To better understand the control of apoptosis during erythropoiesis, this study investigated the role of a novel tumor-associated antigen, RCAS1 (receptor binding cancer antigen expressed on SiSo cells), with regard to the regulation of apoptosis of erythroid progenitor cells. Erythroid colony-forming cells (ECFCs) purified from human peripheral blood were used. Binding experiments of RCAS1 showed that ECFCs abundantly expressed receptors (RCAS1R) for RCAS1 and that the degree of binding of RCAS1 to the receptors diminished rapidly during erythroid maturation in vitro. When the soluble form of RCAS1 was added to the cultures, ECFCs underwent apoptosis, including collapse of the mitochondrial transmembrane potential, and activation of caspases 8 and 3. The addition of an anti-Fas blocking antibody or Fas-Fc failed to reduce the apoptosis induced by RCAS1, thereby indicating that effects of RCAS1 are independent of Fas activation. When binding of RCAS1 to normal bone marrow cells was analyzed, RCAS1R was evident on cells with an immature erythroid phenotype (transferrin receptor+/glycophorin A−) but not with a mature phenotype (transferrin receptor−/glycophorin A+). Histochemical staining revealed the expression of RCAS1 in the cytoplasm of bone marrow macrophages. These findings indicate that RCAS1, which is mainly produced by macrophages in hematopoietic tissue, may have a crucial role in controlling erythropoiesis by modulating apoptosis of erythroid progenitor cells via a Fas-independent mechanism.
Introduction
Homeostasis of hematopoietic tissue is maintained by a balance between cell death and cell proliferation. Erythropoiesis is tightly regulated by an interacting network of various hematopoietic growth factors, such as erythropoietin (EPO), interleukin-3 (IL-3), insulinlike growth factor I (IGF-I), and stem cell factor (SCF),1 all of which support survival of purified erythroid progenitor cells by reducing apoptosis, as well as by regulating cellular differentiation and proliferation.2,3In vitro growth of erythroid progenitor cells depends on EPO, and deprivation of this growth factor from the culture medium results in immediate apoptosis of the cells, which means that erythrocyte production is partly regulated by controlling apoptosis of erythroid progenitor cells.4,5 Fas (CD95/APO-1) is a member of tumor necrosis factor (TNF) family and Fas stimulation results in apoptosis of target cells.6 As functional Fas and Fas ligand (FasL) are expressed by erythroid cells, the Fas/FasL system has a role in maintaining hematopoietic homeostasis by modulating apoptosis.6,7 However, the Fas knock-out mouse did not show evidence of abnormal regulation of hematopoiesis, such as erythrocytosis.5 8-10 Therefore, there may be regulators of apoptosis of erythroid progenitor cells other than Fas/FasL.
A tumor-associated antigen, RCAS1 (receptor binding cancer antigen expressed on SiSo cells), was found to induce apoptosis of activated T cells that express the RCAS1-receptor (RCAS1R).11 RCAS1 is a type II membrane protein isolated from a human uterine adenocarcinoma cell line, SiSo.12 The expression of RCAS1, or RCAS1R, has been confirmed in a variety of tumor cell lines.13 K562, a human leukemic cell line with the potential for erythroid differentiation, expresses functional RCAS1R.11 This evidence prompted us to evaluate the expression of RCAS1R on normal human erythroid progenitor cells.
We investigated the expression of RCAS1R and the effects of its ligand on human erythroid progenitor cells, isolated from human peripheral blood by negative panning and subsequent in vitro expansion, using a combination of hematopoietic growth factors. Our observations revealed that RCAS1 is involved in regulating of apoptosis of erythroid progenitor cells, and a critical role in controlling erythropoiesis can be considered.
Materials and methods
Reagents
Recombinant human erythropoietin (rhEPO) was provided by Chugai Pharmaceutical (Tokyo, Japan); recombinant human interleukin 3 (rhIL-3) and recombinant human stem cell factor (rhSCF) were provided by Kirin-Brewery (Tokyo, Japan). Anti-Fas neutralizing monoclonal antibody (MoAb) (clone 4B4-3B) and Fas-Fc (a chimeric protein containing the extracellular region of human Fas and the Fc portion of human IgG1) were provided by Mochida Pharmaceutical (Tokyo, Japan). Mouse anti-IgG1 antibody was purchased from Life Technologies (Rockville, MD). The soluble form of RCAS1 (nRCAS1) was purified by gel filtration from culture supernatants of SiSo cells, as described.11 The specificity of the nRCAS1 was confirmed by absorption with the 22-1-1 antibody.11
Purification and expansion of ECFCs
The ECFCs were prepared using documented methods.14-16 Light-density mononuclear cells were obtained from peripheral blood buffy coats from normal, healthy Japanese volunteers, using density centrifugation. Adherent cells were depleted by a 1-hour incubation in a polystyrene tissue culture flask at 4°C. Nonadherent cells were then collected and 2 cycles of negative selection were run, using anti-CD3, CD11b, CD15, and CD45RA antibodies and immunomagnetic beads with Vario-MACS columns (Miltenyi Biotech, Auburn, CA). The remaining cells were then cultured in Iscoves modified Dulbecco medium (IMDM; Gibco BRL, Grand Island, NY) containing 15% heat- inactivated fetal calf serum (FCS; Commonwealth Serum Laboratories, Melbourne, Australia), 15% pooled human AB serum, 2 U/mL rhEPO, 20 ng/mL rhSCF, 10 ng/mL rhIL-3, 100 U/mL, penicillin, and 100 μg/mL streptomycin (Gibco BRL) at 37°C in a high-humidity 5% CO2, 95% air incubator (day 0). On day 3, the cells, referred to as day 3 ECFCs, were centrifuged over lymphocyte separation medium (LSM), then collected and incubated under the same conditions, but without rhIL-3. The cultured cells, collected on day 7, are referred to as day 7 ECFCs, and were used in the following experiments. The purity of the day 7 ECFCs, with proerythroblastlike features, was 95% ± 3%, as determined by cytospin preparations. Cell purity was assessed in each experiment.
Serum-free liquid culture of ECFCs
The cells (day 6 ECFCs, 1.0 × 105cells/mL) were incubated in serum-free liquid medium containing 50% IMDM/50% F-12 medium (Sigma Chemical, St Louis, MO) with 1% detoxified bovine serum albumin (BSA; Stem Cell Technologies, Vancouver, BC), 300 μg/mL iron-saturated transferrin (652202, Boehringer Mannheim, Mannheim, Germany), lipid suspension prepared, as described,15 and 10 U/mL rhEPO at 37°C in a high-humidity 5% CO2, 95% air incubator. Then, 10 ng/mL rhIL-3 and 100 ng/mL rhSCF were added, as indicated.
Flow cytometry
To determine expression of the transferrin receptor (TfR) and glycophorin A, cells (1 × 106) were washed twice with phosphate-buffered saline (PBS) and resuspended in PBS with 0.05% BSA. Fluorescein isothiocyanate (FITC)–conjugated antiglycophorin A MoAb (D2.10, IM0772; Immunotech, Marseilles, France) and phycoerythrin (PE)-conjugated anti-CD71 MoAb (YDJ1.2.2, IM2001; Immunotech) were added, followed by incubation on ice for 30 minutes. Samples were analyzed using the Epics Elite ESP flow cytometer (Coulter, Miami, FL). To detect expression of Fas (CD95) on ECFCs, the cells (1 × 106) were washed twice with PBS, then suspended in 0.1 mL PBS prior to incubation on ice for 60 minutes with either FITC-conjugated murine anti-Fas MoAb (UB2, 1506; Immunotech) at 2 μL. PBS (400 μL) was added and the cells were analyzed using the Epics Elite ESP flow cytometer (Coulter).
Expression of RCAS1 and RCAS1R on ECFCs was analyzed at the indicated times by flow cytometry, as described.11 To determine expression of RCAS1R, the cells (1 × 105) were washed with cold washing buffer (0.5% BSA, 0.05% NaN3 in PBS) and incubated with 50 μL blocking buffer (40% normal goat serum in washing buffer) for 15 minutes on ice. Next, 1.0 μg/mL glutathione-S–transferase (GST), as a control, or recombinant RCAS1-GST fusion protein, prepared as described,11 was added and the preparation incubated on ice for 30 minutes. Cells were washed 3 times with washing buffer and incubated with 0.5 μg/mL affinity-purified rabbit antibody against GST for 30 minutes. Cells were next washed 3 times with washing buffer and incubated with PE-conjugated goat F(ab′)2 IgG antibody against rabbit IgG, preabsorbed with human and mouse serum (Southern Biotech, Birmingham, AL), mouse FITC-conjugated antiglycophorin A MoAb, or mouse FITC-conjugated anti-CD71 MoAb. Then samples were washed 3 times with washing buffer, followed by analysis on a Coulter Epics XL flow cytometer (Coulter, Hialeah, FL). To determine expression of RCAS1, cells (1 × 105) were washed with cold washing buffer (0.5% BSA, 0.05% NaN3 in PBS) and incubated with 50 μL blocking buffer for 15 minutes on ice. Then 2.5 μg/mL control mouse IgM (GC323, IM1268; Immunotech) or 2.5 μg/mL purified IgM anti-RCAS1 antibody (22-1-1; provided by Dr M. Nakashima) was added and incubated on ice for 30 minutes. Cells were again washed 3 times with washing buffer and incubated with 2.5 μg/mL PE-conjugated F(ab′)2 goat antimouse IgM antibody (IM0555; Immunotech, preabsorbed by human serum) in washing buffer containing 5% normal goat serum for 30 minutes on ice. The stained cells were washed 3 times with washing buffer. Washing buffer (400 μL) was added and the cells were analyzed using a Coulter Epics XL flow cytometer.
Measurement of the uptake of 3H-thymidine
The ECFCs were incubated at 37°C, 5% CO2 with or without nRCAS1 for 24 hours. For the last 6 hours of the culture period, the cells were pulsed with 1 μCi [3H]-thymidine and collected onto glass filters. The cell counts were adjusted prior to the addition of [3H]-thymidine to negate effects of differences in cell number. The incorporated radioactivity was measured using a Beta plate reader. Viability of the cells was determined by trypan blue exclusion, using a hemocytometer.
Plasma clot assay
The erythroid colony-forming capacity of ECFC was determined by the plasma clot method.2 Day 7 ECFCs were incubated at 37°C in liquid culture, with or without nRCAS1, in the presence of EPO (2 U/mL) and for the indicated times. The cells were then collected and 1 mL of medium consisting of IMDM, 20% FCS, 1% BSA, 10 ng/mL rhSCF, 2 U/mL rhEPO, and 10% pooled, citrated, human AB plasma, containing 600 cells, was plated in triplicate 35-mm culture dishes and incubated at 37°C in a high-humidity 5% CO2, 95% air incubator for 7 days. The clots were then fixed and stained with 3,3′-dimethoxybenzidine, and colonies of 8 or more hemoglobinized cells were defined as colony-forming unit-erythroid (CFU-E).
Apoptosis assay
For assessment of apoptosis we measured membrane redistribution of phosphatidylserine, using an annexin V–FITC apoptosis detection kit (Immunotech, Marseilles, France) according to the manufacturer's protocol. Briefly, cells were washed twice with ice-cold PBS and cell pellets were resuspended in 490 μL binding buffer. The cell suspension was incubated for 30 minutes on ice with FITC-conjugated annexin V antibody and propidium iodide (PI; final concentration, 2.5 μg/mL). Cells were analyzed using the Epics Elite ESP flow cytometer (Coulter, Miami, FL) and the annexin V+ fraction was delineated as apoptotic cells.
Analysis of DNA fragmentation
Whole DNA was extracted from day 7 ECFCs cultured in liquid culture for 24 hours with or without nRCAS1, in the presence of EPO (2 U/mL). Cells (1 × 106) were collected and washed with ice-cold PBS, then were lysed in 500 μL of a hypotonic buffer containing 50 mM Tris/HCl, pH 8.0, 100 mM NaCl, 100 mM EDTA, and 1.0% sodium dodecyl sulfate (SDS). The lysates were treated with 200 μg/mL RNAase A at 37°C for 1 hour and 400 μg/mL proteinase K at 55°C for 2 hours. Samples were extracted with phenol/chloroform and then only chloroform. DNA was then precipitated with ethanol, and nucleic acids were dissolved in 10 mM Tris/HCl, pH 7.5, 1 mM EDTA, and 1% SDS at 60°C for 10 minutes. The samples were electrophoresed at 100 V for 1.5 hours on 1.6% agarose gel in Tris-acetate, EDTA (TAE) and stained with ethidium bromide to visualize DNA. A 1-kb DNA ladder (15615-016; Gibco BRL) was used as a molecular marker.
Evaluation of caspase 3 activity
Activation of caspase 3 was assessed using the PhiPhiLux G1D2 caspase 3 activity detection kit (AK304R1G Oncoimmunin, College Park, MD), according to the manufacturer's instructions. Briefly, 1 × 106 cells were washed twice with PBS, and 50 μL substrate solution (10 mM; GDEVDGI [single-letter amino acid codes]) was added, followed by incubation for 60 minutes in a 5% CO2, 95% air incubator at 37°C. Then, 500 μL cold flow cytometry solution was added to each sample followed by analysis, using a FACScan flow cytometer at 488-nm FL1 channel.
Evaluation of capase 8 activity
Activation of caspase 8 was evaluated using a FLICE/caspase 8 fluorometric protease assay kit (BV-K112 Medical and Biological Laboratories, Nagoya, Japan), according to the manufacturer's instructions. Briefly, lysates from 1 × 106 cells were incubated with fluorogenic substrate (IETDAFC) for 60 minutes at 37°C in buffer containing 5 mM dithiothreitol. Samples were then analyzed using an ARVO multilabel counter (Wallac Oy, Turku, Finland) at 535 nm.
Cytofluorometric analysis of mitochondrial transmembrane potentials
To measure the mitochondrial transmembrane potential (ΔΨm) we used a flow cytometer, as described.17 18 Cells (1.0 × 105) were incubated with 3,3′-dihexyloxacarbocyanine(DiOC6; 31842-6, Aldrich Chemical, Milwaukee, WI; final concentration, 40 nM) for 15 minutes at 37°C. Cells were pelleted by centrifugation and resuspended in 500 μL PBS, followed by analysis on a FACScan flow cytometer.
Blocking of the stimulation of Fas
To block signaling by Fas, cells were preincubated with 1.0 μg/mL hamster anti-Fas neutralizing antibody (clone 4B4-3B)19 for 1 hour at 37°C and nRCAS1 was added as described above. To confirm the specificity of nRCAS1, human Fas-Fc (1 μg/mL; hFas-Fc)20 was preincubated at 4°C with nRCAS1 to block the binding to Fas for 1 hour prior to addition to the cell suspension. After 36 hours of each incubation, analysis of apoptosis was made based on annexin V binding.
Analysis of expression of RCAS1R on human bone marrow cells
To examine the expression of RCAS1R on bone marrow cells, light-density mononuclear cells were obtained from bone marrow aspirates from normal volunteers by density centrifugation using LSM, then were suspended in IMDM containing 15% FCS, 15% human AB serum, 2 U/mL rhEPO, 20 ng/mL rhSCF, 10 ng/mL rhIL-3, and 50 ng/mL recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF). After incubation for 16 hours at 37°C, the expression of RCAS1R, glycophorin A, and TfR (CD71) was determined as described above.
Bone marrow colony assay
CD34+ cells (5 × 105/mL) from bone marrow from healthy volunteers were purified using Direct CD34 Isolation Kit (Miltenyi Biotech) with Vario-MACS. The cells were incubated, with or without 40 μg/mL nRCAS1, for 24 hours, in IMDM containing 20% FCS, 1% BSA, 2 U/mL rhEPO, 50 ng/mL rhSCF, 20 ng/mL rhIL-3, 10 ng/mL rhGM-CSF, and 10 ng/mL thrombopoietin (TPO) at 37°C in a high-humidity 5% CO2, 95% air incubator. The cells were then collected, and 3 × 103 cells with methylcellulose containing media with growth factors (Methocult H4433, Stem Cell Technologies) were plated in triplicate 35-mm culture dishes. After 18 days of culture, burst-forming unit erythroids (BFU-E), colony-forming unit granulocyte macrophages (CFU-GM), and colony-containing granulocytes, erythroids, macrophages, and megakaryocytes (CFU-GEMM) were scored, using an inverted microscope and standard criteria for identification.
Immunohistochemical staining of RCAS1 on human bone marrow
For detection of RCAS1, the 22-1-1 hybridoma culture supernatants, which were diluted 1:20 in PBS, were applied, as described.12 Biotinylated goat F(ab′)2antimouse IgM antibody, absorbed with human and mouse tissues, was used as the second antibody.
Statistical analysis
The Student t test was used to determine significant differences between the groups and results are expressed as the mean ± SD.
Results
Expression of RCAS1 and RCAS1R on ECFCs
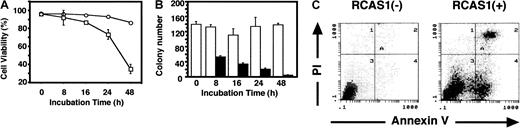
The purified erythroid progenitor cells (morphologically determined purity = 95% ± 3%) formed erythroid colonies in semisolid culture system (colony forming capacity = 69.5% ± 3.9%). These progenitor cells were designated “erythroid colony-forming cells” (ECFCs), as previously defined.14 15 In liquid culture, ECFCs undergo terminal erythroid maturation, as characterized by great changes in the surface expression of glycophorin A and TfR (Figure1A, upper panel). On day 6 of the liquid cultures in the presence of EPO and SCF, 96.6% of ECFCs expressed highly abundant TfRs on the cell surface; then the TfR expression gradually decreased during the culture period, as seen in Figure 1A. The expression of glycophorin A was low on day 6, but gradually increased during culture period of days 6 to 13, whereas the expression of TfR was decreasing. To examine the expression of RCAS1R on ECFCs, a binding assay using rRCAS1-GST was done. As shown in Figure 1, panels A and B, most of the day 6 ECFCs (98.0% ± 1.8%) from 2 independent experiments showed abundant expression of RCAS1R and the percentage of RCAS1R+ cells gradually decreased during erythroid maturation. On day 13, only 4.8% of the cells, with the morphology of mature erythroblasts and enucleated erythrocytes (data not shown), showed RCAS1 binding (Figure 1A, middle and lower panels, B). FACS analysis of the expression of RCAS1R, glycophorin A, and TfR demonstrated that cells with an immature erythroid phenotype (TfR+/glycophorin A−) expressed abundant RCAS1R, whereas mature erythroid cells (TfR−/glycophorin A+) had very little RCASIR (Figure 1A, middle and lower panels). On the other hand, ECFCs did not express RCAS1, determined using 22-1-1 MoAb, at any stage of erythroid maturation (Figure 1B). Histochemical staining confirmed that RCAS1 was not expressed in the cytoplasm of ECFC (data not shown). The percentage of these cells that expressed Fas also was higher in immature ECFC, as compared to mature cells, and it decreased during liquid culture, in the same manner as seen with RCAS1R (Figure 1B).
Erythroid progenitor cells express functional RCAS1R.
(A) Expression of RCAS1R, glycophorin A, and TfR on ECFCs was determined by flow cytometry at indicated times during erythroid maturation in liquid culture with 15% FCS, 15% human AB serum, 20 ng/mL rhSCF, and 2 U/mL rhEPO. Upper panels show the time profile of the expression of glycophorin A and TfR during erythroid maturation. Middle panels show glycophorin A versus RCAS1R expression by 2-color cytometry. Lower panels show TfR versus RCAS1R expression. Data are representative of 2 independent experiments. (B) Expressions of Fas (■), RCAS1 (○), and RCAS1R (●) on ECFCs were determined by flow cytometry, at indicated times, in liquid culture. The expression of Fas is derived from 3 independent experiments. (C) Effect of nRCAS1 on DNA synthesis of ECFCs was determined by [3H]-thymidine uptake. ECFCs were incubated at 37°C, 5% CO2 with or without nRCAS1 for 24 hours. For the last 6 hours of the culture period, cells were pulsed with 1 μCi [3H]-thymidine and collected onto glass filters. The incorporated radioactivity was measured using a Beta plate reader. Percentage inhibition was calculated by dividing radioactivity of the cells incubated with nRCAS1 by that without nRCAS1.
Erythroid progenitor cells express functional RCAS1R.
(A) Expression of RCAS1R, glycophorin A, and TfR on ECFCs was determined by flow cytometry at indicated times during erythroid maturation in liquid culture with 15% FCS, 15% human AB serum, 20 ng/mL rhSCF, and 2 U/mL rhEPO. Upper panels show the time profile of the expression of glycophorin A and TfR during erythroid maturation. Middle panels show glycophorin A versus RCAS1R expression by 2-color cytometry. Lower panels show TfR versus RCAS1R expression. Data are representative of 2 independent experiments. (B) Expressions of Fas (■), RCAS1 (○), and RCAS1R (●) on ECFCs were determined by flow cytometry, at indicated times, in liquid culture. The expression of Fas is derived from 3 independent experiments. (C) Effect of nRCAS1 on DNA synthesis of ECFCs was determined by [3H]-thymidine uptake. ECFCs were incubated at 37°C, 5% CO2 with or without nRCAS1 for 24 hours. For the last 6 hours of the culture period, cells were pulsed with 1 μCi [3H]-thymidine and collected onto glass filters. The incorporated radioactivity was measured using a Beta plate reader. Percentage inhibition was calculated by dividing radioactivity of the cells incubated with nRCAS1 by that without nRCAS1.
RCAS1 suppressed cell proliferation of ECFCs
Next we asked if RCAS1 would affect cellular proliferation of ECFCs. Addition of nRCAS1 to the cultures strongly suppressed cellular proliferation of day 6 ECFCs, as determined by [3H]-thymidine uptake, reducing it to 6% of that seen in control cultures (Figure 1C). The rate of inhibition gradually decreased during the culture period, as the percentage of RCAS1R positivity decreased (Figure 1B,C).
SCF delayed decrease of expression of RCAS1R during maturation of ECFCs
Decrease in the number of RCAS1R+ cells during erythroid maturation was not affected by incubation with IL-3, compared with incubation with EPO alone, but SCF significantly delayed the disappearance of RCAS1R on ECFCs during erythroid maturation (Table1, versus with EPO alone,P = .015, versus EPO+IL-3, P = .021).
Effect of interleukin-3 and stem cell factor on expression of RCAS1-receptor
| Culture conditions . | Day 8 ECFCs . |
|---|---|
| EPO alone | 100.0% ± 21.1% |
| EPO + IL-3 | 112.4% ± 12.9% |
| EPO + SCF | 264.7% ± 127.7%* |
| EPO + IL-3 + SCF | 265.4% ± 118.2%† |
| Culture conditions . | Day 8 ECFCs . |
|---|---|
| EPO alone | 100.0% ± 21.1% |
| EPO + IL-3 | 112.4% ± 12.9% |
| EPO + SCF | 264.7% ± 127.7%* |
| EPO + IL-3 + SCF | 265.4% ± 118.2%† |
To determine the effect of IL-3 and SCF on the expression of RCAS1R, 3 independent experiments were done, using ECFCs purified from 3 donors. Day 6 ECFCs (1 × 105 cells/mL) were incubated in serum-free condition media containing 10 U/mL rhEPO at 37°C in a high-humidity 5% CO2, 95% air incubator. Then 10 ng/mL rhIL-3 and 100 ng/mL rhSCF were added, as indicated. After 48 hours, the expression of RCAS1R on the cell surface was detected, using flow cytometry. Values are expressed as the percentage against the mean of EPO alone, and are mean ± SD from triplicate determinations.
EPO indicates erythropoietin; IL-3, interleukin-3; SCF, stem cell factor.
Versus EPO alone, P = .016, versus EPO + IL-3, P = .022.
Versus EPO alone, P = .015, versus EPO + IL-3, P = .021.
RCAS1 induced apoptosis of ECFCs
To determine if RCAS1 would affect survival of ECFCs, trypan blue exclusion tests were done. The majority of cells in control cultures with EPO remained viable throughout 48 hours of culture, then the viability gradually decreased during liquid culture in the presence of nRCAS1, and the value was significantly smaller than that seen in control culture (P < .05) at 24 hours and (P < .001) at 48 hours (Figure2A). To further measure the significance of these events, we investigated the effect of nRCAS1 on the colony-forming capacity of ECFCs, by a 2-step culture method. nRCAS1 was added to the liquid cultures of ECFCs, then aliquots of the cells (200 cells/clot) were transferred for plasma clot assays, at the indicated times. As seen in Figure 2B, the addition of nRCAS1 significantly reduced the colony-forming capacity of ECFCs, in a time-dependent manner (P < .001at 48 hours).
RCAS1 induces apoptosis of ECFCs.
Day 7 ECFCs (5 × 105cells/mL) were incubated with or without nRCAS1 (40 μg/mL) in the presence of rhEPO and rhSCF, and viability, colony-forming capacity, and apoptosis were measured at the indicated times. (A) Viability of ECFCs with (■) or without (○) nRCAS1 was determined by trypan blue exclusion, using a hemocytometer. (B) Plasma clot assays for colony-forming capacity of day 7 ECFCs after incubation in liquid media with (▪) or without (■) nRCAS1. After incubation for indicated periods, the cells were transferred for plasma clot assays and incubations were carried out for another 7 days. The number of colonies was measured after benzidine staining. (C) After liquid culture for 36 hours with (right panel) or without (left panel) nRCAS1, apoptotic cells were detected by measuring membrane redistribution of phosphatidylserine, using annexin V–FITC, and staining with PI. Figure 2C shows representative data from 10 independent experiments.
RCAS1 induces apoptosis of ECFCs.
Day 7 ECFCs (5 × 105cells/mL) were incubated with or without nRCAS1 (40 μg/mL) in the presence of rhEPO and rhSCF, and viability, colony-forming capacity, and apoptosis were measured at the indicated times. (A) Viability of ECFCs with (■) or without (○) nRCAS1 was determined by trypan blue exclusion, using a hemocytometer. (B) Plasma clot assays for colony-forming capacity of day 7 ECFCs after incubation in liquid media with (▪) or without (■) nRCAS1. After incubation for indicated periods, the cells were transferred for plasma clot assays and incubations were carried out for another 7 days. The number of colonies was measured after benzidine staining. (C) After liquid culture for 36 hours with (right panel) or without (left panel) nRCAS1, apoptotic cells were detected by measuring membrane redistribution of phosphatidylserine, using annexin V–FITC, and staining with PI. Figure 2C shows representative data from 10 independent experiments.
Apoptotic cells were delineated with annexin V, which binds externalized phosphatidylserine at an early stage of apoptosis, and PI, which stains cellular DNA at late stages in the process of apoptosis. In the presence of nRCAS1, an increase in the number of cells both at the early (annexin V+/PI−) and the late (annexin V+/PI+) stage of apoptosis was evident after 16 hours of incubation (Figure 2C, right), compared to findings in control cultures (Figure 2C, left). Analysis of the percentage of apoptotic cells, determined by annexin V binding in 10 experiments, revealed that the total number of apoptotic cells (annexin V+/PI+ and −) was 37.9% ± 9.4% after incubation with nRCAS1, and 10.0% ± 1.7% in control cultures (P < .001).
To confirm that RCAS1 induced apoptosis of ECFCs, cytospin specimens were prepared after incubation for 24 hours with or without nRCAS1. Cells cultured without nRCAS1 showed morphologic features of immature erythroid cells (Figure 3A), whereas cells incubated with nRCAS1 showed typical apoptotic features, such as nuclear condensation, fragmentation, and reduction in cell size (Figure3B). These morphologic changes were seen in 25.6% (range, 19.0%-33.0%; n = 10) of cells incubated with nRCAS1, whereas only 6.5% (range, 3.0%-11.0%; n = 10) of the cells incubated without nRCAS1 showed an apoptotic morphology. Induction of apoptosis by nRCAS1 was further confirmed by analysis of cellular DNA, using agarose gel electrophoresis (Figure 3C). DNA fragmentation was evident in cells cultured with nRCAS1, although the ladder pattern was quite faint in ECFCs, as described previously.2
Apoptotic morphology and DNA fragmentation of ECFCs incubated with nRCAS1.
Day 7 ECFCs were incubated for 24 hours at 37°C in liquid media with 15% FCS, 15% human AB serum, rhSCF (20 ng/mL) and rhEPO (2 U/mL) with, or without nRCAS1. Then, cytospin specimens of the cells were prepared and stained with May-Giemsa. (A) Immature erythroid cells after liquid culture without nRCAS1. (B) Apoptotic cells after liquid culture with nRCAS1. (C) DNA samples were isolated from day 7 ECFCs incubated with, or without nRCAS1 for 24 hours. DNA fragmentation of ECFCs exposed to RCAS1 was shown by neutral pH and 1.6% agarose gel electrophoresis, and stained with 0.5% ethidium bromide. Lane 1 displays size markers; lane 2 displays control cultures with rhEPO (2 U/mL), but without nRCAS1; lane 3 displays cultures with rhEPO plus nRCAS1.
Apoptotic morphology and DNA fragmentation of ECFCs incubated with nRCAS1.
Day 7 ECFCs were incubated for 24 hours at 37°C in liquid media with 15% FCS, 15% human AB serum, rhSCF (20 ng/mL) and rhEPO (2 U/mL) with, or without nRCAS1. Then, cytospin specimens of the cells were prepared and stained with May-Giemsa. (A) Immature erythroid cells after liquid culture without nRCAS1. (B) Apoptotic cells after liquid culture with nRCAS1. (C) DNA samples were isolated from day 7 ECFCs incubated with, or without nRCAS1 for 24 hours. DNA fragmentation of ECFCs exposed to RCAS1 was shown by neutral pH and 1.6% agarose gel electrophoresis, and stained with 0.5% ethidium bromide. Lane 1 displays size markers; lane 2 displays control cultures with rhEPO (2 U/mL), but without nRCAS1; lane 3 displays cultures with rhEPO plus nRCAS1.
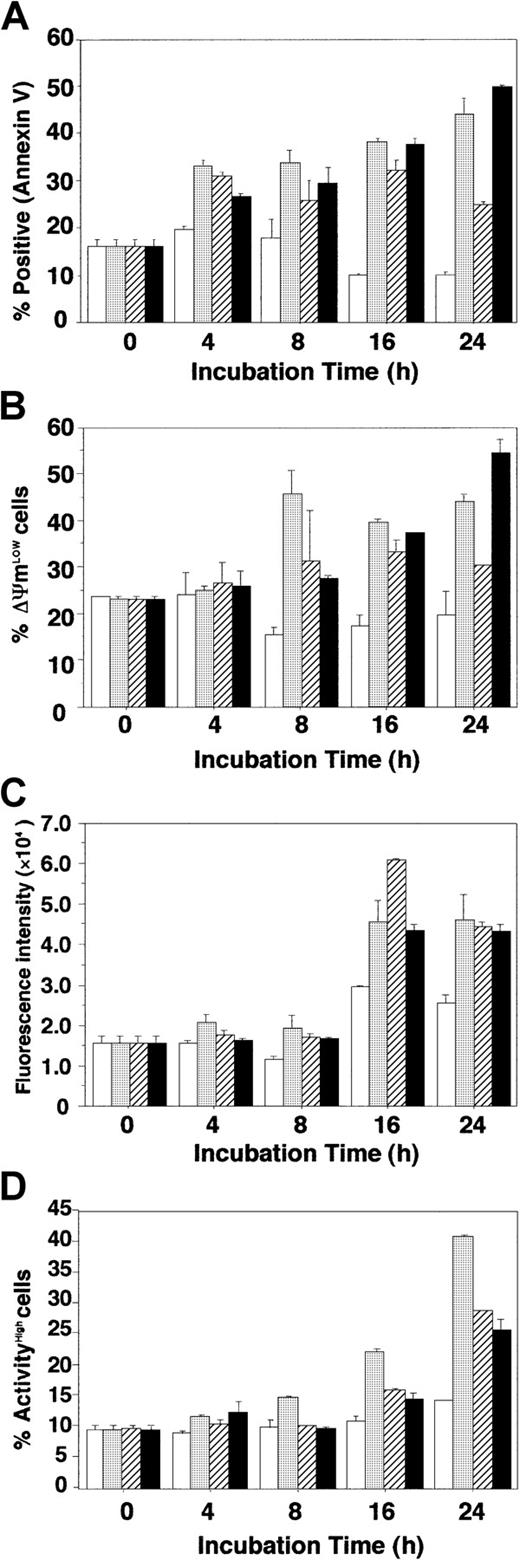
RCAS1 induced apoptosis in ECFCs via modulation of mitochondrial transmembrane potential and caspases
To investigate the mechanism by which RCAS1 induces apoptosis of ECFCs, we examined the kinetics of apoptosis, transmitochondrial potential (ΔΨm), and activation of caspases 8 and 3, in the presence of nRCAS1, and compared these indices to those observed after EPO deprivation or Fas stimulation using a Fas-stimulating antibody. When the percentage of apoptotic cells was determined by flow cytometry using annexin V, the ECFCs cultured with nRCAS1 showed an apoptotic phenotype, in a time-dependent manner, similar to events seen with EPO deprivation or Fas stimulation (Figure4A). Both disruption of ΔΨm, and the subsequent activation of caspases 8 and 3, were evident in cells incubated with nRCAS1, as well as those seen in the case of EPO deprivation, or with Fas-stimulating antibody (CH-11) (Figure 4B-D). Early collapse of ΔΨm was seen in case of EPO deprivation, but differences from the case with Fas-stimulating antibody (CH-11) and with nRCAS1 were not significant (Figure 4B). The kinetics of activation of caspases was similar among incubations without EPO, with Fas-stimulating antibody (CH-11), and with nRCAS1 (Figure 4C, D). The kinetics of intracellular signaling of apoptosis was similar among incubations without EPO, with Fas-stimulating antibody (CH-11), and with nRCAS1 (Figure 4B-D). To confirm that RCAS1-induced apoptosis is independent of Fas-stimulation, anti-Fas neutralizing antibody (4B4-3B), which reduces Fas-mediated apoptosis by occupying Fas, or Fas-Fc chimeric protein, which reduces FasL binding to membrane-bound Fas by attaching to the ligand, were added to cultures together with nRCAS1. As can be seen from Table 2, both additives significantly reduced the apoptosis induced by Fas stimulation, but did not influence apoptosis of the cells incubated with nRCAS1.
Kinetics of RCAS1 signaling in ECFCs.
Day 7 ECFCs were cultured with (■) or without (░) rhEPO (2 U/mL), or with CH-11 (500 ng/mL; ▨) plus rhEPO, or with nRCAS1 (40μg/mL; ▪) plus rhEPO. Each point shows the mean ± SD of triplicates. (A) Detection of apoptotic cells. Apoptotic cells were determined using annexin V staining at the indicated times. (B) Loss of mitochondrial transmembrane potential (ΔΨm). Cells (1.0 × 105) were incubated with 40 nM 3,3′-dihexyloxacarbocyanine(DiOC6) for 15 minutes at 37°C, and pelleted by centrifugation. Cells resuspended in 500 μL PBS were analyzed by flow cytometry. (C) Activation of caspase 8. Lysates from 1 × 106 cells were incubated with fluorogenic substrate (IETDAFC) for 60 minutes at 37°C in buffer containing 5 mM dithiotreitol. Samples were then analyzed using a multilabel counter at 535 nm. (D) Activation of caspase 3. Cells (1 × 106) were washed twice with PBS, and 50μL substrate solution (10 mM; GDEVDGI) was added, prior to incubation for 60 minutes in a 5% CO2, 95% air incubator at 37°C. Ice-cold flow cytometry solution (500 μL) was added to each sample followed by flow cytometry analysis.
Kinetics of RCAS1 signaling in ECFCs.
Day 7 ECFCs were cultured with (■) or without (░) rhEPO (2 U/mL), or with CH-11 (500 ng/mL; ▨) plus rhEPO, or with nRCAS1 (40μg/mL; ▪) plus rhEPO. Each point shows the mean ± SD of triplicates. (A) Detection of apoptotic cells. Apoptotic cells were determined using annexin V staining at the indicated times. (B) Loss of mitochondrial transmembrane potential (ΔΨm). Cells (1.0 × 105) were incubated with 40 nM 3,3′-dihexyloxacarbocyanine(DiOC6) for 15 minutes at 37°C, and pelleted by centrifugation. Cells resuspended in 500 μL PBS were analyzed by flow cytometry. (C) Activation of caspase 8. Lysates from 1 × 106 cells were incubated with fluorogenic substrate (IETDAFC) for 60 minutes at 37°C in buffer containing 5 mM dithiotreitol. Samples were then analyzed using a multilabel counter at 535 nm. (D) Activation of caspase 3. Cells (1 × 106) were washed twice with PBS, and 50μL substrate solution (10 mM; GDEVDGI) was added, prior to incubation for 60 minutes in a 5% CO2, 95% air incubator at 37°C. Ice-cold flow cytometry solution (500 μL) was added to each sample followed by flow cytometry analysis.
Inhibition of RCAS1-induced apoptosis by anti-Fas neutralizing antibody and Fas-Fc
| Culture additives . | Experiment 1 . | Experiment 2 . | ||
|---|---|---|---|---|
| (−) . | + 4B4-3B . | (−) . | + Fas-Fc . | |
| Control | 7.6 ± 0.4 | 6.8 ± 0.1 | 15.4 ± 3.1 | 17.2 ± 0.8 |
| + CH-11 | 47.6 ± 1.4 | 9.1 ± 0.8* | 26.9 ± 1.9 | 16.3 ± 0.9† |
| + nRCAS1 | 40.9 ± 1.1 | 42.8 ± 2.0 | 26.7 ± 2.4 | 24.2 ± 0.9 |
| Culture additives . | Experiment 1 . | Experiment 2 . | ||
|---|---|---|---|---|
| (−) . | + 4B4-3B . | (−) . | + Fas-Fc . | |
| Control | 7.6 ± 0.4 | 6.8 ± 0.1 | 15.4 ± 3.1 | 17.2 ± 0.8 |
| + CH-11 | 47.6 ± 1.4 | 9.1 ± 0.8* | 26.9 ± 1.9 | 16.3 ± 0.9† |
| + nRCAS1 | 40.9 ± 1.1 | 42.8 ± 2.0 | 26.7 ± 2.4 | 24.2 ± 0.9 |
Two experiments to inhibit the stimulation of Fas were done on day 7 ECFCs. Experiment 1: Anti-Fas neutralizing MoAb (clone 4B4-3B, 1.0 μg/mL) was added to the liquid cultures, which were incubated for 1 hour at 37°C. After this, Fas-stimulating antibody (0.5 μg/mL; clone CH-11) or nRCAS1 (40 μg/mL) was added and incubation was carried out for a further 24 hours. Experiment 2: Fas-Fc chimeric protein, which reduces Fas-mediated apoptosis by occupying FasL (100 ng/mL), was preincubated with CH-11 or nRCAS1 for 1 hour at 37°C and were then added to the culture media. In both experiments, apoptotic cells were detected by annexin V binding assay. Values are mean ± SD from triplicate determinations.
nRCAS1 indicates a soluble form of RCAS1; MoAb, monoclonal antibody. See Table 1 for other abbreviations.
Versus without 4B4-3B, P < .001.
Versus without Fas-Fc, P < .001.
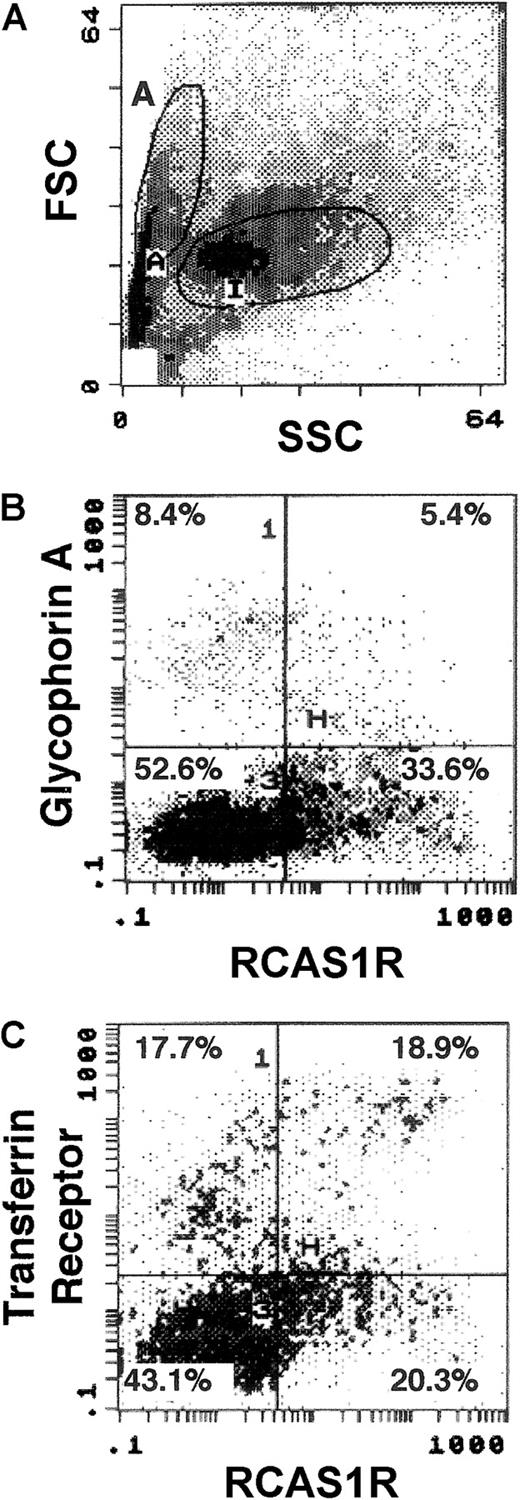
Expression of RCAS1R on human bone marrow cells
The role of the RCAS1 system in regulating physiologic hematopoiesis was investigated by examining the expression of the RCAS1R on bone marrow cells from healthy volunteers, using 2-color cytometry. A forward side scatter (FSC) versus side scatter (SSC) dot plot (Figure 5A) on region A cells, which contained the majority of erythroid progenitor cells, showed that 39.0% of the bone marrow cells expressed RCAS1R. Two-color staining of glycophorin A and RCAS1R revealed that glycophorin A+/RCAS1R+ cells exist but the majority of RCAS1R+ cells were negative for glycophorin A. This indicates that mature erythroid cells express RCAS1R, but the number is fewer than in immature ones, as seen in case of in vitro expanded ECFCs (Figure 5B). We then did 2-color staining of TfR and RCAS1R on bone marrow cells (Figure 5C) and found that majority of TfRHigh cells, which correspond to immature erythroid progenitor cells, possess abundant RCAS1R, whereas TfRLowcells, which correspond to mature erythroid cells, had less RCAS1R. These observations clearly indicate that bone marrow erythroid progenitor cells have RCAS1R, and that the expression is modulated during erythroid maturation, as was seen in in vitro culture of ECFCs. However, unlike the results with blood ECFCs, the TfR−/RCAS1R+ subpopulation was present in bone marrow cells (Figure 5C), which suggests the possibility that hematopoietic progenitor cells other than the erythroid lineage also might possess RCAS1R.
Expression of RCAS1R on bone marrow cells from a healthy volunteer.
The expression of RCAS1R/glycophorin A/TfR was examined on bone marrow cells from normal volunteers. The analysis was performed after incubation for 16 hours in IMDM containing 15% FBS, 15% human AB serum, rhEPO (2 U/mL), rhSCF (20 ng/mL), rhIL-3 (100 ng/mL), and rhGM-CSF (50 ng/mL). Flow cytometry analysis was performed after 2-color staining of RCAS1R versus glycophorin A, or RCAS1R versus TfR. Region A was first gated by selecting the population that contained abundant erythroid progenitor cells in a FSC versus SSC dot plot (A). Then, expressions of glycophorin A versus RCAS1R (B) and TfR (CD71) versus RCAS1R (C) were assessed in region A cells. The results shown are for one experiment representative of the 4 performed.
Expression of RCAS1R on bone marrow cells from a healthy volunteer.
The expression of RCAS1R/glycophorin A/TfR was examined on bone marrow cells from normal volunteers. The analysis was performed after incubation for 16 hours in IMDM containing 15% FBS, 15% human AB serum, rhEPO (2 U/mL), rhSCF (20 ng/mL), rhIL-3 (100 ng/mL), and rhGM-CSF (50 ng/mL). Flow cytometry analysis was performed after 2-color staining of RCAS1R versus glycophorin A, or RCAS1R versus TfR. Region A was first gated by selecting the population that contained abundant erythroid progenitor cells in a FSC versus SSC dot plot (A). Then, expressions of glycophorin A versus RCAS1R (B) and TfR (CD71) versus RCAS1R (C) were assessed in region A cells. The results shown are for one experiment representative of the 4 performed.
RCAS1 suppressed colony formation of CFU-GM from normal bone marrow cells
To determine the effect of RCAS1 on growth of nonerythroid hematopoietic progenitor cells, methylcellulose colony assays of CD34+ cells from human bone marrow were done. Incubation of CD34+ cells with nRCAS1 for 24 hours significantly reduced CFU-GM formation (P = .0052), but numbers of BFU-E and CFU-GEMM were not affected (Table 3).
Effect of nRCAS1 on multilineage colony formation of normal bone marrow cells
| . | Colonies/dish . | |
|---|---|---|
| Control . | With nRCAS1 . | |
| BFU-E | 24.0 ± 13.3 | 22.1 ± 14.1 |
| CFU-GM | 16.7 ± 4.8 | 10.8 ± 4.43-150 |
| CFU-GEMM | 2.8 ± 1.6 | 3.0 ± 2.3 |
| . | Colonies/dish . | |
|---|---|---|
| Control . | With nRCAS1 . | |
| BFU-E | 24.0 ± 13.3 | 22.1 ± 14.1 |
| CFU-GM | 16.7 ± 4.8 | 10.8 ± 4.43-150 |
| CFU-GEMM | 2.8 ± 1.6 | 3.0 ± 2.3 |
CD34+ cells (5 × 105/mL) purified from bone marrow cells of healthy volunteers were incubated with or without 40 μg/mL nRCAS1 for 24 hours, in IMDM containing 20% FCS, 1% BSA, 2 U/mL rhEPO, 50 ng/mL rhSCF, 20 ng/mL rhIL-3, 10 ng/mL rhGM-CSF, and 10 ng/mL TPO at 37°C in a high-humidity 5% CO2, 95% air incubator. The cells were then collected, and 3 × 103 cells in methylcellulose containing media with growth factors were plated in triplicate 35-mm culture dishes. Those dishes were incubated at 37°C in a high-humidity 5% CO2, 95% air incubator. After 18 days of culture, BFU-E, CFU-GM, and CFU-GEMM were scored, using an inverted microscope and standard criteria for identification. Values are mean ± SD from four independent experiments.
BFU-E indicates burst-forming unit erythroids; CFU-GM, colony-forming unit granulocyte macrophages; CFU-GEMM, colony-containing granulocytes, erythroids, macrophages, and megakaryocytes; IMDM, Iscoves modified Dulbecco medium; FCS, fetal calf serum; BSA, bovine serum albumin; TPO, thrombopoietin.
Versus control (without nRCAS1), P = .0052.
Immunohistochemical staining of normal bone marrow cells
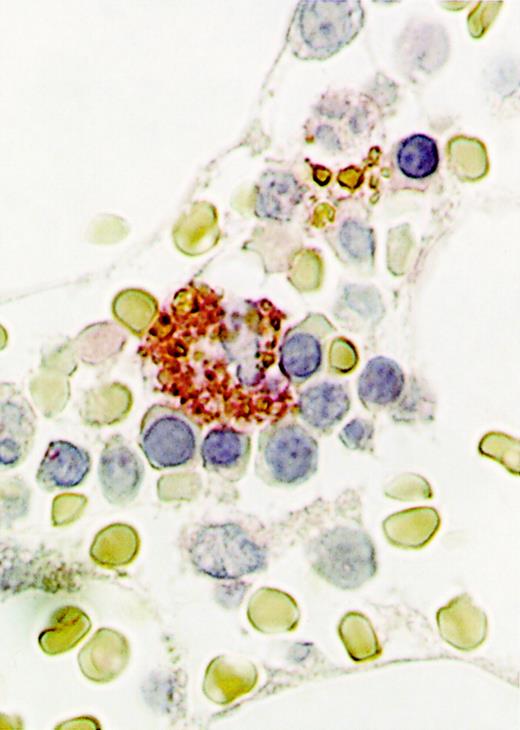
To determine the expression of RCAS1 in bone marrow cells, human bone marrow clot specimens from healthy donors were immunochemically stained by 22-1-1 antibody, which recognizes RCAS1. Among various kinds of bone marrow cells, no cell expressed RCAS1 on the surface. However, cytoplasm of the macrophages was strongly stained by 22-1-1 antibody, as shown in Figure 6. Surrounding erythroblasts and erythrocytes were negative for RCAS1. On the other hand, monocytes purified from human peripheral blood were stained in the focal cytoplasmic pattern, and after stimulation by lipopolysaccharide, the cytoplasm of activated monocytes, with the morphology of macrophages was strongly stained and the pattern was diffuse (data not shown).
Expression of RCAS1 in the cytoplasm of bone marrow macrophages.
Bone marrow clots were obtained by needle aspiration, then immunohistochemical staining was done using 22-1-1, an anti-RCAS1 antibody. For the second antibody, biotinylated goat F(ab)′2 antimouse IgM antibody, absorbed with human and mouse tissues, was used. A representative analysis of 3 performed from 3 different donors is shown. Note the cytoplasmic staining seen in a macrophage, surrounded by erythroblasts (original magnification, ×1000).
Expression of RCAS1 in the cytoplasm of bone marrow macrophages.
Bone marrow clots were obtained by needle aspiration, then immunohistochemical staining was done using 22-1-1, an anti-RCAS1 antibody. For the second antibody, biotinylated goat F(ab)′2 antimouse IgM antibody, absorbed with human and mouse tissues, was used. A representative analysis of 3 performed from 3 different donors is shown. Note the cytoplasmic staining seen in a macrophage, surrounded by erythroblasts (original magnification, ×1000).
Discussion
The concept of a control mechanism for cellular growth through modulation of apoptosis has recently come to include a wide variety of tissue systems, including hematopoietic cells. Change in the balance between cell survival and death are clear signs of development of hematologic disorders such as the myelodysplastic syndromes21,22 and chronic myelogenous leukemia.23 Therefore, tight regulation of apoptosis needs to maintain hematopoietic homeostasis. The apoptosis of hematopoietic progenitor cells is regulated both positively and negatively by an interacting network of various cytokines and adhesive molecules.24 EPO, SCF, and IGF-I reduced apoptosis of purified human erythroid progenitor cells, suggesting that control of apoptosis by each of these factors has a prominent role in the regulation of erythropoiesis.1-4 Although various kinds of hematopoietic growth factors have been reported to reduce apoptosis of target cells, several cytokines promote apoptotic death of hematopoietic progenitor cells. Interferon-γ (IFN-γ) enhances apoptosis of immature purified erythroid progenitor cells,25,26 although it delays apoptosis of mature ones in the absence of EPO.16 Transforming growth factor-β and TNF-α reduce survival of primitive hematopoietic progenitors, which is maintained in the presence of flt3 ligand.27
Fas is a transmembrane type I glycoprotein that belongs to the TNF receptor superfamily and plays an essential role in down-regulation of the immune response by inducing apoptosis of activated T cells. Functional expression of Fas was noted on hematopoietic progenitor cells in various lineages,2,3,6,25,28-30 and the up-regulation of Fas is seen in hematopoietic cells prior to the process of apoptosis induced by TNF-α or IFN-γ.25These data indicate that the Fas system might serve as a “switch” for apoptotic cell death in the regulation of hematopoietic cell growth by various cytokines.
Recently, TNF-related apoptosis-inducing ligand (TRAIL), was found to induce apoptosis in normal human erythroid progenitor cells expanded in vitro.31 TRAIL, a member of the TNF family of proteins, has a structure similar to that of FasL.32 Furthermore, TRAIL induces apoptosis via Fas-associated death domain (FADD), a death adapter molecule functioning downstream in Fas signaling.33 Thus, TRAIL may be also involved in the regulation of apoptosis of erythroid progenitor cells, in cooperation with the Fas-Fas ligand system.
A tumor-associated antigen RCAS1 induced apoptosis of activated T-lymphocytes.11 RCAS1 is a type II membrane protein with an N-terminal transmembrane segment plus a coiled-coil structure in the C-terminal portion; it is expressed in various human cancer cells, especially in cases of invasive disease with poor prognosis.11,34,35 Thus, it is conceivable that the expression of RCAS1 might be involved in the mechanism by which cancer cells escape from surveillance by immune cells possessing RCAS1R.11 Using a binding assay with a recombinant RCAS1-GST fusion protein, it has been revealed that RCAS1-receptors are expressed on surfaces of hematopoietic cell lines, such as K562 (a chronic myelocytic leukemia cell line) and CCRF-CEM (an acute lymphoblastic leukemia cell line).11 We demonstrated here that normal human erythroid progenitor cells expressed RCAS1R and that triggering of RCAS1R by nRCAS1 resulted in apoptosis of these cells. Abundant RCAS1R was expressed on day 6 and day 7 ECFCs, which have a higher proliferative capacity than mature cells,14 and this expression diminished during erythroid maturation. Accordingly, day 7 ECFCs were more susceptible to the apoptotic signal induced by RCAS1, than were the mature cells. The immature erythroid progenitor, BFU-E, was less sensitive to RCAS1, compared to CFU-E, as determined by colony assay (Figure 2A and Table 3). Therefore, expression of RCAS1R might be restricted to the certain stage of erythroid maturation. As seen with flow cytometry, the kinetics of expression of RCAS1R during in vitro culture of erythroid progenitor cells was similar to that seen in the case of Fas. The survival of day 7 ECFCs depends on a variety of growth factors, especially EPO,4 and withdrawal of EPO from the culture medium results in immediate apoptosis of cells. These observations indicate that during erythropoiesis the level of apoptotic cell death is finely modulated by positive and negative regulatory factors mainly at specific stages of cellular maturation, which are predominantly at a high proliferative capacity. We also found that SCF delayed the disappearance of RCAS1R on ECFCs. It has been reported that SCF supports cell proliferation and cell survival and delays the maturation of erythroid progenitor cells.1-3 Thus, this effect of SCF on expression of RCAS1R might be due to inhibition of maturation of ECFCs and the regulation of expression of RCAS1R in erythroid progenitor cells may be closely linked with cellular maturation.
In lpr mice, which carry a homozygous mutation of the gene for Fas, abnormalities in colony-forming capacities of both erythroid and myeloid progenitor cells are not evident in the bone marrow, although marked lymphoid proliferation is noted.7 A study using gld Fas ligand-deficient mice also revealed that the number of committed myeloid progenitor cells did not increase significantly, and the potential of hematopoietic cells to undergo spontaneous apoptosis was not affected.9 Although possible counterregulation by an increase of a humoral factor, such as EPO, might exist in these knock-out models, the data do indicate that the Fas system is not the sole “switch” for hematopoietic cell apoptosis. A combination of receptors for various kinds of growth factors and distinct molecules, including Fas, TRAIL, and RCAS1, appear to control death signals for hematopoietic cells.
Recent advances in understanding molecular mechanisms underlining the process of apoptosis makes feasible a comparison of intracellular events induced by different death stimuli. On Fas activation, formation of the death-inducing signaling complex (DISC) activates caspase 8, an event followed by the activation of caspase 3.36 Loss of the mitochondrial transmembrane potential through a variety of stimuli, including Fas engagement, has been considered a key phenomenon in the process of apoptotic cell death.36-38 The kinetics of activation of caspases and collapse of mitochondrial transmembrane potential was similar between RCAS1 and Fas initiation. In the apoptotic signaling pathway of Fas, FADD is a molecule upstream of caspase 8. It has been reported that FADD is constitutively expressed in human erythroid progenitors.26 Therefore, RCAS1, which activates caspase 8 as demonstrated here, might induce apoptosis of erythroid progenitors via FADD. IFN-γ also activates caspases 3 and 8, when it induces apoptosis of immature erythroid progenitor cells.26 Together with our data, it is conceivable that this intracellular effector mechanism of apoptosis is shared by different kinds of death stimuli.
Although the data presented here strongly indicate involvement of the RCAS1 system in the regulation of erythropoiesis, the true relevance of this system to normal and pathologic hematopoiesis in vivo has yet to be determined. The binding assay performed with normal bone marrow mononuclear cells provides evidence that in vivo erythropoiesis may be partly regulated via modulation of apoptosis of erythroid progenitor cells through the RCAS1 system. Moreover, we found some TfR− cells among normal bone marrow cells expressing RCAS1R. This suggests that nonerythroid hematopoietic cells, as well as erythroid progenitor cells, possess RCAS1R. Addition of nRCAS1 also reduced formation of granulocyte-macrophage colonies from CD34+ cells purified from normal bone marrow (Table 3). Thus, apoptosis of granulocyte-macrophage progenitor cells as well as erythroid progenitor cells might be regulated in part by RCAS1.
The RCAS1 ligand was demonstrated by immunohistochemistry in the cytoplasm of normal bone marrow macrophages, which have a dual role in regulating of erythropoiesis. Whereas macrophages stimulate growth of erythroid progenitor cells in vitro by cell-cell interaction,39 cytokines mainly produced by macrophages, such as TNF-α and IFN-γ, have been shown to suppress the growth of erythroid cells.26,40 Wang and colleagues reported that removal of macrophages from murine-bone marrow cell cultures resulted in a highly significant increase in BFU-E colony formation.41 Thus, bone marrow macrophages, which reside in erythroblastic islands consisting of central macrophages surrounded by primitive erythroid precursors,42 might provide negative signals for growth of erythroid progenitor cells by inducing apoptosis by RCAS1 and cancer cells might produce myelophthisis, the marrow failure seen in cancer patients, through a similar mechanism.
In summary, we obtained evidence that purified human erythroid progenitor cells express functional RCAS1R and that RCAS1 induces apoptosis of these cells through an intracellular cascade shared by the Fas system. These observations mean that RCAS1 has a role in erythropoiesis and pave the way for further investigations on mechanisms related to hematopoiesis by the RCAS1 system.
This research was done in part at the Kyushu University Station for Collaborative Research. We express our deep appreciation to S. Aoki, M. Hirakawa, S. Kitamura, and S. Isewaki for excellent technical assistance. We are indebted to Dr S. B. Krantz (Department of Medicine-Hematology/Oncology, Vanderbilt University School of Medicine, Nashville, TN) for critical reading of the manuscript.
Supported in part by Kyushu University P&P grant.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Koichiro Muta, Department of Medicine and Bioregulatory Science, Graduate School of Medical Science, Kyushu University, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan; e-mail: mmmmm@intmed3.med.kyushu-u.ac.jp.

![Fig. 1. Erythroid progenitor cells express functional RCAS1R. / (A) Expression of RCAS1R, glycophorin A, and TfR on ECFCs was determined by flow cytometry at indicated times during erythroid maturation in liquid culture with 15% FCS, 15% human AB serum, 20 ng/mL rhSCF, and 2 U/mL rhEPO. Upper panels show the time profile of the expression of glycophorin A and TfR during erythroid maturation. Middle panels show glycophorin A versus RCAS1R expression by 2-color cytometry. Lower panels show TfR versus RCAS1R expression. Data are representative of 2 independent experiments. (B) Expressions of Fas (■), RCAS1 (○), and RCAS1R (●) on ECFCs were determined by flow cytometry, at indicated times, in liquid culture. The expression of Fas is derived from 3 independent experiments. (C) Effect of nRCAS1 on DNA synthesis of ECFCs was determined by [3H]-thymidine uptake. ECFCs were incubated at 37°C, 5% CO2 with or without nRCAS1 for 24 hours. For the last 6 hours of the culture period, cells were pulsed with 1 μCi [3H]-thymidine and collected onto glass filters. The incorporated radioactivity was measured using a Beta plate reader. Percentage inhibition was calculated by dividing radioactivity of the cells incubated with nRCAS1 by that without nRCAS1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/2/10.1182_blood.v98.2.313.h8000313_313_321/5/m_h81411276001.jpeg?Expires=1769101829&Signature=OEJNA81smlvE4izTcn6H6~uIZjYE2USRlImRqrezP4j~yuAyhrJpuqOBXe16OlvUejUH22hfxpiZ-LO5lzE18RYA0h3ex9Bw3CThFhORUeJNxmtsjzjLlKWnb9XhCSeZh1Zd9CQcM50q5c7mMlad-s-3bK8c20ZObDmvsynqF8782s2u371B36WWzEn9SvEc8G91iesvPk1cntWdqMsZWIgtsk7A2hl9krGkgprhXZsXSEDzGaR1DkUjz46KNAUPFkgTV33PhCjd7G5QGI59iVYxjeY43XUsQZwwcbWe0khPYMCdKdHWxQl4r3OqY9KAgvEA1wUkrl8u8jVI9TLaHQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal