Abstract
Using heparinized whole blood and flow conditions, it was shown that adenosine 5′-diphosphate (ADP) receptors P2Y12 and P2Y1 are both important in direct shear-induced platelet aggregation and platelet aggregation subsequent to initial adhesion onto von Willebrand factor (vWf)–collagen. In the viscometer, whole blood was subjected to shear rates of 750, 1500, and 3000 s−1 for 30 seconds at room temperature. The extent of aggregation was determined by flow cytometry. The P2Y12antagonist AR-C69 931MX (ARMX) reduced shear-induced aggregation at these rates by 56%, 54%, and 16%, respectively, compared to control samples. Adenosine 3′,5′-diphosphate (A3P5P; P2Y1antagonist) inhibited shear-induced aggregation by 40%, 30% and 29%, respectively, compared to control samples. Blockade of both ADP receptors at 3000 s−1 with ARMX plus A3P5P further reduced the platelet aggregation by 41% compared to the addition of ARMX alone (57% compared to control samples). Using a parallel-plate flow chamber, whole blood was perfused over bovine collagen type 1 at a wall shear rate of 3000 s−1 for 60 seconds. Platelet deposition was quantified with epifluorescence video microscopy and digital image processing. Blockade of P2Y12 alone or blockade of P2Y1 alone did not reduce thrombus formation on vWf–collagen. In contrast, blockade of both P2Y12 and P2Y1 reduced platelet deposition by 72%. These results indicate that combinations of antagonists of the ADP receptors P2Y12 and P2Y1 are effective inhibitors of direct shear-induced platelet aggregation and of platelet aggregation subsequent to initial adhesion under flow conditions. Inhibitors of these pathways are potentially useful as antiarterial thrombotic agents.
Introduction
Direct fluid shear stress-induced platelet aggregation under arterial flow conditions requires the binding of large or unusually large von Willebrand factor (vWf) multimers to the glycoprotein (GP) Ibα component of GPIb-IX-V complexes, followed by the attachment of these large vWf forms to activated GPIIb-IIIa integrins.1-3 Adenosine 5′-diphosphate (ADP) is also essential in this type of platelet aggregation.4
Platelets express at least 3 distinct purinergic receptors that bind ADP—the ligand-gated ion channel P2X1 receptor and 2 distinct G-protein–linked P2 receptors, P2Y12 and P2Y1. (P2Y12 is also called P2YADP, P2YAC, P2Ycyc, and P2TAC.) ADP binding to the P2X1 receptor causes the movement of Ca+2 from the exterior of the platelet to the cytoplasm.5-7 Binding of ADP to P2Y1 activates platelet phospholipase C and mobilizes Ca+2 from intracellular stores, leading to the activation of protein kinase C and the aggregation of washed platelets.6 The P2Y1receptor has been cloned, and it has been determined that washed platelets from P2Y1 receptor-null mice are unable to aggregate in response to ADP.8
A patient was reported by Leon et al9 with severely impaired aggregation in response to ADP. This patient had normal P2Y1 and P2X1 mRNA expression and normal functioning P2Y1 receptors. It was presumed, therefore, that the other ADP P2 receptor, P2Y12, was responsible for the ADP aggregation defect.
ADP binding to P2Y12 inhibits platelet adenylyl cyclase, lowers platelet cyclic adenosine monophosphate levels, and potentiates ADP-induced aggregation.9-15 This receptor has recently been cloned by Hollopeter et al.16 P2Y12 is the target of the structurally similar thienopyridine antithrombotic drugs ticlopidine and clopidogrel. These compounds inhibit ADP-induced platelet activation and prevent Gi-protein association with the platelet membrane. Neither drug inhibits ADP-induced platelet shape change or calcium flux. Although the precise mechanism of action is unknown, the thienopyridines or their metabolites may block the P2Y12 receptor.17 No previous study has investigated the involvement of ADP receptors in shear-induced platelet aggregation in whole blood.
Although the role of the P2X1 receptor remains undefined, the activation of both ADP P2 receptors is essential for ADP-induced platelet aggregation.6,13,18 These data were acquired, however, in washed platelet suspensions using exogenous ADP. Physiologically relevant whole blood systems were not tested. We describe here the relative importance of the different ADP receptor subtypes, P2Y12 and P2Y1, in 2 types of platelet aggregation under arterial flow conditions: direct shear-induced platelet aggregation in a cone-and-plate viscometer and platelet adhesion, followed by aggregation, on an insolubilized vWf–collagen 1 surface using a whole blood perfusion system.2,4,19 These systems simulate the platelet aggregation that occurs in partially occluded arteries with intact endothelial cells or in arteries with exposed extracellular matrix secondary to damage and removal of endothelial cells.20,21We used antagonists specific for each receptor—adenosine 3′5′ diphosphate (A3P5P, a P2Y1 antagonist)6 and AR-C69931MX (ARMX), which is an adenosine triphosphate analogue that specifically inhibits P2Y12.22 Another well-established inhibitor of platelet aggregation, the GP IIb-IIIa antibody abciximab, was included in the study for comparison.23 24
Materials and methods
Blood collection
Whole blood anticoagulated with 10 U/mL unfractionated porcine sodium heparin (Elkins-Sinn, Cherry Hill, NJ) was drawn by venipuncture from healthy unmedicated donors. Platelet-rich plasma (PRP) was prepared by centrifuging heparinized whole blood for 15 minutes at 150g at room temperature. Informed consent was provided according to the Declaration of Helsinki.
Compounds
ARMX (AR-C69931MX; AstraZeneca, Loughborough, United Kingdom) is a specific ADP P2Y12 antagonist. ARMX is a synthetic derivative of adenosine triphosphate that is resistant to hydrolysis by ectonucleotidases because of a dichloromethylene substitution for the anhydride oxygen between β and γ phosphates.22Viscosimetry experiments showed that 0.5 μM ARMX produced maximal inhibition of shear-induced aggregation. In perfusion studies, ARMX was used at 0.5, 1, 50, and 100 μM.
A3P5P (adenosine 3′, 5′-diphosphate; Sigma, St Louis, MO) is known to be an ADP P2Y1 antagonist.6 Other investigators have shown, in washed platelet suspensions, approximately 50% inhibition of ADP-induced platelet aggregation by 100 μM A3P5P.25 In our experiments, A3P5P was used at 10 μM and 100 μM in the viscosimeter and at 0.5, 1, 10, 50, and 100 μM in perfusion studies.
Abciximab (c7E3, Reo-pro; Centocor, Malvern, PA) is a murine–human chimeric monoclonal antibody fragment directed against platelet GPIIb-IIIa.26 Abciximab was used at 5 μg/mL in perfusion and viscometry studies. This concentration is approximately the current dosage given to patients undergoing angioplasty procedures.24
Cone-and-plate viscometry
Whole blood mixed with saline (control), abciximab, ARMX, A3P5P, or a combination of ARMX and A3P5P was subjected to shear rates of 750, 1500, and 3000 s−1 for 30 seconds at room temperature in a cone-and-plate viscometer. Samples taken before and after application of shear stress were immediately fixed in 1% formaldehyde–phosphate-buffered saline (PBS) and were incubated with anti-CD42a–fluorescein isothiocyanate (FITC; anti-GPIX; Becton Dickinson Immunosystems) as the platelet-specific reporting antibody to distinguish the platelet population. The extent of aggregation was quantified by determining the percentage of unaggregated single platelets remaining after each viscosimeter run by flow cytometry (FACScan; Becton Dickinson, San Jose, CA). Each fixed, labeled platelet sample was acquired for 60 seconds with equal volumes of sample for both the control (unsheared) and the sheared sample. It has been shown that platelet aggregation can be quantified by monitoring the disappearance of single platelets.27 The same protocol was followed for experiments using PRP, except that the shear rate was 10 000 s−1 to obtain approximately the same shear stress as the 3000 s−1 whole blood experiments.
Perfusion studies
Whole blood containing 10 μM mepacrine was incubated with saline (control), abciximab, ARMX, A3P5P, or ARMX plus A3P5P for 5 minutes at 37°C and was perfused over bovine type 1 collagen-coated slides in a parallel-plate flow chamber at a wall shear rate of 3000 s−1 for 1 minute at 37°C. Real-time videotapes of fluorescent platelet adherence and aggregation were observed, recorded, and digitally analyzed as previously described.19 28 The calibration process assumes that the integral of the intensity of the image over the area of the thrombus is proportional to the number of platelets in the thrombus. Platelet aggregation is the total intensity of each thrombus (area × average intensity) multiplied by a value determined by dividing the number of single platelets in the 2-second image by the sum of the total intensities of these single platelets (the inverse of the average intensity per platelet).
Statistical analysis
Results are reported as the mean ± SEM. Statistical significance with 95% confidence was determined by analysis of variance using the Fisher protected least significant difference test.
Results
Cone and plate viscometry
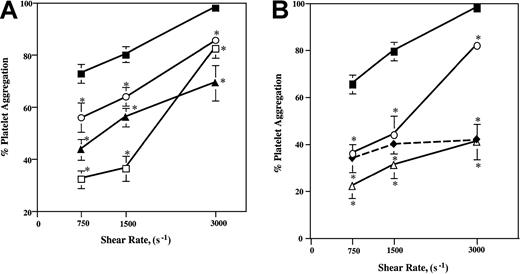
Whole blood was subjected to shear rates of 750, 1500, and 3000 s−1 for 30 seconds at room temperature. The addition of 0.5 μM ARMX (P2Y12 antagonist) reduced shear-induced aggregation at these rates by 56%, 54%, and 16%, respectively, compared to control samples without ARMX (Figure1A). This concentration of ARMX reduced aggregate formation as effectively as the addition of 5 μg/mL abciximab at all 3 shear rates (Figure 1B). The P2Y1antagonist, A3P5P, added at either 10 or 100 μM, also reduced shear-induced aggregation. At these shear rates, blood mixed with 100 μM A3P5P inhibited shear aggregation by 40%, 30%, and 29%; the addition of 10 μM A3P5P reduced shear aggregation by 23%, 20%, and 13%, respectively (Figure 1A).
Platelet aggregation induced by increasing shear rates.
(A, B) Heparinized whole blood was subjected to shear rates of 750, 1500, and 3000 s−1 (shear stresses, approximately 30, 60, and 120 dynes/cm2) in a cone-and-plate viscosimeter for 30 seconds at room temperature. Presheared and postsheared samples were fixed in 1% formaldehyde–PBS. Platelets in whole blood samples were labeled with anti–CD42a-FITC (anti-GPIX), and the extent of platelet aggregation was determined by flow cytometry. Values are mean ± SEM. *Significant difference from control; P < .05. (A) Control, closed squares; 10 μM A3P5P, open circles; 100 μM A3P5P, closed triangles; 0.5 μM ARMX, open squares. (B) Control, closed squares; 5 μg/mL abciximab, open circles; 0.5 μM ARMX + 10 μM A3P5P, open triangles; 0.5 μM ARMX + 100 μM A3P5P, closed diamonds.
Platelet aggregation induced by increasing shear rates.
(A, B) Heparinized whole blood was subjected to shear rates of 750, 1500, and 3000 s−1 (shear stresses, approximately 30, 60, and 120 dynes/cm2) in a cone-and-plate viscosimeter for 30 seconds at room temperature. Presheared and postsheared samples were fixed in 1% formaldehyde–PBS. Platelets in whole blood samples were labeled with anti–CD42a-FITC (anti-GPIX), and the extent of platelet aggregation was determined by flow cytometry. Values are mean ± SEM. *Significant difference from control; P < .05. (A) Control, closed squares; 10 μM A3P5P, open circles; 100 μM A3P5P, closed triangles; 0.5 μM ARMX, open squares. (B) Control, closed squares; 5 μg/mL abciximab, open circles; 0.5 μM ARMX + 10 μM A3P5P, open triangles; 0.5 μM ARMX + 100 μM A3P5P, closed diamonds.
Blockade of both P2Y12 and P2Y1 receptors, using the combination of A3P5P and ARMX, inhibited shear-induced aggregation by 50% to 70% at the 3 shear rates tested (Figure 1B). Blood incubated with 10 or 100 μM A3P5P plus 0.5 μM ARMX reduced shear-induced aggregation about as effectively as either 0.5 μM ARMX addition alone (Figure 1A) or 5 μg/mL abciximab (45%-54%) alone (Figure 1B) at shear rates of 750 and 1500 s−1. At the highest shear rate of 3000 s−1, the combination of 0.5 μM ARMX and either 10 or 100 μM A3P5P reduced aggregate formation by 57% compared to control values, whereas 5 μg/mL abciximab alone or 0.5 μM ARMX alone reduced platelet aggregation at this shear rate by only 16% (Figure 1A-B).
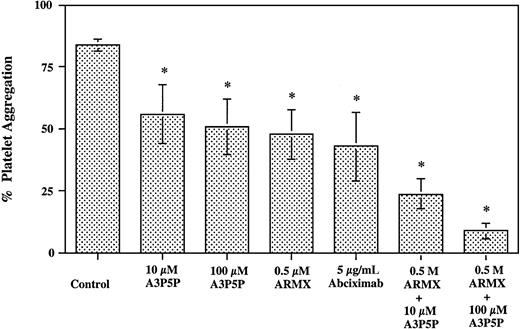
Platelet-rich plasma was subjected to a shear rate of 10 000 s−1 for 30 seconds at room temperature. Blockade of GPIIb-IIIa with the addition of 5 μg/mL abciximab inhibited shear aggregation by 49%. The addition of 0.5 μM ARMX to PRP reduced shear-induced aggregation by 44%. The addition of 10 or 100 μM A3P5P reduced aggregate formation by 33% and 39%, respectively. The combination of 0.5 μM ARMX plus either 10 or 100 μM A3P5P inhibited shear-induced aggregation by 71% and 89%, respectively. These data indicate that blocking both ADP platelet P2 receptors was about twice as effective at inhibiting shear-induced aggregation in PRP samples as either of the ADP receptor blockers alone (Figure2).
Shear-induced platelet aggregation in PRP.
PRP prepared from heparinized whole blood was subjected to a shear rate of 10 000 s−1 in a cone-and-plate viscometer for 30 seconds at room temperature. Presheared and postsheared samples were fixed in 1% formaldehyde–PBS. Platelet samples were labeled with anti–CD42a-FITC (anti-GPIX), and the extent of platelet aggregation was determined by flow cytometry. *Significant difference from control;P < .05.
Shear-induced platelet aggregation in PRP.
PRP prepared from heparinized whole blood was subjected to a shear rate of 10 000 s−1 in a cone-and-plate viscometer for 30 seconds at room temperature. Presheared and postsheared samples were fixed in 1% formaldehyde–PBS. Platelet samples were labeled with anti–CD42a-FITC (anti-GPIX), and the extent of platelet aggregation was determined by flow cytometry. *Significant difference from control;P < .05.
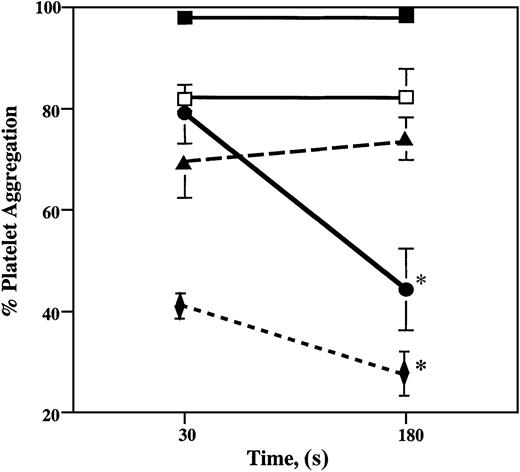
In partially occluded arteries, blood briefly encounters areas of stenosis where fluid shear stress levels may reach 3000 dynes/cm2 and may induce platelet aggregation. Platelet clumps may then enter arterial regions of lower shear stress. An ideal antithrombotic agent would not only inhibit platelet aggregation but also promote platelet disaggregation. We have simulated these arterial regions of high and low fluid shear stress by subjecting whole blood in the viscometer to a shear rate of 3000 s−1 for 30 seconds and then to 100 s−1 for 150 seconds.
Under high shear rates, 98% of platelets from control samples aggregated and remained aggregated after the application of low shear stress (Figure 3). Blood samples with 0.5 μM ARMX had 19% fewer platelet aggregates than control samples after 30 seconds of high shear stress (120 dynes/cm2) and 44% fewer platelet aggregates after an additional 150 seconds at low shear stress (5 dynes/cm2). Abciximab (5 μg/mL) inhibited platelet aggregation induced by high shear stress by 16%, without promoting disaggregation during the next 150 seconds of low shear stress. Platelets in blood samples with 100 μM A3P5P aggregated 30% less than control samples after 30 seconds at high shear stress, also without promoting disaggregation during the subsequent 150 seconds at low shear. The combination of 0.5 μM ARMX plus 100 μM A3P5P inhibited aggregation by 57% after 30 seconds at high shear stress and by 70% after an additional 150 seconds at low shear stress (Figure 3).
Shear-induced platelet aggregation at high shear stress and low shear stress.
Heparinized whole blood was subjected to a shear rate of 3000 s−1 (120 dynes/cm2) for 30 seconds, followed by 150 seconds at 100 s−1 (5 dynes/cm2) in a cone-and-plate viscometer at room temperature. Samples taken before shearing, after 30 seconds at 3000 s−1, and after 180 seconds were immediately fixed in 1% formaldehyde–PBS. Fixed whole blood samples were incubated with anti–CD42a-FITC (anti-GPIX), and the extent of platelet aggregation was determined by flow cytometry. Values are mean ± SEM. *Significant difference from the percentage of platelet aggregation after 30 seconds; P < .05. Control, closed squares; 100 μM A3P5P, closed triangles; 5 μg/mL abciximab, open squares; 0.5 μM ARMX, closed circles; 0.5 μM ARMX + 100 μM A3P5P, closed diamonds.
Shear-induced platelet aggregation at high shear stress and low shear stress.
Heparinized whole blood was subjected to a shear rate of 3000 s−1 (120 dynes/cm2) for 30 seconds, followed by 150 seconds at 100 s−1 (5 dynes/cm2) in a cone-and-plate viscometer at room temperature. Samples taken before shearing, after 30 seconds at 3000 s−1, and after 180 seconds were immediately fixed in 1% formaldehyde–PBS. Fixed whole blood samples were incubated with anti–CD42a-FITC (anti-GPIX), and the extent of platelet aggregation was determined by flow cytometry. Values are mean ± SEM. *Significant difference from the percentage of platelet aggregation after 30 seconds; P < .05. Control, closed squares; 100 μM A3P5P, closed triangles; 5 μg/mL abciximab, open squares; 0.5 μM ARMX, closed circles; 0.5 μM ARMX + 100 μM A3P5P, closed diamonds.
Perfusion studies
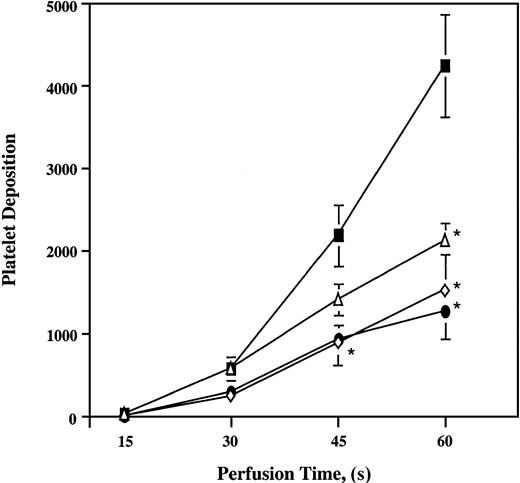
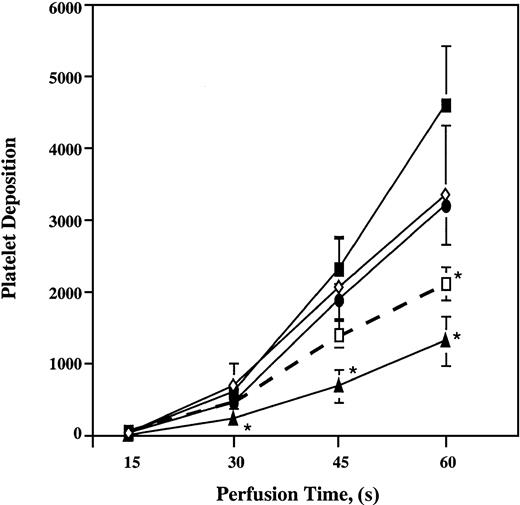
Perfusion of whole blood with increasing concentrations of ARMX (0.5, 1, 50, and 100 μM) or A3P5P (10, 50, and 100 μM) did not significantly reduce platelet deposition onto the vWf–collagen 1 surface compared with untreated samples (data not shown). In contrast, platelet thrombus formation in samples containing both ARMX and A3P5P was inhibited on collagen 1 at 3000 s−1. The combination of 100 μM A3P5P plus either 0.5, 1, or 100 μM ARMX significantly reduced platelet deposition by 50%, 64%, and 70%, respectively, onto vWf–collagen 1 during 60 seconds of flow (Figure4). In the presence of 0.5 μM ARMX, at least 10 μM A3P5P was required for significant inhibition of platelet deposition onto vWf–collagen 1 during 60 seconds of perfusion. Samples containing 0.5 μM ARMX plus 10 μM A3P5P reduced platelet deposition by 72%, whereas combinations with lower concentrations of A3P5P (0.5 and 1 μM) did not affect deposition significantly (Figure5).
Platelet deposition onto vWf–collagen 1 surfaces in the presence of a constant A3P5P concentration and increasing concentrations of ARMX.
Heparinized whole blood was incubated at 37°C for 5 minutes with saline (control, closed squares) or 100 μM A3P5P + either 0.5 μM ARMX (open triangles), 1 μM ARMX (open diamonds), or 100 μM ARMX (closed circles) before perfusion over a vWf–collagen 1 surface at 3000 s−1 for 1 minute at 37°C. Mepacrine-labeled fluorescent platelets were videotaped in real time using an inverted epi-fluorescence microscope with an attached SIT video camera. Images were digitized to quantify mural thrombus formation. Values reported are mean ± SEM of the total number of platelets in a single view of 37 000 μm2. *Significant difference from control;P < .05.
Platelet deposition onto vWf–collagen 1 surfaces in the presence of a constant A3P5P concentration and increasing concentrations of ARMX.
Heparinized whole blood was incubated at 37°C for 5 minutes with saline (control, closed squares) or 100 μM A3P5P + either 0.5 μM ARMX (open triangles), 1 μM ARMX (open diamonds), or 100 μM ARMX (closed circles) before perfusion over a vWf–collagen 1 surface at 3000 s−1 for 1 minute at 37°C. Mepacrine-labeled fluorescent platelets were videotaped in real time using an inverted epi-fluorescence microscope with an attached SIT video camera. Images were digitized to quantify mural thrombus formation. Values reported are mean ± SEM of the total number of platelets in a single view of 37 000 μm2. *Significant difference from control;P < .05.
Platelet deposition onto vWf–collagen 1 surfaces in the presence of a constant ARMX concentration and increasing concentrations of A3P5P.
Heparinized whole blood was incubated at 37°C for 5 minutes with saline (control, closed squares), 0.5 μM ARMX + either 0.5 μM A3P5P (open diamonds), 1 μM A3P5P (closed circles), 10 μM A3P5P (closed triangles), or 0.5 μM ARMX + 100 μM A3P5P (open squares) before perfusion over a vWf–collagen 1 surface at 3000 s−1 for 1 minute at 37°C. Mepacrine-labeled fluorescent platelets were videotaped in real time using an inverted epi-fluorescence microscope with an attached SIT video camera. Images were digitized to quantify mural thrombus formation. Values reported are mean ± SEM of the total number of platelets in a single view of 37 000 μm2. *Significant difference from control;P < .05.
Platelet deposition onto vWf–collagen 1 surfaces in the presence of a constant ARMX concentration and increasing concentrations of A3P5P.
Heparinized whole blood was incubated at 37°C for 5 minutes with saline (control, closed squares), 0.5 μM ARMX + either 0.5 μM A3P5P (open diamonds), 1 μM A3P5P (closed circles), 10 μM A3P5P (closed triangles), or 0.5 μM ARMX + 100 μM A3P5P (open squares) before perfusion over a vWf–collagen 1 surface at 3000 s−1 for 1 minute at 37°C. Mepacrine-labeled fluorescent platelets were videotaped in real time using an inverted epi-fluorescence microscope with an attached SIT video camera. Images were digitized to quantify mural thrombus formation. Values reported are mean ± SEM of the total number of platelets in a single view of 37 000 μm2. *Significant difference from control;P < .05.
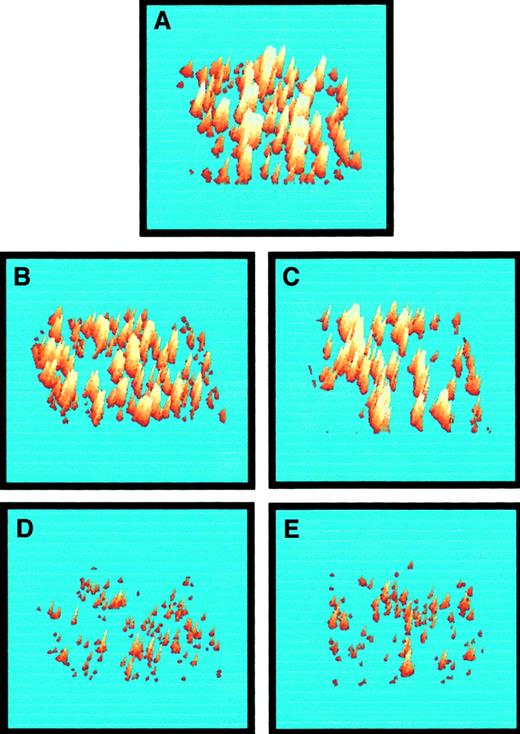
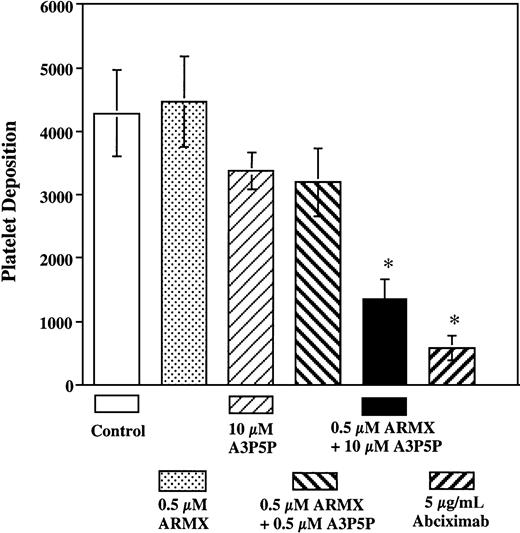
Three-dimensional representations of platelet thrombi on vWf–collagen 1 after 60 seconds of flow at 3000 s−1 in the presence of saline, ARMX, A3P5P, abciximab, and ARMX + A3P5P are shown in Figure 6. Perfusion experiments are summarized in Figure 7. Low concentrations (0.5 μM) of ARMX had no effect on platelet deposition onto vWf–collagen 1. Platelet accumulation was reduced, but insignificantly, with 10 μM A3P5P alone or with 0.5 μM A3P5P plus 0.5 μM ARMX (20% and 22% reduction, respectively). Blockade of both P2Y12 and P2Y1 receptors with 0.5 μM ARMX plus 10 μM A3P5P caused significant inhibition (72% reduction), as did blocking GPIIb-IIIa with abciximab (85% reduction).
Three-dimensional representations of platelet deposition from whole blood onto vWf–collagen 1 after 60 seconds of perfusion at 3000 s−1.
Images were acquired in the presence of (A) saline (control), (B) 0.5 μM ARMX, (C) 10 μM A3P5P, (D) 5 μg/mL abciximab, and (E) 0.5 μM ARMX + 10 μM A3P5P. Images were digitized, and 3-dimensional structures were inferred from their 2-dimensional structures. Fluorescence intensity is proportional to the platelet number; therefore, the height of the thrombus is proportional to the intensity of the image at any point within the thrombus.28
Three-dimensional representations of platelet deposition from whole blood onto vWf–collagen 1 after 60 seconds of perfusion at 3000 s−1.
Images were acquired in the presence of (A) saline (control), (B) 0.5 μM ARMX, (C) 10 μM A3P5P, (D) 5 μg/mL abciximab, and (E) 0.5 μM ARMX + 10 μM A3P5P. Images were digitized, and 3-dimensional structures were inferred from their 2-dimensional structures. Fluorescence intensity is proportional to the platelet number; therefore, the height of the thrombus is proportional to the intensity of the image at any point within the thrombus.28
Platelet deposition onto vWf–collagen 1 after 60 seconds of perfusion at 3000 s−1.
Heparinized whole blood was incubated at 37°C for 5 minutes with saline (control), or with each compound or combination tested, before perfusion over vWf–collagen 1 surface at 3000 s−1 for 60 seconds at 37°C. Mepacrine-labeled fluorescent platelets were videotaped in real time using an inverted epi-fluorescence microscope with an attached SIT video camera. Images were digitized to quantify mural thrombus formation. Values reported are mean ± SEM of the total number of platelets deposited in a 37 000 μm2area. *Significant difference from control;P < .05.
Platelet deposition onto vWf–collagen 1 after 60 seconds of perfusion at 3000 s−1.
Heparinized whole blood was incubated at 37°C for 5 minutes with saline (control), or with each compound or combination tested, before perfusion over vWf–collagen 1 surface at 3000 s−1 for 60 seconds at 37°C. Mepacrine-labeled fluorescent platelets were videotaped in real time using an inverted epi-fluorescence microscope with an attached SIT video camera. Images were digitized to quantify mural thrombus formation. Values reported are mean ± SEM of the total number of platelets deposited in a 37 000 μm2area. *Significant difference from control;P < .05.
Discussion
At high arterial shear rates (750 and 1500 s−1) in the cone-and-plate viscometer, both the P2Y12 ADP receptor antagonist ARMX and the P2Y1 antagonist A3P5P were effective at inhibiting shear-induced platelet aggregation in heparinized whole blood. At a shear stress associated with arterial stenosis (approximately 120 dynes/cm2, corresponding to shear rates of 3000 s−1 in whole blood and 10 000 s−1 in PRP), blockade of both ADP P2 receptors reduced shear-induced aggregation by 2-fold compared to single ADP receptor blockade. On the vWf–collagen surface at high wall shear rates (3000 s−1), platelet aggregation (subsequent to initial GPIbα-vWf–mediated adhesion) was inhibited only with blockade of the P2Y1 and P2Y12 ADP receptors. Increased inhibition in the presence of both inhibitors is consistent with findings by several groups6 18 using washed platelets. These studies conclude that parallel signaling through Giand Gq is essential for platelet aggregation to occur. Any contribution by P2X1 is minimal.
Using whole blood subjected to shear rates of 750 and 1500 s−1 in the viscometer, aggregation was inhibited in the presence of a low concentration of either P2 receptor antagonist. At the lower shear rates of 750 and 1500 s−1, ADP may be released from erythrocytes or other blood cells. One of the purposes of experiments using whole blood is to see the effects of all blood components concomitantly. At these shear rates, exogenous ADP and large vWf multimers in the plasma mediate shear-induced platelet aggregation.4 In whole blood at a shear rate of 3000 s−1 or in PRP at a shear rate of 10 000 s−1, shear-activated platelets secrete endogenous ADP from dense granules and unusually large vWf multimers from α-granules.4Blockade of both ADP P2-type receptors suppresses the potentiation of shear-aggregation by ADP, whether from erythrocytes or activated platelets.
ARMX was more effective than A3P5P at inhibiting direct, high shear-induced aggregation. ARMX is a pharmacologically designed compound targeted for the P2Y12 receptor, with chemical modifications that limit degradation by ectonucleases abundant in whole blood.10,11,22 In washed platelet systems, A3P5P totally inhibits ADP-induced platelet aggregation, shape change, and calcium movement.6,18 29 In contrast, in whole blood the lability of the pyrophosphate bonds in A3P5P may account for reduced effectiveness compared to ARMX.
ADP receptors P2Y12 and P2Y1 may be important in the stabilization of platelet–platelet interactions induced by shear stress. Platelet aggregates formed under high shear stress remained aggregated in untreated blood samples and in blood containing abciximab after exposure to low shear stress for 150 seconds. Platelets with P2Y12 ADP receptors blocked in blood samples subjected to high shear stress disaggregated during the extended application of low shear stress. This suggests that the inhibition of adenylyl cyclase may be especially important in stabilizing the formation of platelet aggregates in shear fields. These results are compatible with data from Storey et al,30 who showed that ARMX addition to hirudin-anticoagulated whole blood reduced the extent and the rate of aggregation in response to ADP.
Studies by Cattaneo et al31 showed that ADP stabilized platelet aggregates in thrombin-stimulated washed platelets. The mechanism was unknown at the time; however, it was postulated that another component, possibly from platelet granules, was required for stable platelet aggregation because ADP alone did not produce stabile aggregates.31 Our shear experiments suggested that shear-induced vWf binding to GPIbα is a possible pathway to aggregation potentiated through ADP signaling. We observed reduced aggregation formed under high shear stress with the blockade of platelet GPIIb-IIIa receptors by abciximab. However, the availability of the platelet P2Y12 receptor pathway maintained those platelet aggregates during extended application of low shear stress.
Our results also indicate that platelet ADP P2 receptors are involved in high shear-induced aggregation subsequent to the initial platelet adhesion onto vWf–collagen 1 under arteriallike flow conditions. This platelet aggregation and mural thrombus formation depend on the activation of platelet GPIIb-IIIa complexes and the presence of bridging ligands.19,32 Collagen stimulation is a platelet agonist that does not require the activation of G-protein receptors. Contrary to this, P2Y1-receptor null mice have reduced aggregation responses to low dosages of collagen, and thromboembolisms do not result from intravenous collagen injection.8
In this study, we found that the blockade of P2Y12 by ARMX or the blockade of P2Y1 with A3P5P caused little suppression of mural thrombosis. In contrast, the blockade of P2Y12 and P2Y1 receptors with low concentrations of ARMX and A3P5P in combination suppressed platelet–platelet cohesion (Figures 3, 4). The vWf–collagen 1 surface, in the presence of both agents, was covered with small platelet thrombi.
Our results suggest that under arteriallike flow conditions (in the absence of ADP receptor inhibition), the coactivation of G-proteins linked to both ADP P2 receptors promotes platelet–platelet cohesion after initial platelet adhesion onto vWf–collagen 1. Under these conditions, the synergistic effect of decreased platelet adenylyl cyclase activity and increased intraplatelet Ca+2 supports GPIIb-IIIa activation, P-selectin expression, ligand binding, and platelet aggregation.
Most data investigating the role of platelet ADP receptors in aggregation have been collected with washed platelets. Using these systems, the hydrolysis of pyrophosphate bonds in ADP by enzymes present in plasma and by ectonucleotidases on the surfaces of red cells can be avoided.10 33 The whole blood system described in this report has the advantages of maintaining physiological ion and protein concentrations and of avoiding centrifugation steps that can promote platelet activation and ADP release.
In whole blood, the simultaneous presence of both ADP P2 antagonists reduced high shear stress-induced direct platelet aggregation and the platelet aggregation that occurred subsequent to initial platelet adhesion onto vWf–collagen. These results suggest potential antithrombotic uses for ARMX combined with a P2Y1blocker.
We thank Drs Jonathon D. Turner and Robert Humphries of AstraZeneca for kindly providing the ARMX.
Supported by National Institutes of Health grants HL-18584 (J.L.M.), HL-54169 (J.L.M.), and HL-18670 (L.V.M.), and by Robert A. Welch Foundation grant c-938 (L.V.M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Larry V. McIntire, Department of Bioengineering, MS 142, Rice University, PO Box 1892, Houston, TX 77251; e-mail:mcintire@rice.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal