Abstract
The conditioning regimen prior to stem cell transplantation in 36 patients with high-risk acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) was intensified by treating patients with a rhenium 188–labeled anti-CD66 monoclonal antibody. Dosimetry was performed prior to therapy, and a favorable dosimetry was observed in all cases. Radioimmunotherapy with the labeled antibody provided a mean of 15.3 Gy of additional radiation to the marrow; the kidney was the normal organ receiving the highest dose of supplemental radiation (mean 7.4 Gy). Radioimmunotherapy was followed by standard full-dose conditioning with total body irradiation (12 Gy) or busulfan and high-dose cyclophosphamide with or without thiotepa. Patients subsequently received a T-cell–depleted allogeneic graft from a HLA-identical family donor (n = 15) or an alternative donor (n = 17). In 4 patients without an allogeneic donor, an unmanipulated autologous graft was used. Infusion-related toxicity due to the labeled antibody was minimal, and no increase in treatment-related mortality due to the radioimmunoconjugate was observed. Day +30 and day +100 mortalities were 3% and 6%, respectively, and after a median follow-up of 18 months treatment-related mortality was 22%. Late renal toxicity was observed in 17% of patients. The relapse rate of 15 patients undergoing transplantation in first CR (complete remission) or second CR was 20%; 21 patients not in remission at the time of transplantation had a 30% relapse rate.
Introduction
The prognosis of patients with acute myeloid leukemia (AML) with favorable cytogenetics has improved considerably in recent years thanks to the introduction of aggressive consolidation chemotherapy.1,2 Patients with high-risk cytogenetics refractory to induction chemotherapy and who have relapsed after achieving a remission are essentially incurable by currently available chemotherapy, and the same is true for patients with high-risk myelodysplastic syndrome (MDS) (refractory anemia with excess blasts [RAEB]; refractory anemia with excess blasts in transition [RAEB-T]).3-5 Some of these high-risk AML and MDS patients can be cured by myeloablative chemoradiotherapy with stem cell support,6-9 but the outcome remains poor, with only 20% to 40% achieving long-term disease-free survival (DFS).10-13 This poor outcome is the result of both a significantly higher relapse rate and a higher transplant-related mortality.
Intensification of the chemotherapy used in the conditioning regimen has not improved outcome.14 In view of the high radiation sensitivity of leukemic cells, it is not surprising that several investigators have attempted to reduce the relapse rate after bone marrow transplantation (BMT) by increasing the dose of total body irradiation (TBI).15,16 Increasing the TBI dose from 12 to 15.75 Gy significantly reduced the relapse rate in patients with both AML in first complete remission (first CR) and chronic myeloid leukemia in chronic phase with cyclosporine/methotrexate as graft-versus-host (GVH) disease prophylaxis. This did not translate into an improved survival because patients treated with the higher dose of external beam radiation had a higher incidence of GVH disease and more hepatotoxicity, which resulted in a higher transplant-related mortality.15 16
A different experience was reported by Papadopoulos et al, who treated patients with AML in first CR with 15.75 Gy plus chemotherapy and transplanted T-cell–depleted grafts. They reported a relapse rate of 3% and a low rate of transplant-related mortality resulting in a DFS of 80%.17 No data on the feasibility of this approach have been reported in patients with advanced acute leukemia because of concerns about the importance of the GVH disease–associated graft-versus-leukemia effect in curing advanced leukaemias.18 19
An alternative approach would be to target the radiation to the marrow using radiolabeled antibodies and thus avoiding excessive organ toxicity. This approach has been pioneered in both animal models and patients by investigators from Seattle and New York using antibodies with specificity for CD33 or CD45 labeled with iodine 131 (131I).20-23 These studies clearly demonstrated the feasibility of this approach in heavily pretreated patients, and the recent update of the Seattle studies provided encouraging results.24
For the study reported here we chose an anti-CD66 (a, b, c, e) monoclonal antibody labeled with rhenium 188 (188Re). The anti-CD66 antibody (antigranulocyte, anti–nonspecific cross-reacting antigen 95, BW 250/183) is well characterized with respect to biokinetic data and clinical application in bone marrow scintigraphy and localization of infections.25-27 The CD66 antigen is expressed at a high density (2 × 105 molecules per cell) on normal myelopoietic cells from the promyelocyte onward but not on AML blasts.28-31 Aberrant expression is found on a significant fraction of CD10+ acute lymphoblastic leukemia (ALL) blasts.31,32 The CD66 molecule is neither internalized nor shed.27 The high affinity of the antibody for its target epitope and the abundant expression of the epitope in the marrow results in the accumulation of approximately 50% of the antibody in the marrow within 2 hours.25,26,33188Re is an almost pure β emitter with just enough γ radiation to permit imaging and a physical half-life of 17 hours.34 These properties substantially reduce the radioprotection requirements compared with 131I. We report our initial experience of combining the strategies of radioimmunotherapy with 188Re-labeled anti-CD66 antibody and T-cell depletion of the graft in 36 patients at high risk of both relapse and transplant-related death.
Patients, materials, and methods
Patients
The aim of the study was to include patients with a relapse risk of at least 40% to 50% after a conventional stem cell transplant. Thus, we included patients with AML beyond first CR, with AML in first CR if high-risk cytogenetic features were present or there was a poor response to primary induction chemotherapy, and patients with high-risk MDS (RAEB, RAEB-T). Patients were required to be in remission or good partial remission (PR) defined as no blasts in the periphery and 25% or fewer blasts in the marrow. The age limits were 16 to 65 years. We accepted HLA-identical and mismatched family members or a haploidentical sibling or parent as well as matched unrelated volunteers as donors. If no allogeneic donor was available, autologous peripheral blood progenitor cells (PBPCs) were acceptable as an alternative stem cell source. Patients were also required to be free of medical conditions excluding them from high-dose chemoradiotherapy and to have a favorable dosimetry. Favorable dosimetry was defined as a marrow or spleen dose higher than that of any other normal organ.
Over a period of 2 years, 36 patients were recruited (Table1). The patient population consisted of 15 males and 21 females; the median age was 48 years (range 17-63). The diagnosis was AML in 32 patients and MDS in 4 patients (Table 1). Of the 32 patients with AML, 23 were beyond first CR (17 in PR, 6 in second CR) and 9 patients with high-risk cytogenetic features (abn(3q),−5/5q−,−7/7q−,abn(12p),17p−, complex karyotype) and/or poor response to primary induction chemotherapy underwent transplantation in first CR.3 4 Primary study end points were feasibility and toxicity of the procedure, and secondary end points were the incidence of acute and chronic GVH disease and the frequency of relapses. The protocol for the study was approved by the Ethics Committee of Ulm University, and all patients and donors gave their written, informed consent.
Patient characteristics and outcome
| UPN . | Age . | Sex . | Disease . | Disease state . | Stem cell source . | Conditioning . | Outcome . |
|---|---|---|---|---|---|---|---|
| 623 | 38 | F | AML | PR | HLA-id fam | TBI/CTX | Alive, CR |
| 626 | 51 | M | AML | PR | HLA-id fam | TBI/CTX | Dead, relapse |
| 627 | 44 | M | AML | second CR | Mism fam | TBI/CTX | Alive, CR |
| 651 | 26 | F | AML | PR | Haplo-id fam | TBI/TT/CTX | Dead, toxicity |
| 656 | 49 | M | AML | second CR | HLA-id fam | TBI/CTX | Alive, CR |
| 660 | 35 | F | AML | second CR | MUD | TBI/CTX | Dead, toxicity |
| 664 | 33 | F | AML | PR | Haplo-id fam | TBI/TT/CTX | Alive, CR |
| 686 | 19 | F | AML | first CR | Autologous | TBI/CTX | Alive, CR |
| 689 | 59 | M | AML | PR | HLA-id fam | Bu/CTX | Dead, relapse |
| 696 | 40 | F | Secondary AML | first CR | MUD | TBI/CTX | Alive, CR |
| 697 | 51 | M | AML | first CR | Autologous | TBI/CTX | Dead, relapse |
| 698 | 47 | F | Secondary AML | PR | Mism fam | TBI/CTX | Alive, CR |
| 706 | 51 | M | AML | PR | Haplo-id fam | TBI/TT/CTX | Dead, toxicity |
| 709 | 60 | F | Secondary AML | PR | HLA-id fam | Bu/CTX | Alive, CR |
| 711 | 41 | M | AML | first CR | Autologous | TBI/CTX | Alive, CR* |
| 715 | 45 | M | AML | first CR | HLA-id fam | TBI/CTX | Dead, relapse |
| 717 | 47 | M | MDS | NC | HLA-id fam | TBI/CTX | Alive, CR |
| 718 | 55 | F | MDS | RAEB-T | HLA-id fam | Bu/CTX | Alive, CR |
| 729 | 50 | M | AML | PR | MUD | TBI/CTX | Dead, relapse |
| 732 | 36 | M | AML | first CR | HLA-id fam | TBI/CTX | Alive, CR |
| 733 | 23 | M | AML | PR | HLA-id fam | TBI/CTX | Dead, toxicity |
| 738 | 17 | M | AML | PR | HLA-id fam | TBI/CTX | Alive, CR |
| 740 | 57 | F | AML | first CR | Syngeneic | Bu/CTX | Alive, CR |
| 742 | 43 | M | AML | PR | MUD | TBI/CTX | Dead, relapse |
| 744 | 63 | M | MDS | RAEB-T | HLA-id fam | Bu/CTX | Dead, toxicity |
| 748 | 46 | M | MDS | NC | MUD | TBI/CTX | Dead, relapse |
| 749 | 45 | M | AML | PR | MUD | TBI/CTX | Dead, relapse |
| 754 | 60 | F | AML | second CR | Autologous | Bu/CTX | Alive, CR |
| 757 | 38 | M | AML | second CR | HLA-id fam | TBI/CTX | Alive, CR |
| 764 | 32 | F | AML | PR | MUD | TBI/CTX | Dead, toxicity |
| 784 | 59 | F | AML | first CR | HLA-id fam | Bu/CTX | Alive, CR |
| 788 | 33 | F | AML | first CR | MUD | TBI/CTX | Alive, CR |
| 795 | 33 | F | AML | PR | Haplo-id fam | TBI/TT/CTX | Dead, toxicity |
| 803 | 47 | F | Secondary AML | PR | MUD | TBI/CTX | Dead, toxicity |
| 807 | 50 | F | Secondary AML | PR | HLA-id fam | Bu/CTX | Alive, CR |
| 811 | 53 | F | AML | second CR | MUD | Bu/CTX | Alive, CR |
| UPN . | Age . | Sex . | Disease . | Disease state . | Stem cell source . | Conditioning . | Outcome . |
|---|---|---|---|---|---|---|---|
| 623 | 38 | F | AML | PR | HLA-id fam | TBI/CTX | Alive, CR |
| 626 | 51 | M | AML | PR | HLA-id fam | TBI/CTX | Dead, relapse |
| 627 | 44 | M | AML | second CR | Mism fam | TBI/CTX | Alive, CR |
| 651 | 26 | F | AML | PR | Haplo-id fam | TBI/TT/CTX | Dead, toxicity |
| 656 | 49 | M | AML | second CR | HLA-id fam | TBI/CTX | Alive, CR |
| 660 | 35 | F | AML | second CR | MUD | TBI/CTX | Dead, toxicity |
| 664 | 33 | F | AML | PR | Haplo-id fam | TBI/TT/CTX | Alive, CR |
| 686 | 19 | F | AML | first CR | Autologous | TBI/CTX | Alive, CR |
| 689 | 59 | M | AML | PR | HLA-id fam | Bu/CTX | Dead, relapse |
| 696 | 40 | F | Secondary AML | first CR | MUD | TBI/CTX | Alive, CR |
| 697 | 51 | M | AML | first CR | Autologous | TBI/CTX | Dead, relapse |
| 698 | 47 | F | Secondary AML | PR | Mism fam | TBI/CTX | Alive, CR |
| 706 | 51 | M | AML | PR | Haplo-id fam | TBI/TT/CTX | Dead, toxicity |
| 709 | 60 | F | Secondary AML | PR | HLA-id fam | Bu/CTX | Alive, CR |
| 711 | 41 | M | AML | first CR | Autologous | TBI/CTX | Alive, CR* |
| 715 | 45 | M | AML | first CR | HLA-id fam | TBI/CTX | Dead, relapse |
| 717 | 47 | M | MDS | NC | HLA-id fam | TBI/CTX | Alive, CR |
| 718 | 55 | F | MDS | RAEB-T | HLA-id fam | Bu/CTX | Alive, CR |
| 729 | 50 | M | AML | PR | MUD | TBI/CTX | Dead, relapse |
| 732 | 36 | M | AML | first CR | HLA-id fam | TBI/CTX | Alive, CR |
| 733 | 23 | M | AML | PR | HLA-id fam | TBI/CTX | Dead, toxicity |
| 738 | 17 | M | AML | PR | HLA-id fam | TBI/CTX | Alive, CR |
| 740 | 57 | F | AML | first CR | Syngeneic | Bu/CTX | Alive, CR |
| 742 | 43 | M | AML | PR | MUD | TBI/CTX | Dead, relapse |
| 744 | 63 | M | MDS | RAEB-T | HLA-id fam | Bu/CTX | Dead, toxicity |
| 748 | 46 | M | MDS | NC | MUD | TBI/CTX | Dead, relapse |
| 749 | 45 | M | AML | PR | MUD | TBI/CTX | Dead, relapse |
| 754 | 60 | F | AML | second CR | Autologous | Bu/CTX | Alive, CR |
| 757 | 38 | M | AML | second CR | HLA-id fam | TBI/CTX | Alive, CR |
| 764 | 32 | F | AML | PR | MUD | TBI/CTX | Dead, toxicity |
| 784 | 59 | F | AML | first CR | HLA-id fam | Bu/CTX | Alive, CR |
| 788 | 33 | F | AML | first CR | MUD | TBI/CTX | Alive, CR |
| 795 | 33 | F | AML | PR | Haplo-id fam | TBI/TT/CTX | Dead, toxicity |
| 803 | 47 | F | Secondary AML | PR | MUD | TBI/CTX | Dead, toxicity |
| 807 | 50 | F | Secondary AML | PR | HLA-id fam | Bu/CTX | Alive, CR |
| 811 | 53 | F | AML | second CR | MUD | Bu/CTX | Alive, CR |
UPN indicates unique patient number; AML, acute myeloid leukemia; PR, partial remission; HLA-id fam, HLA-identical family donor; TBI/TT/CTX, 6 × 2 Gy + 10 mg/kg thiotepa + 120 mg/kg cyclophosphamide; CR, complete remission; Mism fam, mismatched family donor; Haplo-id fam, haploidentical family donor; MUD, matched unrelated donor; Bu, busulfan; MDS, myelodysplastic syndrome; NC, not classifiable.
After MUD transplant.
Antibody labeling
The antibody used for radioimmunotherapy was the anti-CD66 (a, b, c, e) monoclonal antibody (antigranulocyte, Scintec Diagnostics, Zug, Switzerland). This is a mouse immunoglobulin G1 antibody with a high affinity for the CD66 antigen (2 × 109M/L).33188Re was obtained from a188W/188Re radionuclide generator as a solution of sodium perrhenate in saline. The generator was supplied by the Oak Ridge National Laboratory (Oak Ridge, TN). Full details of generator performance have been published elsewhere.35 The labeling procedure for the antibody has been previously described.36 Quality control included high-pressure liquid chromatography with simultaneous monitoring of protein (ultraviolet absorption at 280 nm wavelength) and radioactivity (NaI γ detector) to characterize the labeled product as well as instant thin-layer chromatography to determine the amount of colloids. 188Re incorporation was more than 95% in the final product with less than 3% unbound 188Re perrhenate and less than 2% colloid. Immunoreactivity of the antibody after labeling was evaluated by fluorescence-activated cell sorter analysis and was determined to be 99.3%.
Dosimetry
The methodology used for dosimetry in our study has been published recently.37 In all patients, individual dosimetry was performed after intravenous infusion of 1 to 2 mg anti-CD66 antibody labeled with 1.2 ± 0.6 GBq. Biodistribution of radioimmunoconjugates was measured with whole body imaging by means of a γ camera (Whole Body Imager, Siemens, Erlangen, Germany) in anterior and posterior projections at 1.5, 3, 20, 26, and 44 hours postinjection. Radioactivity excretion in the urine was quantitatively determined until 48 hours postinjection. Because the radioimmunoconjugates used are not excreted with the feces, no sampling of stool was performed. Percent injected dose in organs was determined by the geometric mean of count rates sampled from anterior and posterior regions of interest by γ camera images of respective tissue or organs. After calibration and subtraction of radioactivity excreted with urine, whole body radioactivity measured by γ camera was normalized to the injected dose. Radioactivity in the remainder of the body was calculated by subtracting the sum of organ radioactivity from whole body radioactivity. Decay-corrected radioactivity of organs with significant radioactivity retention (bone marrow, liver, spleen, kidneys) and the remainder of the body were fitted with up to 3 coupled exponential functions with up to 4 parameters. Organ residence times were determined and radioactive exposure calculated using MIRDOSE 3 software.38
Radioimmunotherapy
Patients with a favorable biodistribution as defined above were treated. The intention of the study was to give each patient the highest tolerable dose. No dose escalation study was performed. The dose injected was determined by the results of the biodistribution studies, the activity of the generator, and the type of conditioning. For patients receiving additional TBI with 12 Gy, the limiting organ doses for the bone marrow and liver were derived from the studies of Matthews et al and set at 25 Gy and 7 to 10 Gy, respectively.22,24 The upper limit for the kidney was defined as 12 Gy based on published studies on the radiation tolerance of the kidneys.39,40 For patients not receiving TBI, the dose limits were 35 Gy for the marrow, 19 to 24 Gy for the liver, and 20 Gy for the kidney.40 The therapeutic antibody was given intravenously over a period of 10 minutes in 1 to 2 fractions of 1 to 2 mg labeled antibody 24 to 48 hours after the completion of dosimetry and on day 14 relative to the transplant to ensure elimination of the nuclide. Dose fractionation had no influence on the biodistribution of the antibody.37 To prevent 188Re uptake into the thyroid gland and gastric mucosa, all patients were treated with 3 × 480 mg perchlorate (Irenat, Bayer, Leverkusen, Germany) beginning 24 hours before dosimetry and continuing for 1 week after the last antibody infusion. Radioimmunotherapy was performed in radiation isolation rooms, and patients usually remained there for 48 hours as required by German radioprotection regulations.
Conditioning
All patients were given additional conditioning therapy after radioimmunotherapy. Three protocols were used. Patients with matched family donors, matched unrelated donors, and receiving autologous PBPCs were treated with either TBI 12 Gy plus cyclophosphamide 120 mg/kg (n = 24) or intravenous busulfan 12.8 mg/kg plus cyclophosphamide 120 mg/kg (n = 8). Patients with haploidentical family donors (n = 4) were conditioned with TBI 12 Gy plus thiotepa 10 mg/kg plus cyclophosphamide 120 mg/kg. In all patients receiving TBI, renal shielding was used to reduce the radiation exposure of the kidneys from TBI to 6 Gy. In patients with mismatched family donors or receiving a graft from a matched unrelated donor, conditioning was intensified by adding antithymocyte globulin 5 mg/kg from day −4 to day −1 (Fresenius, Bad Homburg, Germany) to prevent graft rejection.
Donors and grafts
Thirty patients received granulocyte colony-stimulating factor (G-CSF)-mobilized PBPC grafts; bone marrow was the source of stem cells in 6 cases. PBPCs were obtained by treating donors with G-CSF 2 × 6 μg/kg/d for 4 to 6 days. Between days 4 and 6 of G-CSF treatment, 1 to 3 leukaphereses were performed using the Cobe Spectra (Cobe, Lakewood, CO) cell separator. Bone marrow was harvested by multiple aspirations under general anesthesia. Thirty-one patients received an allogeneic PBPC or bone marrow graft, 1 patient syngeneic PBPCs, and 4 patients unmanipulated autologous PBPCs. The donors for the 32 patients receiving an allogeneic transplant were HLA-identical siblings in 15 cases, matched unrelated donors in 10 cases, a mismatched family donor in 2 cases, a haploidentical family member in 4 cases, and a twin in 1 case.
GVH disease prophylaxis
Thirty of the 32 allogeneic stem cell grafts were T-cell–depleted. Two methods of T-cell depletion were employed depending on the risk of GVH disease. In patients with HLA-compatible family donors, 15 of 17 grafts (13 PBPC, 2 bone marrow) were T-cell–depleted by adding the humanized anti-CD52 monoclonal antibody Campath 1H to the leukaphereses.41 Depending on the nucleated cell count, 10, 20, or 30 mg Campath 1H was added to each PBPC or bone marrow graft, and the antibody and cell suspension were gently mixed for 30 minutes at room temperature. In patients receiving a PBPC graft from a matched unrelated donor or a haploidenical family donor, T-cell depletion was performed by CD 34+ selection using the immunomagnetic CliniMACS device (Miltenyi Biotec, Bergisch Gladbach, Germany).42 The target T-cell doses were less than 1 × 105/kg and CD3+ cells less than 5 × 104/kg. Bone marrow from matched unrelated donors was T-cell–depleted using the Campath 1H in-the-bag approach in 3 cases.43 T-cell depletion was the sole GVH disease prophylaxis in 26 patients. In 1 patient given a matched unrelated bone marrow graft, we gave cyclosporine, mycophenolate mofetil, and steroids as GVH disease prophylaxis, and 3 patients were given additional cyclosporine.
Supportive care
Patients were treated in single rooms. Irradiated erythrocytes and platelets were transfused if the hemoglobin level dropped below 80g/L (8 g/dL) or the platelet count below 20 × 109/L (20 000/μL). Cytomegalovirus (CMV)-seronegative blood donors were used if both stem cell donor and recipient were CMV-seronegative. All patients were given prophylactic ofloxacin, fluconazole, acyclovir, and cotrimoxazole. CMV-seropositive patients (n = 18) received prophylactic gancyclovir (5 mg/kg/d intravenously) from day +7 to day +21. Preemptive therapy with gancyclovir 2 × 5 mg/kg intravenously or foscarnet 3 × 60 mg/kg was instituted if patients became positive in the CMV antigenemia test.44Nine patients (25%) were treated with amphotericin for definite or very probable aspergillus pneumonia during induction chemotherapy and required amphotericin prophylaxis (0.5 mg/kg) during the transplant period. Prophylaxis with acyclovir, fluconazole, and cotrimoxazole was maintained until patients had achieved CD4+ T cells numbering more than 0.20 × 109/L (200/μL). Thirty-one patients were treated with G-CSF 5 μg/kg from day + 7 until a stable neutrophil count of more than 1.0 × 109/L (1000/μL) was reached.
Diagnosis of GVH disease
Patients were evaluable for acute GVH disease if they engrafted and survived at least 21 days and for chronic GVH disease if they survived more than 90 days after transplantation. The diagnosis of acute and chronic GVH disease was established by using standard clinical or histopathological criteria.45
Evaluation of regimen-related toxicity
Evaluation of regimen-related toxicity was performed using the Bearman scale, which was specifically designed to evaluate organ toxicity after high-dose chemoradiotherapy and which excludes the effects of infections, GVH disease, and bleeding.46
Results
Dosimetry
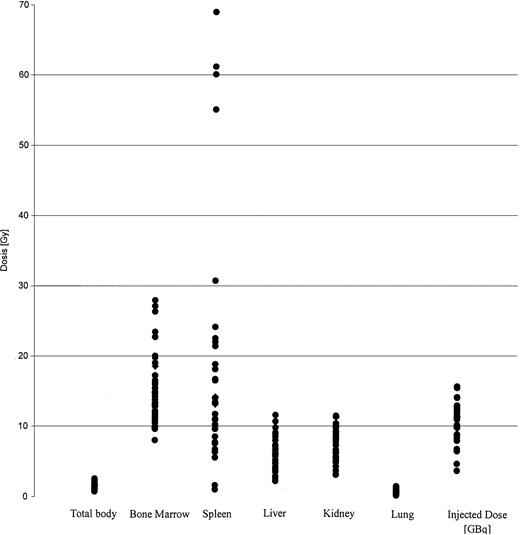
The results of dosimetry for some of these patients have been previously published.37 The tracer dose consisted of 1 to 2 mg anti-CD66 antibody labeled with 1.2 ± 0.6 GBq188Re. The application of the tracer dose was tolerated without any significant reaction by all 36 patients. All of these patients had a favorable dosimetry as defined above and proceeded to transplantation. For therapy a mean of 11.1 ± 2.2 GBq was injected in 1 to 2 fractions. A typical example is given in Figure1. The detailed results of dosimetry are shown in Figure 2 and Table2. The total doses shown in Table 2include the radiation exposure due to the dosimetric dose. The mean red marrow dose achieved was 15.3 ± 4.8 Gy, and the median dose was 14.9 Gy, ranging from 8.1 to 28 Gy. The kidney was the normal organ receiving the highest dose in 22 patients; the liver in 10 patients; and equivalent doses to kidney and liver were observed in 4 patients. Thus, the radiation absorbed doses were red marrow, 1.5 ± 0.6; spleen, 1.6 ± 1.3; liver, 0.5 ± 0.2; kidney, 0.7 ± 0.2; and lung, 0.1 ± 0.1 Gy/GBq. These values therefore give the following marrow:organ ratios: marrow:liver, 3:1; marrow:kidney, 2.1:1; and marrow:lung, 15:1. We observed no significant differences between the marrow doses of patients in complete remission and those in PR. The acute toxicity of the therapeutic antibody application was very mild, with two-thirds of patients complaining of mild nausea; no episodes of severe toxicity were observed.
Favorable dosimetry in a patient with AML in PR (unique patient number 664).
Biodistribution of 1 mg 188Re-labeled anti-CD66 monoclonal antibody after 40 hours, in anteroposterior and posteroanterior projection.
Favorable dosimetry in a patient with AML in PR (unique patient number 664).
Biodistribution of 1 mg 188Re-labeled anti-CD66 monoclonal antibody after 40 hours, in anteroposterior and posteroanterior projection.
Biodistribution of 188 Re-labeled anti-CD66 antibody.
Dose distribution of 188 Re-labeled anti-CD66 antibody in all 36 patients, in Gy per organ. The injected dose of188Re is given in GBq.
Biodistribution of 188 Re-labeled anti-CD66 antibody.
Dose distribution of 188 Re-labeled anti-CD66 antibody in all 36 patients, in Gy per organ. The injected dose of188Re is given in GBq.
Results of dosimetry
| Organ . | Radiation absorbed dose, Gy/GBq . | Total dose in Gy, mean ± SD . | Total dose in Gy, median (range) . |
|---|---|---|---|
| Bone marrow | 1.5 ± 0.6 | 15.3 ± 4.9 | 14.9 (8.1-28) |
| Spleen | 1.7 ± 1.3 | 19.5 ± 17.5 | 14.2 (1.1-69) |
| Liver | 0.5 ± 0.2 | 6.0 ± 2.3 | 6.1 (2.3-11.7) |
| Kidney | 0.7 ± 0.2 | 7.4 ± 2.3 | 7.2 (3.3-11.6) |
| Lung | 0.1 ± 0.1 | 0.9 ± 0.8 | 0.9 (0.3-1.6) |
| Total body | 0.1 ± 0.04 | 1.5 ± 0.4 | 1.5 (0.8-2.6) |
| Organ . | Radiation absorbed dose, Gy/GBq . | Total dose in Gy, mean ± SD . | Total dose in Gy, median (range) . |
|---|---|---|---|
| Bone marrow | 1.5 ± 0.6 | 15.3 ± 4.9 | 14.9 (8.1-28) |
| Spleen | 1.7 ± 1.3 | 19.5 ± 17.5 | 14.2 (1.1-69) |
| Liver | 0.5 ± 0.2 | 6.0 ± 2.3 | 6.1 (2.3-11.7) |
| Kidney | 0.7 ± 0.2 | 7.4 ± 2.3 | 7.2 (3.3-11.6) |
| Lung | 0.1 ± 0.1 | 0.9 ± 0.8 | 0.9 (0.3-1.6) |
| Total body | 0.1 ± 0.04 | 1.5 ± 0.4 | 1.5 (0.8-2.6) |
Engraftment and hematologic reconstitution
All 36 patients achieved rapid and stable engraftment. The median time to achieve more than 0.5 × 109/L (500/μL) neutrophils was 11 days, and the median time to more than 20.0 × 109/L (20 000/μL) thrombocytes was 12 days.
Regimen-related toxicity
The regimen-related toxicity observed after this intensified conditioning regimen was moderate and not significantly different from that observed after conventional conditioning in patients receiving T-cell–depleted grafts. Stomatitis was the most common toxicity, with 2 patients developing grade 1 stomatitis and 34 patients grade 2 stomatitis. The second most common toxicity was gastrointestinal, with 19 patients suffering from diarrhea, grade 1 in 16 patients and grade 2 in 3 patients. Grade 1 renal toxicity was observed in 9 patients, grade 2 in 2 patients, and grade 3 in 1 patient. The more severe renal toxicity developed more than 6 months after transplantation. Renal biopsies are available for the patient requiring dialysis and for one of the patients with grade 2 toxicity. In the case of the patient with end-stage renal disease, the renal pathologist suggested a viral etiology (CMV, BK virus); in the second case a radiation nephropathy was diagnosed. Overall, 5 patients showed the clinical syndrome of bone marrow radiation nephropathy with hypertension, raised serum creatinine level, anemia, and urinary abnormalities.47 Grade 1 hepatic toxicity was seen in 5 patients, and 1 patient developed grade 3 veno-occlusive disease. Finally, 1 case of grade 2 cardiac toxicity was observed.
Graft-versus-host disease
No patient developed severe acute GVH disease. Clinically relevant grade 2 acute GVH disease developed in 3 patients (9%), and mild grade 1 acute GVH disease was observed in 40% of patients. Extensive mild to moderate chronic GVH disease was documented in 9 patients (36%).
Relapse
After a median follow-up of 18 months (range 6-30 months), 9 of 35 (26%) evaluable patients have relapsed, 2 of 4 autologous cases (50%) and 7 of 32 (23%) allogeneic cases. The relapse rate was 20% for patients undergoing transplantation in remission and 30% for patients not in remission at the time of transplantation. Most of the relapses have occurred between 6 and 12 months after transplantation; none of the patients who have survived more than 1 year (n = 10) have so far relapsed. Three of the 9 relapses occurred primarily in extramedullary sites (skin, lymph node, muscle).
Transplant-related mortality
Overall, 8 of 36 (22%) patients have died of transplant-related toxicity. In the subgroup of patients receiving an allogeneic transplant (n = 32), transplant-related mortality was 24%. One patient died within the first 30 days (3%) and a total of 2 patients (6%) within the first 100 days. The risk of dying of transplant-related toxicity was dependent on the type of graft. None of the patients with an autologous graft died of toxicity, whereas transplant-related mortality was 11% with a compatible family donor, 40% with a matched unrelated donor, and 75% after a haploidentical transplant.
Causes of death
Eight of the 9 patients who relapsed have died; 1 patient who relapsed after an autologous transplant is still alive after a matched unrelated donor (MUD) transplant. For the 8 patients who died of transplant-related toxicity, the causes of death were cerebral hemorrhage (n = 1), sepsis (n = 1), pneumonia (1 influenza, 1 CMV, 1 aspergillus, 1 unknown pathogen), Epstein-Barr virus–lymphoproliferative disease (n = 1), and viral encephalitis (n = 1).
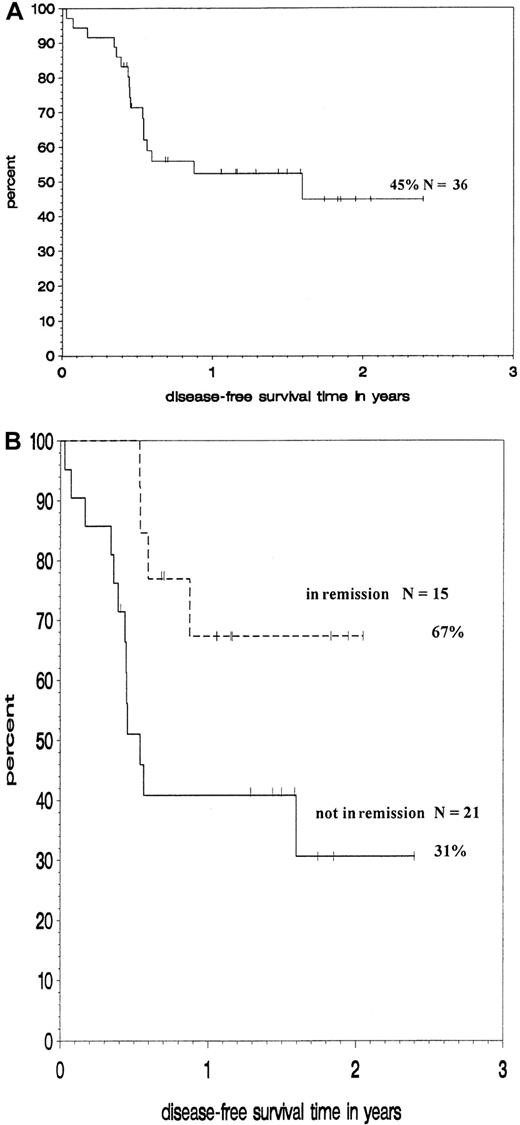
Survival and disease-free survival
Figure 3 shows Kaplan-Maier estimates for DFS at 18 months for the whole group and for subgroups according to their remission status. DFS for the whole group is 45%. Patients undergoing transplantation in remission had a significantly higher probability of DFS (67%) than those undergoing transplantation while not in remission (31%, P = .02 by log-rank test).
Outcome of patients treated with188Re-labeled anti-CD66 antibody.
Kaplan-Meier analysis of DFS of all patients (A) and of subgroups according to remission status at transplantation (B).
Outcome of patients treated with188Re-labeled anti-CD66 antibody.
Kaplan-Meier analysis of DFS of all patients (A) and of subgroups according to remission status at transplantation (B).
Discussion
The primary aim of this study was to evaluate the feasibility and safety of intensifying the conditioning regimen for patients with high-risk myeloid malignancies prior to stem cell transplantation by adding a 188Re-labeled anti-CD66 (a, b, c, e) monoclonal antibody. The concept of using radiolabeled monoclonal antibodies for the treatment of leukemias was pioneered by workers in Seattle and New York in the early 1990s.21-23 48 Feasibility problems have restricted the application of this approach to very few large transplant centers. One major problem has been the choice of therapeutic nuclide. Practically all the published studies have used131I as nuclide, and since 131I emits a significant amount of γ radiation and has a long physical half-life of 8 days this necessitates prolonged isolation of the patient in lead-lined radiation isolation rooms.
We have attempted to improve the feasibility of radioimmunotherapy in the context of stem cell transplantation by replacing 131I with 188Re as the therapeutic nuclide and by using the anti-CD66 monoclonal antibody. 188Re was chosen as the therapeutic nuclide because it is an almost pure β emitter (85%) and because of the high energy of its β emissions (2.1 MeV) and the short half-life of 17 hours.34 These favorable properties should make it possible to limit the radioprotective measures required. The anti-CD66 antibody was chosen for a number of reasons, and feasibility was a major consideration. The antibody has been extensively used in immunoscintigraphy over the past 10 years and is commercially available as a kit. In these diagnostic studies no relevant binding to normal tissues other than bone marrow has been observed.25,26 Thanks to the large number of target sites and its high affinity, the antibody rapidly and quantitatively accumulates in the marrow, thus ensuring a potent crossfire effect.24,25,33,49,50 We felt that these advantageous properties outweighed potential disadvantages such as a lack of binding to myeloid leukaemic blasts.28-30 32 This reliance on the crossfire effect clearly imposes certain limitations on the kinds of patients that can be treated with this approach. Thus, we restricted ourselves to treating patients in remission or PR.
The results presented in this paper clearly confirm our hypothesis with respect to the feasibility of our approach. A favorable dosimetry was observed in all patients tested, and we were able to recruit 36 patients over a period of 2 years.
The acute toxicity of the application of both the tracer and the therapeutic antibody dose compares favorably with that reported by Matthews et al. In contrast to the Seattle experience, no cytokine release reaction was observed in our cohort after infusion of the anti-CD66 antibody. This difference is probably due to the very much lower amount of foreign protein infused into our patients. The total dose of anti-CD66 injected ranged from 2 to 4 mg, compared with 50 to 74 mg 131I-labeled anti-CD45 antibody in the Seattle study.22 24
The biodistribution studies showed that it was possible to deliver on average an additional 15.3 Gy to the marrow while exposing liver, kidney, and lung to mean of 6 Gy, 7.4 Gy, and 0.9 Gy, respectively. Taken at face value, these data suggest that we have more than doubled the therapeutic radiation dose to the marrow compared with standard TBI-based conditioning regimens. This statement must be qualified, however, in view of the limitations of the dosimetric techniques available for estimating the red marrow dose and the uncertainty concerning the biological efficacy of low-dose rate radiation.51-54
The mean therapeutic ratio of radiation delivered to the marrow as compared to the liver of 3:1 was very similar to that reported by the Seattle team, whereas the therapeutic ratios for the lung (15:1) and for the kidney (2.1:1) were substiantially different, favoring the anti-CD66 antibody in the case of the lungs and the anti-CD45 antibody in the case of the kidneys.22,24 For the anti-CD45 antibody, the liver was the dose-limiting organ in all but one of the patients. These differences in biodistribution are probably the result of choosing 188Re as the therapeutic nuclide. Radiometals such as 188Re tend to accumulate in the kidney by the tubular reabsorption of labeled peptides.55
Acute regimen-related organ toxicity was low and no higher than anticipated for this group of high-risk patients. Only one patient experienced grade 3 liver toxicity, and the day +30 (3%) and day +100 (6%) mortalities observed are considerably lower than expected from data reported by the International Bone Marrow Transplant Registries for similar patients.56 This is also true for early renal toxicity. No patient experienced more than grade 1 toxicity, and the incidence of 10% is at the lower end of what is reported in the literature.57 More worrisome is the fact that a rise in serum creatinine level was observed in 6 patients (17%) 6 to 12 months after transplantation. This late renal toxicity was mild (grade 1) in 2 patients, moderate (grade 2) in 3 patients, and severe (grade 3) in 1 patient. Chronic radiation nephritis was identified as the cause by renal biopsy in only 1 patient; however, 4 of the 5 patients fulfill the diagnostic criteria for BMT nephropathy as defined by Cohen et al, ie, a rise in creatinine occurring more than 100 days after BMT with concurrent hypertension and anemia in the absence of an identifiable nephrotoxin.47 In the most severely affected patient, who developed end-stage renal disease, no evidence for the typical histologic features of radiation nephritis could be found in 2 sequential renal biopsies, and the chronic renal failure was ascribed to drug toxicity (gancyclovir) and infection of the kidney with polyomavirus and CMV.
BMT nephropathy is a fairly common complication after both autologous and allogeneic stem cell transplantation, with an incidence ranging from 5% to 50%.47,58-61 Although patients after stem cell transplantation are exposed to a multitude of nephrotoxins, such as aminoglycosides, amphotericin, vancomycin, or cyclosporine, several studies in recent years have identified TBI as the main cause.47,57,62 The most convincing evidence for this hypothesis is the fact that BMT nephropathy is practically never observed after conditioning regimens excluding TBI, that there is a clear dose-response relationship and, finally, that the risk of BMT nephropathy can be reduced by renal shielding.59,63 64
We had anticipated that some renal toxicity and renal shielding was used in all patients receiving TBI. This reduced the radiation exposure of the kidney due to TBI to 6 Gy. The mean kidney dose due to the radiolabeled antibody was 7.0 Gy, resulting in a mean total dose to the kidney of 13.0 Gy. The mean kidney dose of the 5 patients with the diagnosis of BMT nephropathy was 14.7 Gy. The incidence of BMT nephropathy in patients with a kidney dose of more than 12 Gy was 29% compared with 5% in those with a kidney dose of less than 12 Gy. The incidence of BMT nephropathy we have observed so far is thus similar to that in patients being conditioned with TBI doses of more than 12 Gy reported in other studies.59 64 So far, no case of BMT nephropathy has been observed in patients conditioned with busulfan/cyclophosphamide. The incidence of BMT nephropathy in our study could be reduced by replacing TBI by busulfan or by restricting total radiation exposure of the kidney to 12 Gy.
Engraftment was rapid and durable in all 36 patients, and we therefore have no evidence of any stromal damage due to the radioimmunoconjugates, although marrow doses of up to 40 Gy were observed. The speed of engraftment is the result of using G-CSF–mobilized peripheral blood stem cells in most patients and the routine application of G-CSF from day +7.
The intensified conditioning regimen has had no negative impact on other important variables of outcome after allogeneic stem cell transplantation such as the incidence of GVH disease and transplant-related mortality. In our study only 9% of patients developed clinically relevant acute GVH disease; no cases of severe acute GVH disease were observed. Slightly more than one-third of the patients developed mild to moderate chronic GVH disease. Given the fact that more than 50% of patients received allogeneic stem cell grafts from alternative donors, the incidence of acute and chronic GVH disease is low.65,66 This low incidence of clinically relevant GVH disease is due to the use of in vivo/ex vivo T-cell depletion, which effectively reduces the risk of acute and chronic GVH disease while ensuring stable engraftment.17,67 68
Transplant-related mortality is mainly determined by the age of the patient, the stage of disease, the type of donor, and the intensity of the conditioning regimen.10,46,56 If one takes these variables into account, one would anticipate a transplant-related death rate of 30% to 40% for our cohort of high-risk patients. We therefore consider the overall incidence of transplant-related deaths of 22% to be encouraging, especially because Matthews et al reported a transplant-related mortality of 20% for a cohort of 34 patients given either HLA-identical sibling or autologous stem cell grafts after intensification of the conditioning regimen with131I-labeled anti-CD45 antibody.24 The risk of transplant-related death in our study was mainly determined by the stem cell donor, ranging from 11% for a HLA-compatible sibling donor to 75% for a haploidentical family donor. Overall, our results provide no evidence that intensification of the conditioning regimen with188Re-labeled anti-CD66 monoclonal antibody has increased the risk of treatment-related death, although the potential long-term impact of the late renal toxicity remains to be determined.
Although antileukemic efficacy was not the primary end point of this phase I-II study, the impact of using T-cell depletion as GVH disease prophylaxis in this cohort of patients at high risk of relapse should be considered. In patients given standard conditioning regimens, T-cell depletion has been associated with an increased risk of early relapse in both early and advanced AML.18 In our cohort of patients we have observed a relapse rate of 20% in patients undergoing transplantation in first or second remission and of 30% for those undergoing transplantation while not in remission. Because the International Bone Marrow Transplant Registries reports relapse rates of 40% and 60% to 70%, respectively, at 1 year for these 2 subgroups of patients after T-cell–depleted HLA-identical sibling transplants, we are confident that our T-cell depletion strategy has not had a major negative impact on relapse rates.
In summary, we report a feasible approach to the intensification of conditioning for patients with high-risk myeloid malignancies using a188Re-labeled anti-CD66 antibody.
We thank Dr G. Hale and Prof H. Waldmann (Oxford University) for providing Campath 1H. We also acknowledge the efforts of the technical staff of the Red Cross Bloodbank in Ulm as well as the technical staff in the Department of Nuclear Medicine. Above all, we thank the nursing staff of the Adult BMT Unit for its outstanding patient care.
Supported by Deutsche Krebshilfe grant 70-2388-Bu 1.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Donald Bunjes, Dept of Haematology/Oncology, Ulm University Hospital, Robert-Koch-Strasse 8, 89081 Ulm, Germany; e-mail:donald.bunjes@medizin.uni-ulm.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal