Abstract
Interferon α/β plays an important role in the first-line defense against viral infections and can modulate cytokine responses by T-helper cells. Type 1 interferons (IFNs) are clinically important in infectious diseases and in the treatment of leukemia and lymphomas. Many different cell types have the capacity to produce IFN-α after encounter with virus and bacteria. The major, natural type 1 IFN–producing cell in humans was recently described as the plasmacytoid T cell, or pDC2, and it can differentiate into dendritic cells (DCs) on culture. This study describes the murine natural IFN-α–producing cell, or pDC2, that shares morphologic features with its human counterpart but has some distinct phenotypical characteristics. Murine plasmacytoid DCs can be differentially isolated based on their expression of CD11c, B220 (CD45R), and Thy1.2 (CD90). They lack expression of myeloid (eg, CD11b) antigens and CD8α, a marker used to isolate lymphoid DCs. Like human pDC2, murine plasmacytoid DCs exhibit their maximal type 1 IFN–producing capacity at a precursor stage; pDCs isolated from bone marrow responded to viral stimulation with higher IFN-α production than cells of the same phenotype isolated from spleen. Mobilization of mice with Flt3 ligand (Flt3L) or Flt3L and granulocyte-macrophage colony-stimulating factor, hematopoietic factors that specifically enhance DC growth, resulted in strikingly increased numbers of pDC in bone marrow and spleen. The isolation of this novel murine DC subset may serve as a useful tool in the study of viral immunobiology and for the design of treatments for murine malignancies.
Introduction
Dendritic cells (DCs) are rare sentinel cells that provide a first defense against invading microbial and viral pathogens.1,2 Encounter with these antigens induces DC maturation and allows for the subsequent activation of naive, antigen-specific T cells and B cells.2,3 DCs can be divided into distinct subsets based on differential phenotype and function. In humans, myeloid DCs (or DC1) express “myeloid” markers CD13, CD33, high levels of granulocyte-macrophage colony-stimulating factor (GM-CSF) receptors, and accessory molecules. They can induce T-cell proliferation and TH1-like cytokine profiles.2Human lymphoid DCs or DC24,5 do not express the CD11c antigen; instead, they express high levels of interleukin-3R (IL-3R), CD68, CD4, CD45R, and the inhibitory receptor IL-T3.6 Type 1 interferons are clinically important in infectious diseases and in the treatment of leukemia and lymphoma. Many different cell types have the capacity to produce interferon α (IFN-α) after encounter with virus and bacteria. Human precursors of DC2—plasmacytoid (p)DC2—produce large amounts of type 1 IFN in response to viral contact,7 and viruses can induce their differentiation into mature DC-2, with the resultant antigen-presenting cells (APCs) stimulating CD4+ T cells to produce IFN-γ and IL-10. IL-3, but not GM-CSF, also induces the maturation of pDC2. Once mature, these APCs can promote TH2 cytokine production (ie, IL-4, IL-5, and IL-10).8
In contrast to human DCs, mouse DC subsets described so far express CD11c.9,10 Murine myeloid DCs can be isolated based on the coexpression of CD11c and CD11b and can induce a strong allogeneic mixed-lymphocyte reaction (MLR).11 The lymphoid-related DC expresses CD11c and CD8α as a homodimer,12 was originally isolated from thymus, and was therefore termed lymphoid based on the expression of this T-lineage marker. Lymphoid-related DCs have been shown to express Fas ligand13 and may serve a regulatory function because they induce a comparatively low MLR.13,14 Moreover, recent data suggest that although DCs can be generated from common lymphoid precursors and hence are of lymphoid origin, DCs generated from myeloid precursor cells can give rise to CD8α-expressing DC upon adoptive transfer, suggesting expression of CD8α may be more related to maturation than to serving as a lineage marker.15 However, CD8α may serve as a tool to isolate this distinct DC subset.
The origin of plasmacytoid DCs remains controversial. In humans, a recent study on immunoglobulin (Ig) gene arrangement suggests a lymphoid origin.16 However, as their original terms—plasmacytoid T cells17 and plasmacytoid monocytes18—indicate, these cells could give rise to lymphomas with myeloid and lymphoid characteristics.18
To identify the mouse natural IFN–producing cell, or pDC, total leukocyte populations were isolated from different tissues and stimulated with herpes simplex virus (HSV), and culture supernatants were analyzed for IFN-α by specific enzyme-linked immunosorbent assay (ELISA). In addition, mice were treated with Flt3 ligand (Flt3L), an agent known to mobilize DCs in vivo,9 11 either alone or with GM-CSF. Flt3L with or without GM-CSF mobilization resulted in a dramatic elevation in the number of DCs capable of producing IFN-α.
Using multicolor fluorescence-activated cell sorting (FACS), the phenotype murine pDC could be determined. It was found to exhibit a phenotype slightly different from that of its human counterpart in that it expressed CD11c, but they were similar in expressing CD45R and low levels of CD4 and IL-3R. Short-term culture of murine pDC in media containing IL-3 and anti-CD40 antibodies induced maturation, resulting in the evolution of long cellular protrusions and high expression of major histocompatibility complex class 2 and accessory molecules.
Materials and methods
Mice
Balb/c and C57BL/6 6- to 8-week-old female mice were purchased from Jackson Laboratory (Bar Harbor, ME). Animals were maintained according to institutional guidelines in the Central Animal Facility of the Biomedical Science Tower at the University of Pittsburgh under an Institutional Animal Care & Use Committee (IACUC)–approved protocol.
Reagents
Recombinant murine (rm) Flt3L and rmGM-CSF for in vivo use were the generous gifts of Pharmacia (St Louis, MO). rmGM-CSF and rmIL-4 for in vitro culture were the kind gifts of Schering-Plough (Kenilworth, NJ). Herpes simplex virus (KOS strain) was a kind gift from Dr N. DeLuca (Department of Molecular Biology and Genetics, University of Pittsburgh). Stimulatory cytosine poly-guanine (CpG) motifs specific for the induction of IFN-α (GGGGTCAACGTTGAGGGGGG), IL-12 (TCGTCGTTTTGTCGTTTTGTCGTT), and a control CpG motif (CTGGGCTTTCTGGTTTTTTTCTGG) were the kind gifts of Dr A. Krieg (University of Iowa). rmIL-3 was purchased from R&D Systems (Minneapolis, MN). Staphylococcus aureus Cowan strain I (SAC; Pansorbin) was purchased from Calbiochem (La Jolla, CA), lipopolysaccharide (LPS) was obtained from Sigma, and stimulatory anti-CD40 (IgM) antibodies were from Pharmingen (San Diego, CA). All antibodies for cell separation and fluorochrome-conjugated antibodies for FACS and analysis were purchased from Pharmingen. Brefeldin A was obtained from Sigma. MACS magnetic beads directly conjugated with antibodies to CD11c or CD11b or with anti-rat immunoglobulin were purchased from Miltenyi Biotec (Auburn, CA).
Cytokine mobilization
Mice were mobilized with recombinant Flt3L ± GM-CSF or combinations thereof at a concentration of 20 μg/mouse per day in phosphate-buffered saline (PBS) by subcutaneous injections in the neck for 7 consecutive days under an IACUC-approved protocol.
Cell preparation
Bone marrow cells were obtained by flushing femurs and tibias with PBS using a 23-gauge needle. Red cells were hypotonically lysed, and the residual cells were washed and kept on ice until used. Spleens were isolated, and a single-cell suspension was made by passing the spleen through a nylon cell strainer (Falcon; Becton Dickinson, Franklin Lakes, NJ). After lysis of red cells by ammonium chloride solution, cells were incubated with antibodies to block FcR interactions (FcR-block, CD16/CD32, Pharmingen, at 1 μg/1 × 106 cells at 10 × 106/mL in PBS) for 15 minutes on ice. For isolation of CD11c+ cells using directly conjugated MACS magnetic beads (Miltenyi Biotec), CD11c beads were added according to the manufacturer's instructions. After incubation for 20 minutes at 4°C, cells were washed and passed over a MACS column. Positively selected cells were isolated and suspended in appropriate buffer. Purity was checked routinely by FACS and was found to be greater than 97% (not shown). For analysis by FACS, cells were stained with directly conjugated antibodies.
Fluorescence-activated cell sorting
Splenocytes were isolated as described above. After blocking of FcR-binding sites, T and B cells were depleted using antibodies to CD3 and surface immunoglobulin, respectively. After incubation, goat anti-rat–coated magnetic beads (MACS) were added, and, after further incubation, cells were passed over a MACS column. The negative fraction was collected, washed, and stained with antibodies to CD3–fluorescein isothiocyanate (FITC), sIgM-FITC, CD11c–phycoerythrin (PE), and CD11b-APC. Cells were sorted on a FACS Star Plus (Becton Dickinson, Mountain View, CA). Gates were set to exclude debris and dead cells. T and B cells were further excluded by gating on the FITC-negative population. CD11c+CD11b+ and CD11c+CD11b− cells were collected. Cell purity usually exceeded 96% (data not shown). Alternatively, total DCs were isolated using directly conjugated CD11c antibody-coated magnetic beads. After they were washed, DCs were stained with antibodies to CD8α-PE, B220-APC, and a cocktail of FITC-labeled antibodies (CD11b, sIg, CD3). Gates were set to exclude monocytes, T cells, and B cells, and DCs were sorted based on the expression of CD8α and B220. Purity exceeded 96% (data not shown).
Cell culture
All cell cultures were performed in complete RPMI-1640 (Gibco Life Technologies, Grand Island, NY) supplemented with 5% fetal calf serum (Gibco), 2 mM L-glutamine, penicillin, streptomycin, and 1 mM HEPES buffer (Gibco).
ELISA for detection of IFN-α2 and IL-12
ELISA was performed using standard procedures. In brief, for the detection of IFN-α2, 96-well flat-bottomed plates (Costar, Corning, NY) were coated with 5 μg/mL sheep anti–mouse IFN-α/β (a kind gift from Schering-Plough Research Institute) overnight at 4°C. After blocking with 3% bovine serum albumin in PBS-Tween 20 for 1 hour at 37°C, plates were washed, and standard rmIFN-α2 (a kind gift from Schering-Plough) and culture supernatants were added (standard rmIFN-α2 had been titrated in parallel against a known rmIFN-α2 standard provided by the National Institute of Allergy and Infectious Diseases reference reagent repository). Plates were then incubated at 4°C overnight. After washing, a rat anti–mouse IFN-α2antibody (4E-A1 [IgG1]; Seikagaku America, Falmouth, MA) was added, and plates were incubated at room temperature for 2 hours. After washing, peroxidase-conjugated goat anti–rat immunoglobulin antibody (Jackson Laboratory) was added, and plates were further incubated for 1 hour. After washing, plates were developed using tetramethylbenzidine (TMB) substrate (Kirkegaard & Perry, Gaithersburg, MD) and were stopped by the addition of 1 M sulfuric acid. For determination of IL-12 (p70), plates were coated with 5 μg/mL rat anti–mouse IL-12 (p70) (Pharmingen) in PBS overnight. After blocking with bovine serum albumin, supernatants and standard (rmIL-12, a kind gift from the Genetics Institute, Cambridge, MA) was added. After incubation overnight at 4°C, a biotinylated rat anti–mouse IL-12 (p40/p70) antibody was added, and plates were incubated for 2 hours at room temperature. After washing, extravidin-peroxidase (Sigma) was added and plates were further incubated for 1 hour. Finally, plates were developed using TMB substrate as above.
Electron microscopy
Transmission electron microscopy was performed on sorted, CD11c+B220+ DCs. Cells were fixed in 2.5% glutaraldehyde in cacodylate buffer and postfixed in 1% OsO4 solution. Cells were dehydrated in a series of alcohol solutions and embedded in epoxide. Sections were examined using a JEOL 1210 microscope (JEOL, Peabody, MA).
Staining for intracellular cytokines
Cells were cultured for 24 hours together with HSV (10 plaque-forming units [PFU]/cell), and Brefeldin A was added during the last 5 hours of culture. For preparation and staining of cytospins, cells were spun onto slides and fixed for 20 minutes with 2% formaldehyde. Thereafter, cells were permeabilized for 20 minutes using PBS containing 0.55% saponin, 5% fetal calf serum (FCS), and 2 mM HEPES. All subsequent steps were performed in buffer containing saponin. After washing and blocking with 10% goat serum and 5% FCS for 30 minutes, primary rat anti–mouse IFN-α (4E-A1; Seikagaku) or an isotype-matched control antibody was added, and slides were incubated overnight. Rabbit anti–rat immunoglobulin antibodies (Dako a/s, Glostrup, Denmark) were added, slides were incubated for 30 minutes and washed, and rat APAAP (Dako) was added. Incubation with rabbit anti–rat immunoglobulin and rat APAAP was repeated. Finally, slides were developed using Fast Red substrate (Dako).
For intracellular staining of IL-12 (p40), total CD11c+ DCs separated using MACS magnetic beads were cultured for 24 hours together with stimulatory anti-CD40 antibodies (25 μg/mL), SAC (10 μg/mL), or LPS (10μg/mL), and Brefeldin A (10 μg/mL) was added during the last 5 hours of culture. Surface staining of cells was performed using directly FITC-conjugated antibodies to CD11c, CD11b, and B220. After washing, cells were fixed in 2% PFA for 20 minutes and then permeabilized for 30 minutes in PBS containing 0.55% saponin, 5% FCS, and 2 mM HEPES. Directly PE-conjugated rat anti–mouse IL-12 (p40) antibodies (5 μg/mL) were added, and cells were further incubated for 30 minutes. After washing, cells were analyzed by flow cytometry.
Measurement of T-cell stimulation
For alloreaction, serial dilutions of irradiated DC (2000 cGy) that had been preactivated for 24 hours with stimulatory CD40 antibodies (IgM subclass; Pharmingen), and 2 × 105 total allogeneic CD4+ T cells were cultured for 6 days in round-bottomed, 96-well plates at a final volume of 200 μL.3H-thymidine (1 μCi/well [0.037 MBq/well]) was added during the last 18 hours of culture. Proliferation was measured in a β-scintillation counter.
Results
Isolation of murine plasmacytoid dendritic cells from spleen and bone marrow
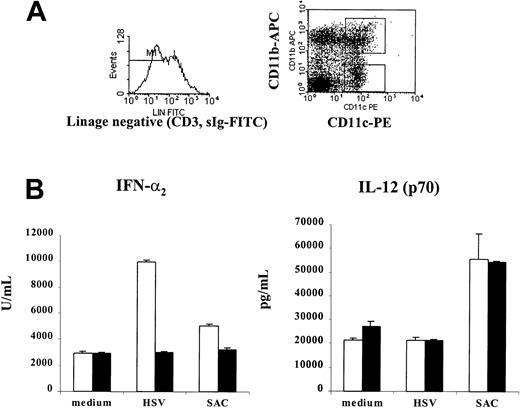
Mice were mobilized for 7 days with Flt3L, a hematopoietic growth factor known to specifically expand DCs in vivo,9 11 and were killed 1 day after the last cytokine injection. Bone marrow cells were obtained by flushing resected femurs with PBS. Spleens were harvested and dispersed into single-cell suspensions. Total bone marrow and spleen cells were analyzed by flow cytometry and were found to express high numbers of CD11c+CD11b+ (myeloid) and CD11c+CD11b− (lymphoid) DCs, respectively (data not shown). Because a functional characteristic of human plasmacytoid DCs (pDC2) is their high production of type 1 interferon after encounter with pathogens, isolated bone marrow and spleen cells were cultured overnight in the presence or absence of HSV or SAC. Cells maintained in medium alone were used as control. After 24 hours of culture, supernatants were harvested and analyzed for their IFN-α2 content by specific ELISA. As shown in Figure1, bone marrow and, to a lesser degree, spleen cells from mice that had been mobilized with Flt3L showed greater production of IFN-α2 in response to HSV than nontreated mice. SAC induced low but reproducible levels of IFN-α2. A significantly greater number of mobilized DCs could be detected when Flt3L was combined in vivo with GM-CSF, a factor known to support DC generation in vitro and also found to have an additive effect when coadministered with Flt3L (P.B. et al, unpublished observation, October 1999). Thus, the combination of Flt3L+GM-CSF was used throughout the rest of this study.
Elevated levels of IFN-α in Flt3L-treated mice.
Mobilization of mice with Flt3L results in elevated levels of IFN-α2 production from (A) BM and (B) spleen cell populations. Total leukocyte populations from BM and spleen from untreated mice or mice mobilized for 7 days with Flt3L were cultured for 24 hours in medium alone (white columns) or with HSV (black columns, 5 PFU/cell) or SAC (gray columns, 10 μg/mL). Supernatants were harvested and analyzed for their IFN-α2 content by specific ELISA. Data from 1 of at least 3 experiments yielding similar results are shown. (C) BM cells and (D) splenocytes were prepared from Flt3L+GM-CSF–treated mice, with either CD11c+ (white columns) or CD11b+ (black columns) cells isolated using directly conjugated magnetic beads (MACS). Cells were cultured for 24 hours in medium alone or with HSV or SAC. Supernatants were collected and analyzed for IFN-α2 by ELISA. Data from 1 of at least 3 experiments yielding comparable results is shown.
Elevated levels of IFN-α in Flt3L-treated mice.
Mobilization of mice with Flt3L results in elevated levels of IFN-α2 production from (A) BM and (B) spleen cell populations. Total leukocyte populations from BM and spleen from untreated mice or mice mobilized for 7 days with Flt3L were cultured for 24 hours in medium alone (white columns) or with HSV (black columns, 5 PFU/cell) or SAC (gray columns, 10 μg/mL). Supernatants were harvested and analyzed for their IFN-α2 content by specific ELISA. Data from 1 of at least 3 experiments yielding similar results are shown. (C) BM cells and (D) splenocytes were prepared from Flt3L+GM-CSF–treated mice, with either CD11c+ (white columns) or CD11b+ (black columns) cells isolated using directly conjugated magnetic beads (MACS). Cells were cultured for 24 hours in medium alone or with HSV or SAC. Supernatants were collected and analyzed for IFN-α2 by ELISA. Data from 1 of at least 3 experiments yielding comparable results is shown.
Bone marrow cells and splenocytes derived from mice mobilized with Flt3L+GM-CSF were isolated with magnetic beads directly conjugated with antibodies to CD11c or CD11b. Cells were cultured in medium alone or together with HSV or SAC, and supernatants were collected after 24 hours. The major IFN-α2 activity was attributed to the CD11c+ cell population (Figure 1C-D).
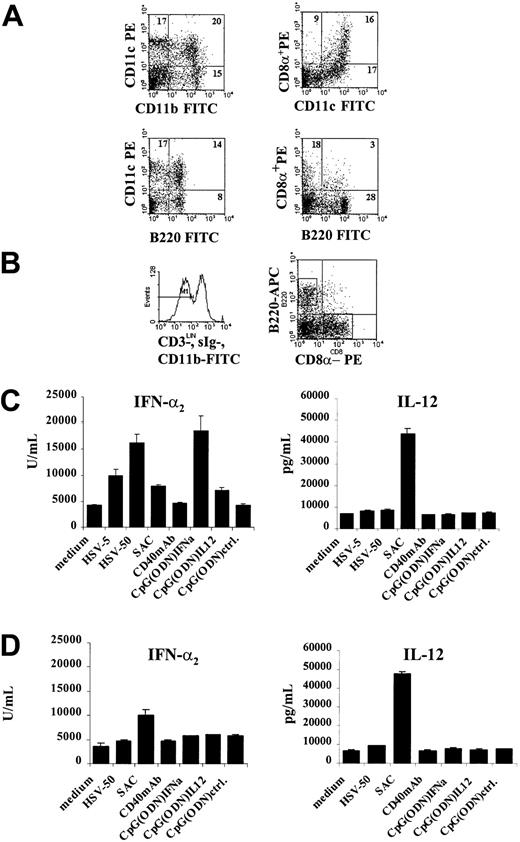
Isolated splenocytes from day 7 Flt3L+GM-CSF–mobilized mice were then sorted by FACS based on their expression of the CD11c+CD11b+ (myeloid) and CD11c+CD11b− (lymphoid) phenotypes using fluorochrome-labeled antibodies. Gates were set to exclude dead cells and debris, and B and T cells were further excluded by staining with antibodies to sIg-FITC and CD3-FITC. The purity of sorted cells exceeded 97% (Figure 2A). Sorted cells were cultured overnight in the presence or absence of HSV or SAC. Supernatants were collected and analyzed for their IFN-α2content by specific ELISA. CD11c+CD11b−, but not CD11c+CD11b+, DCs were found to produce IFN-α after stimulation with virus (Figure 2B). SAC induced lower but significant IFN-α production yet strongly promoted the production of IL-12 from both CD11c+CD11b−(lymphoid) and CD11c+CD11b+ (myeloid) DC subsets.
Murine IFN-α–producing cells are present in the lymphoid, CD11c+CD11b− DC population.
(A) Myeloid (CD11c+CD11b+) and lymphoid (CD11c+CD11b−) DCs were sorted from spleens of mice mobilized for 7 days with Flt3L+GM-CSF using the directly conjugated CD11c-PE and CD11b-APC antibodies. T and B cells depleted by antibodies to CD3 and surface immunoglobulin and directly conjugated goat anti–rat IgG–coated magnetic beads. FITC-labeled antibodies to CD3 and sIgM were used to further exclude contaminating cells in FACS sorting. Gates were set to exclude dead cells and debris. Purity of sorted cells typically exceeded 97%. (B) CD11c+CD11b+ (black columns) and CD11c+CD11b− (white columns) DCs were cultured for 24 hours in medium alone or with HSV (5 PFU/cell) or SAC (10 μg/mL). Supernatants were collected and analyzed for IFN-α2 and IL-12 (p70) by specific ELISA. Lymphoid CD11c+CD11b− contained the major IFN-α activity after stimulation with HSV. SAC induced IFN-α and IL-12 production. Data from 1 of 3 experiments with similar results are shown.
Murine IFN-α–producing cells are present in the lymphoid, CD11c+CD11b− DC population.
(A) Myeloid (CD11c+CD11b+) and lymphoid (CD11c+CD11b−) DCs were sorted from spleens of mice mobilized for 7 days with Flt3L+GM-CSF using the directly conjugated CD11c-PE and CD11b-APC antibodies. T and B cells depleted by antibodies to CD3 and surface immunoglobulin and directly conjugated goat anti–rat IgG–coated magnetic beads. FITC-labeled antibodies to CD3 and sIgM were used to further exclude contaminating cells in FACS sorting. Gates were set to exclude dead cells and debris. Purity of sorted cells typically exceeded 97%. (B) CD11c+CD11b+ (black columns) and CD11c+CD11b− (white columns) DCs were cultured for 24 hours in medium alone or with HSV (5 PFU/cell) or SAC (10 μg/mL). Supernatants were collected and analyzed for IFN-α2 and IL-12 (p70) by specific ELISA. Lymphoid CD11c+CD11b− contained the major IFN-α activity after stimulation with HSV. SAC induced IFN-α and IL-12 production. Data from 1 of 3 experiments with similar results are shown.
Isolation of the major type 1 IFN–producing dendritic cell subset
The CD11c+CD11b− DC subset contains a population of CD11c+CD8α+ DCs that has been previously shown to be the major IL-12–producing DC subset11,19; however, CD11c+CD8α+DCs do not produce significant levels of IFN-α after HSV infection. Because this suggested that there was heterogeneity within the CD11c+CD11b− subset responsible for the production of IFN-α versus IL-12, DCs were sorted in a manner biased by the phenotype recently reported for human pDC2.7 Human pDC2 expresses CD45R, CD4, and the receptor for IL-3,7 but FACS staining of murine CD11c+CD11b− DCs using antibodies recognizing IL-3R or CD4 showed only weak expression of these antigens (Table 1). However, antibodies to B220 (mouse CD45R) could be readily used to subdivide the DC population further given that flow cytometry of total CD11c+ DCs revealed a distinct subpopulation of CD11c+B220+ cells in day 7 Flt3L+GM-CSF–mobilized mice (Figure 3A) This was also observed to a lesser extent in mice treated with Flt3L alone (data not shown). This CD11c+B220+population could be found in bone marrow (BM), peripheral blood, spleen, and lymph nodes of mobilized mice. Total CD11c+ cells were isolated by directly conjugated anti-CD11c magnetic beads and were sorted by flow cytometry using fluorochrome-labeled antibodies to CD11b-FITC, CD8α-PE, and B220-APC. Anti–CD3- and sIg–FITC-labeled antibodies were used to further exclude contaminating T and B cells. CD11b+ DCs (myeloid) were excluded, and gates were set on CD8α+- and B220+- expressing cells, respectively (Figure 3B). FACS analysis of sorted DCs showed that though both populations expressed the CD11c antigen, the CD8α+ population was typically CD11cbright and the B220+ population was CD11cdim (Table 1). B220+CD11c+cells expressed Thy1.2 and low, but detectable, levels of IL-3R and CD4. Furthermore, they expressed MHC class 1 (H2-Kd) and class 2 (I-Ad/b) and accessory and adhesion molecules CD86, CD11a, and CD54, but no myeloid antigens or CD19. The expression of Gr-1 varied and was low or undetectable on freshly isolated cells, but it was slightly up-regulated after overnight culture. The CD8α+CD11c+ DC population expressed high levels of the MHC class II and CD86 molecules but lacked expression of the CD4, IL-3R, and Thy1.2 antigens. Table 1 provides a summary of the phenotype of the distinct murine DC subpopulations either directly after FACS sorting or after overnight culture in complete medium.
Phenotype of splenic plasmacytoid, lymphoid, and myeloid murine DCs
| Cell surface marker . | Plasmacytoid DC CD11c+CD11b−CD8α−B220+ . | Lymphoid DC CD11c+CD11b−CD8α+B220− . | Myeloid DC CD11c+CD11b+CD8α−B220− . | |||
|---|---|---|---|---|---|---|
| Day 0 . | Day 1 . | Day 0 . | Day 1 . | Day 0 . | Day 1 . | |
| CD2 | − | − | − | − | − | − |
| CD4 | + | ± | − | − | − | ± |
| CD5 | − | − | − | − | − | − |
| CD8α | − | − | +++ | +++ | − | − |
| CD11a | ++ | ++ | +++ | +++ | +++ | +++ |
| CD11b | − | − | − | − | +++ | +++ |
| CD11c | + | + | +++ | +++ | +++ | +++ |
| CD19 | − | − | − | − | − | − |
| CD40 | + | ++ | ++ | +++ | + | ++ |
| CD54 | +++ | ++ | +++ | +++ | +++ | +++ |
| CD62L | ++ | − | ++ | − | ± | − |
| CD86 | + | ++ | ++ | ++ | +++ | +++ |
| CD95 (Fas) | ± | ± | ++ | ND | ND | ND |
| B220 (CD45R) | ++ | ++ | − | ± | − | ± |
| Thy1.2 | ++ | ++ | − | − | − | − |
| Gr-1 | ± | + | − | − | − | + |
| H-2Kd (MHC class I) | ++ | ++ | +++ | +++ | +++ | +++ |
| I-Ad(MHC class II) | + | ++ | ++ | +++ | +++ | +++ |
| IL-3R | + | ± | − | − | ± | ± |
| Cell surface marker . | Plasmacytoid DC CD11c+CD11b−CD8α−B220+ . | Lymphoid DC CD11c+CD11b−CD8α+B220− . | Myeloid DC CD11c+CD11b+CD8α−B220− . | |||
|---|---|---|---|---|---|---|
| Day 0 . | Day 1 . | Day 0 . | Day 1 . | Day 0 . | Day 1 . | |
| CD2 | − | − | − | − | − | − |
| CD4 | + | ± | − | − | − | ± |
| CD5 | − | − | − | − | − | − |
| CD8α | − | − | +++ | +++ | − | − |
| CD11a | ++ | ++ | +++ | +++ | +++ | +++ |
| CD11b | − | − | − | − | +++ | +++ |
| CD11c | + | + | +++ | +++ | +++ | +++ |
| CD19 | − | − | − | − | − | − |
| CD40 | + | ++ | ++ | +++ | + | ++ |
| CD54 | +++ | ++ | +++ | +++ | +++ | +++ |
| CD62L | ++ | − | ++ | − | ± | − |
| CD86 | + | ++ | ++ | ++ | +++ | +++ |
| CD95 (Fas) | ± | ± | ++ | ND | ND | ND |
| B220 (CD45R) | ++ | ++ | − | ± | − | ± |
| Thy1.2 | ++ | ++ | − | − | − | − |
| Gr-1 | ± | + | − | − | − | + |
| H-2Kd (MHC class I) | ++ | ++ | +++ | +++ | +++ | +++ |
| I-Ad(MHC class II) | + | ++ | ++ | +++ | +++ | +++ |
| IL-3R | + | ± | − | − | ± | ± |
Expression ranges from ± (low) to +++ (strong). Lymphoid DCs are characterized by a CD11c+CD11b−CD8α+ phenotype, and myeloid DCs exhibit a CD11c+CD11b+CD8α− phenotype. Cells were analyzed directly after FACS sorting or after overnight culture in complete medium.
ND indicates not determined.
Major murine IFN-α–producing DC subset expresses a CD11clow, B220+ phenotype and lacks CD8α expression.
(A) Staining of total splenocytes derived from Flt3L+GM-CSF–treated mice show that, in addition to myeloid CD11c+CD11b+ DC, a subset of CD11c+DC coexpresses the B220+ antigen. This population is distinct from CD11c+CD8α+ lymphoid DCs. (B) The CD11c+CD11b− DC subset was further subdivided using antibodies to CD8α-PE and B220-APC. Total CD11c+ cells were isolated from the spleens of Flt3L+GM-CSF–treated mice using CD11c-conjugated magnetic beads. FITC-conjugated antibodies to CD11b, CD3, and sIg were used to exclude myeloid DCs, T cells and B cells in FACS sorting. Sorted cells were typically more than 96% pure. (C) Sorted splenic CD11c+B220+ DCs were cultured for 24 hours alone or with HSV, SAC, CD40 antibodies, or 2 μg/mL CpG (ODN). Supernatants were harvested and examined for their IFN-α and IL-12 content by specific ELISA. Major HSV-responsive, IFN-α2–producing cells were found in the CD11c+B220+ subset. These cells exhibited the capacity to produce IFN-α and IL-12 in response to SAC. (D) CD11c+CD8α+ lymphoid DCs did not produce IFN-α on stimulation with HSV, but SAC induced low levels of IFN-α and high levels of IL-12. At least 5 experiments performed gave comparable results.
Major murine IFN-α–producing DC subset expresses a CD11clow, B220+ phenotype and lacks CD8α expression.
(A) Staining of total splenocytes derived from Flt3L+GM-CSF–treated mice show that, in addition to myeloid CD11c+CD11b+ DC, a subset of CD11c+DC coexpresses the B220+ antigen. This population is distinct from CD11c+CD8α+ lymphoid DCs. (B) The CD11c+CD11b− DC subset was further subdivided using antibodies to CD8α-PE and B220-APC. Total CD11c+ cells were isolated from the spleens of Flt3L+GM-CSF–treated mice using CD11c-conjugated magnetic beads. FITC-conjugated antibodies to CD11b, CD3, and sIg were used to exclude myeloid DCs, T cells and B cells in FACS sorting. Sorted cells were typically more than 96% pure. (C) Sorted splenic CD11c+B220+ DCs were cultured for 24 hours alone or with HSV, SAC, CD40 antibodies, or 2 μg/mL CpG (ODN). Supernatants were harvested and examined for their IFN-α and IL-12 content by specific ELISA. Major HSV-responsive, IFN-α2–producing cells were found in the CD11c+B220+ subset. These cells exhibited the capacity to produce IFN-α and IL-12 in response to SAC. (D) CD11c+CD8α+ lymphoid DCs did not produce IFN-α on stimulation with HSV, but SAC induced low levels of IFN-α and high levels of IL-12. At least 5 experiments performed gave comparable results.
Freshly isolated and sorted spleen CD11c+B220+ or CD11c+CD8α+ cells were cultured overnight with or without HSV, SAC, anti-CD40 antibodies, and CpG oligodeoxynucleotides (ODN) motifs to analyze the impact of these stimuli on cytokine production. Culture supernatants were then harvested and examined for their content of secreted IFN-α2 and IL-12 (p70). Figure 3C shows that splenic CD11c+B220+ DCs produced IFN-α2after viral stimulation. Interestingly, SAC induced both IL-12 and IFN-α from this DC subset. However, CD11c+CD8α+ lymphoid DCs did not produce IFN-α after HSV stimulation (Figure 3D), even at high viral multiplicity of infection (ie, 50 PFU/cell), but they could produce low levels after stimulation with SAC. SAC were also the only stimuli that induced the production of IL-12 from lymphoid, myeloid, and CD11c+B220+ DCs as measured by intracellular staining and flow cytometry. The major IL-12–producing DC subset was the CD11c+CD8α+ lymphoid DC that also responded to stimulatory anti-CD40 antibodies or LPS activation (Figure 4).
Production of IL-12 (p40) by DC subsets.
Total CD11c+ DCs separated by directly conjugated magnetic beads were stimulated for 24 hours with stimulatory anti-CD40 antibodies (CD40 mAb), LPS (10 μg/mL), or SAC. For the detection of intracellular IL-12, DCs were stained with PE-conjugated antibodies recognizing IL-12 (p40/p70) and directly FITC-labeled antibodies to CD11c, CD11b, and B220. Percentage positive cells are indicated in each quadrant. At least 4 similar experiments were performed.
Production of IL-12 (p40) by DC subsets.
Total CD11c+ DCs separated by directly conjugated magnetic beads were stimulated for 24 hours with stimulatory anti-CD40 antibodies (CD40 mAb), LPS (10 μg/mL), or SAC. For the detection of intracellular IL-12, DCs were stained with PE-conjugated antibodies recognizing IL-12 (p40/p70) and directly FITC-labeled antibodies to CD11c, CD11b, and B220. Percentage positive cells are indicated in each quadrant. At least 4 similar experiments were performed.
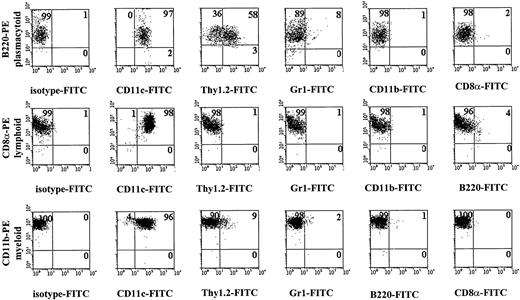
The key markers used to isolate these 3 different mouse DC subsets are detailed in Figure 5. To obtain myeloid DCs, PE-conjugated antibodies to CD11b were used and CD8α-FITC–expressing cells were excluded.
FACS profiles showing key markers for isolation of murine plasmacytoid, lymphoid, and myeloid DCs.
Myeloid DCs were obtained by changing the settings for the FACS sorting so that CD11c+CD8α+ cells were excluded and CD11c+CD11b+ were included. For more detailed analysis, see Table 1.
FACS profiles showing key markers for isolation of murine plasmacytoid, lymphoid, and myeloid DCs.
Myeloid DCs were obtained by changing the settings for the FACS sorting so that CD11c+CD8α+ cells were excluded and CD11c+CD11b+ were included. For more detailed analysis, see Table 1.
Mouse pDC develops into dendritic cells after culture and acquires the capacity to stimulate T cells
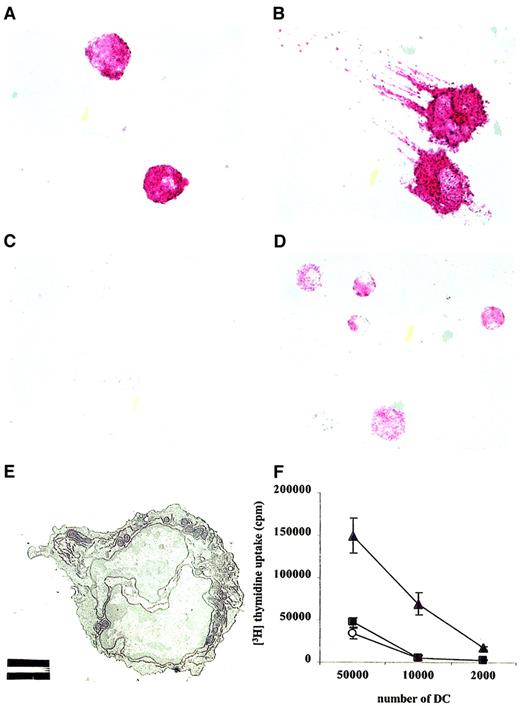
Visual inspection of cytospins of the CD11c+B220+ DC subset revealed round cells lacking the characteristic dendritic morphology (Figure6A). Human pDC2 differentiates into typical DC after culture in IL-3 and anti-CD40 antibodies.4 The culture of CD11c+B220+ DCs in media containing IL-3 and anti-CD40 for 2 days similarly revealed “classical” DCs with long protrusions and high MHC class II expression (Figure 6B). Interferon-α could be detected by intracellular staining in CD11c+B220+ DCs after culture with HSV overnight (Figure 6C-D). IFN-α–producing cells were clearly discernible by their strong cytoplasmic staining. Transmission electron microscopy on freshly isolated, CD11c+B220+ DC confirmed a well-developed endoplasmic reticulum and multiple mitochondria (Figure 6E). Of note, CD11c+B220+DCs did not proliferate significantly in culture, regardless of the combination of stimulating agents evaluated (ie, IL-3, SAC, CD40, HSV; data not shown).
IFN-α–producing plasmacytoid cells differentiate into DCs after culture in IL-3.
Cytospins of sorted CD11c+B220+ cells were stained using antibodies to MHC class II, a biotinylated goat anti–rat IgG antibody, and then by streptavidin-alkaline phosphatase and were developed using Fast Red substrate. These data demonstrate that (A) directly after sorting, cells are round and lack DC attributes and that (B) after 2 days in culture with rmIL-3 and activating anti-CD40 antibodies, many cells develop DC morphology with long protrusions and strong MHC class II expression. (C) Cytospins of CD11c+B220+ DCs were infected for 24 hours with HSV (10 PFU/cell) and then stained with isotype control or (D) rat anti–mouse IFN-α2 antibodies and were developed using Fast Red substrate. (E) Transmission electron microscopy of FACS-sorted CD11c+B220+ DCs. White bar represents 2 μm. Original magnification, × 2500. (F) Irradiated CD11c+CD11b+ myeloid DCs (filled triangles) CD11c+B220+ pDC (filled circles) and CD11c+CD8α+ lymphoid DCs (open circles) were cultured with total allogeneic CD4+ T cells (200 000/well) for 6 days in triplicate in 96-well, round-bottomed plates, and3H-thymidine was added for the last 18 hours of culture. Cells were harvested, and proliferation was measured in a scintillation counter. Data are representative of 3 experiments yielding comparable data.
IFN-α–producing plasmacytoid cells differentiate into DCs after culture in IL-3.
Cytospins of sorted CD11c+B220+ cells were stained using antibodies to MHC class II, a biotinylated goat anti–rat IgG antibody, and then by streptavidin-alkaline phosphatase and were developed using Fast Red substrate. These data demonstrate that (A) directly after sorting, cells are round and lack DC attributes and that (B) after 2 days in culture with rmIL-3 and activating anti-CD40 antibodies, many cells develop DC morphology with long protrusions and strong MHC class II expression. (C) Cytospins of CD11c+B220+ DCs were infected for 24 hours with HSV (10 PFU/cell) and then stained with isotype control or (D) rat anti–mouse IFN-α2 antibodies and were developed using Fast Red substrate. (E) Transmission electron microscopy of FACS-sorted CD11c+B220+ DCs. White bar represents 2 μm. Original magnification, × 2500. (F) Irradiated CD11c+CD11b+ myeloid DCs (filled triangles) CD11c+B220+ pDC (filled circles) and CD11c+CD8α+ lymphoid DCs (open circles) were cultured with total allogeneic CD4+ T cells (200 000/well) for 6 days in triplicate in 96-well, round-bottomed plates, and3H-thymidine was added for the last 18 hours of culture. Cells were harvested, and proliferation was measured in a scintillation counter. Data are representative of 3 experiments yielding comparable data.
T-cell costimulation
A defining characteristic for most DC subsets is T-cell stimulatory capacity. CD11c+B220+ DCs were examined for their capacity to stimulate T-cell proliferation. Isolated DCs were cultured overnight with stimulatory anti-CD40 antibodies (25 μg/mL). After washing, DCs were irradiated (2000 cGy), and serial dilutions of these APCs were cultured with total allogeneic CD4+ T cells (2 × 105/well). T-cell cocultures with CD11c+CD11b+ and CD11c+CD8α+ DCs were generated in parallel. As shown in Figure 6F, CD40-activated pDC induced T-cell proliferation to levels similar to those observed for CD11c+CD8α+ lymphoid DCs. Human cultured pDC has also been shown to induce a more potent T-cell stimulation than freshly isolated.4 As expected, myeloid DCs induced comparably strong T-cell stimulation.
In summary, the mouse plasmacytoid DCs exhibited a CD11cdimB220+Thy1.2+ phenotype but did not express the CD8α lymphoid or CD11b myeloid DC markers. They are the major producers of type 1 interferon after viral stimulation but can also produce both IFN-α and IL-12 when activated by bacterial proteins. Table 2 outlines the characteristics of murine and human DC subsets. Murine pDC are not the strong stimulators of allogeneic T cells their human counterparts are and may possibly serve a regulatory function similar to that of murine lymphoid DCs.
Summary of the characteristics of mouse and human DC subsets
| Mouse . | Human . |
|---|---|
| Myeloid CD11c+CD11b+(CD8α−) induce strong MLR. Primarily T-cell stimulatory function. | Myeloid DC1 major IL-12–producing DCs, CD11c+IL3R−, induce strong MLR. Primarily T-cell stimulatory function. |
| Lymphoid CD11c+CD11b−(CD8α+) can express Fas ligand, produce high amounts of IL-12, and induce weak MLR. Primarily T-cell regulatory function. | |
| Plasmacytoid CD11c+CD11b−CD8α−B220+Thy1.2+, but low expression of IL-3R Major type I IFN–producing DCs, induce weak MLR and differentiate into DCs upon culture with CD40 antibodies and IL3. Primarily T-cell regulatory function? | Plasmacytoid DC2 major type I IFN–producing cells, CD11c−IL3R+CD45R+CD4+, induce strong MLR and mature into DCs with Th2-stimulating capacity on culture with CD40 antibodies and IL3. T-cell stimulatory and regulatory functions. |
| Mouse . | Human . |
|---|---|
| Myeloid CD11c+CD11b+(CD8α−) induce strong MLR. Primarily T-cell stimulatory function. | Myeloid DC1 major IL-12–producing DCs, CD11c+IL3R−, induce strong MLR. Primarily T-cell stimulatory function. |
| Lymphoid CD11c+CD11b−(CD8α+) can express Fas ligand, produce high amounts of IL-12, and induce weak MLR. Primarily T-cell regulatory function. | |
| Plasmacytoid CD11c+CD11b−CD8α−B220+Thy1.2+, but low expression of IL-3R Major type I IFN–producing DCs, induce weak MLR and differentiate into DCs upon culture with CD40 antibodies and IL3. Primarily T-cell regulatory function? | Plasmacytoid DC2 major type I IFN–producing cells, CD11c−IL3R+CD45R+CD4+, induce strong MLR and mature into DCs with Th2-stimulating capacity on culture with CD40 antibodies and IL3. T-cell stimulatory and regulatory functions. |
Discussion
The current study describes the identification of the murine natural type 1 IFN–producing cell20,21 or plasmacytoid DC2,7 with the capacity to produce IFN-α after viral challenge. Murine pDC can also produce IL-12 in response to bacterial antigen such as SAC. The kinetics of the pDC response to SAC is different from that of lymphoid CD11c+CD8α+DC, the major IL-12 producing DC subset,19 in that it is immediate and transient (not shown). Activating anti-CD40 antibodies were unable to induce IL-12 production in murine pDC, whereas lymphoid CD11c+CD8α+ DCs responded readily to this stimuli and to stimulation using LPS. Moreover, as shown for human natural IFN–producing cells,22 murine pDC produced low but significant levels of IFN-α in response to SAC. This may have in vivo relevance because both IFN-α and IL-12 have been reported to promote CD8+ cytotoxic T-lymphocyte and natural killer activity,20 21 thereby providing a link between innate and adaptive immunity. Experiments are under way to analyze what signals regulate IFN-α versus IL-12 production in murine pDC.
All murine DC subsets described to date express the CD11c antigen, whereas those for human DCs do not. Murine pDC also express the CD45R (B220) antigen but at lower levels of CD4 and IL-3R than its human counterpart. In addition, murine pDC expressed Thy1.2, a marker expressed on T cells, but did not express the CD8α antigen under any conditions evaluated in this study. Recently, CD8α has been found on activated, myeloid DCs,15 23 suggesting it may not be a hallmark of lymphoid DC. Instead, expression of CD11c in the absence of CD11b might serve as a more reliable index for distinguishing lymphoid DCs from myeloid DCs.
The existence of the natural IFN–producing cell has been known for some time.24 Although its hematopoietic origin is still a matter of debate, a recent study identified certain transcription factors that regulate T- and B-cell development and pDC2 development, but not DC1, suggesting a lymphoid origin for pDC2.16 Murine pDC express markers associated with lymphoid lineage cells but lack expression of myeloid markers (ie, CD11b) and express low levels of Gr-1 when isolated from BM but not from spleen. However, a low expression of Gr-1 could be found on splenic pDC after overnight culture. A BM-derived cell with the phenotype CD4lowGr-1low that also express low amounts of B220 and Thy-1 was isolated previously25 and found to give rise to both B- and T-lineage cells and to myeloid cells on adoptive transfer. Whether these are the same cells as the plasmacytoid DC described here merits further study. Murine pDC also do not express CD19, a marker that can be found coexpressed with CD11c on a small subset of cells in BM26; this may define an additional subset of DCs.
Flt3L with or without GM-CSF was found to induce and enhance the frequency of the CD11c+B220+DC subset that was correlated with the mobilization of the IFN-α–producing cell population. In nontreated mice these cells were found principally in the BM, whereas in Flt3L-treated mice, much of the IFN-α production was relocated to the spleen. This finding may prove to be of considerable importance in clinical trials of Flt3L, in which the migration of pDC to peripheral sites is desired.
Type 1 interferons produced by pDC would be anticipated to have multiple effects on the immune system, including the up-regulation of MHC class I on all cell types and functional activation of macrophages and natural killer cells.20,21 IFN-α also has proven antiapoptotic effects on CD4+ and CD8+ T cells through the direct induction of BCL-xL and the indirect induction of IL-15.20 TRAIL27 is also up-regulated on activated T cells by treatment with IFN-α and is associated with enhanced cytolytic effector function. Each of these effects would be expected to benefit immunotherapies designed to enhance cytotoxic T-lymphocyte viability and activity, such as in patients with cancer.
Most recent data suggest the involvement of pDC in allergic asthma28 and in the autoimmune condition systemic lupus erythematosus.29 Targeting pDC in murine models for these diseases may provide important insights in their pathogenesis.
Dr W. J. Storkus is greatly acknowledged for support, help, and fruitful discussions. I thank Dr L. D. Falo for support, Mr. R. Lakomy for expert help with FACS sorting, and Drs S. Watkins and A. Bursick at the Center for Biological Imaging for help with electron microscopy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Pia Björck, Department of Dermatology, University of Pittsburgh, BST W 1546, 200 Lothrop St, Pittsburgh, PA 15213; e-mail: bjorckp@msx.upmc.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal