the near absence of S phase (Figure 3A) and a substantial increase in G2/M (4.5- to 8-fold) (Figure 3B). All cells cultured in IL-3 remained asynchronous, with only a modest increase in G2/M after γ-IR.
The p85 recruitment site of EPO-R is required for EPO-dependent suppression of γ-IR–induced cell-cycle arrest.
(A) 32D cells expressing wild-type (wt) or mutated EPO-R were cultured in medium that contained either 1.4 ng/mL IL-3 or 5 U/mL EPO. Subsequently, cultures were exposed to 4 Gy γ-IR (+γ) or were left untreated (−γ). Twenty-four hours after irradiation, cells were stained with PI and analyzed by FACS. Before PI staining, the viability of the cells was determined by trypan blue exclusion and was 94% or greater in all experiments. Percentage of S-phase cells is indicated for each culture. (B) Percentage of G2/M cells in γ-IR–treated cultures versus nonirradiated cultures shown in panel A was determined, and the fold increase in G2/M was calculated (G2+γ/G2−γ). Values presented represent the average of 3 independent experiments. Similar results were obtained with at least 3 independent clones of each cell type. ▪, EPO; ■, IL-3.
The p85 recruitment site of EPO-R is required for EPO-dependent suppression of γ-IR–induced cell-cycle arrest.
(A) 32D cells expressing wild-type (wt) or mutated EPO-R were cultured in medium that contained either 1.4 ng/mL IL-3 or 5 U/mL EPO. Subsequently, cultures were exposed to 4 Gy γ-IR (+γ) or were left untreated (−γ). Twenty-four hours after irradiation, cells were stained with PI and analyzed by FACS. Before PI staining, the viability of the cells was determined by trypan blue exclusion and was 94% or greater in all experiments. Percentage of S-phase cells is indicated for each culture. (B) Percentage of G2/M cells in γ-IR–treated cultures versus nonirradiated cultures shown in panel A was determined, and the fold increase in G2/M was calculated (G2+γ/G2−γ). Values presented represent the average of 3 independent experiments. Similar results were obtained with at least 3 independent clones of each cell type. ▪, EPO; ■, IL-3.
PI-3K activity is necessary for cytokines to override γ-irradiation–induced growth arrest
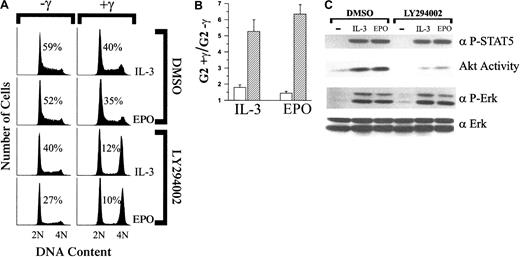
The inability of EPO to override DNA damage–induced growth arrest resulting from the mutation of tyrosine 479 implies a requirement for the activation of PI-3K. To confirm this requirement, the effects of γ-IR on cell-cycle status were assessed for 32D–EPO-R(wt) cells cultured in IL-3 or EPO and the presence or absence of the PI-3K inhibitor, LY294002 (Figure 4A). In the absence of irradiation, 32D–EPO-R(wt) cells continued to proliferate when cultured in EPO or IL-3 plus LY294002, though there was a reduction of S-phase cells compared to DMSO (vehicle)-treated controls. By contrast, γ-IR of LY294002-treated 32D–EPO-R(wt) cells caused arrest in G1 and G2/M despite treatment with EPO or IL-3 (Figure 4A-B). For all experiments, cells remained at least 94% viable regardless of treatment conditions (data not shown).
Inhibition of PI-3K–dependent signaling pathways prevents IL-3 and EPO from overriding γ-IR–induced growth arrest.
(A) 32D–EPO-R(wt) cells were cultured in medium that contained either 1.4 ng/mL IL-3 or 5 U/mL EPO. Cultures were then supplemented with either DMSO (0.2%) or LY294002 (10 μM). Subsequently, cultures were exposed to 4 Gy γ-IR (+γ) or were left untreated (−γ). Twenty-four hours after irradiation, cells were stained with PI and analyzed by FACS. Before PI staining, viability of the cells was determined by trypan blue exclusion and was 94% or greater in all experiments. Percentage of S-phase cells is indicated for each culture. (B) Percentage of G2/M cells in γ-IR–treated cultures versus nonirradiated cultures shown in panel A was determined, and the fold increase in G2/M was calculated (G2+γ/G2−γ). Values presented represent the average of 3 independent experiments. ■, DMSO; ▨, LY294002. (C) 32D–EPO-R(wt) cells treated with either DMSO (0.2%) or LY294002 (10 μM) were left unstimulated (−) or were stimulated with 1.4 ng/mL IL-3 or 5 U/mL EPO for 8 minutes. Akt catalytic activity was assayed from cell lysates in an in vitro kinase assay (see “Materials and methods). Alternatively, whole cell lysates were Western blotted with anti–phospho-Erk (α P-Erk) or anti–phospho-Stat-5A/B (α P-STAT5) antibodies. As a control for loading, lysates were probed with anti-Erk2 (α Erk) antiserum.
Inhibition of PI-3K–dependent signaling pathways prevents IL-3 and EPO from overriding γ-IR–induced growth arrest.
(A) 32D–EPO-R(wt) cells were cultured in medium that contained either 1.4 ng/mL IL-3 or 5 U/mL EPO. Cultures were then supplemented with either DMSO (0.2%) or LY294002 (10 μM). Subsequently, cultures were exposed to 4 Gy γ-IR (+γ) or were left untreated (−γ). Twenty-four hours after irradiation, cells were stained with PI and analyzed by FACS. Before PI staining, viability of the cells was determined by trypan blue exclusion and was 94% or greater in all experiments. Percentage of S-phase cells is indicated for each culture. (B) Percentage of G2/M cells in γ-IR–treated cultures versus nonirradiated cultures shown in panel A was determined, and the fold increase in G2/M was calculated (G2+γ/G2−γ). Values presented represent the average of 3 independent experiments. ■, DMSO; ▨, LY294002. (C) 32D–EPO-R(wt) cells treated with either DMSO (0.2%) or LY294002 (10 μM) were left unstimulated (−) or were stimulated with 1.4 ng/mL IL-3 or 5 U/mL EPO for 8 minutes. Akt catalytic activity was assayed from cell lysates in an in vitro kinase assay (see “Materials and methods). Alternatively, whole cell lysates were Western blotted with anti–phospho-Erk (α P-Erk) or anti–phospho-Stat-5A/B (α P-STAT5) antibodies. As a control for loading, lysates were probed with anti-Erk2 (α Erk) antiserum.
To confirm the specific inhibitory activity of LY294002 in 32D cells, we compared the ability of IL-3 or EPO to activate Akt, Erk, and Stat-5 in the presence or absence of the inhibitor. As shown in Figure 4C, treatment of 32D–EPO-R(wt) cells with 10 μM LY294002 did not completely abolish Akt activity but did suppress both IL-3 and EPO-induced Akt activation by more than 2-fold. LY294002 treatment had no detectable effect on cytokine-dependent Erk or Stat-5 phosphorylation.
Expression of a dominant-negative Akt prevents cytokines from overriding γ-irradiation–induced growth arrest
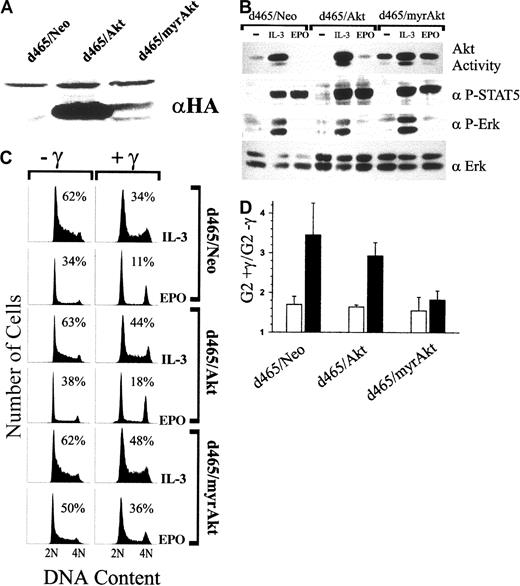
Activation of Akt is a well-characterized consequence of PI-3K activation. To assess the requirement for Akt activity in overriding the DNA damage–induced arrest, 32D cells were prepared that stably overexpressed wild-type Akt (32D-Akt) or a dominant-negative Akt (32D-Akt[KM]). As shown in Figure 5A, both Akt and Akt(KM) were expressed at higher levels in multiple clones than endogenous Akt. 32D-Akt or 32D-Akt(KM) proliferated normally when cultured in IL-3 (Figure 5B), as previously reported.22Similar to parental 32D cells, IL-3 was able to prevent γ-IR–induced growth arrest in 32D-Akt. Conversely, the dominant-negative form of Akt blocked the ability of IL-3 to override these checkpoints because 32D-Akt(KM) cells cultured in IL-3 were dramatically arrested in G1 and G2/M after γ-IR.
Overexpression of a dominant-negative Akt (Akt[KM]) impairs the ability of IL-3 to override γ-IR–induced cell-cycle arrest.
(A) Whole cell lysates from 32D-Neo (lane 1) or 2 clones of 32D-Akt (lanes 2 and 3) or 32D-Akt(KM) (lanes 3 and 4) cells were Western blotted with anti-HA antibodies (αHA) or anti-Akt/PKB PH domain antiserum (αAkt). (B) 32D-Neo, 32D-Akt, and 32D-Akt(KM) cells were cultured in medium that contained 70 pg/mL IL-3. Subsequently, cultures were exposed to 4 Gy γ-IR (+γ) or were left untreated (−γ). Twenty-four hours after irradiation, cells were stained with PI and analyzed by flow cytometry. Before PI staining, cell viability was determined by trypan blue exclusion and was 94% or greater in all experiments. Percentage of S-phase cells is indicated for each culture.
Overexpression of a dominant-negative Akt (Akt[KM]) impairs the ability of IL-3 to override γ-IR–induced cell-cycle arrest.
(A) Whole cell lysates from 32D-Neo (lane 1) or 2 clones of 32D-Akt (lanes 2 and 3) or 32D-Akt(KM) (lanes 3 and 4) cells were Western blotted with anti-HA antibodies (αHA) or anti-Akt/PKB PH domain antiserum (αAkt). (B) 32D-Neo, 32D-Akt, and 32D-Akt(KM) cells were cultured in medium that contained 70 pg/mL IL-3. Subsequently, cultures were exposed to 4 Gy γ-IR (+γ) or were left untreated (−γ). Twenty-four hours after irradiation, cells were stained with PI and analyzed by flow cytometry. Before PI staining, cell viability was determined by trypan blue exclusion and was 94% or greater in all experiments. Percentage of S-phase cells is indicated for each culture.
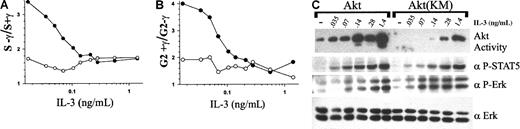
It was noted in the previous experiments that higher concentrations of IL-3 could overcome the dominant-negative activity of Akt(KM). Therefore, the dose dependence of IL-3 suppression of γ-IR–induced arrest was compared for 32D-Akt(KM) versus 32D-Akt cells. As shown in Figure 6, IL-3 was able to override γ-IR–induced cell-cycle arrest of 32D-Akt(KM) cells in a dose-dependent manner. However, significantly higher concentrations of IL-3 were required to override γ-IR–induced growth arrest in 32D-Akt(KM) than in 32D-Akt cells. Specifically, when cultured in low concentrations (less than 100 pg/mL) of IL-3, 32D-Akt(KM) cells experienced a substantial reduction in S phase after γ-IR (3.4-fold at 17.5 pg/mL IL-3). IL-3 concentrations less than 17.5 pg/mL were insufficient to maintain viability of 32D-Akt(KM) cell cultures after γ-IR. In contrast, 32D-Akt(KM) cells treated with IL-3 concentrations greater than 150 pg/mL resulted in less than a 2-fold reduction in S phase after γ-IR. By comparison, the slight reductions in S phase (1.5-1.8 fold) after γ-IR of 32D-Akt cells was not altered by increasing IL-3 concentrations. A similar dose dependence was observed for IL-3 suppression of γ-IR–induced increases in the G2/M population for 32D-Akt(KM) versus 32D-Akt cells (Figure 6B). Notably, treatment with 10 μM LY294002 completely abolished the ability of 280 pg/mL IL-3 to override γ-IR–induced arrest in 32D-Akt(KM) cells (3.75-fold decrease in S phase and 8.3-fold increase in G2/M after irradiation). Thus, the ability of higher concentrations of IL-3 to override γ-IR–induced cell-cycle arrest of 32D-Akt(KM) cells requires PI-3K activity and is not indicative of aberrant activation of an alternative PI-3K–independent pathway.
DNA damage–induced growth arrest of IL-3–treated cells is correlated with a lack of IL-3 induced Akt activity.
(A, B) 32D-Akt (○) or 32D-Akt(KM) (●) cells were cultured over a range of IL-3 concentrations. Percentage of S-phase cells in γ-IR–treated cultures versus nonirradiated cultures was determined, and the fold reduction in S phase was calculated (S−γ/S+γ) (A). Alternatively, the percentage of G2/M cells in the γ-IR–treated versus the nonirradiated cultures was determined, and the fold increase in G2/M was calculated (G2+γ /G2−γ) (B). (C) 32D-Akt or 32D-Akt(KM) cells were left unstimulated (−) or were stimulated with the indicated concentration of IL-3 for 8 minutes. Akt catalytic activity was assayed from cell lysates in an in vitro kinase assay (see “Materials and methods”). Alternatively, whole cell lysates were Western blotted with anti–phospho-Stat-5 (α P-STAT5) or anti–phospho-Erk (α P-Erk) antibodies. As a control for loading, lysates were probed with anti-Erk2 (α Erk) antiserum.
DNA damage–induced growth arrest of IL-3–treated cells is correlated with a lack of IL-3 induced Akt activity.
(A, B) 32D-Akt (○) or 32D-Akt(KM) (●) cells were cultured over a range of IL-3 concentrations. Percentage of S-phase cells in γ-IR–treated cultures versus nonirradiated cultures was determined, and the fold reduction in S phase was calculated (S−γ/S+γ) (A). Alternatively, the percentage of G2/M cells in the γ-IR–treated versus the nonirradiated cultures was determined, and the fold increase in G2/M was calculated (G2+γ /G2−γ) (B). (C) 32D-Akt or 32D-Akt(KM) cells were left unstimulated (−) or were stimulated with the indicated concentration of IL-3 for 8 minutes. Akt catalytic activity was assayed from cell lysates in an in vitro kinase assay (see “Materials and methods”). Alternatively, whole cell lysates were Western blotted with anti–phospho-Stat-5 (α P-STAT5) or anti–phospho-Erk (α P-Erk) antibodies. As a control for loading, lysates were probed with anti-Erk2 (α Erk) antiserum.
To confirm that the increased IL-3 concentrations required to override γ-IR–induced checkpoints in 32D-Akt(KM) cells correlated with activation of endogenous Akt, Akt kinase activity was measured in cells treated over a range of IL-3 concentrations. As shown in Figure 6C, 32D-Akt(KM) cells contained no detectable Akt activity when treated with less than 140 pg/mL IL-3, but they contained increasing levels of Akt activity with higher doses of IL-3. By comparison, 32D-Akt cells contained significant Akt activity when treated with as little as 35 pg/mL IL-3, and they reached maximum activity with 140 pg/mL IL-3. As a control, levels of IL-3–induced Erk and Stat-5 phosphorylation were found to reach maximum levels with approximately 140 pg/mL IL-3 in both 32D-Akt and 32D-Akt(KM) cells.
Expression of constitutively active Akt restores the ability of EPO-R mutants to override DNA damage checkpoints
To determine whether PI-3 kinase activation by the C-terminal domain of EPO-R is sufficient to override DNA damage–induced checkpoints, 32D–EPO-R(d465) cells were stably transfected with a constitutively active form of Akt (myrAkt). As shown in Figure7A, expression of HA-tagged myrAkt was detectable in clonal cell lines (32D-d465/myrAkt). However, levels of myrAkt expression were substantially lower than those achieved in clones expressing wild-type Akt (32D-d465/Akt). Nonetheless, 32D-d465/myrAkt cells contained constitutive levels of Akt activity in the absence of cytokine or after treatment with EPO (Figure 7B). IL-3 treatment modestly enhanced Akt kinase activity in 32D-d465/myrAkt cells. 32D-d465/Akt cells contained little or no Akt activity in the absence of cytokine or after EPO treatment. In all cell lines, EPO induced Stat-5 phosphorylation but failed to activate Erk. As with parental 32D–EPO-R(d465) cells, EPO treatment of 32D-d465/Akt cells did not prevent γ-IR–induced growth arrest (Figure 7C). However, expression of myrAkt restored the ability of EPO to override these checkpoints because 32D-d465/myrAkt cells cultured in EPO remained asynchronous after γ-IR treatment (Figure 7C-D).
Expression of constitutively active Akt in 32D–EPO-R(d465) restores the ability of EPO to override γ-IR–induced growth arrest.
(A) Whole cell lysates of 32D–EPO-R(d465) clones stably expressing a Neo control plasmid, wild-type Akt, or myrAkt were Western blotted with anti-HA (αHA) antibodies. (B) Cells were left unstimulated (−) or were treated with IL-3 (1.4 ng/mL) or EPO (5 U/mL) for 8 minutes. Akt catalytic activity was assayed from cell lysates in an in vitro kinase assay (see “Materials and methods”). Alternatively, whole cell lysates were Western blotted with anti–phospho-Stat-5 (α P-STAT5) or anti–phospho-Erk (α P-Erk) antibodies. As a control for loading, lysates were probed with anti-Erk2 antiserum (α Erk). (C) Cells were cultured in medium containing either 1.4 ng/mL IL-3 or 5 U/mL EPO. Subsequently, cultures were exposed to 4 Gy γ-IR (+γ) or were left untreated (−γ). Twenty-four hours after irradiation, cells were stained with PI and analyzed by FACS. Before PI staining, cell viability was determined by trypan blue exclusion and was 80% or greater in all experiments. Percentage of S-phase cells is indicated for each culture. (D) Percentage of G2/M cells in γ-IR–treated cultures versus nonirradiated cultures shown in panel C was determined, and the fold increase in G2/M was calculated (G2+γ/G2−γ). Values presented represent the average of 3 independent experiments. Similar results were also obtained from polyclonal populations of each cell type. ■, IL-3; ▪, EPO.
Expression of constitutively active Akt in 32D–EPO-R(d465) restores the ability of EPO to override γ-IR–induced growth arrest.
(A) Whole cell lysates of 32D–EPO-R(d465) clones stably expressing a Neo control plasmid, wild-type Akt, or myrAkt were Western blotted with anti-HA (αHA) antibodies. (B) Cells were left unstimulated (−) or were treated with IL-3 (1.4 ng/mL) or EPO (5 U/mL) for 8 minutes. Akt catalytic activity was assayed from cell lysates in an in vitro kinase assay (see “Materials and methods”). Alternatively, whole cell lysates were Western blotted with anti–phospho-Stat-5 (α P-STAT5) or anti–phospho-Erk (α P-Erk) antibodies. As a control for loading, lysates were probed with anti-Erk2 antiserum (α Erk). (C) Cells were cultured in medium containing either 1.4 ng/mL IL-3 or 5 U/mL EPO. Subsequently, cultures were exposed to 4 Gy γ-IR (+γ) or were left untreated (−γ). Twenty-four hours after irradiation, cells were stained with PI and analyzed by FACS. Before PI staining, cell viability was determined by trypan blue exclusion and was 80% or greater in all experiments. Percentage of S-phase cells is indicated for each culture. (D) Percentage of G2/M cells in γ-IR–treated cultures versus nonirradiated cultures shown in panel C was determined, and the fold increase in G2/M was calculated (G2+γ/G2−γ). Values presented represent the average of 3 independent experiments. Similar results were also obtained from polyclonal populations of each cell type. ■, IL-3; ▪, EPO.
Discussion
Cellular responses to DNA-damaging agents include apoptotic cell death and cell-cycle arrest at specific checkpoints.1 In hematopoietic cells, both these responses can be overridden by growth-promoting cytokines such as EPO or IL-3.5-7 We have previously shown that the abilities of EPO to override DNA damage–induced apoptosis and growth arrest are regulated by distinct signaling pathways.7 EPO-R truncation mutants, lacking as many as 106 C-terminal amino acids (ie, EPO-R[H]), retain the ability to suppress the apoptotic response induced by γ-IR. The membrane-proximal domain retained by EPO-R(H) associates with Jak-2 and is sufficient to mediate EPO-dependent up-regulation of antiapoptotic genes, including Bcl-2 and Bcl-XL. This receptor domain is necessary and sufficient to promote the proliferation of hematopoietic cells under normal culture conditions. However, EPO-dependent activation of EPO-R(H) and Jak-2 is not sufficient to override γ-IR–induced G1 and G2 phase growth arrest. Therefore, the C-terminal domain of EPO-R is dispensable for EPO-induced cell growth under nonstressing conditions but is required to override γ-IR–induced cell-cycle arrest.
In the present study, we have further defined regions within the C-terminal domain of EPO-R that are required for EPO to override the γ-IR–induced cell-cycle arrest. Notably, this activity is shown to require a single C-terminal tyrosine (Y479) that is also required for effective PI-3K activation. Conversion of Y479 to phenylalanine (EPO-R[Y479F]) disrupted EPO-induced Akt activation and abrogated the ability of EPO to override the γ-IR–induced cell-cycle arrest. The lack of Akt activation by EPO-R(Y479F) is consistent with the previous observation that Y479 is a primary recruitment site for the p85 subunit of PI-3K.17 18 Although it remains possible that other signaling molecules may also be recruited to phosphorylated Y479 of EPO-R, the addition of the PI-3K inhibitor, LY294002, to cells expressing wild-type EPO-R similarly abolished the ability of EPO to override γ-IR–induced growth arrest. These data indicate a specific requirement for PI-3K in overriding these DNA damage–induced checkpoints. Moreover, LY294002 also inhibited the ability of IL-3 to override γ-IR–induced growth arrest. Thus, it appears that the activation of PI-3K may be a general requirement for cytokines to override the growth arrest induced by DNA damage.
PI-3K regulates a large number of pleckstrin homology (PH)-domain containing effector proteins, including Akt and Pdk1, through the phosphorylation of inositol lipids at the plasma membrane.19 In the present study, we show that the expression of dominant-negative Akt (Akt[KM]) inhibits the ability of IL-3 to override γ-IR–induced cell-cycle arrest. In contrast, the expression of wild-type Akt at comparable levels to dominant-negative Akt had no effect on the ability of IL-3 to override γ-IR–induced arrest. This demonstrates that the simple overexpression of a PH domain-containing protein (eg, Akt) is insufficient to block a PI-3K–activated pathway needed to override the γ-IR–induced growth arrest. Thus, we conclude that dominant-negative Akt does not inhibit the recruitment of other PH-domain–containing effector proteins to the plasma membrane, nor does it inhibit their subsequent activation. Instead, the ability of cytokines to override damage–induced checkpoints is dependent on the catalytic activity of Akt. Moreover, treatment of Akt(KM) cells with increased concentrations of IL-3 overcame the dominant-negative Akt inhibition of endogenous Akt activity while overriding DNA damage–induced growth arrest. Therefore, the activation of Akt is required for cytokines to override the DNA damage–induced cell-cycle arrest. Furthermore, the inability of the EPO-R(d465) truncation mutant to override these checkpoints can be alleviated by the expression of active Akt, suggesting that restoration of this activity alone is sufficient to override the checkpoint.
In the present study, we found that the EPO-R–(Y479F) mutation had no effect on EPO-induced Erk phosphorylation. Conversely, a previous report found that EPO-induced Erk activation was disrupted by an apparently identical Y479F point mutation.18 Although these studies were performed in different cell lines, the basis for these differing results remains unclear. Ultimately, it was concluded that Erk activation by EPO was dependent on the activation of PI-3K.18 However, it is noteworthy that we also failed to detect any reduction of EPO- or IL-3–induced Erk phosphorylation in cells treated with the PI-3K inhibitor, LY294002. Instead, we found that the truncation of as few as 17 C-terminal amino acids, including Y479, from EPO-R (EPO-R[d465]) abrogated EPO-dependent phosphorylation of Erk1 and Erk2. The lack of Erk phosphorylation resulting from the d465 truncation could stem from loss of a recruitment site within this 17–amino acid domain or a general perturbation of the C-terminal region of EPO-R. In this regard, Klingmuller et al18 found that mutation of the other 7 tyrosines of EPO-R (except Y479) also resulted in the significant loss of EPO-induced Shc phosphorylation, Grb2 association, and Erk activation. Thus, it appears likely that Erk activation by EPO-R may not be associated simply with a single receptor domain. Regardless of the mechanism by which Erk is activated, the observation that the expression of active Akt restores the ability of EPO-R(d465) to override DNA damage–induced growth arrest suggests that activation of the Ras/Erk pathway is not required to override these checkpoints.
In hematopoietic cell lines, such as 32D, γ-IR induces both G1 and G2/M phase arrest. Our previous work demonstrated that cytokines override these cell-cycle checkpoints and promote growth in the face of DNA damage. Here we argue that PI-3K/Akt activation is essential for this activity. However, the targets of this signaling pathway in G1 and G2/M remain to be identified. In many cell types, DNA damage–induced cell-cycle arrest in G1 is thought to be dependent on p53-mediated up-regulation of the cyclin/cdk inhibitor p21Cip1. However, we have previously shown that γ-IR–induced cell-cycle arrest of hematopoietic cell lines occurs in the absence of p53.7 We have not ruled out the possibility that recently identified relatives of p53, p63, and p73 could contribute to DNA damage–induced G1 arrest through the up-regulation of p21Cip1 or GADD45 protein levels.30,31 Other pathways mediating DNA damage–induced cell-cycle arrest have been described, such as the Chk1-mediated phosphorylation and inactivation of Cdc25.32 33Although this pathway has clearly been shown to contribute to G2/M phase arrest, there is no evidence for its involvement in a DNA damage–induced G1 checkpoint. We are investigating whether these or other DNA damage–induced responses are responsible for the cell-cycle arrest observed in hematopoietic cell lines. It is clear, however, that whatever mediates this arrest can be regulated by cytokine-dependent signaling pathways involving PI-3K and Akt.
Evidence suggests a general role for PI-3K signaling pathway in regulating cell-cycle progression. Notably, PI-3K activity can be sufficient to induce G1 transit in fibroblasts34 and is required for IL-2–dependent activation of E2F in T cells.35 Thus, downstream targets of PI-3K/Akt–dependent pathways that regulate normal cell-cycle progression may also participate in overriding γ-IR–induced checkpoints. PI-3K activity has been shown to contribute to induced expression of D-type cyclins36 and to increase cyclin D1 stability through Akt-dependent phosphorylation of GSK-3β.37Alternatively, PI-3K effects on G1-phase progression may be mediated through the down-regulated expression of the Cdk inhibitor, p27kip1, because various inhibitors of PI-3K pathways have been shown to cause enhanced expression of p27kip1protein.35,38 39 Although PI-3K activation does not appear to be required for EPO- or IL-3–dependent proliferation of nonirradiated cells, lack of PI-3K activity in DNA-damaged cells could impair some or all of these events, resulting in growth arrest.
The results of the present study demonstrate that cytokine receptors use multiple signaling pathways to regulate cell growth. Under normal culture conditions, EPO-induced proliferation is primarily dependent on signaling pathways activated through the membrane-proximal domain of EPO-R and is not dependent on the activation of PI-3K. However, the present study demonstrates that cytokine-dependent activation of PI-3K/Akt signaling pathways is specifically required to override the effects of DNA damage on cell-cycle progression. The targets of this pathway are likely to be cell-cycle regulators governing G1/S- and G2/M-phase transit. Identification of these targets should provide a better understanding of how DNA damage–induced cell-cycle arrest is regulated in hematopoietic cells, and it may provide insight into how these checkpoints might be bypassed during leukemogenic development.
We thank Dr Naheed Ahmed (Fox Chase Cancer Center, Philadelphia, PA) for providing Akt cDNA constructs, Ann Friedman for the construction of truncated EPO-R cDNA, Dr James W. Osborne (Department of Radiation Biology, University of Iowa) for assistance in use of the 137Cs source, Justin Fishbaugh and Gene Hess for assistance in the Flow Cytometry Facility (University of Iowa), core facilities of The Diabetes and Endocrinology Research Center at the University of Iowa, and Dr Dawn Quelle for helpful discussion and review of this manuscript.
Supported by a Howard Hughes Medical Institute Biomedical Research Support Program grant and by Public Health Service grant CA-79889 from the National Cancer Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Frederick W. Quelle, Department of Pharmacology, 2-210 Bowen Science Bldg, The University of Iowa College of Medicine, Iowa City, IA 52242; e-mail: frederick-quelle@uiowa.edu.

![Fig. 5. Overexpression of a dominant-negative Akt (Akt[KM]) impairs the ability of IL-3 to override γ-IR–induced cell-cycle arrest. / (A) Whole cell lysates from 32D-Neo (lane 1) or 2 clones of 32D-Akt (lanes 2 and 3) or 32D-Akt(KM) (lanes 3 and 4) cells were Western blotted with anti-HA antibodies (αHA) or anti-Akt/PKB PH domain antiserum (αAkt). (B) 32D-Neo, 32D-Akt, and 32D-Akt(KM) cells were cultured in medium that contained 70 pg/mL IL-3. Subsequently, cultures were exposed to 4 Gy γ-IR (+γ) or were left untreated (−γ). Twenty-four hours after irradiation, cells were stained with PI and analyzed by flow cytometry. Before PI staining, cell viability was determined by trypan blue exclusion and was 94% or greater in all experiments. Percentage of S-phase cells is indicated for each culture.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/3/10.1182_blood.v98.3.834/5/m_h81511366005.jpeg?Expires=1769094474&Signature=sa7oXtJy1KTCMdVCwJgk6hEI7JusVHf2rvaQ0OkNgLi800QksrOk8Ie~9ehVohfr~Fppi5RTtv8HOwe2477GuyNSgEGHVwHCH15A7ktbeYmKaNhpNxj21cmVtwrf8es~xjavXcaIeRn~R7sCCTKrjQSMU~5IOvvbfFpKZTlfNDwszP4pcYgB1gb8XKAheM-U8L88u43m3MDuEreoa6hYDfHX3KXH3dYUVv5zDt3cncU~k8HFdvOT8vk99sbQcf~ToatzAUFtlx7P9gIsHjSCObYhs6geVipdZikk92QOFf~tsYuyy78PJm6Qxy6mes8nL6TvSpao~Nv5YrGRsqNsfA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal