Abstract
Multiple myeloma (MM) is a clonal B-cell malignancy characterized by slow-growing plasma cells in the bone marrow (BM). Patients with MM typically respond to initial chemotherapies; however, essentially all progress to a chemoresistant state. Factors that contribute to the chemorefractory phenotype include modulation of free radical scavenging, increased expression of drug efflux pumps, and changes in gene expression that allow escape from apoptotic signaling. Recent data indicate that arsenic trioxide (As2O3) induces remission of refractory acute promyelocytic leukemia and apoptosis of cell lines overexpressing Bcl-2 family members; therefore, it was hypothesized that chemorefractory MM cells would be sensitive to As2O3. As2O3 induced apoptosis in 4 human MM cell lines: 8226/S, 8226/Dox40, U266, and U266/Bcl-xL. The addition of interleukin-6 had no effect on cell death. Glutathione (GSH) has been implicated as an inhibitor of As2O3-induced cell death either through conjugating As2O3 or by sequestering reactive oxygen induced by As2O3. Consistent with this possibility, increasing GSH levels with N-acetylcysteine attenuated As2O3 cytotoxicity. Decreases in GSH have been associated with ascorbic acid (AA) metabolism. Clinically relevant doses of AA decreased GSH levels and potentiated As2O3-mediated cell death of all 4 MM cell lines. Similar results were obtained in freshly isolated human MM cells. In contrast, normal BM cells displayed little sensitivity to As2O3 alone or in combination with AA. Together, these data suggest that As2O3 and AA may be effective antineoplastic agents in refractory MM and that AA might be a useful adjuvant in GSH-sensitive therapies.
Introduction
Multiple myeloma (MM) is an incurable B-cell malignancy characterized by infiltrating, slow-growing plasma cells in the bone marrow (BM) that produce monoclonal immunoglobulin molecules (reviewed in Hallek et al1). MM accounts for 1% of all cancers and slightly more than 10% of all hematologic cancers, making it more common than Hodgkin disease and acute leukemia.2Current therapies for MM include alkylating agents (melphalan) and steroids (prednisone, dexamethasone) alone or combined with other antineoplastic agents, including plant alkaloids (vincristine, vinblastine) and anthracyclines (Adriamycin).2 High-dose chemotherapy with autologous or allogeneic stem cell transplantation has resulted in some improvement in survival rates,2 and a number of newer therapeutics are under investigation, including thalidomide.3 However, MM has an invariably aggressive course; essentially all (more than 90%) patients progress to a chemoresistant or refractory state. Even with treatment, the median 5-year survival rate for patients with MM is less than 25%.2 Thus, it is clear that new therapeutics are needed for the treatment of refractory MM.
At least 4 distinct mechanisms contribute to the acquisition of the chemoresistant phenotype in MM that must be overcome or circumvented to effectively treat refractory disease.4,5 Cellular response to steroids typically depends on the expression of the glucocorticoid receptor, and resistance to steroid therapies is classically associated with down-regulation or loss of glucocorticoid receptor expression in malignant plasma cells.6,7 The expression drug efflux pumps, such as mdr gene product P-glycoprotein (PgP), is also a common trait of chemoresistant MM cells.8-10 Plasma cells from patients with refractory MM and drug-resistant cell lines overexpress PgP.8-10 Clinically, however, efforts to overcome PgP with calcium-channel blockers, such as verapamil, or cyclosporin analogues have yielded disappointing results.11,12 In addition to limiting the access of antineoplastic agents to drug targets, chemoresistant cells have been selected for the ability to defend against mechanisms of drug action. It is widely held that many classes of chemotherapeutics, including those used in the treatment of MM, converge on apoptotic pathways to kill tumor cells. Concordant with this mode of action, changes in cellular apoptotic threshold are associated with chemoresistance or advanced disease in a number of solid tumors, leukemias, and lymphomas.13 Recently, it was reported that expression of the antiapoptotic protein Bcl-xL is higher in chemoresistant MM cell lines and in samples from patients with refractory MM.14 In addition, it is well established that interleukin-6 (IL-6), either in a paracrine or an autocrine fashion, plays an essential role in the malignant progression of MM by regulating the growth and survival of MM cells.15,16Lastly, chemoresistant MM cells have been reported to metabolize antineoplastic drugs more efficiently than drug-sensitive cells.12,17,18 For example, increases in the expression or activity of glutathione (GSH) and GSH-related enzymes, respectively, in MM cells confers resistance to alkylating agents such as melphalan.17 18 Effective treatments for chemorefractory MM must overcome or bypass the expression of drug efflux pumps, proliferative and survival signals of IL-6, elevated levels of antiapoptotic proteins such as Bcl-xL, and increases in glutathione expression.
In response to recent data that patients with chemorefractory acute promyelocytic leukemia respond to arsenic trioxide (As2O3) therapy,19 studies have been initiated to evaluate the therapeutic potential of As2O3 in other diseases.20-24Mechanistic studies of As2O3 action in leukemia and lymphoid cell lines suggest that antineoplastic activity overcomes Bcl-2 or Bcl-xL expression.20,22,24,25Furthermore, it is unlikely that unconjugated As2O3 would be a substrate for drug efflux pumps. Thus, As2O3 may be a promising antineoplastic for MM, even in PgP-positive plasma cells with increased Bcl-xL expression levels. However, the primary determinant of cellular sensitivity to As2O3 appears to be intracellular GSH levels,23,26-30 and elevated GSH levels have been associated with the MM chemoresistant phenotype.12,17,18 Therefore, optimal therapies for chemoresistant MM should also overcome or bypass increases in GSH expression. Compounds that decrease intracellular GSH levels are likely to increase the sensitivity of chemorefractory MM cells to As2O3. Despite the therapeutic potential of available GSH-depleting compounds such as ethacrynic acid and buthionine sulfoximine, toxicities limit widespread use.31-35 Therefore, new agents that can safely lower GSH levels must be investigated; one promising candidate is ascorbic acid (AA), widely heralded as an antioxidant.36 However, evidence is mounting that AA can also act as an oxidizing agent, particularly in the presence of compounds that increase the production of reactive oxygen species (ROS).37,38 The pro-oxidant effects of AA and the potentiation of cell death induced by free radicals appear to involve the production of hydrogen peroxide (H2O2).29,37 38
These data led us to hypothesize that the combination of As2O3 and AA is likely to be an effective therapy for chemorefractory MM. Consistent with the finding that GSH levels dictate As2O3sensitivity,23 26-30 we report that N-acetylcysteine (NAC) attenuated As2O3-mediated cell death of MM cell lines. Using in vitro models of chemosensitive and chemorefractory MM, we demonstrate that AA depleted intracellular GSH, increased hydrogen peroxide production, and potentiated As2O3cytotoxicity. Significantly, AA also increased As2O3-induced cell death of plasma cells isolated from the bone marrow of patients with MM with little toxicity in nonplasma cell populations.
Materials and methods
Reagents and cell lines
The 8226/S, 8226/Dox40,8,10 and U26639cell lines have been previously described. U266/Bcl-xL is described in more detail elsewhere (Oshiro et al, manuscript submitted). As2O3, AA, propidium iodide (PI), etoposide, Taxol, staurosporine, and metaphosphoric acid were purchased from Sigma Chemical (St Louis, MO); NAC and doxorubicin (Dox) were purchased from Bedford Laboratories (Bedford, OH).
Annexin V–fluorescein isothiocyanate and propidium iodide staining
Cell viability was measured by Annexin V–fluorescein isothiocyanate (FITC) (Biovision, Palo Alto, CA) and PI staining. Cells (2.5 × 105) were treated with the indicated concentration of As2O3 (1-100 μM for dose response experiments; 2 μM for other experiments), NAC (10 μM), AA (100 μM), etoposide (10 μg/mL), Taxol (0.5 μM), staurosporine (0.5 μM), and doxorubicin (200 nM). For the IL-6 studies, cells were pretreated with IL-6 (100 ng/mL) for 1 hour before the addition of As2O3 (2 μM). After the indicated time, cells were washed once in phosphate-buffered saline PBS and were stained with Annexin V–FITC (Biovision) and PI (2 μg/mL) according to manufacturer's instructions. Samples were acquired on a FACScan flow cytometer (Becton Dickinson, San Jose, CA) and analyzed with Cellquest software (Becton Dickinson).
Intracellular glutathione measurements
Intracellular GSH levels were determined in cell lines and in mononuclear cells from BM aspirates. BM aspirates were obtained from consenting patients with MM, and mononuclear cells were isolated by purification over a Ficoll gradient. BM aspirate (approximately 15 mL) was diluted 1:1 with prewarmed (37°C) PBS and overlaid onto 15 mL prewarmed Ficoll-Hypaque (Amersham, Uppsala, Sweden). After centrifugation at 2000 rpm for 20 minutes at room temperature, the mononuclear cells (including plasma cells) were removed, washed again with PBS, counted on a hemacytometer, and plated. Cells (4 × 106) were treated for 48 hours with AA (100 μM), and intracellular GSH was measured using the Glutathione Assay Kit (Calbiochem, San Diego, CA). Briefly, cells were pelleted, resuspended in 500 μL ice-cold 5% metaphosphoric acid, and homogenized with a Teflon pestle (Wheaton, Millville, NJ) and overhead stirrer. After centrifugation at 4°C and 3000g for 10 minutes, 200 μL supernatant, 700 μL buffer, 50 μL solution R1, and 50 μL solution R2 were combined according to the manufacturer's instructions. Samples were incubated at room temperature for 10 minutes in the dark, and final absorbance was measured at 400 nm and compared to a GSH standard curve. To quantitate total protein, the pellet from the above centrifugation was resuspended in 1 N NaOH, and the protein concentration was measured using the Bio-Rad DC Protein Assay (Bio-Rad, Hercules, CA). Intracellular GSH was normalized to total protein content.
Determination of H2O2 production
H2O2 production was measured using H2DCFDA (Molecular Probes, Eugene, OR), an H2O2-sensitive fluorescent dye. Briefly, cells (2 × 105) were incubated in the presence or absence of AA (100 μM) for 24 hours. In the last 30 minutes of incubation time, H2DCFDA (0.5 μM) was added to the cell cultures. Cells were washed in PBS, resuspended in FACS buffer (PBS with 1% BSA and 0.01% sodium azide), and analyzed by FACScan flow cytometry.
Determination of superoxide production
Superoxide production was determined by measuring the conversion of hydroethidine (Molecular Probes) to ethidium. Briefly, cells (2 × 105) were incubated in the presence or absence of As2O3 (2 μM) with or without AA (100 μM) for 24 hours. Cells were washed in PBS, resuspended in 1 mL PBS, and placed on ice. Hydroethidine stock solution (2 mM) was diluted in PBS (25 μL hydroethidine stock into 5 mL PBS) and added (250 μL) to each sample. Samples were immediately vortexed and incubated at 37°C in the dark for 20 minutes. After incubation, cells were stored on ice, protected from light, and analyzed by flow cytometry within 10 minutes.
Flow cytometric analysis of mitochondrial membrane potential)
Mitochondrial membrane potential was measured using the cationic, lipophilic dye tetramethylrhodamine ethyl ester (TMRE; Molecular Probes). Cells (2 × 105) were incubated in the presence or absence of As2O3 (2 μM) ± AA (100 μM) for 48 hours. Cells were washed in PBS, resuspended in FACS buffer supplemented with TMRE (150 nM), and incubated in the dark at 37°C for 20 minutes. Cells were then washed with PBS supplemented with TMRE (15 nM) and resuspended in FACS buffer supplemented with TMRE (15 nM). Samples were analyzed by flow cytometry.
Plasma cell isolation and cell death analysis
Bone marrow aspirates were obtained by consent from patients with chemorefractory and newly diagnosed MM. Mononuclear cells (including plasma cells) were isolated from BM biopsies by purification over a Ficoll gradient. BM aspirates (approximately 15 mL) were diluted 1:1 with prewarmed (37°C) PBS and overlaid onto 15 mL prewarmed Ficoll-Hypaque (Amersham). After centrifugation at 2000 rpm for 20 minutes at room temperature, mononuclear cells were removed, washed again with PBS, counted on a hemacytometer, and plated. Cells (4 × 105) were cultured in the presence or absence of As2O3 (2 μM) with or without AA (100 μM) or etoposide (10 μg/mL). After 48 hours, cells were triple stained with a phycoerythrin-conjugated mouse anti–human CD38 antibody (Becton Dickinson), a CyChrome-conjugated mouse anti–human CD45 antibody (Becton Dickinson), and FITC-conjugated Annexin V (Biovision). Controls included unstained cells, control mouse immunoglobulin G (IgG)–stained cells, and single-stained cells. Malignant plasma cells are defined as cells that express high levels of CD38 and no or low levels of CD45 (CD38+/CD45−).40 Nonplasma cells were defined as cells with low or no CD38 expression (CD38−). Apoptosis was determined by FACScan analysis using Cellquest software.
Statistical analyses
Data are presented graphically as the mean ± standard deviation (SD) or ± mean standard error (SE). Treatment groups were compared by independent t test or paired ttest as appropriate, with P reported in each figure legend. Statistical analyses were performed using Sigmaplot software (Jandel Scientific, Chicago, IL).
Results
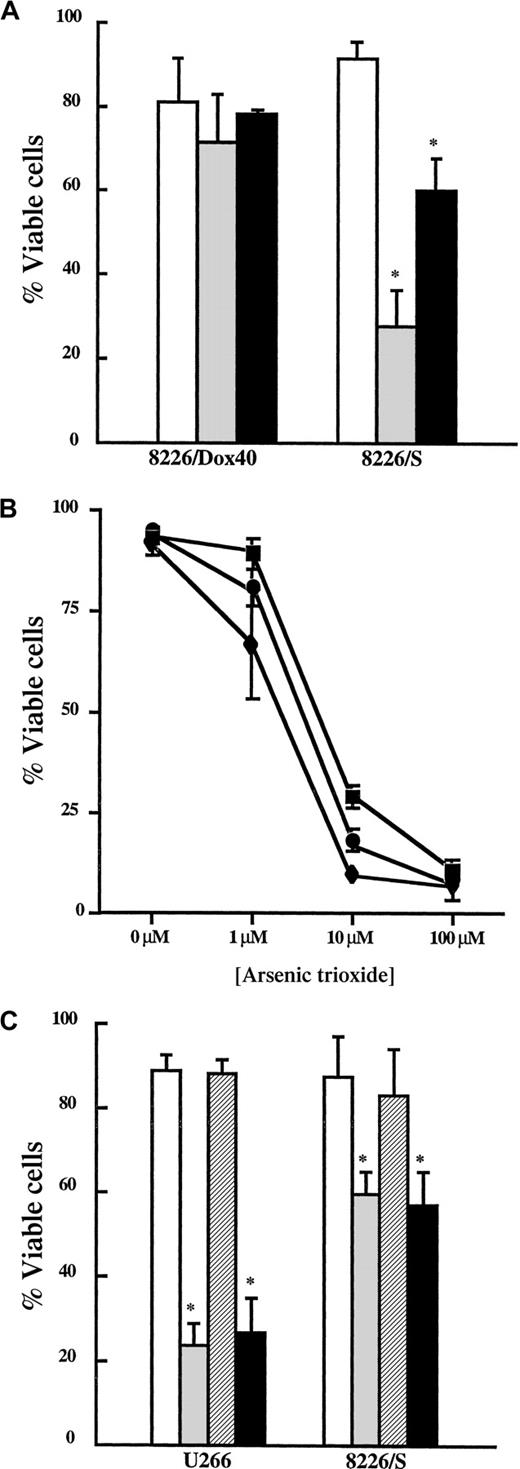
To determine whether As2O3 has therapeutic potential in MM, we first investigated the ability of As2O3 to induce apoptosis in the human MM cell lines, 8226/S, 8226/Dox40, and U266. The 8226/S cell line is an IL-6–independent MM line that is sensitive to chemotherapy-induced apoptosis.10 Selection of 8226/S cells in doxorubicin resulted in the generation of the 8226/Dox40 line; this cell line overexpresses both the drug efflux pump, PgP,10 and the antiapoptotic protein, Bcl-xL.14,39 The 8226/Dox40 cells maintain doxorubicin resistance in the absence of doxorubicin-containing medium and display a multidrug resistance phenotype because they are cross-resistant to etoposide (Figure1A). U266 cells are dependent on an autocrine IL-6 loop and express high levels of Bcl-xL compared to the 8226/S cells.39
MM cells are sensitive to As2O3 at clinically relevant concentrations.
(A) 8226/Dox40 and 8226/S cells were cultured in the absence (control, ■) or the presence of etoposide (10 μg/mL, ░) or doxorubicin (200 nM, ▪) for 48 hours. Viability was assessed as described in “Materials and methods.” Data are presented as the mean ± SD of at least 3 experiments. *Means for the treatment groups were significantly lower than for the control group (P < .0001). (B) Cells were incubated in the presence or absence of the indicated concentration of As2O3(1-100 μM) for 48 hours. Viability was assessed as described in “Materials and methods.” Data are presented as the mean ± SD of 5 experiments with 8226/S (▪) and 8226/Dox40 (♦) cells and of 3 experiments with U266 cells (●). (C) Cells were treated with (▨) or without IL-6 (■, 100 ng/mL) for 1 hour. Cultures were then incubated in the presence or absence of As2O3 (2 μM) for 48 hours. Viability was assessed as described in “Materials and methods.” Data are presented as the mean ± SD of at least 3 experiments per treatment group. *Means for As-treated (░) and As+IL-6–treated (▪) cells were significantly lower than for control cells (P < .003).
MM cells are sensitive to As2O3 at clinically relevant concentrations.
(A) 8226/Dox40 and 8226/S cells were cultured in the absence (control, ■) or the presence of etoposide (10 μg/mL, ░) or doxorubicin (200 nM, ▪) for 48 hours. Viability was assessed as described in “Materials and methods.” Data are presented as the mean ± SD of at least 3 experiments. *Means for the treatment groups were significantly lower than for the control group (P < .0001). (B) Cells were incubated in the presence or absence of the indicated concentration of As2O3(1-100 μM) for 48 hours. Viability was assessed as described in “Materials and methods.” Data are presented as the mean ± SD of 5 experiments with 8226/S (▪) and 8226/Dox40 (♦) cells and of 3 experiments with U266 cells (●). (C) Cells were treated with (▨) or without IL-6 (■, 100 ng/mL) for 1 hour. Cultures were then incubated in the presence or absence of As2O3 (2 μM) for 48 hours. Viability was assessed as described in “Materials and methods.” Data are presented as the mean ± SD of at least 3 experiments per treatment group. *Means for As-treated (░) and As+IL-6–treated (▪) cells were significantly lower than for control cells (P < .003).
Cells were treated with increasing concentrations of As2O3, and viability was monitored by Annexin V–PI staining after 48 hours. As seen in Figure 1B, all 3 cell lines were sensitive to As2O3 in a dose-dependent fashion. Published reports indicate that plasma levels of As2O3 peak at 5 to 7 μM and plateau at 1 to 2 μM.19 41 Therefore, the As2O3cytotoxicity detected between 1 μM and 10 μM occurred at therapeutically achievable doses. Based on the MM cell dose-response curves and the concentration of As2O3achievable in vivo, we performed all subsequent experiments in this report at a 2 μM As2O3 concentration.
IL-6 is the major survival factor for malignant plasma cells.15,16 For example, IL-6 has been shown to protect malignant plasma cells from apoptosis from different stimuli, including dexamethasone, CD95/Fas, serum starvation, and γ-irradiation.39 42-44 To determine whether IL-6 is capable of protecting MM cells from As2O3-induced cell death, cells were incubated in the presence and absence of IL-6 for 1 hour, and then As2O3 (2 μM) was added. IL-6 had no effect on the viability of U266 or 8226/S cells treated with As2O3 compared to control cells (Figure 1C). These data indicate that IL-6 is insufficient to block As2O3-induced cell death in MM cell lines.
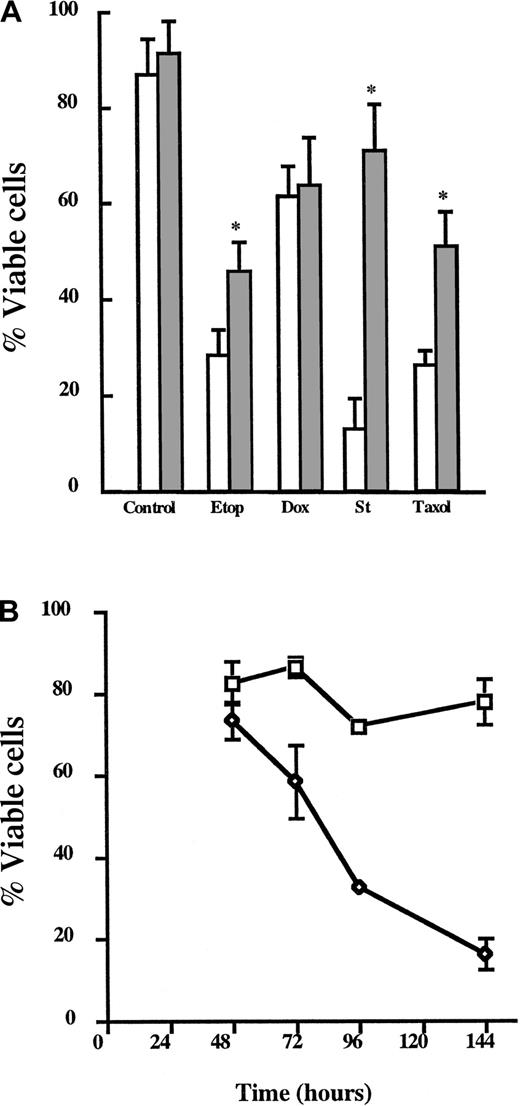
Because 8226/Dox40 cells display multiple mechanisms of chemoresistance (PgP expression and elevated Bcl-xL levels) and because Bcl-xL expression is emerging as a potentially important prognostic indicator of chemorefractory MM,14 45 we next formally determined whether elevated Bcl-xL levels affect As2O3 sensitivity. First, U266 cells stably expressing exogenous Bcl-xL (Oshiro et al, manuscript submitted) were analyzed for resistance to different classes of apoptosis-inducing agents and cell death monitored by Annexin V and PI exclusion. Although there was no difference in the viability of control cells (U266, 87.2% ± 6.6%; U266/Bcl-xL, 92.3% ± 6.3%), U266/Bcl-xL cells were less sensitive than U266 cells after etoposide, staurosporine, or Taxol treatment (P < .001; Figure 2A). U266/Bcl-xL cells were treated with As2O3 (2 μM) and assayed for cell viability at the indicated times (Figure 2B). U266/Bcl-xL cells were initially resistant to As2O3 (73.3% ± 4.3% viable at 48 hours); however, Bcl-xL could only delay death induced by As2O3 because the cells were less than 35% viable at 96 hours. From these studies, we concluded that As2O3 can overcome or bypass IL-6 signaling, the drug efflux pump PgP, and elevated Bcl-xL expression in MM cells.
Bcl-xL delays As2O3-induced cell death in multiple myeloma cells.
(A) U266 (■) and U266/Bcl-xL (░) were incubated in the presence or absence of etoposide (Etop; 10 μg/mL), doxorubicin (Dox; 200 nM), staurosporine (St; 0.5 μM), and Taxol (0.5 μM) for 48 hours. Viability was assessed as described in “Materials and methods.” Data are presented as the mean ± SD of at least 4 experiments per treatment group. *Means for the treated U266 cells were significantly lower than for similarly treated U266/Bcl-xLcells (P < .01). (B) U266/Bcl-xL cells were incubated in the presence (⋄) or absence (■) of As2O3 (2 μM) for the indicated times. Viability was assessed as described in “Materials and methods.” Data are presented as the mean ± SD of at least 4 experiments per time point.
Bcl-xL delays As2O3-induced cell death in multiple myeloma cells.
(A) U266 (■) and U266/Bcl-xL (░) were incubated in the presence or absence of etoposide (Etop; 10 μg/mL), doxorubicin (Dox; 200 nM), staurosporine (St; 0.5 μM), and Taxol (0.5 μM) for 48 hours. Viability was assessed as described in “Materials and methods.” Data are presented as the mean ± SD of at least 4 experiments per treatment group. *Means for the treated U266 cells were significantly lower than for similarly treated U266/Bcl-xLcells (P < .01). (B) U266/Bcl-xL cells were incubated in the presence (⋄) or absence (■) of As2O3 (2 μM) for the indicated times. Viability was assessed as described in “Materials and methods.” Data are presented as the mean ± SD of at least 4 experiments per time point.
Consistent with our in vitro data that only approximately 75% cell death is achieved at a 10 μM dose of As2O3(Figure 1B), clinical studies have recently shown modest responses with As2O3 alone in patients with MM.46Therefore, to optimize MM cell sensitivity to As2O3, we next initiated studies to determine the mechanism of As2O3 action in MM cells. In other cell types, sensitivity to As2O3correlates with intracellular GSH levels.23 24-30 To determine whether increases in GSH levels affect the sensitivity of MM cells to As2O3, we measured the ability of NAC to block As2O3-induced apoptosis. NAC is an antioxidant that functions by donating a cysteine to the de novo synthesis of GSH. Consistent with a crucial role for GSH in determining cellular sensitivity to As2O3, NAC abrogated As2O3-mediated cell death (Figure3).
NAC attenuates As2O3-mediated cell death.
Cells (2.5 × 105) were cultured in the absence (■) or the presence of As2O3 (2 μM; ░) or As2O3 (2 μM) and NAC (10 μM; ▪) for 48 hours. Viability was assessed by Annexin V–FITC and PI staining followed by FACScan analysis. Data are presented as the mean ± SD of at least 3 independent experiments per cell line. *Means of the As-treated cells are significantly lower than those of the As+NAC–treated cells and the control cells (P < .005). **Means of the As-treated cells are lower than those of the As+NAC–treated cells (P < .03) and the control cells (P < .005). #Means of the As+NAC–treated cells are lower than those of the control cells (P < .01).
NAC attenuates As2O3-mediated cell death.
Cells (2.5 × 105) were cultured in the absence (■) or the presence of As2O3 (2 μM; ░) or As2O3 (2 μM) and NAC (10 μM; ▪) for 48 hours. Viability was assessed by Annexin V–FITC and PI staining followed by FACScan analysis. Data are presented as the mean ± SD of at least 3 independent experiments per cell line. *Means of the As-treated cells are significantly lower than those of the As+NAC–treated cells and the control cells (P < .005). **Means of the As-treated cells are lower than those of the As+NAC–treated cells (P < .03) and the control cells (P < .005). #Means of the As+NAC–treated cells are lower than those of the control cells (P < .01).
It follows that if increased GSH synthesis attenuated As2O3-induced cell death, compounds that decrease MM cellular GSH levels are likely to potentiate As2O3-mediated apoptosis. In addition to its well-documented antioxidant effects,36 AA has been shown to have pro-oxidant properties.37,38 Auto-oxidation of AA to dehydroascorbate can result in the production of H2O2.38,47,48 Dehydroascorbate is then rapidly reduced back to AA by glutaredoxin in a GSH-dependent manner. This reduction of dehydroascorbate to AA results in a decrease of reduced intracellular GSH.38,47,48 Because AA is well tolerated in vivo,49 it is possible that the combination of AA and As2O3 would be a more efficient therapy in chemoresistant MM than As2O3 alone. Therefore, we tested the ability of AA to deplete GSH and to augment As2O3 cytotoxicity in MM cell lines.
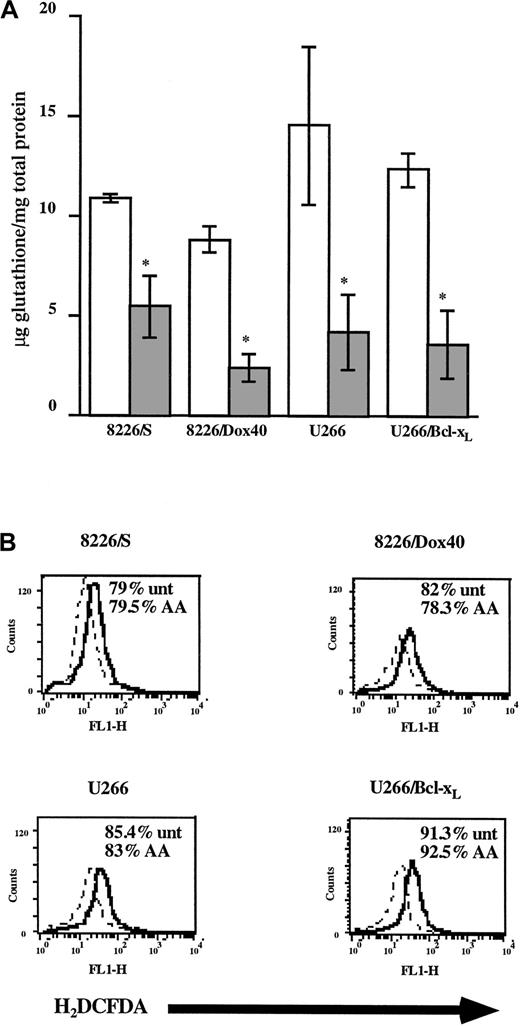
First, we determined whether AA treatment depletes GSH in MM cell lines. As seen in Figure 4A, incubation with AA at a concentration achievable in vivo with a vitamin C supplement (100 μM)49 results in the diminution (more than 2-fold) of GSH levels in all 4 cell lines. Consistent with its proposed pro-oxidant activity, the AA-mediated decrease in intracellular GSH levels correlated functionally with an increase in H2O2 production (Figure 4B). However, AA alone had no effect on cell viability (Figure 4B), suggesting that AA does not produce a sufficient level of H2O2 to initiate oxidative damage. Rather, AA treatment increases basal levels of cellular H2O2.
Ascorbic acid depletes intracellular GSH and increases H2O2 production in MM cell lines.
(A) Cells (4 × 106) were cultured in the absence (■) or the presence of AA (100 μM; ░). GSH levels were determined using the Glutathione Assay kit (Calbiochem) and were normalized to total cellular protein content. Data are presented as mean ± SD of at least 3 independent experiments per cell line. *Means of the AA-treated cells are lower than those of the control cells (P < .001). (B) Cells (2.5 × 105) were cultured for 24 hours in the absence (dashed line) or the presence of AA (100 μM; solid line). Cells were incubated for 30 minutes in 0.5 μM H2DCFDA, washed, and acquired by flow cytometry. Data are representative of at least 3 experiments for each cell line. Percentage cell viability was monitored by PI exclusion and is shown in the inset.
Ascorbic acid depletes intracellular GSH and increases H2O2 production in MM cell lines.
(A) Cells (4 × 106) were cultured in the absence (■) or the presence of AA (100 μM; ░). GSH levels were determined using the Glutathione Assay kit (Calbiochem) and were normalized to total cellular protein content. Data are presented as mean ± SD of at least 3 independent experiments per cell line. *Means of the AA-treated cells are lower than those of the control cells (P < .001). (B) Cells (2.5 × 105) were cultured for 24 hours in the absence (dashed line) or the presence of AA (100 μM; solid line). Cells were incubated for 30 minutes in 0.5 μM H2DCFDA, washed, and acquired by flow cytometry. Data are representative of at least 3 experiments for each cell line. Percentage cell viability was monitored by PI exclusion and is shown in the inset.
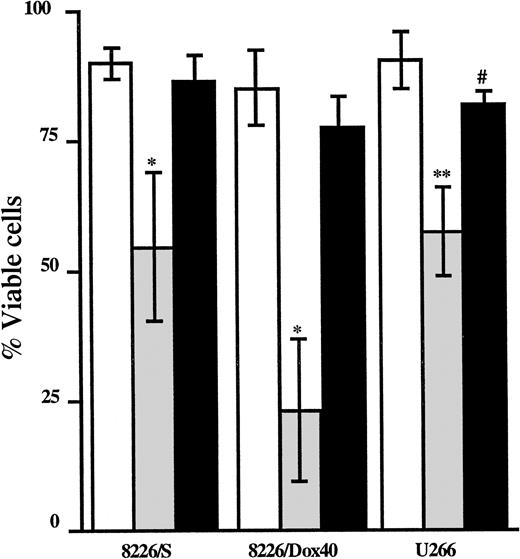
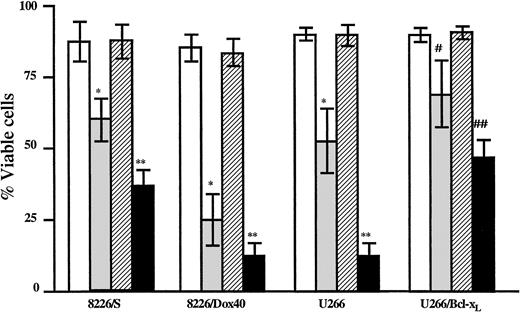
The depletion of GSH and the subsequent elevation of basal H2O2 by AA should render the cell more susceptible to damage from compounds that are metabolized by GSH conjugation. Additionally, AA-mediated decreases in GSH could impair the ability of the cell to recover from oxidative insults. To determine whether AA-mediated decreases in GSH are sufficient to increase MM cell sensitivity to As2O3, MM cells were treated with As2O3 in the presence or absence of AA, and cell viability was measured by flow cytometry. The 8226/S, 8226/Dox40, and U266 cell lines were analyzed after 48-hour incubation; the U266/Bcl-xL cells were assayed after 72 hours of treatment. As2O3 alone induced cell death (between 30% and 60%) in each cell line, as indicated in Figure5. However, the combination of AA and As2O3 resulted in a significant increase in apoptosis compared to As2O3 treatment alone (approximately 30% increase) in all 4 cell lines (Figure 5).
Ascorbic acid potentiates As2O3-mediated cell death in multiple myeloma cell lines.
Cells were cultured in the absence (■) or the presence of As2O3 (2 μM; ░), AA (100 μM; ▨), or As2O3+AA (▪). Viability was assessed as described in “Materials and methods” after 48 hours for the 8226/S, 8226/Dox40, and U266 cell lines or after 72 hours for the U266/Bcl-xL cell line. Data are presented as the mean ± SD of at least 4 experiments per cell line. *Means of the As-treated cells are lower than those of the control cells and of the AA-treated cells (P < .002). **Means of the As+AA-treated cells are lower than of the As-treated cells (P < .02). #Means of the As-treated cells are lower than of the control cells and of the AA-treated cells (P < .01). ##Means of the As+AA-treated cells are lower than of the As-treated cells (P < .02).
Ascorbic acid potentiates As2O3-mediated cell death in multiple myeloma cell lines.
Cells were cultured in the absence (■) or the presence of As2O3 (2 μM; ░), AA (100 μM; ▨), or As2O3+AA (▪). Viability was assessed as described in “Materials and methods” after 48 hours for the 8226/S, 8226/Dox40, and U266 cell lines or after 72 hours for the U266/Bcl-xL cell line. Data are presented as the mean ± SD of at least 4 experiments per cell line. *Means of the As-treated cells are lower than those of the control cells and of the AA-treated cells (P < .002). **Means of the As+AA-treated cells are lower than of the As-treated cells (P < .02). #Means of the As-treated cells are lower than of the control cells and of the AA-treated cells (P < .01). ##Means of the As+AA-treated cells are lower than of the As-treated cells (P < .02).
Taken together, results of the NAC and AA studies are consistent with those from a model in which GSH is a critical determinant of As2O3 sensitivity in MM cells. There is evidence that GSH can conjugate arsenicals, resulting in a complex that is easily excreted by the MRP2/cMOAT transporter.28,50Therefore, depletion of GSH levels could decrease As2O3 efflux, thereby causing an intracellular accumulation of As2O3 and increasing cytotoxicity. However, because neither 8226/S nor 8226/Dox40 cells express MRP2/cMOAT,51-55 it is unlikely that GSH-dependent efflux mechanisms underlie the AA-mediated increase in cell death observed in MM cells.
Alternatively, GSH depletion and consequent elevation in basal H2O2 levels could render the cell more sensitive to oxidative damage. Because AA potentiated As2O3-mediated cell death, it is possible that As2O3 treatment increased ROS production. To directly assess the effects of As2O3 on ROS production, we used flow cytometric techniques to measure superoxide production in MM cell lines after 24 hours of As2O3 treatment (Figure6). The production of superoxide coincided with the initial detection of apoptotic cells (Figure 6). AA alone had no effect on superoxide production (P > .05), but the combination of AA and As2O3 resulted in higher levels of superoxide than did As2O3alone (Figure 6; P < .0003 for 8226/S, 8226/Dox40, U266 cells; P < .0005 for U266/Bcl-xL cells). Superoxide is predominantly produced in the mitochondria and can result in free radical attack of membrane phospholipids, leading to a loss of mitochondrial membrane potential. Therefore, we next determined whether mitochondrial membrane integrity was affected by As2O3 and whether AA could augment an As2O3-mediated loss of integrity. Using flow cytometric techniques, we measured the ability of MM cell mitochondria to maintain a membrane potential in the presence and absence of As2O3. As2O3 treatment resulted in a depolarization of the inner mitochondrial membrane (Figure 7). AA alone had no effect on mitochondrial membrane potential, but the combination of As2O3 and AA resulted in a greater percentage of cells displaying decreased mitochondrial membrane potential than with As2O3 alone (Figure 7). These findings are consistent with As2O3 exerting cytotoxic effects through the generation of ROS, resulting in free radical cellular damage.
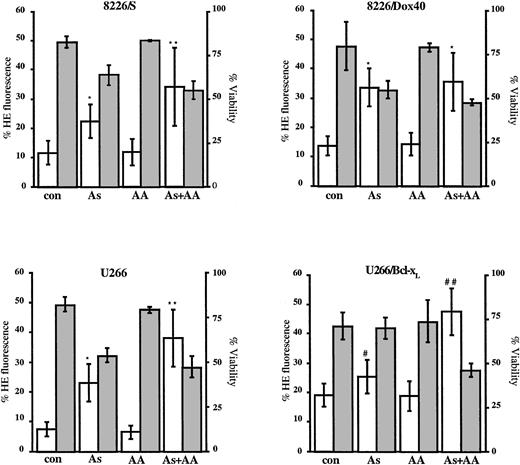
Ascorbic acid potentiates As2O3-mediated increases in the production of superoxide.
Cells (2.5 × 105) were cultured for 24 hours in the absence or the presence of As2O3 (2 μM), AA (100 μM), or As2O3+AA, as indicated. Cells were stained with hydroethidine, as described in “Materials and methods.” Data are presented as the mean ± SD of hydroethidine fluorescence (■, left scale) from at least 6 experiments for each cell line. *Means of the As- or the As+AA–treated cells are higher than those of the AA-treated cells and of control cells (P < .0003). **Means of the As+AA–treated cells are higher than those of the As-treated cells (P < .03). #Means of the As-treated cells are higher than those of the AA-treated and of control cells (P < .03). ##Means of the As+AA–treated cells are higher than those of As-treated cells (P < .0005). Percentage cell viability was monitored by Annexin V–FITC staining and is shown by the gray bars (right scale) as the mean ± SD of at least 6 experiments for each cell line.
Ascorbic acid potentiates As2O3-mediated increases in the production of superoxide.
Cells (2.5 × 105) were cultured for 24 hours in the absence or the presence of As2O3 (2 μM), AA (100 μM), or As2O3+AA, as indicated. Cells were stained with hydroethidine, as described in “Materials and methods.” Data are presented as the mean ± SD of hydroethidine fluorescence (■, left scale) from at least 6 experiments for each cell line. *Means of the As- or the As+AA–treated cells are higher than those of the AA-treated cells and of control cells (P < .0003). **Means of the As+AA–treated cells are higher than those of the As-treated cells (P < .03). #Means of the As-treated cells are higher than those of the AA-treated and of control cells (P < .03). ##Means of the As+AA–treated cells are higher than those of As-treated cells (P < .0005). Percentage cell viability was monitored by Annexin V–FITC staining and is shown by the gray bars (right scale) as the mean ± SD of at least 6 experiments for each cell line.
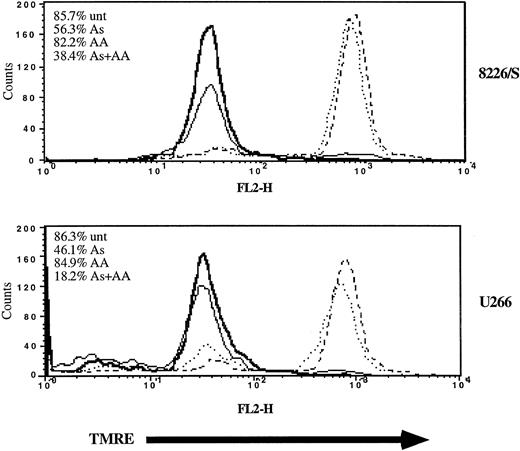
Ascorbic acid potentiates As2O3-mediated disruption of mitochondrial membrane potential.
Cells (2 × 105) were cultured in the absence (dashed line) or the presence of As2O3 (2 μM; solid line), AA (100 μM; dotted), or As2O3+AA (bold) for 48 hours. Mitochondrial membrane potential was measured by TMRE fluorescence and FACScan analysis as described in “Materials and methods.” Cell viability (inset) was monitored by Annexin V–FITC and PI staining followed by FACScan analysis. Data are representative of 3 independent experiments per cell line.
Ascorbic acid potentiates As2O3-mediated disruption of mitochondrial membrane potential.
Cells (2 × 105) were cultured in the absence (dashed line) or the presence of As2O3 (2 μM; solid line), AA (100 μM; dotted), or As2O3+AA (bold) for 48 hours. Mitochondrial membrane potential was measured by TMRE fluorescence and FACScan analysis as described in “Materials and methods.” Cell viability (inset) was monitored by Annexin V–FITC and PI staining followed by FACScan analysis. Data are representative of 3 independent experiments per cell line.
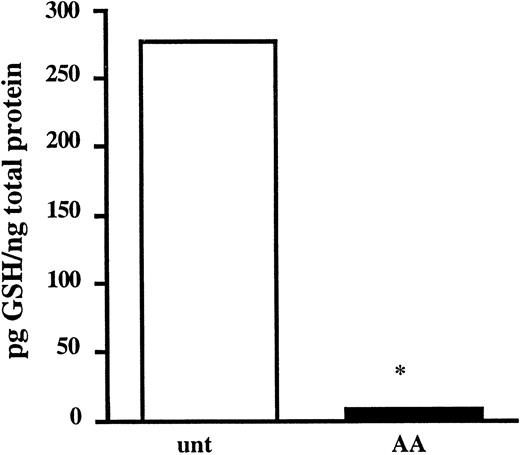
Based on our finding that As2O3 and AA induced apoptosis in drug-sensitive and drug-resistant MM cell lines, we expanded our studies to test the cytotoxicity of As2O3 and the combination of As2O3 and AA in samples from patients with MM. First, to determine whether AA can deplete GSH in freshly isolated cells, mononuclear cells (including plasma cells) isolated from BM aspirates from 2 patients were incubated in the presence or absence of a clinically achievable concentration of AA (100 μM) for 48 hours. Intracellular GSH levels were measured as described in “Materials and methods.” Because of the small number of total cells isolated from the BM aspirates, it was not possible to sort plasma cells (CD38+/CD45−) from nonplasma cells (CD38−) before GSH measurements. However, these BM aspirates contained an average of 40% plasma cells. After 48 hours of AA treatment, a more than 20-fold decrease in GSH level was detected in the total cell population (Figure 8).
Ascorbic acid depletes intracellular GSH in mononuclear cells from patients with multiple myeloma.
Mononuclear cells were isolated from BM aspirates as described in “Materials and methods.” Cells (4 × 106) were incubated in the absence (■) or the presence of AA (100 μM; ▪) for 48 hours. GSH levels were determined as described in “Materials and methods” and are presented as the average of 2 patient samples. *Means of the AA-treated cells are lower than of the control cells (P < .005).
Ascorbic acid depletes intracellular GSH in mononuclear cells from patients with multiple myeloma.
Mononuclear cells were isolated from BM aspirates as described in “Materials and methods.” Cells (4 × 106) were incubated in the absence (■) or the presence of AA (100 μM; ▪) for 48 hours. GSH levels were determined as described in “Materials and methods” and are presented as the average of 2 patient samples. *Means of the AA-treated cells are lower than of the control cells (P < .005).
Additionally, viability studies were performed with mononuclear cells isolated by consent from patients with newly diagnosed (n = 9) and chemorefractory (n = 14) MM. Mononuclear cells from BM aspirates were isolated as described in “Materials and methods.” Cells were cultured in the presence or absence of As2O3with or without AA or etoposide for 48 hours and were stained with anti-CD38 and anti-CD45 antibodies and with Annexin V, and the viability of triple-stained cells was monitored by flow cytometry. Etoposide was included in these studies for 2 reasons. First, it allowed for a comparison to an apoptosis-inducing agent sensitive to drug efflux pumps. Second, because patients with chemorefractory MM were not treated with the same therapeutic protocols, we wanted to use an agent that no patient had been treated with to eliminate any specific drug resistance that might confound our analyses.
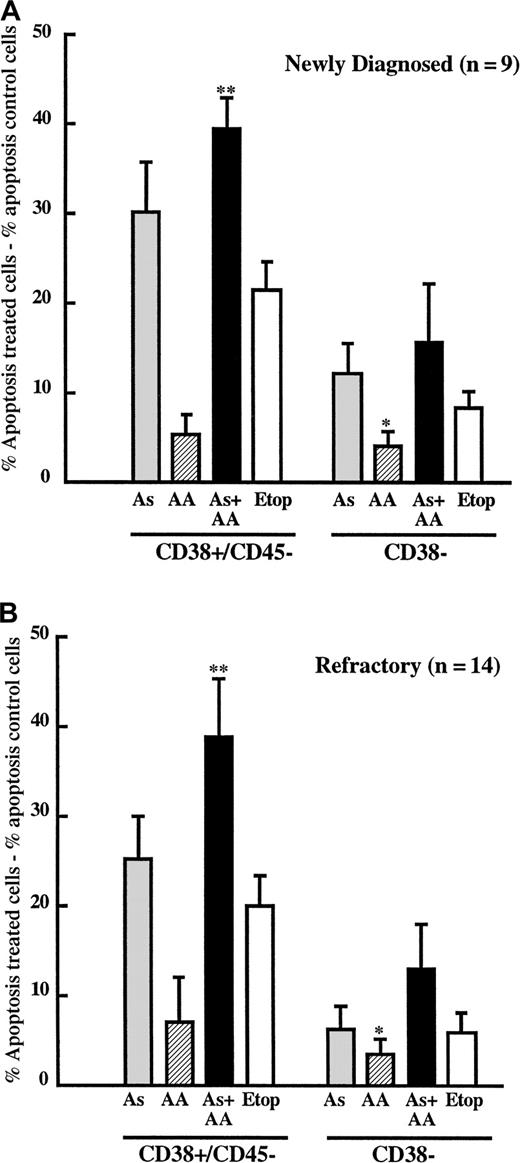
These studies revealed that As2O3 selectively induced apoptosis of the plasma cell population (CD38+CD45−) compared with the nonplasma cell population (CD38−) in samples from patients with de novo and refractory MM (Figure 9A-B). Consistent with studies in the MM cell lines, AA was sufficient to potentiate As2O3 cytotoxicity of malignant plasma cells in BM aspirates from patients with refractory MM (Figure9B; P < .004). Interestingly, results with the combination of AA and As2O3 were not significantly different from those with As2O3alone in the de novo patient samples (Figure 9A;P > .05). However, the sensitivity of plasma cells from patients with newly diagnosed MM showed a trend toward greater sensitivity to As2O3 alone (30.23% ± 5.42%) than did plasma cells from patients with refractory MM (25.12% ± 4.83%). In contrast, cell death induced by As2O3 + AA was nearly identical in the de novo and chemorefractory patient samples (39.40% ± 3.58% vs 38.46% ± 6.34%). Thus, the lack of significant differences between the As2O3 and the As2O3+AA treatment groups appears to be due to a greater sensitivity of the de novo cells to As2O3 alone. It is possible that differences in GSH levels contribute to this apparent difference in sensitivity. Viabilities of nonplasma, CD38− cells treated with As, AA + As, or etoposide were not significantly different in either the de novo or the refractory samples (Figure 9A-B; P > .05). AA treatment alone was not cytotoxic in any cell population, suggesting that AA has the potential to be a safe and effective chemosensitizing agent in As2O3-based therapy.
Plasma cells from patients with multiple myelomas are sensitive to As2O3 and ascorbic acid.
(A) BM mononuclear cells were isolated from patients with newly diagnosed MM (n = 9) and cultured in the absence or presence of As2O3 (2 μM; ░), AA (100 μM; ▨), As2O3+AA (▪), or etoposide (10 μg/mL; ■) for 48 hours, as described in “Material and methods.” Cells were then triple stained with phycoerythrin-conjugated anti-CD38, CyChrome conjugated anti-CD45, and FITC-conjugated Annexin V. Death of CD38/CD45− cells (myeloma cells) and of the CD38− population (nonmyeloma cells) was determined by FACScan analysis. Data are presented as the mean ± SE of the percentage apoptotic (Annexin V–FITC+) cells in treated wells minus percentage apoptotic cells in untreated wells. Statistical analyses were performed using a paired t test, where AA+As differs from AA and etoposide in the CD38/CD45− population (**P < .0006) and As differs from AA in the CD38− population (*P < .03). (B) BM mononuclear cells were isolated from patients with refractory MM (n = 14), treated, and analyzed as described in panel A. Statistical analyses were performed using a paired t test, where AA+As differs from As, AA, and etoposide in the CD38/CD45−population (**P < .02) and AA+As differs from AA in the CD38− population (*P < .03).
Plasma cells from patients with multiple myelomas are sensitive to As2O3 and ascorbic acid.
(A) BM mononuclear cells were isolated from patients with newly diagnosed MM (n = 9) and cultured in the absence or presence of As2O3 (2 μM; ░), AA (100 μM; ▨), As2O3+AA (▪), or etoposide (10 μg/mL; ■) for 48 hours, as described in “Material and methods.” Cells were then triple stained with phycoerythrin-conjugated anti-CD38, CyChrome conjugated anti-CD45, and FITC-conjugated Annexin V. Death of CD38/CD45− cells (myeloma cells) and of the CD38− population (nonmyeloma cells) was determined by FACScan analysis. Data are presented as the mean ± SE of the percentage apoptotic (Annexin V–FITC+) cells in treated wells minus percentage apoptotic cells in untreated wells. Statistical analyses were performed using a paired t test, where AA+As differs from AA and etoposide in the CD38/CD45− population (**P < .0006) and As differs from AA in the CD38− population (*P < .03). (B) BM mononuclear cells were isolated from patients with refractory MM (n = 14), treated, and analyzed as described in panel A. Statistical analyses were performed using a paired t test, where AA+As differs from As, AA, and etoposide in the CD38/CD45−population (**P < .02) and AA+As differs from AA in the CD38− population (*P < .03).
Discussion
MM cells ultimately acquire a chemoresistant phenotype that includes expression of drug efflux pumps, changes in apoptotic threshold, and increased ability to detoxify or metabolize chemotherapeutic agents. Effective therapies for refractory MM must be able to overcome or bypass these adaptive cellular changes. In this report we show that As2O3 is not sensitive to drug efflux pump mechanisms of resistance in MM. The PgP-positive cell line 8226/Dox40 undergoes apoptosis in response to As2O3 in vitro, similar to the drug-sensitive cell line 8226/S (Figure 1B). IL-6 is unable to attenuate As2O3-induced cell death (Figure 1C). Similarly, increased expression of the antiapoptotic protein Bcl-xL does not confer resistance to As2O3 because MM cells that express exogenous Bcl-xL are sensitive to As2O3(Figure 2B). These data are consistent with those from recent studies demonstrating that As2O3 induces apoptosis in leukemic cell lines that overexpress Bcl-2 or Bcl-xL.20,22,24,25 Others have shown that As2O3 overcomes Bcl-2 by down-regulating gene expression22 25; however, we did not detect any changes in Bcl-xL expression after As2O3treatment (J.M.G., N.J.B., and L.H.B., unpublished observation, March 2000). Furthermore, As2O3 treatment induced apoptosis in plasma cells from patients for whom repeated rounds of VAD and melphalan-prednisone therapy failed (Figure 9B). These data indicate that As2O3 is likely to overcome drug efflux mechanisms and the antiapoptotic effects of IL-6 and Bcl-xL in chemorefractory MM.
Our data indicate that ROS production is a critical contributor to As2O3-induced MM cell death. First, As2O3-mediated cell death was abrogated in the presence of NAC (Figure 3). It is relevant to note that As2O3 has been reported to bind to vicinal thiol groups56 and that NAC contains 2 such thiol groups. However, the protective effects of NAC are likely due to increases in GSH levels and not to NAC sequestering of As2O3because the addition of dithiothreitol, at a concentration 5 times higher than that of NAC used in these studies (50 μM dithiothreitol versus 10 μM NAC), could only partially protect against As2O3-induced cell death in MM cells (J.M.G., N.J.B., and L.H.B., unpublished observation, November 1999). Additional evidence supporting a role for oxidative pathways in As2O3-mediated cell death stems from the relation between AA, GSH, and As2O3cytotoxicity. AA has been shown to decrease GSH levels and to potentiate As2O3-mediated cell death in lymphoid and myeloid cells.29
The GSH redox system is known to affect the activity of arsenicals.23,26-30 First, As2O3sensitivity correlates with intracellular GSH levels in other model systems.23 Cells expressing higher levels of GSH23,27 or GSH-associated enzymes26,27 are less sensitive to As2O3 than cells expressing lower levels of these molecules.27,57-59 Moreover, cells with increased GSH levels can be sensitized to As2O3 by agents that deplete intracellular GSH, such as buthionine sulfoximine or ethacrynic acid.29 GSH exerts antioxidant effects by a variety of mechanisms. It can conjugate and thereby inactivate molecules that generate free radicals. In a cell-free system, trivalent arsenic can complex with GSH, forming a transient As(GS)3molecule.30 Additionally, GSH conjugation can render a molecule more easily excreted through drug efflux pumps.28Kala et al28 recently demonstrated that biliary excretion of sodium arsenite requires GSH and the MRP2/cMOAT transporter. However, because MRP2/cMOAT expression is not detected in myeloma cells, including 8226/S and 8226/Dox40,51-55 and because primary MM samples express comparable levels of MRP as normal plasma cells,60 61 this mechanism is not likely to be relevant in MM cells. It is important to note that though our studies indicate that a GSH-dependent efflux of As2O3 or its metabolites is unlikely in MM cells, the possibility remains that GSH conjugation is involved in regulating As2O3sensitivity. GSH can also become oxidized and provide electrons for enzymes such as GSH peroxidase, which reduce H2O2 to H2O. Clearly, the direct conjugation of As2O3 and the inhibition of As2O3-induced ROS production through GSH peroxidase are not mutually exclusive mechanisms because both can contribute to As2O3 detoxification. The relevant mode(s) of action for GSH inhibition of As2O3-induced MM cell death remains to be determined.
The precise determinants of whether AA exerts pro- or antioxidant effects are unclear, but they appear to be temporal.38Cotreatment of AS52 cells with AA (50 μM) and RGS resulted in increased cell death compared to treatment with RGS alone.38 However, when AS52 cells were pretreated for 24 hours with AA and then challenged with RGS, the cells were protected. Therefore, the timing of AA treatment may have an impact on whether AA acts to potentiate or dampen cytotoxicity from oxidative damage.38
Data from our studies with MM cell lines led us to postulate that the combination of As2O3 and AA might be a feasible therapy for patients with refractory and plateau MM. To begin to address this hypothesis, we expanded our in vitro work to include studies using plasma cells from patients with newly diagnosed and refractory MM. Similar to what was determined with the MM cell lines, AA treatment depleted GSH levels in mononuclear cells from the BM of patients with MM. Freshly isolated MM plasma cells were sensitive to As2O3, and AA potentiated the cytotoxicity in refractory patient samples; nonplasma cell populations were relatively unaffected (Figure 9A-B). MM cultures contained a mixed population of mononuclear cells, including BM stromal cells (BMSCs), and the presence of BMSCs in the MM patient cultures might have affected the therapeutic efficacy of As2O3. Interaction with BMSCs has been reported to confer cytoprotection against certain antineoplastic agents and to provide key mitogenic and antiapoptotic growth factors and cytokines.62,63 Additionally, cell adhesion–mediated drug resistance is emerging as a potentially BMSC-related mechanism whereby malignant cells may escape apoptotic signals and allow for the development of more classical drug resistance phenotypes (efflux pumps and so on).64 Taken together, our data with MM cell lines and in MM patient samples suggest that the combination of As2O3 and AA is a strong candidate as a therapy for MM. To determine the safety and efficacy of the combination therapy in patients with refractory and plateau MM, a National Cancer Institute–sponsored phase I/II clinical trial has been opened at the University of Miami.
Because most patients with MM will ultimately progress to a refractory state and succumb to their cancer, it is critical to identify new therapeutics capable of overcoming or circumventing chemoresistance. As2O3 is emerging as an effective agent in the treatment of refractory acute promyelocytic leukemia.19,41,65 Significantly, the safety and efficacy of As2O3 in the treatment of refractory acute promyelocytic leukemia has been demonstrated.19,41,65 The AA and As2O3 concentrations used in this report are well within clinically achievable serum levels and are reportedly well tolerated independently.19,41,49,65 In summary, As2O3 is an exciting new addition to the antineoplastic arsenal and warrants serious investigation as a therapeutic for MM. The mechanism of As2O3-mediated cell death appears to be multifactorial and cell specific.20-22,24,25,56 66 Our studies support a role for GSH and ROS production as critical mediators of As2O3-induced cell death in MM cells. The addition of AA to As2O3-based and other GSH-sensitive regimes can potentially optimize the cytotoxic effects of specific chemotherapeutics with little toxicity.
We thank Kapil Bhalla, Kelvin Lee, Enrique Cepero, and Bryan Johnson for their insight and their helpful comments.
Supported in part by National Institutes of Health grant R29CA77837 (L.H.B.), a seed grant from the Viral Oncology Program of the Sylvester Cancer Center (L.H.B.), an ASCO Young Investigator Award (N.J.B.), and an AICR postdoctoral fellowship (J.M.G.).
J.M.G. and N.J.B. contributed equally to this article.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Lawrence Boise, Department of Microbiology and Immunology, University of Miami School of Medicine, PO Box 016960 (R-138), Miami, FL 33101; e-mail: lboise@med.miami.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal