Abstract
The duration of immunodeficiency following marrow transplantation is not known. Questionnaires were used to study the infection rates in 72 patients surviving 20 to 30 years after marrow grafting. Furthermore, in 33 of the 72 patients and in 16 donors (siblings who originally donated the marrow) leukocyte subsets were assessed by flow cytometry. T-cell receptor excision circles (TRECs), markers of T cells generated de novo, were quantitated by real-time polymerase chain reaction. Immunoglobulin G2 (IgG2) and antigen-specific IgG levels were determined by enzyme-linked immunosorbent assay. Infections diagnosed 15 years after transplantation occurred rarely. The average rate was 0.07 infections per patient-year (one infection every 14 years), excluding respiratory tract infections, gastroenteritis, lip sores, and hepatitis C. The counts of circulating monocytes, natural killer cells, B cells, CD4 T cells, and CD8 T cells in the patients were not lower than in the donors. The counts of TREC+ CD4 T cells in transplant recipients younger than age 18 years (at the time of transplantation) were not different from the counts in their donors. In contrast, the counts of TREC+ CD4 T cells were lower in transplant recipients age 18 years or older, even in those with no history of clinical extensive chronic graft-versus-host disease, compared with their donors. The levels of total IgG2 and specific IgG against Haemophilus influenzae and Streptococcus pneumoniae were similar in patients and donors. Overall, the immunity of patients surviving 20 to 30 years after transplantation is normal or near normal. Patients who received transplants in adulthood have a clinically insignificant deficiency of de novo–generated CD4 T cells, suggesting that in these patients the posttransplantation thymic insufficiency may not be fully reversible.
Introduction
More than 125 000 allogeneic and syngeneic hematopoietic cell transplantations (HCTs) have been performed worldwide for hematologic malignancies, aplastic anemia, and inborn errors of hematolymphopoietic cells.1-3 Immunodeficiency follows HCT and lasts for more than 1 year.4-11 Because HCT only came into general practice in the 1980s, there has been, up until now, no opportunity to observe the immunity of very long-term survivors. There are conflicting views about what might happen to the immunity of these patients. On the one hand, infection rates as well as laboratory parameters of immunity like CD4 T-cell counts gradually improve in the first 5 years after transplantation (reviewed in Parkman and Weinberg4 and Storek and Witherspoon5). Thus, by 20 years, one might expect relatively complete recovery. On the other hand, there is the possibility that the replicative potential of the transplanted immune cells and/or their precursors might be limited.12 This possibility could result in late onset immune deficiency. We have undertaken a study of a unique cohort of patients, the first group surviving 20 to 30 years after transplantation. We have asked the following questions: (1) What are the counts of immune cells in this group of patients? CD4 T cells were of particular interest because low CD4 T-cell counts have been associated with high rates of postengraftment infections and because the duration of CD4 T lymphocytopenia in adult marrow transplant recipients is not known (CD4 T-cell counts remain low for at least 5 years after transplantation).13-15 (2) What are the counts of T cells generated de novo (from hematolymphopoietic cells)? This question is important because concerns have been raised that the grafted hematolymphopoietic cells or the host thymus cannot sustain de novo T lymphopoiesis for more than 14 years after transplantation.12 (3) What are the levels of immunoglobulin (Ig)G2 and IgG against the capsular polysaccharides of commonly encountered encapsulated bacteria? Low levels have been associated with infections in HCT recipients, and the duration of this selective immunoglobulin deficiency is not known (levels remain low for at least 2 to 5 years after transplantation).16-20 (4) How frequently do the very late survivors develop infections? Infections, the incidence of which is the most clinically relevant measure of immunity, have been described to cause significant morbidity and mortality even between 2 and 16 years after transplantation.21 22
Patients, materials, and methods
Patients
Of 389 patients undergoing allogeneic (361) or syngeneic (28) bone marrow grafting in Seattle before January 1, 1978, 72 patients survived for 20 or more years (range, 20-30 years). All 72 patients were evaluable for infections through questionnaires (see “Enumeration of infections” that follows). Thirty-three patients and 16 of their 33 marrow donors also agreed to have blood drawn for the determination of lymphocyte subset counts and antibody levels. The study was approved by the Fred Hutchinson Cancer Research Center Institutional Review Board. Demographic and clinical characteristics of the patients are given in Table 1. Patients received no immunosuppressive drugs, prophylactic antibiotics, or intravenous immunoglobulin beyond year 15 after transplantation, except for one lung transplant recipient (Table 1, footnote). Except for a higher incidence of hepatitis C, the 33 patients who gave blood were generally representative of the whole cohort of the 72 patients (Table 1).
Patient characteristics
| . | All patients (n = 72) . | Patients who gave blood (n = 33)* . |
|---|---|---|
| Patient sex, male/female (no. of patients) | 38/34 | 16/17 |
| Patient age at transplantation, y, median (range) | 17 (3-40) | 17 (5-36) |
| Donor age at transplantation, y, median (range) | 19 (4-50) | 20 (4-42) |
| Patient age at blood draw, y, median (range) | NA | 39 (28-62) |
| Donor age at blood draw, y, median (range) | NA | 42 (26-53) |
| Donor type, HLA-matched sibling/identical twin (no. of patients) | 66/6 | 30/3 |
| Reason for transplantation, leukemia/aplastic anemia (no. of patients) | 29/43† | 10/23 |
| Cytomegalovirus serostatus before transplantation (patient), positive/negative/unknown (no. of patients) | 6/13/53 | 3/6/24 |
| History of splenectomy, yes/no (no. of patients) | 1/71 | 0/33 |
| Conditioning, TBI + Cy/Cy only/other‡ (no. of patients) | 24/36/12 | 7/19/7 |
| T-cell depletion, yes/no (no. of patients) | 0/72 | 0/33 |
| Donor blood buffy coat cells given in addition to marrow,661-153 yes/no (no. of patients) | 9/57 | 7/23 |
| GVHD prophylaxis,1-153methotrexate67/other (no. of patients) | 64/2 | 29/1 |
| Acute GVHD,1-153 grade 2-3/grade 0-1 (no. of patients) | 13/53 | 5/25 |
| Chronic GVHD (clinical),1-153extensive1-155/none or limited/unknown (no. of patients) | 14/51/1 | 6/24/0 |
| Karnofsky performance score at 20 years after transplantation, 90% or greater/under 90%/unknown (no. of patients) | 62/3/7 | 29/2/2 |
| Posttransplantation conditions potentially impairing immunity (no. of patients) | ||
| Relapse of original disease, yes/no | 21-154/70 | 21-154/31 |
| Secondary malignancy,1-159 yes/no | 14/58 | 5/28 |
| Treatment with chemotherapy, yes/no | 2/70 | 1/32 |
| Chronic hepatitis C, yes/no | 231-160/49 | 151-160/18 |
| Treatment with interferon and/or ribavirin, yes/no | 11/61 | 6/27 |
| Diabetes mellitus,1-164yes/no | 7/65 | 2/31 |
| Obstructive lung disease, yes/no | 31-161/69 | 11-161/32 |
| . | All patients (n = 72) . | Patients who gave blood (n = 33)* . |
|---|---|---|
| Patient sex, male/female (no. of patients) | 38/34 | 16/17 |
| Patient age at transplantation, y, median (range) | 17 (3-40) | 17 (5-36) |
| Donor age at transplantation, y, median (range) | 19 (4-50) | 20 (4-42) |
| Patient age at blood draw, y, median (range) | NA | 39 (28-62) |
| Donor age at blood draw, y, median (range) | NA | 42 (26-53) |
| Donor type, HLA-matched sibling/identical twin (no. of patients) | 66/6 | 30/3 |
| Reason for transplantation, leukemia/aplastic anemia (no. of patients) | 29/43† | 10/23 |
| Cytomegalovirus serostatus before transplantation (patient), positive/negative/unknown (no. of patients) | 6/13/53 | 3/6/24 |
| History of splenectomy, yes/no (no. of patients) | 1/71 | 0/33 |
| Conditioning, TBI + Cy/Cy only/other‡ (no. of patients) | 24/36/12 | 7/19/7 |
| T-cell depletion, yes/no (no. of patients) | 0/72 | 0/33 |
| Donor blood buffy coat cells given in addition to marrow,661-153 yes/no (no. of patients) | 9/57 | 7/23 |
| GVHD prophylaxis,1-153methotrexate67/other (no. of patients) | 64/2 | 29/1 |
| Acute GVHD,1-153 grade 2-3/grade 0-1 (no. of patients) | 13/53 | 5/25 |
| Chronic GVHD (clinical),1-153extensive1-155/none or limited/unknown (no. of patients) | 14/51/1 | 6/24/0 |
| Karnofsky performance score at 20 years after transplantation, 90% or greater/under 90%/unknown (no. of patients) | 62/3/7 | 29/2/2 |
| Posttransplantation conditions potentially impairing immunity (no. of patients) | ||
| Relapse of original disease, yes/no | 21-154/70 | 21-154/31 |
| Secondary malignancy,1-159 yes/no | 14/58 | 5/28 |
| Treatment with chemotherapy, yes/no | 2/70 | 1/32 |
| Chronic hepatitis C, yes/no | 231-160/49 | 151-160/18 |
| Treatment with interferon and/or ribavirin, yes/no | 11/61 | 6/27 |
| Diabetes mellitus,1-164yes/no | 7/65 | 2/31 |
| Obstructive lung disease, yes/no | 31-161/69 | 11-161/32 |
NA indicates not applicable; TBI, total body irradiation; Cy, cyclophosphamide.
Data on late hematopoiesis in 17 patients have been reported.35 In 15 of 16 patients studied more than 97% of donor-derived hematopoiesis was documented, whereas 1 patient had indeterminate chimerism.
One patient had paroxysmal nocturnal hemoglobinuria with hypoplastic marrow.
TBI + Cy denotes total body irradiation (10 Gy) plus cyclophosphamide (120 mg/kg).67 Cy only denotes cyclophosphamide (200 mg/kg).66 Other conditioning regimens contained combinations of chemotherapeutic agents, TBI, and/or antithymocyte globulin.
Allogeneic patients only.
Patients who developed clinical extensive chronic GVHD were treated with glucocorticoids and/or chemotherapy (azathioprine, procarbazine, or cyclophosphamide) for up to 8 years after transplantation. After that they were presumed to be free of clinical extensive chronic GVHD, except for 1 patient (see “Results,” “Deaths”). In 3 of the 14 patients with clinical extensive chronic GVHD, the chronic GVHD was preceded by grade 2-3 acute GVHD.
One patient who underwent transplantation to treat aplastic anemia developed pancytopenia of unknown cause at 26 years after transplantation (the year of the blood draw for our study); his blood cells were of donor origin.35 One patient who underwent transplantation to treat chronic myelogenous leukemia relapsed at 21 years after transplantation (1 year after the blood draw for our study) and received a new transplant; for the purpose of this study the follow-up for infections was stopped at the time of the second transplantation.
Basal cell or squamous cell cancer of the skin in 6 patients, breast cancer in 2 patients, oral and/or esophageal cancer in 2 patients, melanoma in 1 patient, metastatic cancer of unknown primary in 1 patient, and multiple malignancies in 2 patients (thyroid cancer, meningioma, basal cell skin cancer and breast cancer in 1 patient, and basal cell skin cancer and breast cancer in the other patient).
With cirrhosis in 1 patient.
Transient diabetes during glucocorticoid therapy was not counted.
None of the patients had a history of clinical extensive chronic GVHD. One patient underwent lung transplantation for presumed bronchiolitis obliterans at 24 years after transplantation; between the time of lung transplantation and the time of death from chronic lung rejection at 28 years after marrow transplantation, the patient received prednisone, cyclosporine, azathioprine, and sulfamethoxazole/trimethoprim.
Enumeration of mononuclear cell subsets
Blood specimens were drawn at a median of 20 years after transplantation (range, 20-27 years). Enumeration of mononuclear cell subsets (Table 2) was done by 3-color flow cytometry as described.11,23 24
Definitions of mononuclear cell subsets
| MNC subset . | Definition . |
|---|---|
| B cells | MNCs* expressing CD19 or CD20 and not brightly expressing CD3, CD13, CD14, CD16, CD56, CD10, or CD34 |
| Naive (IgD+) | B cells (defined as above) expressing surface IgD |
| Memory (IgD−) | B cells (defined as above) not expressing surface IgD |
| CD4 T cells (total) | CD3+ CD4+ CD8− MNCs |
| Naive (CD45RAhigh) | MNCs expressing CD4, brightly expressing CD45RA, and not expressing CD8, CD13, CD14, or CD16 |
| Memory or effector (CD45RAlow/−) | MNCs expressing CD4, dimly expressing or not expressing CD45RA, and not expressing CD8, CD13, CD14, or CD16 |
| Costimulation competent (CD28+) | MNCs expressing CD4, expressing CD28, and not expressing CD8, CD13, CD14, or CD16 |
| Costimulation incompetent (CD28−) | MNCs expressing CD4, not expressing CD28, and not expressing CD8, CD13, CD14, or CD16 |
| CD8 T cells (total) | CD3+ CD4− CD8+ MNCs |
| Naive (CD11alow) | MNCs brightly expressing CD8, dimly expressing CD11a, and not expressing CD4, CD13, CD14, CD16, or CD19 |
| Memory or effector (CD11ahigh) | MNCs brightly expressing CD8, brightly expressing CD11a, and not expressing CD4, CD13, CD14, CD16, or CD19 |
| Costimulation competent (CD28+) | MNCs brightly expressing CD8, expressing CD28, and not expressing CD4, CD13, CD14, or CD16 |
| Costimulation incompetent (CD28−) | MNCs brightly expressing CD8, not expressing CD28, and not expressing CD4, CD13, CD14, or CD16 |
| NK cells | MNCs expressing CD16 or CD56 and not expressing CD3 or CD14 |
| Monocytes | CD14+ MNCs |
| MNC subset . | Definition . |
|---|---|
| B cells | MNCs* expressing CD19 or CD20 and not brightly expressing CD3, CD13, CD14, CD16, CD56, CD10, or CD34 |
| Naive (IgD+) | B cells (defined as above) expressing surface IgD |
| Memory (IgD−) | B cells (defined as above) not expressing surface IgD |
| CD4 T cells (total) | CD3+ CD4+ CD8− MNCs |
| Naive (CD45RAhigh) | MNCs expressing CD4, brightly expressing CD45RA, and not expressing CD8, CD13, CD14, or CD16 |
| Memory or effector (CD45RAlow/−) | MNCs expressing CD4, dimly expressing or not expressing CD45RA, and not expressing CD8, CD13, CD14, or CD16 |
| Costimulation competent (CD28+) | MNCs expressing CD4, expressing CD28, and not expressing CD8, CD13, CD14, or CD16 |
| Costimulation incompetent (CD28−) | MNCs expressing CD4, not expressing CD28, and not expressing CD8, CD13, CD14, or CD16 |
| CD8 T cells (total) | CD3+ CD4− CD8+ MNCs |
| Naive (CD11alow) | MNCs brightly expressing CD8, dimly expressing CD11a, and not expressing CD4, CD13, CD14, CD16, or CD19 |
| Memory or effector (CD11ahigh) | MNCs brightly expressing CD8, brightly expressing CD11a, and not expressing CD4, CD13, CD14, CD16, or CD19 |
| Costimulation competent (CD28+) | MNCs brightly expressing CD8, expressing CD28, and not expressing CD4, CD13, CD14, or CD16 |
| Costimulation incompetent (CD28−) | MNCs brightly expressing CD8, not expressing CD28, and not expressing CD4, CD13, CD14, or CD16 |
| NK cells | MNCs expressing CD16 or CD56 and not expressing CD3 or CD14 |
| Monocytes | CD14+ MNCs |
MNC indicates mononuclear cell.
Cells with forward versus side scatter characteristics of lymphocytes and monocytes.
Quantitation of T-cell receptor excision circle (TREC) levels (number of TREC copies per 100 000 CD3+CD4+ or CD3+CD8+ cells sorted by flow-activated cell sorter) was performed by real-time quantitative polymerase chain reaction (PCR) as described.25 Briefly, sorted cells were lysed in 100 μg/mL proteinase K at 107 cells/mL. The 5′-nuclease (Taqman) assay was performed on 5 μL cell lysate (equivalent to 50 000 cells) with the primers CACATCCCTTTCAACCATGCT and GCCAGCTGCAGGGTTTAGG and the probe FAM-ACACCTCTGGTTTTTGTAAAGGTGCCCACT-TAMRA (MegaBases, Chicago, IL). PCR reactions contained 0.5 U Taq polymerase, 3.5 mM MgCl2, 0.2 mM dNTPs, 500 nM of each primer, 150 nM probe, and Blue-636 reference (MegaBases). The reactions were run at 95°C for 5 minutes, then 95°C for 5 minutes and 60°C for 1 minute for 40 cycles, using ABI7700 system (PE Biosystems, Norwalk, CT). Samples were analyzed in duplicates. A standard curve was plotted, and TREC levels (the number of TREC copies per 100 000 CD4 or CD8 T cells) were calculated by the ABI7700 software. The TREC levels reflect not only the de novo generation of T cells but also the postrearrangement expansion of thymocytes or T cells. For example, the TREC levels drop when peripheral T cells proliferate as a result of an infection. In contrast, the absolute count of TREC-containing (TREC+) T cells (per unit blood volume) is not influenced by postrearrangement proliferation and thus serves as a better indicator of the quantity of de novo–generated T cells. Therefore, we calculated the absolute count of TREC+ CD4 (CD8) T cells (per microliter) as the TREC level (per 100 000 cells) multiplied by the absolute CD4 (CD8) T-cell count (per microliter) and divided by 100 000.
The immunophenotypic subsets were determined in all 33 patients. The counts of TREC+ T cells were determined in 32 patients because of a technical error in one case.
Determination of antibody levels
Serum levels of total IgG2 and IgG specific for tetanus toxoid, for Haemophilus influenzae capsular polysaccharide, or for a mixture of 23 common pneumococcal polysaccharide serotypes were determined by enzyme-linked immunosorbent assay (ELISA), using kits purchased from The Binding Site (Birmingham, United Kingdom). Questionnaires asking whether the patients and the donors had been vaccinated against tetanus, H influenzae, orStreptococcus pneumoniae since transplantation were sent to them at the time of the blood draw. Specific IgG levels of only the unvaccinated patients/donors are presented.
Enumeration of infections
Questionnaires were sent annually (at the end of each posttransplantation year) to the patients and their primary physicians, asking about events (including infections and vaccinations) occurring since the time of the last returned questionnaire and about current medications. We counted infections occurring in the 16th year after transplantation through the last year covered by a questionnaire returned to us for a total of 591 patient-years. We included infections occurring in the 16th to the 19th year after transplantation to expand the number of years of follow-up for infections and thus to make a more accurate determination of the infection rate. (If we evaluated only infections occurring in the 20th year through the last questionnaire year, the infection rate would have been determined based on only 282 patient-years.) Respiratory tract infections, gastroenteritis, and lip sores were not counted because these were minor infections that had probably been underreported. Hepatitis C was not counted, as this infection likely started in the peritransplantation period.
Statistics
Significance of differences between patients and donors was tested by Wilcoxon signed-rank test (paired). It was also tested by Mann-Whitney rank sum test (nonpaired) to allow the inclusion of patients whose donors were not evaluated.
Because of the limited number of the original marrow donors, the patients were also compared with unrelated healthy adult volunteers (unrelated controls) studied in our laboratory (n = 101 for immunophenotypic cell subsets, n = 32 for TRECs, and n = 66 for immunoglobulin levels). These comparisons were done by using Mann-Whitney rank sum test (nonpaired). For the comparison of TREC+ T-cell counts, which used unrelated controls carefully matched for age (1:1), Wilcoxon signed-rank test (paired) was also used. The 32 age-matched unrelated controls were chosen from a pool of 51 controls, whose TREC+ T-cell counts had been determined in our laboratory, according to the following criteria: (1) Match as many patients as possible with controls less than 1 year older or younger than the patient. Such controls were found for 25 of 32 (78%) patients. (2) Match the remaining patients with controls as close in age as possible. Three patients were matched to a 2-year older or younger control, 3 patients were matched to a 3-year older or younger control, and one patient was matched to a 6-year older control. Whenever 2 controls of equal age difference from the patient were available, the control was chosen randomly (by a coin toss). Two-tailedP values are given.
Results
Mononuclear cell subset counts
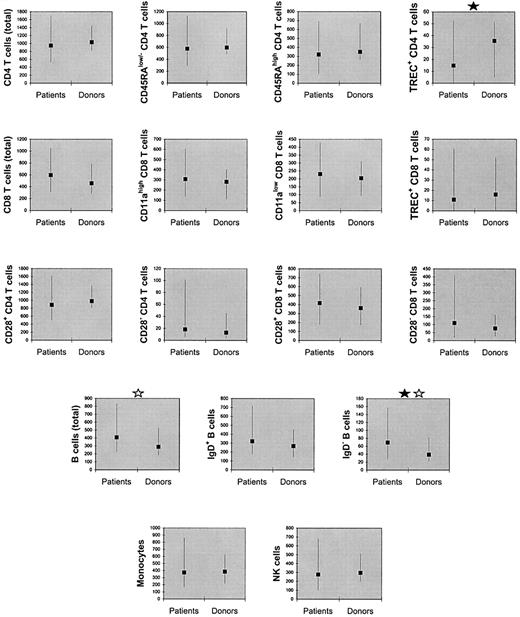
As shown in Figure 1, the counts of monocytes, natural killer (NK) cells, total B cells, IgD+ B cells, IgD− B cells, total CD4 T cells, CD28+CD4 T cells, CD28−CD4 T cells, CD45RAlow/−CD4 T cells, CD45RAhigh CD4 T cells, total CD8 T cells, CD28+ CD8 T cells, CD28−CD8 T cells, CD11ahigh CD8 T cells, CD11alow CD8 T cells, and TREC+ CD8 T-cell counts were not significantly lower in the patients compared with their donors. These counts were also not significantly lower in the patients compared with the unrelated controls (data not shown). TREC+ CD4 T-cell counts were lower in the patients compared with the donors (Ppaired = .005,Pnonpaired = .06) as well as compared with the unrelated controls (Ppaired = .007,Pnonpaired = .008).
Counts of mononuclear cell subsets (per microliter of blood) were not significantly lower in patients compared with their donors, except for TREC-containing CD4 T cells.
The squares denote medians and the error bars denote 10th to 90th percentiles. A black star denotes significant difference (P < .05) by paired (Wilcoxon) signed-rank test. A gray star denotes significant difference (P < .05) by Mann-Whitney rank sum test (nonpaired).
Counts of mononuclear cell subsets (per microliter of blood) were not significantly lower in patients compared with their donors, except for TREC-containing CD4 T cells.
The squares denote medians and the error bars denote 10th to 90th percentiles. A black star denotes significant difference (P < .05) by paired (Wilcoxon) signed-rank test. A gray star denotes significant difference (P < .05) by Mann-Whitney rank sum test (nonpaired).
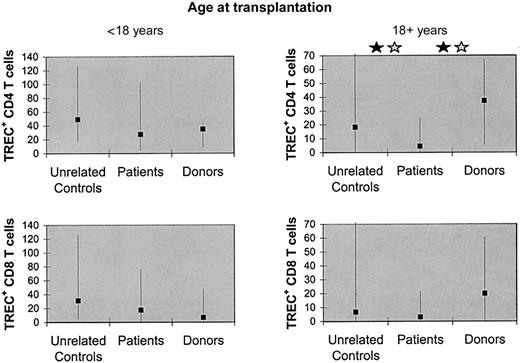
The reconstitution of TRECs might be influenced by irradiation, graft-versus-host disease (GVHD), or patient age (Douek et al,25 Chung et al,26 Weinberg et al,27 and Storek et al68). We observed no significant difference in the counts of TREC+ CD4 or CD8 T cells between patients conditioned with versus without total body irradiation, allogeneic versus syngeneic graft recipients, patients who had versus had not developed grade 2 to 4 acute GVHD, or patients who had versus had not developed clinical extensive chronic GVHD. Also, there was no significant difference between patients with versus without chronic hepatitis C or patients treated versus not treated with interferon. However, there were significant differences between patients who received transplants before the age of 18 years (n = 17) and patients who received transplants at the age of 18 years or older (n = 15) (median TREC+ CD4 T-cell counts, 27.5 versus 4.5 × 106/L, P = .004; median TREC+ CD8 T-cell counts, 17.5 versus 3.2 × 106/L, P = .006). Because even in healthy individuals the quantity of TRECs declines with age,28 we next compared the patients with their sibling donors as well as unrelated age-matched controls (for the size and median age of these subgroups, see the legend to Figure2). In the younger patients, the counts of TREC+ CD4 T cells and TREC+ CD8 T cells were not significantly different from the counts in their donors or the age-matched unrelated controls. In contrast, the older patients' TREC+ CD4 T-cell counts (but not TREC+ CD8 T-cell counts) were significantly lower compared with the donors (Ppaired = .004,Pnonpaired = .005) or to the age-matched unrelated controls (Ppaired = .05,Pnonpaired = .04) (Figure 2). In agreement with that finding, the older patients' (but not the younger patients') CD45RAhigh CD4 T-cell counts were lower compared with the donors' counts (median 203 versus 409 × 106/L, Ppaired = .004,Pnonpaired = .03). For both older and younger patients, the counts of monocytes, NK cells, total B cells, IgD+ B cells, IgD− B cells, total CD4 T cells, CD45RAlow/−CD4 T cells, total CD8 T cells, CD11alow CD8 T cells, and CD11ahigh CD8 T cells were not lower compared with their donors (data not shown).
Counts of TREC-containing CD4 T cells (but not counts of TREC-containing CD8 T cells) were low in patients who received transplants at the age of 18 years or older but not in transplant recipients younger than 18 years.
The patients (n = 17 for the younger patients, n = 15 for the older patients) were compared with their sibling marrow donors (n = 7 for the younger patients, n = 9 for the older patients) as well as to unrelated age-matched controls (n = 17 for the younger patients and n = 15 for the older patients). The median ages of the younger group at the time of the blood draw were 37 years for the patients, 37 years for the marrow donors, and 36 years for the unrelated controls (not significantly different by Mann-Whitney test). The median ages of the older group at the time of the blood draw were 44 years for the patients, 46 years for the marrow donors, and 45 years for the unrelated controls (not significantly different by Mann-Whitney test). The squares denote medians and the error bars denote 10th to 90th percentiles. A black star denotes significant difference (P < .05) by paired (Wilcoxon) signed-rank test. A gray star denotes significant difference (P < .05) by Mann-Whitney rank sum test (nonpaired).
Counts of TREC-containing CD4 T cells (but not counts of TREC-containing CD8 T cells) were low in patients who received transplants at the age of 18 years or older but not in transplant recipients younger than 18 years.
The patients (n = 17 for the younger patients, n = 15 for the older patients) were compared with their sibling marrow donors (n = 7 for the younger patients, n = 9 for the older patients) as well as to unrelated age-matched controls (n = 17 for the younger patients and n = 15 for the older patients). The median ages of the younger group at the time of the blood draw were 37 years for the patients, 37 years for the marrow donors, and 36 years for the unrelated controls (not significantly different by Mann-Whitney test). The median ages of the older group at the time of the blood draw were 44 years for the patients, 46 years for the marrow donors, and 45 years for the unrelated controls (not significantly different by Mann-Whitney test). The squares denote medians and the error bars denote 10th to 90th percentiles. A black star denotes significant difference (P < .05) by paired (Wilcoxon) signed-rank test. A gray star denotes significant difference (P < .05) by Mann-Whitney rank sum test (nonpaired).
Older patients have a higher propensity to develop chronic GVHD than young patients,29 and chronic GVHD and/or its treatment may inhibit de novo T lymphopoiesis.27 To evaluate whether the low TREC+ CD4 T-cell counts in our older patients could be attributed to chronic GVHD and/or its treatment, we next analyzed only the patients who received transplants at the age of 18 years or older who had no history of clinical extensive chronic GVHD (n = 11). Their TREC+ CD4 T-cell counts were lower compared with their donors (median 4.6 versus 38.3 × 106/L,Ppaired = .03,Pnonpaired = .04) and tended to be lower compared with their unrelated age-matched controls (median 4.6 versus 31.0 × 106/L, Ppaired = .12,Pnonpaired = .25). Thus, the long-lasting thymic insufficiency in the older patients was not due to a high incidence of clinical extensive chronic GVHD or its treatment. Because of insufficient data, we could not exclude a role of clinical limited or subclinical chronic GVHD.
Antibody levels
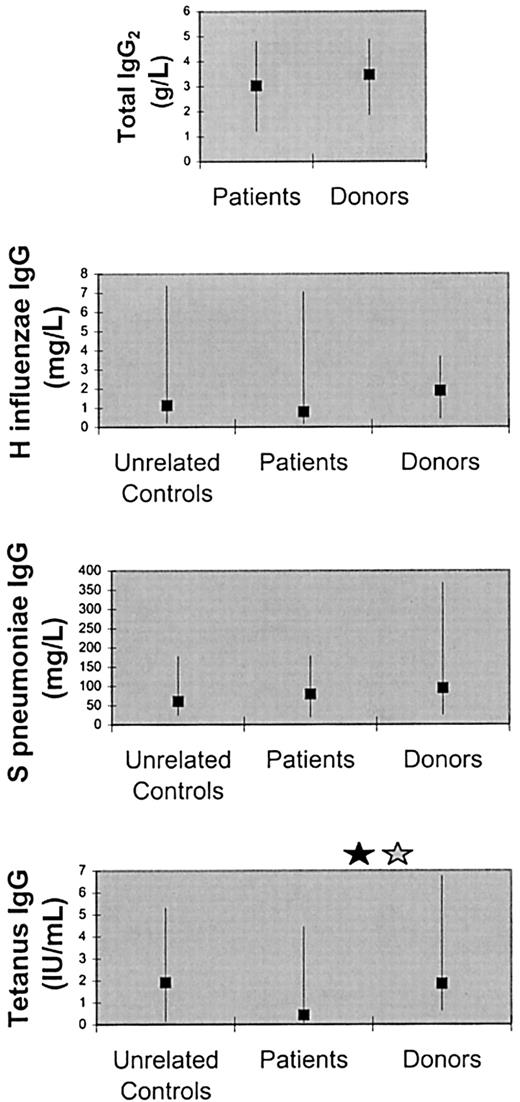
There were no significant differences between the patients and their donors in the serum concentrations of total IgG2,H influenzae polysaccharide-specific IgG (evaluated in only 29 patients and 15 donors not vaccinated since the transplantation), and S pneumoniae polysaccharide-specific IgG (evaluated in only 26 patients and 15 donors not vaccinated since the transplantation) (Figure 3). The following facts constitute further evidence that the IgG2and antipolysaccharide IgG levels in our patients were normal: (1) Median IgG2 level determined in 102 healthy adult individuals by the kit manufacturer (3.57 g/L; 95 percentile range, 1.65-5.45; package insert) was close to the median IgG2level in our patients (3.04 g/L). (We did not determine IgG2 levels in our unrelated controls.) (2) H influenzae and S pneumoniae IgG levels in 66 unrelated controls determined in our laboratory were similar to the levels in the patients (Figure 3), even though some of the unrelated controls may have received H influenzae or S pneumoniaevaccines.
Serum antibody levels in the patients, their marrow donors, and unrelated controls.
The levels of total IgG2 and specific IgG againstStreptococcus pneumoniae and Haemophilus influenzae capsular polysaccharides did not significantly differ between patients and their donors, whereas the levels of tetanus-specific IgG were lower in the patients compared with their donors. The squares denote medians and the error bars 10th and 90th percentiles. A black star denotes significant difference (P < .05) by paired (Wilcoxon) signed-rank test. A gray star denotes significant difference (P < .05) by Mann-Whitney rank sum test (nonpaired).
Serum antibody levels in the patients, their marrow donors, and unrelated controls.
The levels of total IgG2 and specific IgG againstStreptococcus pneumoniae and Haemophilus influenzae capsular polysaccharides did not significantly differ between patients and their donors, whereas the levels of tetanus-specific IgG were lower in the patients compared with their donors. The squares denote medians and the error bars 10th and 90th percentiles. A black star denotes significant difference (P < .05) by paired (Wilcoxon) signed-rank test. A gray star denotes significant difference (P < .05) by Mann-Whitney rank sum test (nonpaired).
Tetanus toxoid–specific IgG levels, evaluated in 8 patients and 10 donors who were not vaccinated since the transplantation, were lower in the patients (Ppaired = .03,Pnonpaired = .04) (Figure 3). The tetanus IgG levels in the patients also tended to be lower compared with those in our 66 unrelated controls (Pnonpaired = .11).
Infections
Infections occurring in the 591 patient-years were described in 361 patient questionnaires and/or 323 physician questionnaires returned to us. It means that 361 of 591 (61%) years were covered by patient questionnaires and 323 of 591 (55%) years by physician questionnaires filled out at the end of the posttransplantation year, whereas the remaining years were covered by questionnaires filled out later. For all 72 patients, we received at least one patient or physician questionnaire.
A total of 41 infections occurred over the 591 patient-years between the 16th and the 30th year after transplantation. Thus, the rate was 0.07 infections per year (one infection every 14 years). In the patients who received transplants before age 18, the rate was 0.06 per year (one infection every 18 years). In the patients who received transplants at age 18 years or older, the rate was 0.09 per year (one infection every 11 years). Details on the infections are given in Table3. It should be noted that infections were rare despite the fact that some patients suffered from conditions known to be associated with increased susceptibility to infections, such as diabetes or cancer (Table 1).
Sites of the infections occurring between 15 years after transplantation and the time of the last questionnaire
| Site of infection . | Number of infections . |
|---|---|
| Ear | 3 |
| Paranasal sinuses | 9 |
| Lungs | 143-150 |
| Urinary tract or kidney | 4 |
| Skin or subcutis | 73-151 |
| Other | 43-152 |
| Site of infection . | Number of infections . |
|---|---|
| Ear | 3 |
| Paranasal sinuses | 9 |
| Lungs | 143-150 |
| Urinary tract or kidney | 4 |
| Skin or subcutis | 73-151 |
| Other | 43-152 |
At least 12 cases were radiologically documented. In one case, the pathogen was known (Pseudomonas aeruginosa in the patient with chronic lung graft rejection).
Cellulitis due to a cat bite (1), cellulitis due to an infected skin biopsy site (1), cellulitis of unclear origin (1), carbuncle (1), balanitis (1), zoster (1), and tinea corporis (1).
Oral thrush (1), bursitis due to Staphylococcus aureus (1), conjunctivitis (1), and sepsis associated with sternal injury (1).
The 72 of 389 very late survivors may have been highly selected for those who had normal or near-normal immunity already early after transplantation. To ascertain whether the 72 patients were immunocompromised early after transplantation, we reviewed their records and counted early postengraftment infections, excluding respiratory tract infections, gastroenteritis, and lip sores. Early postengraftment infections were defined as infections diagnosed between day 30 and day 365 (65 of 72 patients reached sustained absolute neutrophil count > 500 × 106/L by day 30; the remaining 7 patients engrafted by day 43 and did not have an infection between day 30 and the day of engraftment). A total of 85 early postengraftment infections occurred (rate of 1.29 infections per year). This rate is 18 times higher than the rate around 20 years after transplantation (0.07 infections per year). Thus, these patients were immunocompromised early after transplantation.
Deaths
Five deaths occurred between year 20 and the time of the last contact. One was due to pneumonia in a patient who appeared to have had ongoing chronic GVHD (cachexia and advanced scleroderma with leg ulcers and contractures were reported during the last 4 years of life). Four additional deaths were not attributed to infection; one due to lung graft rejection, one due to polycystic liver disease, one due to advanced esophageal cancer, and one due to metastatic cancer of uncertain origin.
Discussion
This is the first study on the immunity of transplant recipients surviving 20 years or more. Given the extremely low infection rates, normal counts of monocytes, NK cells, total B cells, total CD4 T cells and total CD8 T cells, and normal levels of antibodies against commonly encountered encapsulated bacteria, we conclude that the immunity has recovered to normal or near normal by 20 years after transplantation.
In theory, a limited replicative potential of the grafted immune cells and/or their precursors12 or a limited ability of adult patients to generate T cells de novo (Storek et al,15 Mackall et al,30 Jendro et al,31 Weinberg et al,32 Dumont-Girard et al,33 and Storek et al68) could have caused long-lasting immunodeficiency after HCT. Data shown here suggest that the limit of replication extends beyond 20 years after transplantation, as patients surviving 20 years or more did not have low counts of monocytes, NK cells, B cells, CD4 T cells, or CD8 T cells. This finding is in agreement with the recent studies of Rufer et al34and Mathioudakis et al,35 showing that immunocyte telomeres are only slightly shorter in patients surviving 10 to 27 years after transplantation than in their donors and that the slope indicating the shortening of telomeres over time is not steeper. In our study, even the counts of the T-cell subsets expected to have the shortest telomeres (memory/effector cells and CD28−cells36-38) were not low. Collectively, these data suggest that the replicative potential is not severely limited. However, patients who received transplants in adulthood appeared to have a mild deficiency of phenotypically naive CD4 T cells and TREC+CD4 T cells. It is unclear whether this might be due to a limited potential of the grafted adult hematolymphopoietic progenitors to generate T cells (“seed deficiency”) or a limited potential of the adult thymic microenviroenment to support the differentiation of the hematolymphopoietic progenitors to T cells (“soil deficiency”). The fact that in the patients who received transplants in adulthood the counts of monocytes, NK cells, and B cells were not lower than in their donors argues against any seed deficiency. It is possible that the thymic histologic defects observed early after transplantation,39 presumably induced by conditioning or GVHD,26 27 may be only partially reversible in patients who received transplants as adults.
It is not clear why the patients who received transplants in adulthood had a deficiency of de novo–generated CD4 but not CD8 T cells. In mice, extrathymic CD8 but not CD4 T lymphopoiesis de novo has been well documented.40-42 Perhaps, in the patients the putative extrathymic T-lymphopoietic organ was not damaged or has fully recovered by 20 years after transplantation, leading to the normalization of the counts of phenotypically naive and TREC+ CD8 T cells, whereas the thymus has not fully recovered by 20 years after transplantation in the older patients, leading to extremely long-lasting deficiency of phenotypically naive and TREC+ CD4 T cells.
The deficiency of de novo–generated CD4 T cells in the patients 18 year or older was mild (Figure 2) and clinically insignificant (not associated with frequent infections). However, we studied only transplant recipients younger than 40 years of age, because none of the 29 recipients in Seattle who were older than 40 years of age before January 1, 1978, survived 20 years or more. We have not excluded the possibility that, in transplant recipients older than 40 years of age, the deficiency of de novo–generated T cells might be greater. Because de novo generation results in diverse repertoire,43transplant recipients older than 40 years of age might have limited T-cell repertoire. Theoretically, this could result in old HCT recipients' ability to mount T-cell responses against only a limited number of pathogens.
The finding of normal counts of phenotypically naive and TREC+ T cells in our patients who received transplants before age 18 years contrasts with the recent report of Patel et al12 showing that, after an initial increase of phenotypically naive T cells and TREC levels in the first 2 years after transplantation, there was a decline to values approaching zero by 14 years after transplantation. Our patients received T-cell–replete marrow after myeloablative conditioning for treatment of aplastic anemia or leukemia, whereas most of Patel's patients received T-cell–depleted marrow without conditioning for treatment of severe combined immunodeficiency. The former transplant type usually leads to full chimerism (both T and myeloid cells of donor origin)44,45 presumably with the engraftment of abundant donor hematolymphopoietic progenitors. In contrast, the latter transplant type usually leads to selective T-cell chimerism (T cells of donor origin and myeloid cells of host origin)46,47 with the engraftment of only a limited number of donor hematolymphopoietic progenitors (in 2 cases studied ≤ 2% of marrow CD34 cells were donor type48,49). In the patients described by Patel et al12 phenotypically naive T-cell counts and TREC levels increased as the donor hematolymphopoietic progenitors initially differentiated to T cells. The decline of TREC levels to near zero between 2 and 14 years after transplantation suggests that the limited hematolymphopoietic progenitors were not able to sustain T-lymphopoiesis for more than 14 years. In contrast, in our patients, most of whom were full chimeras (Table 1, footnote), the donor stem cells sustained T lymphopoiesis for 20 years or more.
Low TREC+ CD4 T-cell counts with normal total CD4 T-cell counts could be caused by a low rate of de novo T lymphopoiesis or by increased peripheral turnover (expansion and death) of de novo–generated T cells. The association between older patient age and lower TREC+ CD4 T-cell counts favors a low rate of de novo T lymphopoiesis as the more likely explanation. In mice given allogeneic T cells, donor T cells (both alloreactive cells and “innocent bystanders”) undergo significant proliferation and activation-induced cell death during the first 2 weeks after transplantation.50 It is not known whether the high cell turnover can persist long term.
It was not known whether posttransplantation patients recover from the deficiency of total IgG2 and IgG specific for the capsular polysaccharides of commonly encountered encapsulated bacteria. Our results show that they can recover as the total IgG2level and the levels of S pneumoniae and H influenzae IgG were not low. Because only patients not vaccinated since the transplantation were included in our analysis, it is likely that the normalization of the specific IgG levels resulted from natural exposure to H influenzae and S pneumoniae.
After a decline in the first several months after transplantation, levels of specific antibodies to protein antigens frequently encountered after transplantation (eg, cytomegalovirus in patients who were cytomegalovirus-seropositive before transplantation) return to pretransplantation levels within 1 year.51,52 In contrast, antibodies to protein antigens that are unlikely encountered after transplantation (eg, tetanus, measles, and polio) continue to decline.53-57 However, it was not known whether the decline was faster than the natural decline observed in healthy individuals.58 Our results document that the decline is faster, because the levels of tetanus IgG were lower in the patients than in their donors (both not vaccinated since transplantation). Surprisingly, the decline may not lead to complete disappearance of the specific IgG even by 20 years after transplantation, as 8 of 8 patients not vaccinated since transplantation had tetanus IgG levels above the sensitivity level of the ELISA (0.009 IU/mL) and 5 of 8 patients had protective levels (> 0.15 IU/mL59). It is unlikely that this finding could be due to natural exposure to tetanus toxin. Tetanus antibodies have been detected in unvaccinated individuals living in a developing country under primitive conditions60 but not in 119 of 119 unvaccinated individuals living in a developed country.61 The 8 patients we studied lived in developed countries. Therefore, the continued production of tetanus IgG at 20 years or more after transplantation is most likely attributable to the immunization of the donors and/or the patients before transplantation. Given the lower tetanus IgG levels in our patients versus their donors, we support the recommendation of posttransplantation vaccination.62-64
The very low average infection rate (one infection every 14 years) might be attributed to underreporting, because only 361 of 591 (61%) patient-years were covered by patient questionnaires filled out at the end of the posttransplantation year. The remaining 230 patient-years (39%) were covered by patient questionnaires filled out later, usually at the end of the next posttransplantation year. At that time patients may have no longer remembered minor infections from the previous year(s). We believe, however, that any serious infections would have been remembered and recorded by the patients. In addition, physician questionnaires and medical records from the primary physician were used as a further source of information. Thus, we have likely captured a significant majority of serious infections. It would be interesting to directly compare the rates of infections in the patients and their donors or unrelated controls. Unfortunately, we have not been sending the questionnaires to the donors, and a study on infection rates in healthy adult volunteers has not been published to our knowledge.
Because most of the measured laboratory parameters of immunity were normal and because infections occurred rarely in the very long-term survivors, we concluded that their immunity recovered to normal or near normal by 20 years after transplantation. However, there may be an alternative explanation of the results. Instead of gradually improving their immunity over the first 2 decades after transplantation, the patients surviving 20 years or more may have had normal immunity early after transplantation, whereas the patients with poor immunity early died by 20 years after transplantation. Two observations suggest that this situation was not the case. First, among 105 allogeneic marrow recipients we recently studied on day 80 only one had CD4 T-cell count more than 350 × 106/L.65 Second, in the patients surviving 20 to 30 years after transplantation the rate of significant infections between the time of neutrophil engraftment and day 365 after transplantation was 18-fold higher than the rate of infections around 20 years after transplantation. This finding suggests that the very late survivors were severely immunocompromised in the early period after engraftment and that their immunity subsequently improved. Nevertheless, it is possible that the very late survivors were less immunocompromised early after transplantation than patients who died by 20 years after transplantation, as high infection rates between day 50 and day 730 have been associated with high nonrelapse mortality in the first 5 years after transplantation.10
In conclusion, patients surviving 20 years or more after transplantation are no longer significantly immunocompromised. Compared with healthy individuals of similar age, transplant recipients may be more susceptible to rare diseases for which children are commonly vaccinated like tetanus, polio, or measles, unless revaccinated after transplantation. Continued follow-up of patients who received transplants in adulthood is needed to determine whether their counts of de novo–generated T cells will ultimately reach normal levels.
We thank the patients who agreed to participate in the study as well as their physicians who kindly provided us with annual updates on the clinical status of the patients and facilitated the blood draw. We also appreciate the hard work of the staff of the Fred Hutchinson Cancer Research Center Long-Term Follow-Up Department, particularly Judy Campbell, Mary-Joy Lopez, Kathy Erne, and Jane Jocom, who diligently gathered clinical information. We also thank Dr Lawrence Corey and Dr Meei-Li Huang for facilitating the TREC assay. Dr German Espino currently works at Complejo Hospitalario Metropolitano CSS, Panama, Republica de Panama. Dr Keith M. Sullivan currently works at the Division of Medical Oncology and Transplantation, Duke University Medical Center, Durham, NC 27712.
Supported by grants AI46108, CA68496, CA18221, CA18029, CA15704, HL36444 from the National Institutes of Health and by a Leukemia and Lymphoma Society Translational Research Award 6540-00.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jan Storek, FHCRC, D1-100, 1100 Fairview Ave N, Seattle, WA 98109-1024; e-mail: jstorek@fhcrc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal