Abstract
Adoptive cellular immunotherapy has proven to be a successful approach in preventing and curing cytomegalovirus infection and Epstein-Barr virus–associated lymphomas after bone marrow transplantation. Translation of this approach for preventing leukemia relapse after bone marrow transplantation might require ex vivo priming and long-term maintenance of leukemia blast-specific T cells. To accomplish this goal, procedures were optimized for the in vitro priming of naive CD8 using dendritic cells activated by CD40 ligation, interleukin-12 (IL-12), and IL-7. Using T lymphocytes and dendritic cells obtained from HLA-matched allogeneic bone marrow transplantation donors and leukemia blasts as a source of tumor antigens, anti–acute myeloid leukemia cytotoxic T lymphocytes (CTLs) were induced. In these experiments, it was found that though it is possible to induce CTLs using immature dendritic cells, IL-12, and IL-7, obtaining long-term CTLs requires the presence of CD4 T cells in the priming phase. Using this approach, long-term antileukemia CTL lines could be generated from 4 of 4 bone marrow donors. Because this procedure does not require definition of the target antigen and because it selects responding cells from a virgin T-cell repertoire, its general application is suggested in adoptive immunotherapy and in the definition of tumor rejection antigens.
Introduction
Allogeneic bone marrow transplantation (BMT) is an effective treatment for many hematologic malignancies.1Unfortunately, recurrence of original neoplastic disease is still one of the major causes of failure of this therapy, even considering the occurrence of the well-documented graft-versus-leukemia (GVL) effect mediated by donor T cells.2,3 The problem of relapse has been addressed with several immunologic strategies for restoring or enhancing antitumor immunity in patients with leukemia.4-13 In previous studies, we demonstrated that cytotoxic T-lymphocyte precursors (CTLp) directed against the leukemia blasts (LBs) of the bone marrow (BM) recipient emerged and persisted in the peripheral blood of recipients who maintained a state of remission.14,15 In contrast, in BM recipients who experienced leukemic relapse, the frequency of antileukemia CTLp rapidly declined, reaching undetectable levels. These results are strengthened by a recently reported finding demonstrating the strong correlation between antileukemia CTLs in patients with chronic myeloid leukemia who underwent allogeneic BMT and cytogenetic disappearance of the tumor.16 Together these data provide evidence that LB-reactive CTLs generated after allogeneic BMT are important in maintaining the state of remission. Recently, Falkenburg et al17 demonstrated that in a patient with chronic myeloid leukemia (CML) relapsing in accelerated phase after allogeneic BMT and resistant to donor leukocyte infusions, treatment with ex vivo–generated leukemia-reactive CTLs achieved complete remission.
One potentially successful therapeutic approach for treating minimal residual disease in leukemia-affected BMT recipients is the infusion of tumor-specific CTLs, generated and expanded ex vivo. However, leukemia-specific lymphocytes in the BM donor are expected to be naive and low in frequency, and most LB are poorly immunogenic because they express low levels of costimulatory molecules, such as CD40, CD80, and CD86.18-20 For these reasons ex vivo generation, expansion, and long-term maintenance of antileukemia CTLs are difficult and laborious,14 21 especially in patients with acute leukemia.
In the past several years, dendritic cells (DCs) have been identified as the most effective antigen-presenting cells (APCs) for ex vivo T-cell induction.22-24 Currently, one factor hindering ex vivo generation and expansion of acute leukemia-specific CTLs is the absence of known acute leukemia-specific antigens. The finding that DCs can present antigens derived from apoptotic cells25 26prompted us to evaluate the possibility of using apoptotic LBs to induce antileukemia CTLs.
In this study, we have first evaluated various experimental parameters to determine the best conditions for ex vivo priming of purified CD8 lymphocytes. We compared the ability of immature (imm) and lipopolysaccharide (LPS)–, tumor necrosis factor α (TNF-α)–, and CD40 ligand-challenged dendritic cells (imm-DC, LPS-DC, TNF-DC, and CD40L-DC) to induce a CTL response. We found that dendritic cells were able to induce such a response only after CD40 ligation. Interleukin-12 (IL-12) could substitute for CD40 ligation, and IL-7 could further increase the resultant CTL response. We then adapted these conditions for inducing CTL lines specific for acute myeloid leukemia (AML) blasts. We demonstrated that CD8+ antileukemia CTLs could be elicited and efficiently expanded from the BM or from the peripheral blood of the HLA-matched BM donor. Interestingly, we found that the maintenance of long-lived antileukemia CTL lines is dependent on the presence of irradiated CD4 T lymphocytes in the induction phase. Our results strongly suggest the use of antileukemia CD8 cytotoxic T-cell lines, generated and expanded ex vivo in adoptive immunotherapy of minimal residual disease in patients with AML. The procedure we describe can be adapted and applied to adoptive therapy of other tumors or infectious diseases and to the discovery of new rejection antigens.
Patients and methods
Patients
This study included 4 pediatric patients with AML who underwent allogeneic BMT and their donors. All patients had AML M5 subtype, according to the French-American-British classification. At the time of diagnosis, karyotypes were normal and white blood cell counts ranged between 26 × 103/μL and 156 × 103/μL. None of the BM recipients experienced relapse. Follow-up ranged from 23 to 28 months after transplantation. Three patients (patient 1, patient 2, patient 3) received BMT from an HLA-identical sibling, whereas patient 4 received BMT from an HLA-matched unrelated donor. BMT recipients were evaluated for LB-specific cytotoxic activity 6 months after transplantation, when immunosuppressive therapy had been discontinued. The protocol was approved by the local Institutional Review Board.
Stimulator and target cells
The following cell lines were grown in RPMI 1640 media (Gibco, Grand Island, NY) containing 10% fetal calf serum (FCS; Sigma, St Louis, MO), 4 mM L-glutamine, 1% nonessential amino acids, 1 mM sodium pyruvate, 5 × 10−5 M 2-mercaptoethanol, and 50 μg/mL gentamicin (all from Gibco) (RPMI-FCS): K562, a human natural killer (NK)–sensitive cell line; 3A4-721.221, an Epstein-Barr virus (EBV)–transformed cell line mutagenized and selected to be class 1 negative; and Jy, an EBV-transformed HLA-A2 homozygous cell line. CD40L-transfected cells27 were a kind gift from Dr Antonio Lanzavecchia (Bellinzona, Switzerland).
Leukemia blasts, used as stimulators for the ex vivo cultures and as targets for the CTL assay, were prepared from heparinized bone marrow aspirates (more than 90% LB) from each patient at the time of diagnosis. Bone marrow remission cells (BMRC), used as targets in the CTL assay, were isolated from patients before the BMT procedure and after demonstration of complete hematologic remission. Mitogen-stimulated T-cell lines (T-phytohemagglutinin [T-PHA]), used as targets in the CTL assay, were established for each patient by stimulating cryopreserved peripheral blood mononuclear cells (PBMCs) before transplantation with PHA and serial passages in RPMI-FCS supplemented and 100 U/mL recombinant interleukin-2 (rIL-2) (Cetus, Emeryville, CA).
Dendritic cells
DCs were generated from peripheral blood monocytes essentially as described.22 For the initial assessment of the requirements for optimal CD8 induction, HLA-A2–positive PBMCs from healthy blood donors were isolated by Ficoll-Hypaque density gradient centrifugation and suspended at the concentration of 4 × 106 cells/mL in RPMI-FCS medium. Of this suspension, 15-mL aliquots were plated in 150-mm tissue culture dishes. After 90 minutes at 37°C, nonadherent cells were discarded, and plates were washed 5 times with phosphate-buffered saline and subsequently incubated at 37°C for 30 minutes in the presence of phosphate-buffered saline containing 2% FCS and 2% EDTA. Adherent cells were detached by pipetting followed by scraping. Cell suspension was then further depleted of CD2+, CD19+, and CD56+ cells with Dynabeads (Dynal AS, Oslo, Norway) according to the manufacturer's instructions. This cell population (less than 0.5% CD3 and more than 90% CD14) was plated in 6-well plates (1.5 × 106 cells/well) in RPMI-FCS in the presence of 500 U/mL human rIL-4 (Genzyme, Boston, MA) and 800 U/mL human recombinant granulocyte-monocyte colony-stimulating factor (GM-CSF) (Pharmingen, San Diego, CA). After 6 to 7 days, wells were treated with 20 ng/mL TNF-α, (Pharmingen), 20 ng/mL LPS (Sigma), or no additive (immature DCs). After a 40-hour incubation at 37°C, DCs were collected and plated. DCs were challenged with CD40L at the time of culture with the CD8 cells by the addition of irradiated (250 Gy) CD40L-transfected cells per well to the immature DCs. For inducing antileukemia CTL, 500 U/mL human rIL-4 (Genzyme) and 800 U/mL human recombinant GM-CSF (Pharmingen) were added to adherent cells immediately after nonadherent cells were removed.
CD8 lymphocytes
To assess the requirements for optimal CD8 induction, we obtained CD8 lymphocytes from PBMCs of HLA-A2–negative healthy blood donors. CD8+ cells, recovered after positive selection with Dynabeads and Detachabead (Dynal, Lake Success, NY) according to the manufacturer's instructions, were cryopreserved until needed. After thawing, cells were further depleted of CD4, CD14, CD19, and CD56 with Dynabeads. Fluorescence-activated cell sorter analysis showed that this cell population was more than 99% CD8. For inducing antileukemia CTL, CD8 lymphocytes were recovered from PBMC or bone marrow cells (BMC) with Dynabeads after negative selection of CD4 cells, and CD4 cells were recovered after negative selection of CD8 cells.
Induction of HLA-A2–specific CTL
The medium used was RPMI 1640 supplemented with 2 mM L-glutamine, 50 μg/mL gentamicin, 1% nonessential amino acids, 1mM sodium pyruvate, and 5% pooled human serum (RPMI-HS). DCs (104 cells/well) and CD8 cells (105cells/well), prepared as described above, were cultured in 96-well flat-bottomed plates in a final volume of 200 μL. Where indicated, IL-7 (10 ng/mL) and IL-12 (10 pg/mL) (Pharmingen, San Diego, CA) were added. Duplicate plates were prepared for each condition. After 6 days at 37°C, CTL activity was measured by chromium51Cr-release assay.
Induction of antileukemia CTL
The medium used was RPMI-HS supplemented with 10 ng/mL IL-7 and 10 pg/mL IL-12. CD8-enriched cells (0.5 to 1 × 106cells/mL) were added to 48-well plates and were cocultured with irradiated (200 Gy) BMT recipient LB (5 × 105 cells/mL), irradiated (30 Gy) CD8-autologous CD4 lymphocytes (3 to 5 × 105 cells/mL), and CD8-autologous DCs (2 × 105 cells/mL) in a final volume of 1 mL. After 7 to 10 days, cultures were restimulated with irradiated (200 Gy) BMT recipient LB (5 × 105 cells/mL) and irradiated, autologous adherent PBMCs as (30 Gy) feeder cells.28Adherent feeder cells included more than 80% CD14+ cells. Two days later, 25 U/mL rIL-2 was added to the cultures. The same protocol was used for each successive round of stimulation.
Induction of alloreactive CTL
CD8-enriched cells (0.5 to 1 × 106 cells/mL), obtained from a healthy volunteer, were cocultured with irradiated (200 Gy) BMT recipient BMRCs (5 × 105 cells/mL), irradiated (30 Gy) CD8-autologous CD4 lymphocytes (3 to 5 × 105cells/mL), and CD8-autologous DC (2 × 105 cells/mL) in a final volume of 1 mL in the presence of IL-7 and IL-12. Two rounds of restimulation with BMT-recipient BMRCs were performed following the protocol described above for inducing antileukemia CTL lines.
Surface marker analysis
Cytofluorimetric analysis of cell populations was performed by direct immunofluorescence and measured with a flow cytometer (FACScan; BD Biosciences, Mountain View, CA).
51CR-release assay
HLA-A2–specific CTLs were detected by splitting the 96-well culture plate into 2 sets of replicate plates (96-well, U-bottomed). For each set, one plate contained 15 μL and the other plate contained 75 μL of the original culture. One set received51Cr-labeled 221 target cells (104 cells/well), whereas the other set received 51Cr-labeled JY target cells (104 cells/well). To decrease any antigen nonspecific cytotoxic activity caused by NK cell activity, all the wells received 2 × 105 K562 cells. After 6 hours at 37°C, the51Cr content in 100 μL supernatant was measured, and the percentage specific lysis was calculated according to the formula: % lysis = 100 × (experimental release − spontaneous release)/(maximum release − spontaneous release). For proper comparison of the efficiency of the different conditions tested, the data are expressed as LU 30/106 input cells. One LU is defined as the number of input cells required to achieve 30% lysis of 104 target cells in a 6-hour assay. Specific CTL activity is obtained by subtracting the LU obtained from 221 cells from the LU obtained from JY cells.
For detecting antileukemia CTL, target cells included LB, BMRC, and T-PHA obtained from the patient before transplantation. Antileukemia CTL lines were tested in an 8-hour cytotoxicity assay. Blocking experiments of antileukemia cytolytic activity with monoclonal antibody (mAb) required either preliminary incubation of target cells with anti-HLA class 1 or class 2 mAb (25 μg/mL) for 30 minutes at 4°C or incubation of effector cells with mAb specific for CD4 and CD8 for 30 minutes at 4°C. The same concentration of each mAb was added to cultures after approximately 4 hours from the beginning of the cytotoxicity assay.
To evaluate the role of perforin in CTL-mediated cytotoxicity, we pretreated effector cells with concanamycin A (CMA; Sigma, St Louis, MO), an inhibitor of vacuolar type H+-ATPase, at concentrations of 1, 10, and 100 nM/L for 2 hours. After CMA pretreatment, effector cells were incubated with target cells in the presence of CMA in an 8-hour cytotoxicity assay. The role of the granule exocytosis pathway in CTL-mediated cytotoxicity was evaluated by treating effector cells with strontium chloride (SrCl2) (Sigma), which induces degranulation of CTL. Effector cells were pretreated with SrCl2 at a concentration of 5, 25, and 50 mM/L for 18 hours, washed twice, and then incubated with target cells in an 8-hour cytotoxicity assay.29
Cell-mediated inhibition assay of hematopoietic progenitor cells
Nonstimulated PBMCs and antileukemia CTL lines obtained from PBMCs of patient 2 and patient 4 after the third or fourth stimulation were also tested for their capacity to suppress the growth of nonleukemic bone marrow–derived clonogenic progenitor cells derived from recipient bone marrow cells before transplantation (patient 2) or bone marrow cells after transplantation (patient 4). After centrifugation over Ficoll-Hypaque, light-density bone marrow cells (LDBMCs) were incubated in liquid culture with either PBMCs or CTL (or medium alone as control) at a ratio of 1:3, respectively. This ratio was chosen based on our preliminary experiments and on previously published results.17 After 4- and 7-hour incubations at 37°C, cells were washed once and plated in a classical clonogenic assay in semisolid medium. To assess the susceptibility of nonleukemic bone marrow–derived clonogenic progenitor cells to be inhibited in the clonogenic assay, we used alloreactive CTL lines derived from PBMCs of a healthy volunteer and directed against the BMRC of patients 2 and 4.
Clonogenic assay
LDBMCs (2 × 104) were plated in 30-mm culture dishes in 1 mL Iscoves modified Dulbecco medium containing 30% FCS (Hyclone, Logan, UT), 10 ng IL-3, 10 ng GM-CSF, 50 ng stem cell factor (all from Peprotec, EC, London, United Kingdom), 3 IU erythropoietin, and 0.9% (wt/vol) methylcellulose. Assays were performed in duplicate. Cultures were incubated at 37°C, 5% CO2 in a fully humidified incubator for 14 days. At the end of incubation, colonies were identified and scored according to standard criteria.
Results
Requirements for in vitro CTL activation by dendritic cells
To determine the optimal conditions for the in vitro induction of human CTL, we used HLA-A2 DCs to activate non–HLA-A2 purified CD8 T lymphocytes (more than 99% CD8+). We chose a major histocompatibility complex class 1 antigen as a target antigen because of the high precursor frequency of CD8 cells specific for these antigens and, thus, the possibility of detecting a CTL response after only one round of in vitro stimulation. To investigate the effect different maturation signals have on CD8 activation, we tested the ability of DCs challenged with LPS, TNF-α, or CD40L to induce CTL activity in purified CD8 cells. Of the many optimization experiments we performed for CTL induction, we present here the results obtained in 4 experiments. As shown in Figure 1A, of the maturation signals tested, only CD40L DCs were able to induce cytotoxic activity from purified CD8 T cells. CTL activity was detected in 3 of 4 CD8 cell populations tested. To compare the antigen presentation capabilities of monocytes, immature DCs, and mature DCs, we measured the expression levels of class 1 molecules, transporter associated with antigen processing, PA28 (an immunoproteasome regulator), and immunoproteasomes by flow cytometry and immunoprecipitation with appropriate antibodies. After treatment with GM-CSF and IL-4, the resultant immature DCs increased expression of class 1 molecules, transporter associated with antigen processing, PA28, and immunoproteasomes by 4- to 5-fold (data not shown) in comparison with the untreated monocytes. Furthermore, the levels of class 1 expression increased by another 2-fold after additional treatment with LPS, CD40L, or TNF-α (data not shown). Regardless of treatment, mature DCs expressed similar levels of class 1 molecules. It seems, therefore, unlikely that the different abilities in CTL induction by DCs are the result of differences in antigen processing and presentation capacity.
Only CD40L-DC induces cytotoxic activity from CD8 cells.
DCs were derived from adherent PBMCs obtained from 4 HLA-A2–positive donors. Adherent cells were purified through negative selection with CD2 and CD19 Dynabeads. Resultant monocytes were cultured in GM-CSF and IL-4 for 6 to 8 days. Maturation was induced with LPS, TNF-α, or CD40L. CD8 cells from 4 HLA-A2–negative donors were purified by positive selection with CD8 Dynabeads and cryopreserved until needed. On the day DCs were collected, CD8 cells were thawed and further purified by negative selection with CD4, CD19, CD14, and CD56. CD8 lymphocytes and DCs were cultured for 6 days and were assayed on JY (A2+) and 221 (A2−) 51Cr-labeled target cells. Cytotoxic activity is expressed in LU 30/106input cells. (A) Cytotoxic activity induced by imm-DC, LPS-DC, TNF-DC, or CD40L-DC. (B) Cytotoxic activity induced by imm-DC or CD40L-DC in the presence of IL-12, IL-7, or IL-12 plus IL-7. (Each bar pattern represents data for one combination of DC and CD8.)
Only CD40L-DC induces cytotoxic activity from CD8 cells.
DCs were derived from adherent PBMCs obtained from 4 HLA-A2–positive donors. Adherent cells were purified through negative selection with CD2 and CD19 Dynabeads. Resultant monocytes were cultured in GM-CSF and IL-4 for 6 to 8 days. Maturation was induced with LPS, TNF-α, or CD40L. CD8 cells from 4 HLA-A2–negative donors were purified by positive selection with CD8 Dynabeads and cryopreserved until needed. On the day DCs were collected, CD8 cells were thawed and further purified by negative selection with CD4, CD19, CD14, and CD56. CD8 lymphocytes and DCs were cultured for 6 days and were assayed on JY (A2+) and 221 (A2−) 51Cr-labeled target cells. Cytotoxic activity is expressed in LU 30/106input cells. (A) Cytotoxic activity induced by imm-DC, LPS-DC, TNF-DC, or CD40L-DC. (B) Cytotoxic activity induced by imm-DC or CD40L-DC in the presence of IL-12, IL-7, or IL-12 plus IL-7. (Each bar pattern represents data for one combination of DC and CD8.)
CD40 ligation of DCs has been shown to be the only stimulus able to induce high production of IL-12.27 In addition, IL-12 is a cytokine known to have an important role in promoting CTL development and function.30 31 Therefore, we decided to investigate whether the induction of cytotoxicity by CD40L-DCs could be substituted by IL-12. As seen in Figure 1B, in 3 of 4 purified CD8 cell populations, the addition of IL-12 to immature DCs was able to substitute for CD40L. IL-7 had no effect on immature DCs, but it enhanced the cytotoxic activity that could be induced by CD40L-DC and imm-DC plus IL-12 by approximately 3-fold (Figure 1C). Furthermore, in the presence of IL-7 and IL-12, the 4 CD8 populations developed cytotoxic activity (Figure 1D). We tested the induction of CTL in a variety of systems, including the CTL response against the superantigen TSST, against the A2-restricted matrix peptide derived from the influenza A virus, and against the A2-restricted tyrosinase peptide (data not shown). In all systems tested, combined treatment with CD40L-DC and IL-7 resulted in the most consistent induction of cytotoxic response for all CD8 donors.
Induction of antileukemia CD8 CTL lines
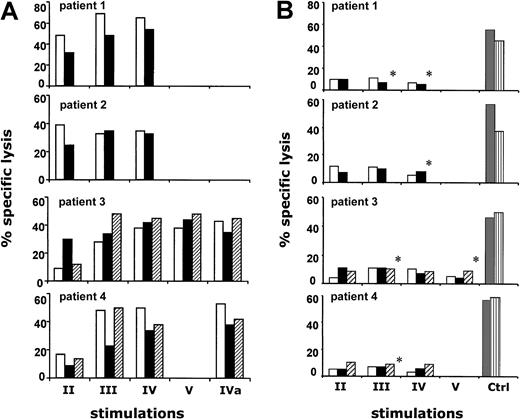
On the basis of these results, we developed a procedure for inducing antileukemia CTL. Because of the low amount of blood available from BMT donors and recipients, we modified the previous procedure as follows. To generate DCs, instead of extensively purifying monocytes, we added GM-CSF and IL-4 directly to adherent PBMCs. After a 7-day culture period, DCs expressed a pattern of activation antigens similar to the phenotype for resting DCs activated by mechanical manipulation, recently described by Gallucci et al.26 We obtained CD8 cells by negative depletion of CD4 cells. In a first set of experiments, CD8 cells (CD4, less than 1%) were primed using LBs plus DCs together with IL-7 and IL-12. We assessed CD8 cells of 2 BMT donors (don) (patient 1–don and patient 2–don), and we were able to generate antileukemia CTL after the second stimulation. Unfortunately, these cultures were difficult to maintain because of their low propensity to expand (data not shown). To provide additional help to CD8 cells, we reverted to adding, during the priming phase, CD40L, which we had determined to be critical in the previous procedure. However, we reasoned that it would be more physiologic to add autologous CD4 cells as the source of CD40L instead of using transfected cells. This approach was applied to generate antileukemia CTL lines from CD8 cells derived from either peripheral blood or bone marrow of 4 BMT donors (patient 1–don, patient 2–don, patient 3–don, and patient 4–don). No cytolytic activity was observed after the primary stimulation. However, after the second stimulation (14 to 16 days after the initial culturing), antileukemia cytotoxicity was observed in all cultures tested and was maintained or augmented after further restimulation (Figure 2A). The same kinetics and magnitude of CTL activation were observed when CD8 cells were obtained from PBMCs of the 4 BMT recipients (patient 1, patient 2, patient 3, and patient 4) 6 months after transplantation, when hematopoietic and immune systems were of donor origin. The cytotoxic capacity of antileukemia CTL lines was not affected by cryopreservation (Figure 2). In view of their potential use for adoptive immunotherapy, we tested all antileukemia CTL lines, obtained after the second and subsequent stimulations, against nonleukemic cells from patients before transplantation as an in vitro control for the graft-versus-host reaction (GVHR). At the highest E/T ratio tested (up to 40:1), all cultures showed low reactivity against nonleukemic BMRCs or T-PHA blasts that were obtained from patients before BMT and, therefore, autologous to the LBs (Figure 2B). Leukemic and nonleukemic targets were simultaneously tested in the same experiments. The susceptibility to lysis of nonleukemic BMRCs and T-PHA blasts was assessed by using alloreactive CTL lines as effector cells (Figure 2B).
Cytolytic activity of antileukemia CTL.
(A) CTL lines were derived from PBMCs of BMT recipient (■), from BMCs of BMT donor (▪), and from PBMCs of BMT donor (▨). Cultures were assessed by stimulating responder cells with LB-pulsed DCs as described, followed by weekly restimulations with LBs and autologous irradiated adherent feeder cells. Cultures were tested 7 days after each subsequent stimulation against recipient LB at an effector-target (E/T) ratio of 5:1. The number of cell culture stimulations is represented by II, III, IV, and V. Cytolytic activity of thawed antileukemia CTL, cryopreserved after the fourth restimulation, is represented as IVa. The 4 panels refer to patient 1, patient 2, patient 3, and patient 4 donor-recipient pairs, respectively. (B) CTL lines derived from PBMCs of BMT recipient (■), from BMCs of BMT donor (▪), and from PBMCs of BMT donor (▨) were simultaneously tested against pretransplantation recipient T-PHA blasts or BMRCs (*) at an E/T ratio of 40:1. Ctrl refers to alloreactive CTL lines derived from PBMCs of a healthy volunteer stimulated with autologous DC pulsed with patient 1, patient 2, patient 3, and patient 4 BMRCs, respectively. Alloreactive CTL lines were tested against pretransplantation recipient T-PHA blasts (░) or BMRCs (▥).
Cytolytic activity of antileukemia CTL.
(A) CTL lines were derived from PBMCs of BMT recipient (■), from BMCs of BMT donor (▪), and from PBMCs of BMT donor (▨). Cultures were assessed by stimulating responder cells with LB-pulsed DCs as described, followed by weekly restimulations with LBs and autologous irradiated adherent feeder cells. Cultures were tested 7 days after each subsequent stimulation against recipient LB at an effector-target (E/T) ratio of 5:1. The number of cell culture stimulations is represented by II, III, IV, and V. Cytolytic activity of thawed antileukemia CTL, cryopreserved after the fourth restimulation, is represented as IVa. The 4 panels refer to patient 1, patient 2, patient 3, and patient 4 donor-recipient pairs, respectively. (B) CTL lines derived from PBMCs of BMT recipient (■), from BMCs of BMT donor (▪), and from PBMCs of BMT donor (▨) were simultaneously tested against pretransplantation recipient T-PHA blasts or BMRCs (*) at an E/T ratio of 40:1. Ctrl refers to alloreactive CTL lines derived from PBMCs of a healthy volunteer stimulated with autologous DC pulsed with patient 1, patient 2, patient 3, and patient 4 BMRCs, respectively. Alloreactive CTL lines were tested against pretransplantation recipient T-PHA blasts (░) or BMRCs (▥).
Antileukemia CTL lines derived from PBMCs of patient 2–donor and patient 4–donor were also tested in a clonogenic assay for their capacity to inhibit the growth of nonleukemic hematopoietic progenitor cells. Results showed that CTL lines, obtained after the third and fourth stimulations, did not affect the growth of progenitor cells derived from pretransplantation recipient BM (patient 2) or posttransplantation BM recipient BM (patient 4). As shown in Table1, the number of clonogenic progenitor cells (erythroid burst-forming unit [BFU-E]; granulocyte–monocyte colony-forming unit [CFU-GM]; granulocyte–erythroid–monocyte–megakaryocyte colony-forming unit [CFU-GEMM]) grown in the control cultures did not substantially differ from the number of clonogenic progenitor cells grown after incubation with either CTL line or unstimulated PBMCs. To show that the colonies were susceptible to inhibition, we used alloreactive CTL lines derived from a healthy volunteer. As shown in Table 1, the in vitro growth of the same BMT progenitors was strongly inhibited.
Effect of antileukemia CTL lines on the in vitro growth of clonogenic progenitor cells
| . | Patient 2 . | Patient 4 . | ||||
|---|---|---|---|---|---|---|
| BFU-E . | CFU-GM . | CFU-GEMM . | BFU-E . | CFU-GM . | CFU-GEMM . | |
| Experiment 1 | ||||||
| Control | 36 | 30 | 1 | 99 | 80 | 2 |
| PBL, 4 h | 38 | 28 | 1 | 81 | 68 | 1 |
| PBL, 7 h | 33 | 24 | 2 | 100 | 79 | 2 |
| CTL3, 4 h | 29 | 32 | 1 | ND | ND | ND |
| CTL3, 7 h | 30 | 24 | 1 | 86 | 70 | 1 |
| CTL4, 4 h | 33 | 25 | 0 | 79 | 71 | 2 |
| CTL4, 7 h | 29 | 25 | 1 | 79 | 74 | 1 |
| Experiment 2 | ||||||
| Control | 33 | 52 | 2 | 105 | 95 | 3 |
| CTL allo, 4 h | 10 | 17 | 0 | 29 | 19 | 0 |
| CTL allo, 7 h | 6 | 11 | 0 | 12 | 8 | 0 |
| . | Patient 2 . | Patient 4 . | ||||
|---|---|---|---|---|---|---|
| BFU-E . | CFU-GM . | CFU-GEMM . | BFU-E . | CFU-GM . | CFU-GEMM . | |
| Experiment 1 | ||||||
| Control | 36 | 30 | 1 | 99 | 80 | 2 |
| PBL, 4 h | 38 | 28 | 1 | 81 | 68 | 1 |
| PBL, 7 h | 33 | 24 | 2 | 100 | 79 | 2 |
| CTL3, 4 h | 29 | 32 | 1 | ND | ND | ND |
| CTL3, 7 h | 30 | 24 | 1 | 86 | 70 | 1 |
| CTL4, 4 h | 33 | 25 | 0 | 79 | 71 | 2 |
| CTL4, 7 h | 29 | 25 | 1 | 79 | 74 | 1 |
| Experiment 2 | ||||||
| Control | 33 | 52 | 2 | 105 | 95 | 3 |
| CTL allo, 4 h | 10 | 17 | 0 | 29 | 19 | 0 |
| CTL allo, 7 h | 6 | 11 | 0 | 12 | 8 | 0 |
LDBMCs were incubated in RPMI 1640 and 10% FCS in the presence of PBMCs or CTLs at a ratio of 1:3, respectively. After 4- and 7-hour incubations at 37°C, cells were washed and plated in methylcellulose in a classical clonogenic assay. Numbers represent the mean colony number of a clonogenic assay performed in duplicate.
CTL3 indicates CTL obtained after 3rd stimulation; CTL4, CTL obtained after 4th stimulation; CTL allo, CTL obtained after induction of a healthy volunteer's PBMCs with autologous DCs pulsed with irradiated patient 2 or patient 4 BMRCs, respectively; ND, not done.
After repeated stimulation, CTL lines showed, along with an incremental increase in cytotoxic activity, a variable but continuous expansion of the absolute number of cultured cells. Compared with cells seeded at the beginning of the cultures, total cells were expanded 9 to 20 times after 4 rounds of stimulation and 40 times after 5 rounds of stimulation (Figure 3).
Cell numbers increase after each in vitro stimulation.
Results of cell expansion of antileukemia CTL lines derived from BMT recipient PBMCs (▴), BMT donor PBMCs (▪), or BMT donor BMCs (♦). Data represent a theoretical calculation based on the expansion rates of the CTL lines. Expansion rates of the CTL lines were calculated as follows: expansion rate = total number of the cells after restimulation/number of restimulated cells. The initial number of CD8-enriched responder cells was 106.
Cell numbers increase after each in vitro stimulation.
Results of cell expansion of antileukemia CTL lines derived from BMT recipient PBMCs (▴), BMT donor PBMCs (▪), or BMT donor BMCs (♦). Data represent a theoretical calculation based on the expansion rates of the CTL lines. Expansion rates of the CTL lines were calculated as follows: expansion rate = total number of the cells after restimulation/number of restimulated cells. The initial number of CD8-enriched responder cells was 106.
Characterization of antileukemia CTL lines
Phenotypic analysis was performed at the time of each cytotoxicity assay. Representative results obtained from primary cultures and after the fourth stimulation are reported in Table2. In primary cultures, when antileukemia cytotoxic activity was undetectable, CD3 and CD8 lymphocytes ranged from 32% to 79%, and a considerable proportion of CD56 lymphocytes (ranging from 20% to 66%) was present. After the fourth stimulation, most effector cells were CD3 and CD8 lymphocytes, whereas the proportion of CD56 cells decreased to less than 20%. Interestingly, CD4 cells also increased from less than 1% before stimulation to approximately 10% at the fourth round of stimulation.
Surface phenotype of antileukemia CTL lines
| . | Effector cells after primary stimulation . | Effector cells after 4 stimulations . | ||
|---|---|---|---|---|
| % . | (Range) . | % . | (Range) . | |
| CTL lines BMT recipients | ||||
| CD3 | 48 | (42 -57) | 94 | (93 -96) |
| CD8 | 45 | (32 -55) | 82 | (73 -88) |
| CD4 | 5 | (4 -6) | 8 | (7 -10) |
| CD56 | 50 | (36 -66) | 14 | (11 -20) |
| γδ | 10 | (5 -14) | 30 | (13 -65) |
| CTL lines* BMT donors | ||||
| CD3 | 66 | (58 -79) | 92 | (87 -97) |
| CD8 | 50 | (41 -62) | 84 | (80 -88) |
| CD4 | 6 | (3 -10) | 11 | (8 -15) |
| CD56 | 28 | (20 -30) | 9 | (6 -15) |
| γδ | 8 | (5 -16) | 21 | (3 -52) |
| . | Effector cells after primary stimulation . | Effector cells after 4 stimulations . | ||
|---|---|---|---|---|
| % . | (Range) . | % . | (Range) . | |
| CTL lines BMT recipients | ||||
| CD3 | 48 | (42 -57) | 94 | (93 -96) |
| CD8 | 45 | (32 -55) | 82 | (73 -88) |
| CD4 | 5 | (4 -6) | 8 | (7 -10) |
| CD56 | 50 | (36 -66) | 14 | (11 -20) |
| γδ | 10 | (5 -14) | 30 | (13 -65) |
| CTL lines* BMT donors | ||||
| CD3 | 66 | (58 -79) | 92 | (87 -97) |
| CD8 | 50 | (41 -62) | 84 | (80 -88) |
| CD4 | 6 | (3 -10) | 11 | (8 -15) |
| CD56 | 28 | (20 -30) | 9 | (6 -15) |
| γδ | 8 | (5 -16) | 21 | (3 -52) |
Obtained from PBMCs.
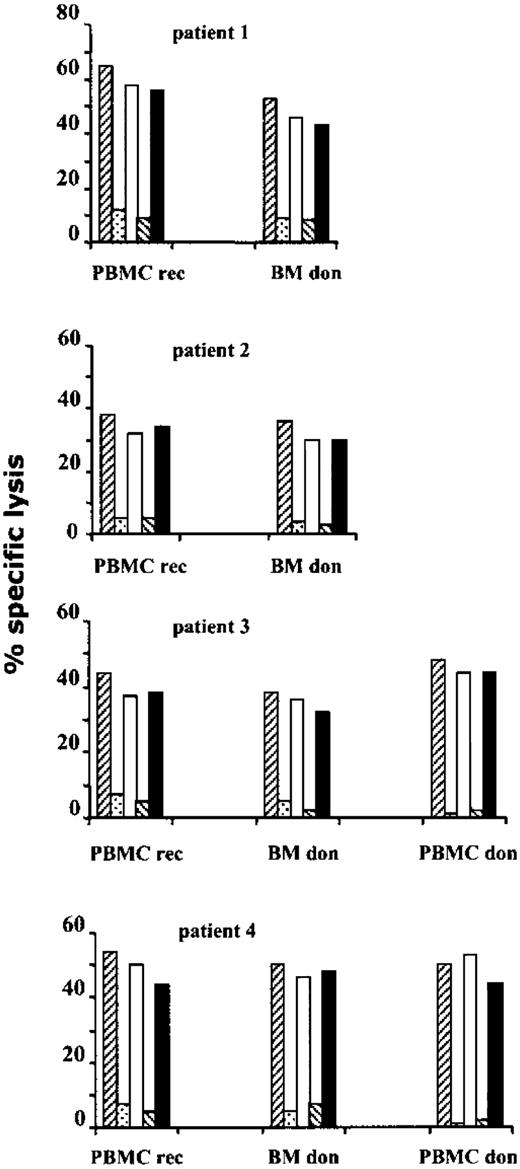
Blocking experiments of cytolytic activity indicated that, in all cases, antileukemia lysis was inhibited by anti-HLA class 1 and anti-CD8 mAb but not by anti-HLA class 2 and anti-CD4 mAb (Figure4). After repeated stimulation, CD4 cells contained in antileukemia CTL lines ranged from 5% to 12%. Thus, depletion experiments of CD4 cells were performed before cytotoxicity assays to definitely exclude the involvement of this subpopulation in mediating cytolytic activity. These results demonstrate that antileukemia cytotoxic activity was unaffected by depletion of CD4+ effector cells (data not shown).
Antileukemia CTL lines are CD8+ class 1 restricted.
After 3 (patient 3 and patient 4) or 4 (patient 1 and patient 2) in vitro stimulations, CTL lines, obtained from PBMCs of BMT recipients (PBMC rec) and from bone marrow cells (BM don) or PBMCs (PBMC don) of BMT donors, were tested for CTL activity, at an E/T ratio of 10:1, against LB target cells in the presence of media only (▨), anti-HLA class 1 (░), anti-HLA class 2 (■), anti-CD8 (▧), and anti-CD4 (▪) mAbs.
Antileukemia CTL lines are CD8+ class 1 restricted.
After 3 (patient 3 and patient 4) or 4 (patient 1 and patient 2) in vitro stimulations, CTL lines, obtained from PBMCs of BMT recipients (PBMC rec) and from bone marrow cells (BM don) or PBMCs (PBMC don) of BMT donors, were tested for CTL activity, at an E/T ratio of 10:1, against LB target cells in the presence of media only (▨), anti-HLA class 1 (░), anti-HLA class 2 (■), anti-CD8 (▧), and anti-CD4 (▪) mAbs.
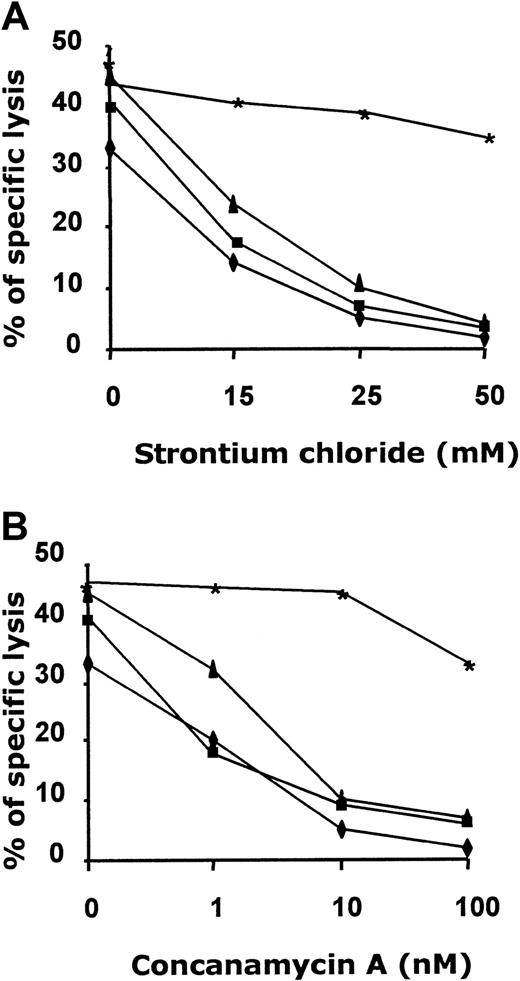
To identify the mechanism responsible for target lysis in antileukemia cytotoxic activity, we analyzed the role of the granule exocytosis pathway by using either CMA, an inhibitor of vacuolar type H+-ATPase, or SrCl2, which causes degranulation and release of granule contents from effector cells. Our results demonstrate that antileukemia cytotoxicity was inhibited by treatment with either CMA or SrCl2 (mean inhibition, 87%; Figure5). Because these reagents selectively block perforin-based target lysis, we conclude that target lysis is perforin dependent.29 We tested the specificity of CMA and SrCl2 inhibition using, as effector cells, a perforin-negative, CD4+ Epstein-Barr virus (EBV)–specific CTL line (P.C. et al, unpublished observations, January 2000). As shown in Figure 5, the addition of CMA and SrCl2 did not affect cytolytic activity of the EBV-specific CTL line against autologous EBV-LCL.
CTL-mediated LB lysis is perforin dependent.
Effect of CMA and SrCl2 on cytotoxic activity displayed from antileukemia CTLs obtained from PBMCs of recipient patient 3 (▪), BMCs of donor patient 3 (▴), PBMCs of donor patient 3 (♦) after 3 stimulations, and a perforin-negative, EBV-specific CTL line (∗) (see “Results” for details). (A) CTL lines were incubated with CMA at a concentration of 1, 10, and 100 nM/L for 2 hours. (B) CTL lines were incubated with SrCl2 at a concentration of 15, 25, and 50 mM/L for 18 hours. CTLs were then cocultured with 51Cr-labeled recipient LB or EBV-LCL for 8 hours at E/T ratios of 20:1, 10:1, and 5:1. The figure shows results from an E/T ratio of 5:1.
CTL-mediated LB lysis is perforin dependent.
Effect of CMA and SrCl2 on cytotoxic activity displayed from antileukemia CTLs obtained from PBMCs of recipient patient 3 (▪), BMCs of donor patient 3 (▴), PBMCs of donor patient 3 (♦) after 3 stimulations, and a perforin-negative, EBV-specific CTL line (∗) (see “Results” for details). (A) CTL lines were incubated with CMA at a concentration of 1, 10, and 100 nM/L for 2 hours. (B) CTL lines were incubated with SrCl2 at a concentration of 15, 25, and 50 mM/L for 18 hours. CTLs were then cocultured with 51Cr-labeled recipient LB or EBV-LCL for 8 hours at E/T ratios of 20:1, 10:1, and 5:1. The figure shows results from an E/T ratio of 5:1.
Discussion
The rationale for pursuing cancer immunotherapy is based mainly on 2 sets of discoveries: (1) the effectiveness demonstrated by adoptive cellular immunotherapy of cytomegalovirus (CMV) infection and EBV-associated lymphomas in patients who underwent BMT21 and (2) the proof, obtained in rodents, of the existence of tumor-rejection antigens.31 32 However, attempts to treat patients with tumors by adoptive transfer of cytotoxic T lymphocytes have met with only limited success. A striking difference between success and failure consists in the origin of the cells used. Cells used for cellular therapy of CMV and EBV were memory cells that had been generated against multiple antigens and were able to control the disease in the BM donor. On the contrary, for the adoptive therapy of tumors, cells were obtained from patients affected by the disease. In this case, the CTLs that were present had already failed to control tumor growth and were likely representative of a diminished and tolerized repertoire. An analogous difference exists in the definition of tumor rejection antigens. Cells used for determining tumor rejection antigens in the mouse were memory cells that had rejected the tumor in vivo, whereas in the human system, the antigens were defined by cells that failed to control the disease.
On the basis of this analysis, we attempted to raise a CTL response against the whole tumor cell, starting from a naive repertoire (the BM donor has never had contact with the leukemia blasts from the BM recipient). We have demonstrated that in the presence of DCs and CD4 T cells, it is possible to raise a primary, self-renewing antileukemia CD8 response using the apoptotic LB as the source of antigen. In the absence of DCs, stimulation with LB and IL-2 is adequate to elicit antileukemia CTLs, but most of them are HLA-unrestricted CTLs characterized by a low propensity to expand.14 In the current study, we have demonstrated that CD4 cells are required in the priming phase, not only for induction but, interestingly, also for long-term maintenance of CTLs in vitro. Generated against AML blasts, these self-renewing CD8 HLA class 1–restricted antileukemia CTL lines can be induced from the peripheral blood and from the bone marrow of the BM donor and from the peripheral blood of the BM recipient 6 months after transplantation.
The importance of helper T cells in CTL induction was first described in the late 1970s. Zinkernagel et al33 and von Boehmer and Haas34 reported that the activation of specific CD8 lymphocytes in vivo was dependent on the presence of CD4 helper cells. Later, this concept was elegantly extended by Cassell and Forman,35 who showed that for CTLs to develop in vivo, CD4 and CD8 cells must recognize antigen on the same APC; antigen presented by 2 distinct APCs did not lead to CTL activation. Consistent with these findings, in previous studies, we have shown that peptide priming is helper dependent and that peptide-specific CD8 cells are more effectively primed if the helper epitope and the CTL epitope are linked, suggesting the need for presentation by the same APC.28 More recently, studies in mice have unambiguously shown that CD4 T cell help to CTLs is delivered through DCs by way of the CD40L-CD40 interaction.36-38 This interaction between the helper cell and the APC enables the APC to activate the CTL. Recent data by Lu et al39 demonstrated that helper CD4 T cells also use CD40-independent pathways for the priming of CD8 CTLs. Our current data establish that CD40-induced maturation of human monocyte–derived DCs is necessary and sufficient for in vitro induction of antigen-specific CD8 cytotoxicity, thus confirming and extending previously reported data on the involvement of CD40 ligation for activating human leukemia–specific CTLs.12Stimulation of DCs with LPS or TNF-α did not elicit a CTL response. Like the activation of DCs through CD40L, the activation of DCs with LPS or TNF-α also induces DC maturation, dramatically increasing the ability of imm-DC to induce a primary in vitro CD4 T-cell response.27 In the current study, we could not detect any differences regarding molecules involved in class 1 antigen presentation among the 3 types of mature DCs. Instead, all 3 mature DCs exhibited a high level of class 1 expression and contained an abundance of potent immunoproteasomes for antigen degradation. These data indicate that all 3 types of maturation stimuli induce an enormous capability to process and present antigens to CD8 class 1–restricted cells. Together with the finding that IL-12 can substitute for CD40 ligation, these data suggest that the difference between CD4 help and other DC maturation signals does not rest with the mechanisms responsible for antigen processing and presentation. As for the synergistic effect of IL-7 with CD40L-DC, we believe it results mainly from the ability of IL-7 to prevent apoptosis of activated cells.40
We were surprised to discover that in the absence of CD4 lymphocytes in the induction phase, we were unable to maintain the CTLs despite the continuous addition of IL-2. The evidence that only the addition of CD4 cells allows for long-term expansion of CD8 T cells indicates that CD4 cells are important not only for priming CD8 T cells through the ligation of CD40 on DCs but also for establishing self-renewing CD8 T cells. These results are reminiscent of data reported by Borrow et al41 and Whitmire et al,42 who demonstrated that CD40L-deficient (−/−) mice are able to develop virus-specific CTL during acute infection, but, because they lack effective CD4 help, they have an impaired memory CTL response. Our results are also consistent with data from Greenberg and Riddell,43demonstrating that maintenance of adoptively transferred CD8+ cell immunity to CMV and EBV infection requires a concurrent CD4+ T-helper response or an adoptive transfer of both CD8+ and CD4+ virus-specific T cells
LB antigens are likely to be presented to CTLs by DCs through the same mechanism responsible for the in vivo phenomenon known as cross-priming44 rather than by direct presentation by LB. Cross-presentation of apoptotic cells by DCs has been shown to be responsible for inducing activation or tolerance.25,26,45-47 Our data are in agreement with murine in vivo studies that have also demonstrated that the induction and maintenance of CTL through cross-priming require CD4 help.48
CTLs generated ex vivo with LBs plus DCs are likely to recognize more than a single antigen, as has been shown for the in vivo induction of CTLs using DCs fused to tumor cells.49 In that study, in vivo priming with the fusion cell resulted in generating CTLs specific to multiple CTL epitopes, which included known and unknown epitopes. The possibility of generating CTLs using the whole tumor cell allows epitopes to be selected that are immunogenic in the context of the individual CTL repertoire. Moreover, the generation of CTLs with multiple specificities should diminish the possibility of selecting for escape variants by the poor immunogenicity of LBs in vivo. Although we did not identify the target antigens recognized by antileukemia CTLs, our results do suggest that these antigens are poorly immunogenic in vivo. In fact, the requirement for 2 or 3 rounds of stimulation in the presence of IL-2 for detecting antileukemia CTLs indicates that the precursors to these cells are present in low frequency in both the donor and the BM recipient. Furthermore, we did not observe a difference in the magnitude and kinetics of the generation of antileukemia CTL between CD8 cells derived from BM donors (who are unprimed to recipient antigens) and BM recipients (who could have been primed by minimal residual disease). On the whole, these observations confirm the usefulness of inducing these CTLs in vitro.
From recently reported data on animal models,50 the concern has arisen that using DCs to induce CTLs could result in life-threatening autoimmune reactions. In our system, increased GVHR is also of potential concern. However, antileukemia CTLs induced using DCs did not result in cytotoxicity against patient nonleukemic cells. Moreover, these CTL lines did not affect the growth of normal hematopoietic progenitor cells of either donor or recipient origin.
We conclude that the procedure we have defined for the ex vivo induction of antitumor T-cell lines offers several advantages over currently available immunologic strategies intended to restore or enhance antitumor immunity in patients with cancer. Significantly, this procedure does not require the definition of a specific tumor antigen, and it allows for the selection of the best combination of antigen and available T-cell repertoire. These observations suggest that it may be possible to adapt this methodology for the immunotherapy of a wide range of human cancers and chronic infectious diseases.
Supported in part by grants from Associazione Italiana Ricerca sul Cancro, Ministero dell'Università e della ricerca Scientifica e Tecnologica, and Istituto di Ricovero e Cura a Carattere Scientifico Policlinico S. Matteo (R.M., F.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Daniela Montagna, Laboratorio di Immunologia, Dipartimento di Scienze Pediatriche, Università di Pavia, IRCCS Policlinico San Matteo, P.le Golgi 2, 27100 Pavia, Italy; e-mail: d.montagna@smatteo.pv.it; Antonella Vitiello, R. W. Johnson PRI, 3210 Merryfield Row, San Diego, CA 92121; e-mail:avitiell@prius.jnj.com.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal