Abstract
A peptide from the C-terminal domain of thrombospondin-1 (Arg-Phe-Tyr-Val-Val-Met-Trp-Lys; known as 4N1-1) has been reported to induce platelet aggregation and to bind to the integrin-associated protein (IAP), which is also known as CD47. In this study, it was discovered that 4N1-1 or its derivative peptide, 4N1K, induces rapid phosphorylation of the Fc receptor (FcR) γ chain, Syk, SLP-76, and phospholipase C γ2 in human platelets. A specific inhibitor of Src family kinases, 4-amino-4-(4-methylphenyl)-7-(t-butyl) pyrazola[3,4-d]pyrimidine, prevented phosphorylation of these proteins, abolished platelet secretion, and reduced aggregation by approximately 50%. A similar inhibition of aggregation to 4N1-1 was obtained in the presence of Arg-Gly-Asp-Ser in mouse platelets deficient in FcR γ chain or SLP-76 and in patients with type I Glanzmann thrombasthenia. These results show that 4N1-1 signals through a pathway similar to that used by the collagen receptor glycoprotein (GP) VI. The αIIbβ3-independent aggregation induced by 4N1-1 was also observed in fixed platelets and platelets from patients with Bernard-Soulier syndrome, which are deficient in GPIbα. Surprisingly, the ability of 4N1-1 to stimulate aggregation and tyrosine phosphorylation was not altered in platelets pretreated with anti-IAP antibodies and in IAP-deficient mice. These results show that the C-terminal peptide of thrombospondin induces platelet aggregation through the FcR γ-chain signaling pathway and through agglutination. The latter pathway is independent of signaling events and does not use GPIbα or αIIbβ3. Neither of these pathways is mediated by IAP.
Introduction
Platelet aggregation and clot formation are initiated when platelets are activated by soluble activators such as thrombin or by binding to components of the subendothelial matrix, such as von Willebrand factor (vWF) or collagen. These events lead to a change in platelet shape and secretion of dense and α granules. Platelet aggregation is supported by binding of fibrinogen to activated αIIbβ3 and by binding of vWF to αIIbβ3 and the glycoprotein (GP) Ib-IX-V complex.1
Thrombospondin 1 (TSP1) was first discovered as a glycoprotein associated with the surface of thrombin-stimulated platelets.2 TSP1 is stored in α granules and is released on activation, after which it becomes bound to the surface of the activated platelet. Anti-TSP1 antibodies can inhibit platelet aggregation induced by thrombin and collagen, and it has been proposed that TSP1 acts by stabilizing platelet aggregates initiated by interactions between fibrinogen and αIIbβ3.3-5
TSP1 binds to a large number and wide variety of receptors on the platelet surface. Proteolytic digestion of TSP1 and expression of domains as recombinant proteins or synthetic peptides has enabled identification of several sequences that support these interactions, including a heparin-binding domain in the N-terminal region, which binds to proteoglycans6; a region within the type 1 repeats, which binds to CD367; and an Arg-Gly-Asp sequence within the last of the type III repeats, which bind αvβ3 and αIIbβ3 integrins.8,9 Additionally, the C-terminal domain of TSP1, named the C-terminal cell-binding domain, and a minimal sequence from this domain (Arg-Phe-Tyr-Val-Val-Met-Trp-Lys), named 4N1-1, bind to a widely expressed 50-kd membrane protein identified as integrin-associated protein (IAP) or CD47.10-12 IAP has an extracellular immunoglobulin domain, 5 membrane-spanning segments, and a short hydrophilic tail. Gene-targeted mice deficient in IAP have a strongly reduced host defense system that is thought to result from a defect in granulocyte migration.13 The IAP receptor associates with several integrins, including αvβ3, α2β1, and αIIbβ3.14-16 In several cells, functions dependent on αvβ3 and α2β1 are positively modulated by the C-terminal cell-binding domain of TSP1 or its derived peptide by means of IAP.12,16 17
In platelets, IAP is associated with αIIbβ3 and α2β1 integrins that bind to fibrinogen and collagen, respectively.15,18It was reported that 4N1-1 and the more soluble peptide 4N1K (Lys-Arg-Phe-Tyr-Val-Val-M-Lys-Lys) induce aggregation19 and spreading of platelets on immobilized fibronectin and collagen.15,18 The ability of the C-terminal peptide of TSP1 to modulate integrin function depends on its interaction with IAP, since spreading of platelets on fibrinogen and collagen was decreased after treatment with an antifunctional IAP antibody and in platelets from mice deficient in IAP.15 18
There is evidence suggesting that the C-terminal peptide of TSP1 regulates integrin function by means of intracellular signaling events. For example, platelets stimulated by 4N1K do not spread on fibrinogen when they are treated by inhibitors of either tyrosine kinases, phosphatidylinositol 3-kinase, or protein kinase C.15 IAP is functionally coupled to a heterotrimeric Gi protein,20and 4N1K induces tyrosine phosphorylation of several proteins in platelets, including Syk and focal adhesion kinase.15 This study was undertaken to examine the functional importance of these signaling events in platelets activated by the C-terminal peptide of TSP1.
Materials and methods
Antibodies and reagents
Antiphosphotyrosine monoclonal antibody (mAb) 4G10 was purchased from Upstate Biotechnology (TCS Biologicals, United Kingdom). Antiphospholipase C γ2 (anti-PLCγ2) and anti-Syk rabbit polyclonal antiserum were gifts of M. Tomlinson (DNAX Research Institute, Palo Alto, CA). Anticonvulxin polyclonal antibody was a gift of Dr M. Leduc and Dr C. Bon. Anti–SLP-76 rabbit polyclonal antibody was generated as described previously. Antihuman and mouse IAP antibodies were generated as described previously.13,21,22 Human vWF was a gift of Dr M. C. Brendt. Ristocetin was from Sigma (Poole, Dorset, United Kingdom). The peptides 4N1-1, 4NGG (Arg-Phe-Tyr-Gly-Gly-Met-Trp-Lys), and 4N1K were synthesized by TANA Laboratories (Houston, TX) and purified with use of a high-performance liquid chromatography column. All peptides were dissolved in dimethyl sulfoxide (concentration, 25 mM). Collagen (native collagen fibrils from equine tendons) was from Nycomed (Munich, Germany). Mice deficient in Fc receptor (FcR) γ chain, SLP-76, and IAP were generated as described previously.13,23 Other reagents were from sources described previously.24 25
Platelet preparation
Blood samples were collected from healthy volunteer donors in a 1:10 volume of 3.8% trisodium citrate (wt/vol). Blood from patients with type I Glanzmann thrombasthenia or Bernard-Soulier syndrome (BSS) was obtained through University College Hospital, London. Absence of expression of αIIbβ3 and GPIbα was confirmed by flow cytometry for type I Glanzmann thrombasthenia and BSS, respectively. Murine blood was obtained by cardiac puncture after asphyxiation using carbon dioxide. Platelets were isolated from platelet-rich plasma (PRP) by centrifugation at 1000g for 10 minutes in the presence of prostacyclin (0.1 μg/mL). The pellet was resuspended in a modified Tyrode-HEPES buffer (134 mM sodium chloride [NaCl], 0.34 mM sodium phosphate [dibasic], 2.9 mM potassium chloride, 12 mM sodium bicarbonate, 20 mM HEPES, 5 mM glucose, and 1 mM magnesium chloride [pH 7.3]) in the presence of prostacyclin (0.1 μg/mL). Platelets were again centrifuged at 1000g for 10 minutes and resuspended at a concentration of 2 × 108 platelets/mL in Tyrode-HEPES buffer. Platelet stimulations were done at 37°C in an aggregometer (PAP4; BioData, Hatboro, PA), with continuous stirring at 1200 rpm. Platelets were fixed by addition of 0.2% formaldehyde. Single platelets were counted with a Coulter counter (Z-series; Coulter Electronics, United Kingdom).
Serotonin (5-HT) secretion assay
PRP was incubated for 1 hour at 37°C with 0.5 μCi/mL (0.0185 MBq) tritium–5-HT. Platelets were prepared from PRP as described above. Stimulation of platelets was terminated by addition of an equal volume of 6% glutaraldehyde in phosphate buffer (20 mM sodium phosphate [monobasic] and 80 mM sodium phosphate [pH 7.3]). After a brief microcentrifugation, the level of tritium–5-HT release into the supernatant was determined by scintillation spectrometry. The amount of tritium–5-HT released was expressed as a percentage of total tissue content after subtraction of the amount released under basal conditions, as described previously.26
Scanning electron microscopy
Scanning electron microscopy was done as described previously.27 Briefly, basal or stimulated platelets at a concentration of 2 × 108 platelets/mL were fixed with an equal volume of 4% glutaraldehyde in phosphate buffer. The platelets were collected on polycarbonate filters by using gentle suction. Filters were dehydrated by washing with increasing concentrations of ethanol. The filters were then subjected to critical-point drying, coated with gold, and analyzed on a scanner (Philips 515; FEI United Kingdom, Cambridge, United Kingdom).
Glutathione-S-transferase precipitation, immunoprecipitation, and immunoblotting
Platelets (5 × 108 cells/mL) were lysed with an equal volume of ice-cold Nonidet P-40 (NP-40) buffer (20 mM Tris, 300 mM NaCl, 2 mM EDTA, 2% [vol/vol] NP-40, 1 mM phenylmethylsulfonyl fluoride, 2 mM sodium orthovanadate, 10 μg/mL leupeptin, 10 μg/mL aprotinin, and 1 μg/mL pepstatin A [pH 7.3]). Nonlysed cells and debris were removed by centrifugation. Cell lysates were precleared for 1 hour at 4°C with glutathione-agarose or protein A–Sepharose for Glutathione-S-transferase (GST) precipitation and immunoprecipitation, respectively. For GST precipitation, lysates were incubated overnight at 4°C with 5 μg GST, Syk, and Src homology 2 (SH2) immobilized on agarose. For immunoprecipitation, platelet lysates were incubated overnight at 4°C with 3 μL anti-Syk, anti-PLCγ2, or anti–SLP-76 antibodies, with constant rotation. For GPVI precipitation, platelet lysates were incubated for 2 hours with 10 μg convulxin and 2 hours with 3 μL anticonvulxin antibody. Protein A–Sepharose was added, and the samples were rotated for an additional 60 minutes. The pellet of protein A–Sepharose or glutathione-agarose was washed once in lysis buffer and 3 times in 10 mM Tris, 160 mM NaCl, and 0.1% Tween 20 (pH 7.3); Laemmli buffer was added; and the mixture was boiled for 2 minutes. Proteins were separated by sodium dodecyl–sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride membrane. Blots were developed by using an enhanced chemiluminescence detection system.
Results
4N1-1 stimulates early tyrosine phosphorylation
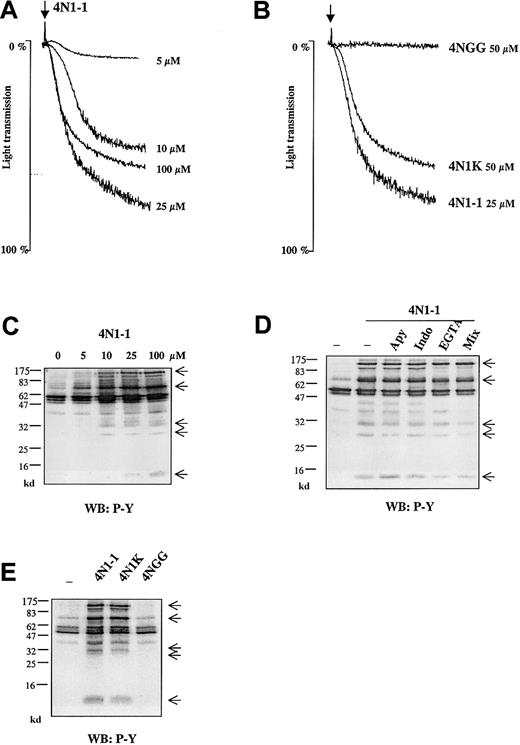
4N1-1 peptide from the C-terminal domain of TSP1 stimulated aggregation of platelets in a concentration-dependent manner, with a maximal response at 25 μM (Figure 1A). In all donor samples, the response to 100 μM 4N1-1 was lower than that to 25 μM 4N1-1. The peptide 4N1K, which was modified to improve solubility,11 also induced aggregation, with a maximal response at 50 μM (Figure 1B). Similar to the results with 4N1-1, higher concentrations of 4N1K produced a decreased response (data not shown). The mutated peptide 4NGG12 did not induce aggregation (Figure 1B).
4N1-1 induces phosphorylation events independently of secondary platelet activation.
(A) Washed human platelets (2 × 108/mL) were stimulated with increasing concentrations of 4N1-1. (B) Washed human platelets were stimulated with 25 μM 4N1-1, 50 μM 4NGG, and 50 μM 4N1K. Aggregation was monitored by light transmission. Arrows indicate addition of agonist. (C) Platelets were stimulated with increasing concentrations of 4N1-1. (D) Human platelets were pretreated for 10 minutes with 2 U/mL apyrase (apy), 10 μM indomethacin (indo), 1 mM EGTA, or all 3. Mix platelets were then stimulated with 100 μM 4N1-1 for 2 minutes. (E) Human platelets, preincubated as described in the legend for panel D with apyrase, indomethacin, and EGTA, were stimulated with 100 μM 4N1-1, 100 μM 4NGG, and 100 μM 4N1K for 2 minutes. (C-E) Platelets were lysed by addition of NP-40 buffer. Proteins of whole-cell lysate (35 μL) were resolved by 12.5% SDS-PAGE and analyzed with Western blotting using an antiphosphotyrosine antibody (WB: P-Y). Molecular-weight markers are on the left. Arrows on the right indicate the position of prominent phosphorylated bands. Results are representative of an experiment using samples from a minimum of 3 different donors.
4N1-1 induces phosphorylation events independently of secondary platelet activation.
(A) Washed human platelets (2 × 108/mL) were stimulated with increasing concentrations of 4N1-1. (B) Washed human platelets were stimulated with 25 μM 4N1-1, 50 μM 4NGG, and 50 μM 4N1K. Aggregation was monitored by light transmission. Arrows indicate addition of agonist. (C) Platelets were stimulated with increasing concentrations of 4N1-1. (D) Human platelets were pretreated for 10 minutes with 2 U/mL apyrase (apy), 10 μM indomethacin (indo), 1 mM EGTA, or all 3. Mix platelets were then stimulated with 100 μM 4N1-1 for 2 minutes. (E) Human platelets, preincubated as described in the legend for panel D with apyrase, indomethacin, and EGTA, were stimulated with 100 μM 4N1-1, 100 μM 4NGG, and 100 μM 4N1K for 2 minutes. (C-E) Platelets were lysed by addition of NP-40 buffer. Proteins of whole-cell lysate (35 μL) were resolved by 12.5% SDS-PAGE and analyzed with Western blotting using an antiphosphotyrosine antibody (WB: P-Y). Molecular-weight markers are on the left. Arrows on the right indicate the position of prominent phosphorylated bands. Results are representative of an experiment using samples from a minimum of 3 different donors.
Whole-cell extracts from platelets stimulated with 4N1-1 were analyzed by Western blotting using an antiphosphotyrosine antibody. Tyrosine phosphorylation was detected throughout the length of the gel, with prominent bands at 12, 30, 35, 70, and 120 kd (Figure 1C). The 4N1-1–induced increase in tyrosine phosphorylation occurred over the same concentration range and time course as those for aggregation (maximal by 10 seconds and sustained for up to 240 seconds; Figure 1C and Figure 2A).
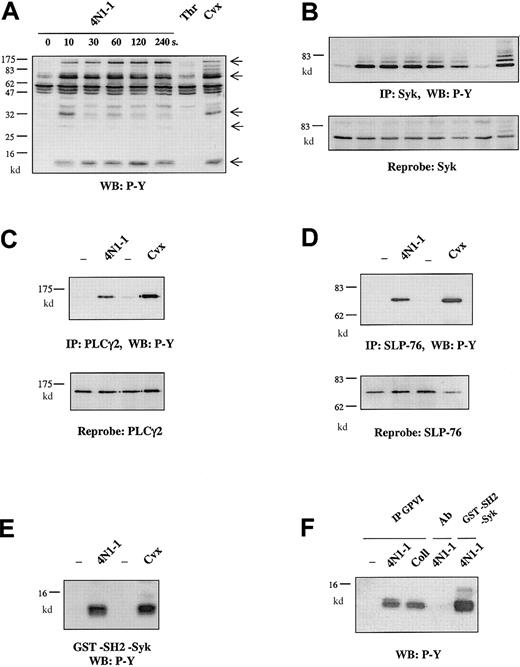
4N1-1 and GPVI activation induce a similar tyrosine phosphorylation pattern.
Human platelets, preincubated as described in the legend for Figure 1with apyrase, indomethacin, and EGTA, were stimulated with 100 μM 4N1-1 from 10 to 240 seconds or for 2 minutes with 1 U/mL thrombin (Thr) or 10 μg/mL convulxin (Cvx). Platelets were then lysed by addition of NP-40 buffer. (A) Proteins of whole-cell lysate were resolved by 12.5% SDS-PAGE and analyzed with Western blotting using an antiphosphotyrosine antibody. Arrows on the right indicate the position of prominent phosphorylated bands. (B) Cell extracts were immunoprecipitated by using the anti-Syk antibody. Immunoprecipitates were resolved by 10% SDS-PAGE and analyzed with Western blotting using an antiphosphotyrosine antibody (top). The filter was dehybridized and reprobed by using the anti-Syk antibody (bottom). (C-E) Human platelets were stimulated with 100 μM 4N1-1 or 10 μg/mL convulxin and lysed 2 minutes later. (C) Cell extracts were immunoprecipitated by using the anti-PLCγ2 antibody. Immunoprecipitates were resolved by 7% SDS-PAGE and analyzed with Western blotting using an antiphosphotyrosine antibody (top). The filter was dehybridized and reprobed by using the anti-PLCγ2 antibody (bottom). (D) Cell extracts were immunoprecipitated by using the anti–SLP-76 antibody. Immunoprecipitates were resolved by 10% SDS-PAGE and analyzed with Western blotting using an antiphosphotyrosine antibody (top). The filter was dehybridized and reprobed by using the anti–SLP-76 antibody (bottom). (E) Cell extracts were precipitated by using GST fusion protein containing the tandem SH2 domain of Syk. Precipitated proteins were resolved by 15% SDS-PAGE and analyzed with Western blotting using an antiphosphotyrosine antibody. (F) Cell extracts were precipitated by using convulxin and an anticonvulxin antibody (IP GPVI), the anticonvulxin antibody alone (Ab), or the GST-SH2-Syk fusion protein. Precipitated proteins were resolved by 15% SDS-PAGE and analyzed with Western blotting using an antiphosphotyrosine antibody. Coll indicates collagen.
4N1-1 and GPVI activation induce a similar tyrosine phosphorylation pattern.
Human platelets, preincubated as described in the legend for Figure 1with apyrase, indomethacin, and EGTA, were stimulated with 100 μM 4N1-1 from 10 to 240 seconds or for 2 minutes with 1 U/mL thrombin (Thr) or 10 μg/mL convulxin (Cvx). Platelets were then lysed by addition of NP-40 buffer. (A) Proteins of whole-cell lysate were resolved by 12.5% SDS-PAGE and analyzed with Western blotting using an antiphosphotyrosine antibody. Arrows on the right indicate the position of prominent phosphorylated bands. (B) Cell extracts were immunoprecipitated by using the anti-Syk antibody. Immunoprecipitates were resolved by 10% SDS-PAGE and analyzed with Western blotting using an antiphosphotyrosine antibody (top). The filter was dehybridized and reprobed by using the anti-Syk antibody (bottom). (C-E) Human platelets were stimulated with 100 μM 4N1-1 or 10 μg/mL convulxin and lysed 2 minutes later. (C) Cell extracts were immunoprecipitated by using the anti-PLCγ2 antibody. Immunoprecipitates were resolved by 7% SDS-PAGE and analyzed with Western blotting using an antiphosphotyrosine antibody (top). The filter was dehybridized and reprobed by using the anti-PLCγ2 antibody (bottom). (D) Cell extracts were immunoprecipitated by using the anti–SLP-76 antibody. Immunoprecipitates were resolved by 10% SDS-PAGE and analyzed with Western blotting using an antiphosphotyrosine antibody (top). The filter was dehybridized and reprobed by using the anti–SLP-76 antibody (bottom). (E) Cell extracts were precipitated by using GST fusion protein containing the tandem SH2 domain of Syk. Precipitated proteins were resolved by 15% SDS-PAGE and analyzed with Western blotting using an antiphosphotyrosine antibody. (F) Cell extracts were precipitated by using convulxin and an anticonvulxin antibody (IP GPVI), the anticonvulxin antibody alone (Ab), or the GST-SH2-Syk fusion protein. Precipitated proteins were resolved by 15% SDS-PAGE and analyzed with Western blotting using an antiphosphotyrosine antibody. Coll indicates collagen.
Tyrosine phosphorylation was maintained in the presence of indomethacin, apyrase, and ethyleneglycotetraacetic acid (EGTA), used either alone or together (Figure 1D). These results indicate that 4N1-1–induced tyrosine phosphorylation does not depend on release of thromboxanes or adenosine diphosphate (ADP) or αIIbβ3-mediated platelet-platelet interactions. A similar pattern of phosphorylation was observed in response to 4N1K, whereas 4NGG was inactive (Figure1E).
The pattern of protein phosphorylation in response to 4N1-1 was similar to that induced by the GPVI agonist convulxin (Figure 2A). In contrast, the G protein–coupled receptor agonist thrombin induces a minimal increase in tyrosine phosphorylation. We investigated whether 4N1-1 stimulation induces tyrosine phosphorylation of the major proteins involved in the signaling pathway activated by GPVI. In agreement with previous findings,15 4N1-1 stimulated phosphorylation of Syk over the same time course as that for the increase in whole-cell phosphorylation (Figure 2B). Additionally, 4N1-1 stimulated a slightly lower level of tyrosine phosphorylation of FcR γ chain, SLP-76, and phospholipase C γ2 (PLCγ2) compared with convulxin (Figure 2C-E). Furthermore, the phosphorylated FcR γ chain was in a complex with GPVI, since it could be detected after precipitation of GPVI in platelets stimulated with 4N1-1 (Figure 2F). These results show that 4N1-1 induces rapid tyrosine phosphorylation, which includes GPVI-associated FcR γ chain, Syk, SLP-76, and PLCγ2.
Signaling pathway associated with FcR γ chain mediates platelet activation in response to 4N1-1
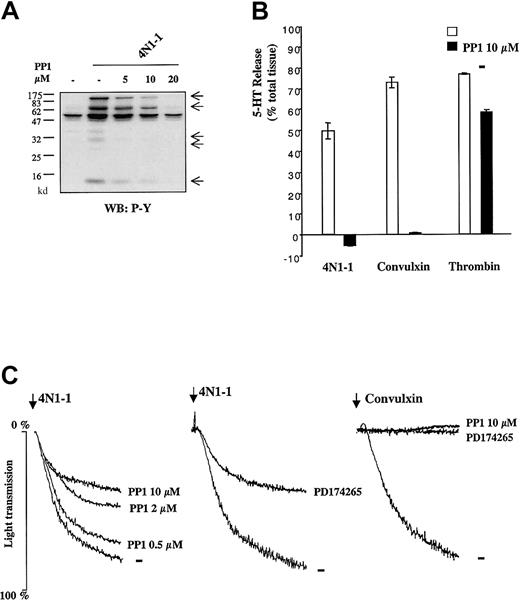
To investigate involvement of the FcR γ-chain–associated signaling pathway in response to 4N1-1, we used the Src family kinase inhibitor 4-amino-4-(4-methylphenyl)-7-(t-butyl) pyrazola[3,4-d]pyrimidine (PP1), which can abolish phosphorylation of the FcR γ chain by GPVI.28 29 PP1 inhibited 4N1-1–induced tyrosine phosphorylation in a concentration-dependent manner as shown in whole-cell lysates (Figure3A) and in FcR γ-chain precipitates (data not shown). Furthermore, 5-HT release induced by 4N1-1 and convulxin was abolished by pretreatment with PP1, whereas the response to thrombin was only slightly inhibited (Figure 3B). These results show that Src kinase activity is crucial for FcR γ-chain phosphorylation and secretion induced by 4N1-1.
Src kinases are crucial for responses induced by 4N1-1 in platelets.
(A) Human platelets were pretreated for 10 minutes with 5, 10, or 20 μM PP1 and stimulated for 2 minutes with 100 μM 4N1-1. Whole-cell lysates were resolved by 12.5% SDS-PAGE and analyzed with Western blotting using an antiphosphotyrosine antibody. Arrows on the right indicate the position of prominent phosphorylated bands. (B) Human platelets (2 × 108/mL) loaded with tritium–5-HT were preincubated for 10 minutes with 10 μM PP1 and then stimulated for 2 minutes with 4N1-1 (25 μM), convulxin (1 μg/mL), or thrombin (0.1 U/mL). Tritium–5-HT release determined by scintillation spectrometry was expressed as a percentage of total tissue content. Each experiment was done with triplicate samples, and results are expressed as means ± SD. (C) Human platelets (2 × 108/mL) were preincubated for 10 minutes with 0.5, 2, or 10 μM PP1 or 5 μM PD174265. Platelets were then stimulated with 25 μM 4N1-1 or 1 μg/mL convulxin. Aggregation was monitored by light transmission. Arrows indicate addition of agonist. Results are representative of experiments using samples from 3 different donors.
Src kinases are crucial for responses induced by 4N1-1 in platelets.
(A) Human platelets were pretreated for 10 minutes with 5, 10, or 20 μM PP1 and stimulated for 2 minutes with 100 μM 4N1-1. Whole-cell lysates were resolved by 12.5% SDS-PAGE and analyzed with Western blotting using an antiphosphotyrosine antibody. Arrows on the right indicate the position of prominent phosphorylated bands. (B) Human platelets (2 × 108/mL) loaded with tritium–5-HT were preincubated for 10 minutes with 10 μM PP1 and then stimulated for 2 minutes with 4N1-1 (25 μM), convulxin (1 μg/mL), or thrombin (0.1 U/mL). Tritium–5-HT release determined by scintillation spectrometry was expressed as a percentage of total tissue content. Each experiment was done with triplicate samples, and results are expressed as means ± SD. (C) Human platelets (2 × 108/mL) were preincubated for 10 minutes with 0.5, 2, or 10 μM PP1 or 5 μM PD174265. Platelets were then stimulated with 25 μM 4N1-1 or 1 μg/mL convulxin. Aggregation was monitored by light transmission. Arrows indicate addition of agonist. Results are representative of experiments using samples from 3 different donors.
PP1 also inhibited 4N1-1–induced aggregation in a concentration-dependent manner (Figure 3C). However, 10 μM PP1, which abolished aggregation in response to convulxin (Figure 3C) and secretion induced by 4N1-1 (Figure 3B), only inhibited 4N1-1–induced aggregation by approximately 50%. Similar results were observed with higher concentrations of PP1 (data not shown) and with a structurally unrelated Src family kinase inhibitor, 4-[(3-bromophenyl) amino]-6-propionylamidoquinazoline (PD174265; Figure 3C). These results show that Src family kinase activity is necessary for tyrosine phosphorylation events and dense granule secretion and is partly involved in aggregation induced by 4N1-1.
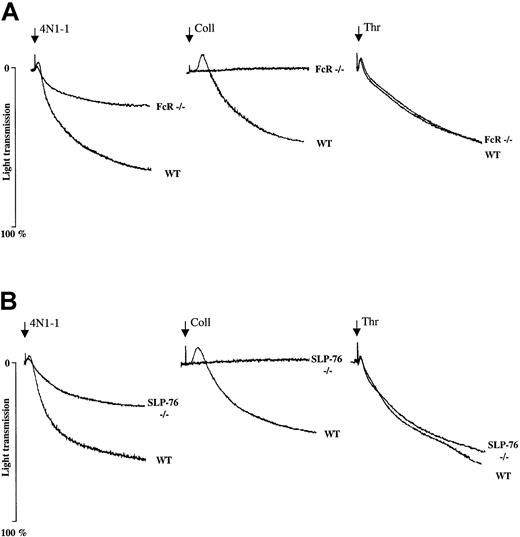
We used genetically modified mice that do not express FcR γ chain or SLP-76 to determine the involvement of these proteins during platelet aggregation induced by 4N1-1. Platelet aggregation induced by 4N1-1 was inhibited by approximately 50% in both types of mice, whereas the response to collagen (Figure 4A and B) was totally abolished and that to thrombin was unaffected (Figure 4A and B). These results show that functional FcR γ chain and SLP-76 are crucial for maximal mouse platelet aggregation induced by 4N1-1.
Aggregation induced by 4N1-1 is strongly reduced in mice deficient in FcR γ chain and SLP-76.
Washed platelets (1 × 108/mL) from WT mice and mice deficient in FcR γ chain and SLP-76 were stimulated with 25 μM 4N1-1, 10 μg/mL collagen, or 0.1 U/mL thrombin. Aggregation was monitored by light transmission. Arrows indicate addition of agonist. Results are representative of experiments using 2 mice for each genotype.
Aggregation induced by 4N1-1 is strongly reduced in mice deficient in FcR γ chain and SLP-76.
Washed platelets (1 × 108/mL) from WT mice and mice deficient in FcR γ chain and SLP-76 were stimulated with 25 μM 4N1-1, 10 μg/mL collagen, or 0.1 U/mL thrombin. Aggregation was monitored by light transmission. Arrows indicate addition of agonist. Results are representative of experiments using 2 mice for each genotype.
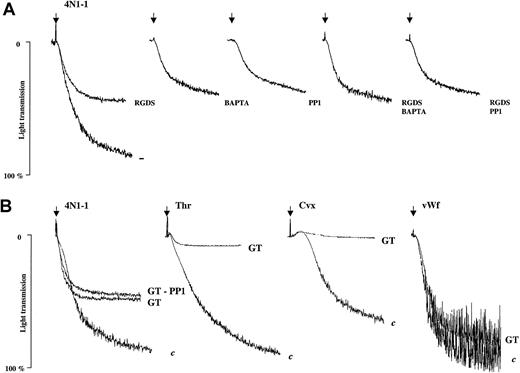
Aggregation in response to 4N1-1 is partly supported by αIIbβ3 integrin
We investigated the involvement of αIIbβ3 during platelet aggregation in response to 4N1-1. The Arg-Gly-Asp-Ser peptide, which binds to αIIbβ3 and prevents interactions with fibrinogen and vWF, inhibited aggregation to 4N1-1 by approximately 50% (Figure5A). In contrast, aggregation induced by thrombin or convulxin was abolished (data not shown). An intracellular chelator of calcium, 2-bis (2-aminophenoxy) ethane-N, N, N, N′, N′-tetraacetic acid (BAPTA-AM), and PP1, used alone or with Arg-Gly-Asp-Ser, produced a degree of inhibition of aggregation that was similar to that observed with 4N1-1 (Figure 5A). Consistent with these results, aggregation of platelets from a patient with type I Glanzmann thrombasthenia, which do not express functional αIIbβ3, was reduced by approximately 50%, whereas aggregation induced by thrombin and convulxin was abolished. Pretreatment of platelets from patients with Glanzmann thrombasthenia with PP1 did not further decrease the aggregation induced by 4N1-1. Agglutination in response to vWF was unaffected in the Glanzmann platelets (Figure 5B). These results show that platelet aggregation induced by 4N1-1 is mediated partly through αIIbβ3. The absence of additive inhibition between αIIbβ3 blockade and PP1 and between Glanzmann platelets and PP1 suggests that the aggregation response supported by αIIbβ3 depends on activation of the FcR γ chain.
Aggregation induced by 4N1-1 is partly mediated by αIIbβ3.
(A) Human platelets were pretreated for 10 minutes with 1 mM Arg-Gly-Asp-Ser, 40 μM BAPTA-AM, 10 μM PP1, or a combination of these inhibitors before stimulation with 25 μM 4N1-1. (B) Platelets from patients with type I Glanzmann thrombasthenia (GT) or control donors (c) were pretreated, when necessary, for 10 minutes with 10 μM PP1 before stimulation with 25 μM 4N1-1, 0.5 μg/mL convulxin, 0.1 U/mL thrombin, or 10 μg/mL vWF activated with 1 mg/mL ristocetin. Aggregation was monitored by light transmission. Arrows indicate addition of agonist. Results are representative of experiments using platelets from 3 different control donors and one patient with GT.
Aggregation induced by 4N1-1 is partly mediated by αIIbβ3.
(A) Human platelets were pretreated for 10 minutes with 1 mM Arg-Gly-Asp-Ser, 40 μM BAPTA-AM, 10 μM PP1, or a combination of these inhibitors before stimulation with 25 μM 4N1-1. (B) Platelets from patients with type I Glanzmann thrombasthenia (GT) or control donors (c) were pretreated, when necessary, for 10 minutes with 10 μM PP1 before stimulation with 25 μM 4N1-1, 0.5 μg/mL convulxin, 0.1 U/mL thrombin, or 10 μg/mL vWF activated with 1 mg/mL ristocetin. Aggregation was monitored by light transmission. Arrows indicate addition of agonist. Results are representative of experiments using platelets from 3 different control donors and one patient with GT.
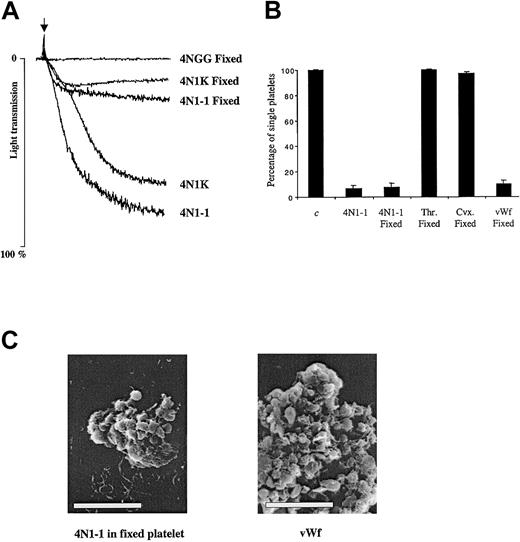
Platelet agglutination is induced by 4N1-1 independent of GPIbα
To define the mechanism underlying the αIIbβ3-independent platelet aggregation induced by the C-terminal peptide of TSP1, we used platelets in which activation was prevented by fixation with formaldehyde. In the fixed platelets, aggregation induced by 4N1-1 and 4N1K was reduced by approximately 70% (Figure6A). We also determined the number of single platelets after aggregation induced in fixed platelets. As shown in Figure 6B, 4N1-1 and vWF induced a comparable decrease in single-platelet counts in untreated and fixed platelets, whereas thrombin and convulxin did not decrease the number of fixed platelets. Scanning electron microscopy showed that 4N1-1 induced formation of small aggregates of fixed platelets and that cell integrity was preserved (Figure 6C). Although the aggregates were much larger in response to vWF, a similar preservation of platelet integrity was observed. These results show that 4N1-1 can induce platelet agglutination.
4N1-1 can induce agglutination of fixed platelets.
(A) Untreated or fixed human platelets were stimulated with 25 μM 4N1-1, 50 μM 4NGG, and 50 μM 4N1K. Aggregation was monitored by light transmission. Arrow indicates addition of agonist. (B) Untreated or fixed human platelets were stimulated with 25 μM 4N1-1, 0.5 μg/mL convulxin, 0.1 U/mL thrombin, or 10 μg/mL vWF activated with 1 mg/mL ristocetin. After full aggregation, the platelets were fixed and the number of single platelets was determined. Results were expressed as a percentage of nonstimulated platelets. Triplicate samples from each donor were used, and means ± SD are shown. Aggregation results and single-platelet counts are from one experiment representative of 3. (C) Fixed and untreated platelets were simulated with 25 μM 4N1-1 and 10 μg/mL vWF activated with 1 mg/mL ristocetin, respectively. Aggregates were fixed and dehydrated for analysis by electron scanning microscopy. The white bar represents 10 μm.
4N1-1 can induce agglutination of fixed platelets.
(A) Untreated or fixed human platelets were stimulated with 25 μM 4N1-1, 50 μM 4NGG, and 50 μM 4N1K. Aggregation was monitored by light transmission. Arrow indicates addition of agonist. (B) Untreated or fixed human platelets were stimulated with 25 μM 4N1-1, 0.5 μg/mL convulxin, 0.1 U/mL thrombin, or 10 μg/mL vWF activated with 1 mg/mL ristocetin. After full aggregation, the platelets were fixed and the number of single platelets was determined. Results were expressed as a percentage of nonstimulated platelets. Triplicate samples from each donor were used, and means ± SD are shown. Aggregation results and single-platelet counts are from one experiment representative of 3. (C) Fixed and untreated platelets were simulated with 25 μM 4N1-1 and 10 μg/mL vWF activated with 1 mg/mL ristocetin, respectively. Aggregates were fixed and dehydrated for analysis by electron scanning microscopy. The white bar represents 10 μm.
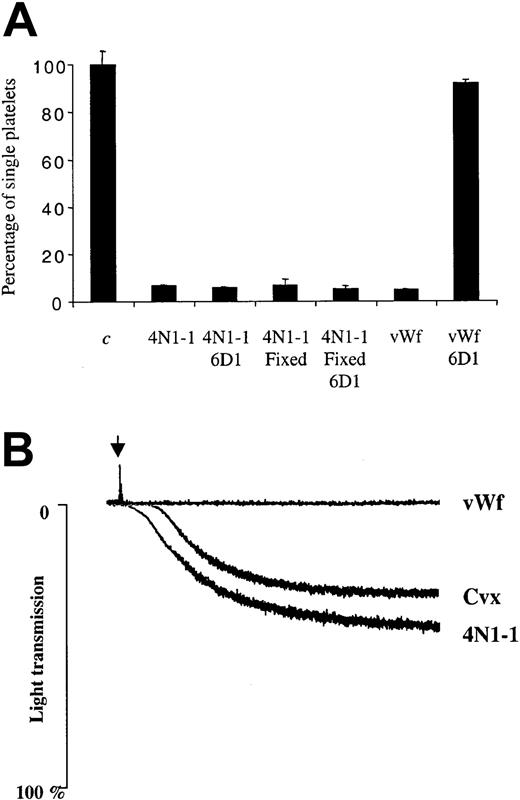
It is known that vWF induces platelet cross-linking and subsequent agglutination through interaction with the GPIb-IX-V complex.30 To investigate the role of this complex during the response to 4N1-1, we used an antifunctional GPIbα antibody, 6D1. As shown in Figure 7A, 6D1 did not inhibit the response to 4N1-1, but it did abolish the response to vWF. Consistent with these results, aggregation to 4N1-1 was maintained in platelets from patients with BSS, who do not express functional GPIb-IX-V complex, whereas vWF was inactive (Figure 7B). Aggregation to 4N1-1 was also observed in platelets from BSS patients pretreated with PP1 (data not shown). The abnormal pattern of aggregation was observed in platelets from 3 different BSS patients and was apparently due to the large size of the platelets. These data show that GPIbα is not essential for the agglutination response to 4N1-1.
GPIbα is not required for aggregation induced by 4N1-1.
(A) Untreated or fixed human platelets were pretreated for 10 minutes with 10 μg/mL of an anti-GPIbα antibody (6D1) before stimulation with 25 μM 4N1-1 or 10 μg/mL vWF activated with 1 mg/mL ristocetin. After full aggregation, platelets were fixed and the number of single platelets was determined. Results were expressed as a percentage of nonstimulated platelets and are representative of experiments using 2 different donors. (B) Platelets from patients with BSS were stimulated with 25 μM 4N1-1, 1 μg/mL convulxin, or 10 μg/mL vWF activated with 1 mg/mL ristocetin. Aggregation was monitored by light transmission. Arrow indicates addition of agonist. Results are representative of one experiment using platelets from 3 different patients with BSS.
GPIbα is not required for aggregation induced by 4N1-1.
(A) Untreated or fixed human platelets were pretreated for 10 minutes with 10 μg/mL of an anti-GPIbα antibody (6D1) before stimulation with 25 μM 4N1-1 or 10 μg/mL vWF activated with 1 mg/mL ristocetin. After full aggregation, platelets were fixed and the number of single platelets was determined. Results were expressed as a percentage of nonstimulated platelets and are representative of experiments using 2 different donors. (B) Platelets from patients with BSS were stimulated with 25 μM 4N1-1, 1 μg/mL convulxin, or 10 μg/mL vWF activated with 1 mg/mL ristocetin. Aggregation was monitored by light transmission. Arrow indicates addition of agonist. Results are representative of one experiment using platelets from 3 different patients with BSS.
IAP does not mediate platelet aggregation induced by 4N1-1
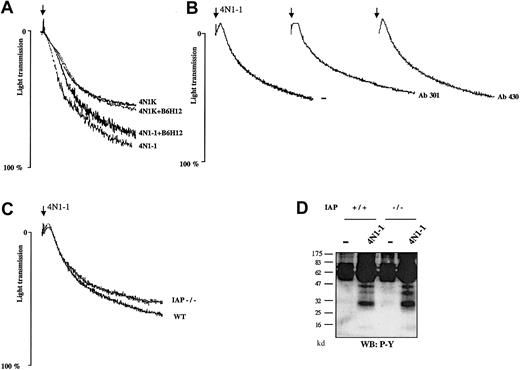
To assess the involvement of IAP during platelet aggregation in response to 4N1-1, platelets were pretreated with antifunctional IAP antibodies. For studies with human platelets, we used the F(ab′)2 fragment of antihuman IAP antibody (B6H12) to avoid activation of the low-affinity immune receptor, FcγRIIA. Aggregation to 4N1-1 and 4N1K were unaffected in human platelets incubated with B6H12 (Figure 8A). There was also no inhibition of response in mouse platelets pretreated with 2 distinct antibodies against mouse IAP (mAb 301 and mAb 430; Figure 8B). The concentrations of antibodies used were higher than those used to abolish IAP function in various other assays.21,22 31Consistent with these results, 4N1-1 induced a similar level of platelet aggregation in wild-type (WT) and IAP-deficient mice (Figure8C). Aggregation induced by thrombin, convulxin, and collagen was also unaffected in platelets from IAP-deficient mice (data not shown). In addition, 4N1-1 induced a similar pattern of phosphorylation in WT and IAP-deficient mice (Figure 8D). These results show that platelet-aggregation induced by 4N1-1 is not mediated by IAP.
Aggregation induced by 4N1-1 is unaffected in IAP-deficient mice and in platelets treated with anti-IAP antibodies.
(A) Human platelets were pretreated for 10 minutes with 50 μg/mL F(ab′)2 fragment of antihuman IAP monoclonal antibody B6H12. (B) Mouse platelets were pretreated for 10 minutes with 50 μg/mL antimouse IAP antibodies mAb 301 and mAb 430. Platelets were then stimulated with 25 μM 4N1-1 or 50 μM 4N1K. (C) Mouse platelets from WT mice and IAP-deficient mice (IAP−/−) were stimulated with 25 μM 4N1-1. Aggregation was monitored by light transmission. Arrows indicate addition of agonist. (D) Mouse platelets from WT and IAP-deficient mice were stimulated with 25 μM 4N1-1. Whole-cell lysates were resolved by 12.5% SDS-PAGE and analyzed with Western blotting using an antiphosphotyrosine antibody. Results are representative of experiments using samples from 3 different donors and 2 different mice.
Aggregation induced by 4N1-1 is unaffected in IAP-deficient mice and in platelets treated with anti-IAP antibodies.
(A) Human platelets were pretreated for 10 minutes with 50 μg/mL F(ab′)2 fragment of antihuman IAP monoclonal antibody B6H12. (B) Mouse platelets were pretreated for 10 minutes with 50 μg/mL antimouse IAP antibodies mAb 301 and mAb 430. Platelets were then stimulated with 25 μM 4N1-1 or 50 μM 4N1K. (C) Mouse platelets from WT mice and IAP-deficient mice (IAP−/−) were stimulated with 25 μM 4N1-1. Aggregation was monitored by light transmission. Arrows indicate addition of agonist. (D) Mouse platelets from WT and IAP-deficient mice were stimulated with 25 μM 4N1-1. Whole-cell lysates were resolved by 12.5% SDS-PAGE and analyzed with Western blotting using an antiphosphotyrosine antibody. Results are representative of experiments using samples from 3 different donors and 2 different mice.
Discussion
In this study, we found that peptide 4N1-1, derived from the C-terminal domain of TSP1, and its more soluble derivative, 4N1K, stimulate platelet aggregation through the FcR γ-chain signaling pathway and platelet agglutination independently of GPIIb-IIIa and GPIbα. We also showed that IAP does not mediate these responses.
Tyrosine phosphorylation of FcR γ chain, Syk, SLP-76, and PLCγ2, along with several other bands, was stimulated by 4N1-1. This was not dependent on secondary events including αIIbβ3 activation or release of thromboxane and ADP. To assess the functional importance of the increase in tyrosine phosphorylation induced by 4N1-1, we used 2 structurally unrelated Src family kinase inhibitors and genetically modified mice. Inhibition of the Src family kinases prevented the increase in phosphorylation of all proteins and blocked secretion, whereas aggregation was reduced by approximately 50%. A similar reduction of aggregation was observed in platelets deficient in FcR γ chain and SLP-76 and in the presence of Arg-Gly-Asp-Ser or the absence of the integrin αIIbβ3.
Collagen and convulxin induce platelet activation through GPVI.32,33 This leads to phosphorylation of the FcR γ chain by the Src kinases Fyn and Lyn, providing a docking site for the tyrosine kinase Syk, whose activation is necessary for tyrosine phosphorylation of the adapter proteins linker for activation of T cells and SLP-76.28,29,34 These adapters are necessary for activation of PLCγ2, which regulates calcium elevation and protein kinase C.35 We showed that 4N1-1 induces a pattern of tyrosine phosphorylation similar to that observed after activation of GPVI. In addition, we found that, as for GPVI, this signaling pathway is crucial for platelet activation induced by 4N1-1. The phosphorylated FcR γ chain in platelets stimulated with 4N1-1 was observed to coimmunoprecipitate with the collagen receptor GPVI. However, 4N1-1 was unable to activate GPVI–FcR γ-chain complex when expressed in K562 erythroleukemic cells (O.B., D.T., and S.W., unpublished data, January 2001), suggesting that 4N1-1 does not activate GPVI.
For collagen and convulxin, platelet aggregation depends totally on the FcR γ-chain–associated signaling pathway and functional αIIbβ3, whereas for 4N1-1, this pathway is only partly involved. Moreover, the αIIbβ3-independent aggregation to 4N1-1 was observed in fixed platelets and is more appropriately described as agglutination. Agglutination to 4N1-1 was maintained in the presence of an antifunctional antibody against GPIbα and in platelets from patients with BSS, which lack detectable GPIbα. The mechanism underlying agglutination to 4N1-1 is not known and may not be receptor dependent, since it is unlikely that the peptide promotes conformational changes in surface proteins of fixed cells.
It is important to consider whether full-length TSP1 can induce a response similar to that of 4N1-1. It was shown previously that TSP1 reinforces platelet aggregation in response to thrombin and collagen.3-5 Furthermore, it was reported that TSP1 induces agglutination of fixed platelets previously activated by thrombin.36 However, we did not observe aggregation or tyrosine phosphorylation in response to purified TSP1 at concentrations up to 100 μg/mL (data not shown). This result is consistent with findings of several other studies that concluded that TSP1 alone cannot induce activation of platelets. Nevertheless, it was reported previously that a complex of TSP1 with heparin induces efficient platelet activation.37 This may be mediated by a conformational change in TSP1 in the presence of heparin.38
We found that platelet aggregation induced by 4N1-1 was not inhibited in IAP-deficient cells or in platelets pretreated with several distinct antifunctional IAP antibodies. However, previous studies showed inhibition of spreading on immobilized fibrinogen of platelets treated with antifunctional IAP antibody (B6H12)15 and inhibition of spreading and formation of aggregates on immobilized collagen in platelets from IAP−/− mice,18 in response to 4N1K. These results led the authors to conclude that IAP regulates αIIbβ3 and α2β1 integrins in response to 4N1K in platelets. The disparity in findings may have resulted from measurement of different functional responses—namely, spreading compared with aggregation—and suggests involvement of IAP during adhesive events.
Our results show that the C-terminal peptide of TSP1 induces platelet aggregation independently of IAP and suggest the presence of another receptor for 4N1-1 in platelets. The activation of the FcR γ-chain–associated signaling pathway raises the possibility that the C-terminal peptide of TSP1 may be an agonist at a receptor, other than GPVI, which is coupled to the FcR γ chain. However, our results do not disprove that IAP is a functional receptor of 4N1-1 in other cell lines.
This study led to the identification of the FcR γ-chain–associated signaling pathway as the intracellular mechanism that mediates platelet activation and subsequent αIIbβ3 activation in response to 4N1-1. In addition, the C-terminal peptide of TSP1 can induce agglutination through a mechanism independent of αIIbβ3 and GPIbα. This dichotomous mechanism of aggregation is original and represents a novel mechanism of platelet activation. The functional importance of these responses, as well as the initial events allowing them, need to be explored.
We thank Drs Mireille Leduc and Cassian Bon for the gift of convulxin and anticonvulxin antibody.
Supported by the British Heart Foundation (BHF). S.P.W. is a BHF Senior Research Fellow.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
David Tulasne, Department of Pharmacology, University of Oxford, Mansfield Road, Oxford OX1 3QT, United Kingdom; e-mail: david.tulasne@pharmacology.oxford.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal