Abstract
Many mutant mice deficient in leukocyte adhesion molecules display altered hematopoiesis and neutrophilia. This study investigated whether peripheral blood neutrophil concentrations in these mice are elevated as a result of accumulation of neutrophils in the circulation or altered hematopoiesis mediated by a disrupted regulatory feedback loop. Chimeric mice were generated by transplanting various ratios of CD18+/+ and CD18−/− unfractionated bone marrow cells into lethally irradiated wild-type mice, resulting in approximately 0%, 10%, 50%, 90%, or 100% CD18 null neutrophils in the blood. The presence of only 10% CD18+/+ neutrophils was sufficient to prevent the severe neutrophilia seen in mice reconstituted with CD18−/− bone marrow cells. These data show that the neutrophilia in CD18−/− mice is not caused by enhanced neutrophil survival or the inability of neutrophils to leave the vascular compartment. In CD18−/−, CD18−/−E−/−, CD18−/−P−/−, EP−/−, and EPI−/− mice, levels of granulocyte colony-stimulating factor (G-CSF) and interleukin-17 (IL-17) were elevated in proportion to the neutrophilia seen in these mice, regardless of the underlying mutation. Antibiotic treatment or the propensity to develop skin lesions did not correlate with neutrophil counts. Blocking IL-17 or G-CSF function in vivo significantly reduced neutrophil counts in severely neutrophilic mice by approximately 50% (P < .05) or 70% (P < .01), respectively. These data show that peripheral blood neutrophil numbers are regulated by a feedback loop involving G-CSF and IL-17 and that this feedback loop is disrupted when neutrophils cannot migrate into peripheral tissues.
Introduction
Adhesion molecule–deficient mice have provided valuable information in elucidating leukocyte recruitment mechanisms. Many of the mice deficient in leukocyte adhesion molecules display secondary phenotypes, including altered hematopoiesis and neutrophilia, which have not been fully investigated. Mice lacking P-selectin, leukocyte function–associated antigen-1, intercellular adhesion molecule-1 (ICAM-1), core-2 glucosaminyltransferase, P- and L-selectin, P-selectin and ICAM-1, or L-selectin and ICAM-1 are mildly neutrophilic.1-7 Mice deficient in multiple leukocyte adhesion molecules, including CD18 integrins; E- and P-selectin; E- and P-selectin and ICAM-1; E-, P-, and L-selectin; E-, P-, and L-selectin and ICAM-1; CD18 and E-selectin; and CD18 and P-selectin show more severe neutrophilia.3,8-14 A few adhesion molecule–deficient mice, including mice lacking Mac-1, E-selectin, or both E- and L-selectin, have normal circulating neutrophil concentrations.2,3 15
Although the existence of physiologic mechanisms controlling peripheral neutrophil counts has been proposed as early as 1991,16the reason for elevated neutrophil counts in adhesion molecule knockout mice is not known. One candidate mechanism for neutrophilia is passive accumulation of circulating neutrophils because of altered neutrophil survival. Although mice lacking Mac-1 show defective apoptosis in transmigrated neutrophils17 and P-selectin–deficient mice show an increased neutrophil half-life,18 there is no evidence that these defects cause elevated neutrophil numbers. Candidate mediators have been implicated in the regulation of circulating neutrophil levels in adhesion molecule–deficient mice. Frenette et al9 showed elevated serum levels of granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-3 (IL-3) in mice lacking E- and P-selectin compared with wild-type and P-selectin–deficient mice. CD18 null mice had increased IL-3 and IL-6 serum levels compared with wild-type mice.8However, no causality has been established between the elevated cytokine levels and neutrophilia in these leukocyte adhesion molecule–deficient mice.
Neutrophil survival, proliferation, differentiation, and function are all regulated by granulocyte colony-stimulating factor (G-CSF).16,19 G-CSF is produced by monocytes, macrophages, endothelial cells, fibroblasts, mesothelial cells, and stromal cells in response to lipopolysaccharide, tumor necrosis factor-α (TNF-α), IL-1, GM-CSF, IL-3, IL-4, interferon-γ (IFN-γ), and IL-17.16,19,20 Previous data have shown that G-CSF regulates both basal and “emergency” hematopoiesis. Mice deficient in G-CSF display chronic neutropenia, with peripheral blood neutrophil levels at 20% to 30% of those in wild-type mice.21Canines treated with human G-CSF developed neutralizing antibodies to human G-CSF that cross-reacted with endogenous canine G-CSF. These dogs developed severe neutropenia, which suggests that G-CSF maintains basal levels of circulating neutrophils.22 G-CSF–deficient mice have an impaired ability to control infection with Listeria monocytogenes, indicating a role for G-CSF in inflammation- and infection-driven granulopoiesis,21 in which high levels of circulating neutrophils are required to combat stress conditions.
IL-17 has been reported to be expressed predominantly by activated CD4+ and CD8+ memory T lymphocytes,23 although its receptor (IL-17R) is ubiquitously expressed.24 In vitro, IL-17 stimulates the production of proinflammatory and hematopoietic cytokines. Recombinant hIL-17 induces the secretion of IL-6, IL-8, prostaglandin E2, and G-CSF from rheumatoid synovial fibroblasts, synoviocytes, endothelial cells, and epithelial cells.23IL-17 released from skin-infiltrating T lymphocytes stimulated the secretion of IL-6 and IL-8 from keratinocytes and also induced the expression of ICAM-1.23 Synovial fibroblasts cultured in the presence of hIL-17 sustained the proliferation of CD34+hematopoietic progenitors and their maturation into neutrophils.20 Cai et al25 showed that IL-17 increased steady-state G-CSF mRNA levels in the murine 3T3 fibroblast cell line. Schwarzenberger et al26 have recently shown that adenovirus-mediated delivery of mIL-17 cDNA to the liver resulted in drastically altered hematopoiesis and increased granulopoiesis in wild-type mice. These mice displayed leukocytosis, splenomegaly, increased cellularity of the spleen, and a rapid rise in serum G-CSF levels. These data indicate that IL-17 mediates granulopoiesis, at least in part through G-CSF stimulation.
Our experiments were designed to test the hypothesis that adhesion molecule–deficient mice with high neutrophil counts have defective neutrophil trafficking, which alters hematopoiesis, neutrophil half-life, and/or survival. We tested whether peripheral neutrophil concentrations in leukocyte adhesion molecule–deficient mice are elevated as a result of (1) accumulation of neutrophils in the circulation because of enhanced neutrophil survival and/or the inability of neutrophils to transmigrate out of the vessels; or (2) altered hematopoiesis mediated by a disrupted regulatory feedback loop, causing the bone marrow to increase neutrophil production. To investigate the mechanism(s) underlying the high neutrophil levels in leukocyte adhesion molecule–deficient mice, we generated chimeric mice reconstituted with varying ratios of CD18+/+ and CD18−/− bone marrow, resulting in corresponding ratios of CD18+/+ and CD18−/− neutrophils in the circulation, and analyzed the serum levels of candidate cytokines relevant to increased granulopoiesis. We measured IL-17 and G-CSF levels in 6 adhesion molecule–deficient mouse lines and used interventional studies to determine the regulatory impact of IL-17 and G-CSF on neutrophilia.
Materials and methods
Animals
Mice lacking E-selectin (E−/−),10CD18 (CD18−/−),8 E- and P-selectin (EP−/−),10 E- and P-selectin and ICAM-1 (EPI−/−),11 CD18 and E-selectin (CD18−/−E−/−),12 CD18 and P-selectin (CD18−/−P−/−),12and C57BL/6 wild-type mice (Hilltop Lab Animals, Scottdale, PA), were maintained at the University of Virginia Health Sciences Center vivarium under specific pathogen-free conditions in a barrier facility. All mice were back-crossed into the C57BL/6 background for at least 6 generations.
Generation of chimeric mice
Bone marrow was harvested from CD18−/− and wild-type mice and transplanted into irradiated wild-type mice, as described previously.12,27 Recipient mice were lethally irradiated in 2 doses of 600 rad each approximately 4 hours apart. Donor mice were killed by lethal injection of sodium pentobarbital (Nembutal; Abbott Laboratories, North Chicago, IL), and bone marrow cells were harvested by flushing both femurs and tibias with RPMI (Gibco, Grand Island, NY) (without phenol red) containing 10% fetal calf serum (FCS; Atlanta Biologicals, Norcross, GA) under sterile conditions. Suspended bone marrow cells were washed and erythrocytes were lysed in 0.15 M NH4Cl lysing solution. Approximately 2 million unfractionated bone marrow cells in 200 μL medium were delivered intravenously through the tail vein of each recipient mouse. Recipient mice underwent transplantation with either wild-type, CD18−/−, or a 1:10, 1:1, or 10:1 mixture of wild-type and CD18−/− unfractionated bone marrow cells. Recipient mice were housed in a barrier facility (individually ventilated cages with high-efficiency particulate air filter) under pathogen-free conditions before and after bone marrow transplantation. After bone marrow transplantation, the mice were maintained on autoclaved water with antibiotics (5 mM sulfamethoxazole, 0.86 mM trimethoprim) (Sigma Chemical, St Louis, MO) and fed autoclaved food. CD18 and E-selectin double mutant mice (CD18−/−E−/−) were generated by injecting lethally irradiated E−/− mice with 2 × 106 unfractionated CD18−/− bone marrow cells, as described.12 Mice were used for experiments after 4 weeks of bone marrow reconstitution.
Blood sampling and flow cytometry
Serum or plasma samples were obtained from peripheral blood collected by tail vein bleeding. Total leukocyte counts and leukocyte differentials were obtained from Kimura-stained blood samples. The percentages of CD18+/+ and CD18−/−neutrophils in the peripheral blood of chimeric mice were determined by direct immunofluorescence using a laser flow cytometer (FACScan; Becton Dickinson, San Jose, CA), as described previously.13 Whole blood was incubated with fluorescein isothiocyanate–labeled monoclonal antibody (mAb) Gr-1 (Pharmingen, San Diego, CA; 0.5 μg/106 cells) to identify granulocytes (Gr-1hi) and monocytes (Gr-1low), and phycoerythrin-labeled mAb C71/16 to label CD18 (Pharmingen; 0.5 μg/106 cells) or isotype control (R35-95, Pharmingen; 0.5 μg/106 cells). Samples were incubated for 30 minutes on ice. Unlabeled antibody was removed by aspiration after centrifugation. Peripheral blood was resuspended in 150 mM NH4Cl, 10 mM NaHCO3, 1 mM Na2 EDTA in deionized distilled water to lyse red blood cells. Neutrophils were identified and gated by expression of Gr-1 antigen. Data are presented as fluorescence histograms of CD18 expression of Gr-1–positive cells on a 4-decade log scale.
AdIL-17RFc
To inhibit IL-17–mediated signaling in vivo, the extracellular domain of the mouse IL-17R was amplified using the primers IL-17R A, 5′-TGGTACCCGGGCTATGGCGATTCGGC-3′; and IL-17R B-TCS, 5′-GGATCCACGCGGAACCAGCCACAGGGGAATGTAGTC-3′; and KlenTaq (Clontech, Palo Alto, CA). The IL-17R B primer incorporated amino acids encoding a thrombin-sensitive cleavage site that serves as a bridge between the IL-17R extracellular domain and immunoglobulin (Ig) G1, as described previously.28,29 A 1680-bp fusion product of IL-17R and mIgG1 CH2 and CH3 was obtained and cloned into PCR2.1 (Invitrogen, Carlsbad, CA), the sequence of the fusion was verified by dideoxynucleotide sequencing, and the product was subcloned into pACCMV PLA.30 To verify protein expression of this construct in vitro, we performed transient transfections with the resultant plasmid pCCMVIL17RFc in 293 cells using lipofectamine (Life Technologies, Gaithersburg, MD). Western blotting of transfected 293 cells revealed a 140-kd product on nonreducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis that reacted with both antimouse IgG1 or antimouse IL-17R (R&D Systems, Minneapolis, MN). IL-17RFc–containing media showed a significant, dose-dependent suppression of IL-6 production in a bioassay, as described.31
IL-17RFc was subcloned into pAd5MCSloxP (provided by Dr Blake Roessler, University of Michigan, Ann Arbor, MI), and a recombinant adenoviral genome was generated by in vitro recombination with Cre recombinase, as described.32 Adenovirus expressing IL-17 receptor-Fc construct (AdIL-17RFc) clones were further screened by polymerase chain reaction, and protein production was confirmed by Western blotting of cell supernatants using an antimouse IgG antibody (Bio-Rad, Hercules, CA). Viruses were propagated in 911 cells using endotoxin-free conditions and purified by CsCl. Virus preparations were screened for replication-competent adenovirus by propagation in A549 cells. This assay has a sensitivity of one contaminant per 108plaque-forming units (PFUs). All viral propagations had a PFU-to-particle ratio of less than 100:1. All lots of recombinant AdIL-17RFc contained less than 1 endotoxin unit/mL, as measured by the Limulus amebocyte lysate assay (BioWhittaker, Walkersville, MD).
mIL-17 and G-CSF assays
The biologic assay previously described by Fossiez et al20 and Schwarzenberger et al26 was used to validate mIL-17 expression in vivo. The concentration of mIL-17 was calculated from standard curves using log-log linear regression. One mIL-17 unit is defined as the amount that results in release of 1 pg/mL mIL-17–dependent mIL-6 secretion in this assay. Serum G-CSF protein concentrations were determined using a specific enzyme-linked immunoassay with antibody pairs purchased from R&D Systems. For this purpose, the wells of a 96-well plate (Nunc Immunoplate Maxisorb, Neptune, NJ) were coated with 2 μg/mL capture antibody and incubated overnight at 4°C. The plates were then washed 5 times with wash buffer (0.05% Tween 20 in phosphate-buffered saline) and blocked with 200 μL 2% bovine serum albumin in wash buffer for 2 hours at room temperature. G-CSF standards and samples were diluted in wash buffer containing 2% FCS. Both standards (31.25 to 1000 pg/mL) and samples (50 μL) were added to wells, and the plates were incubated for 1 hour at 37°C. After washing, 50 μL biotinylated anti–G-CSF (0.1 μg/mL in dilution buffer) was added, and the plates were incubated for 1 hour at 37°C. Wells were then washed and incubated for 1 hour after adding 100 μL of 0.1 μg/mL peroxidase-conjugated streptavidin (Jackson Laboratories, West Grove, PA) in dilution buffer. After washing the plate, we added 100 μL tetramethylbenzidine (TMB) (Sigma) as substrate and allowed color to develop for 30 minutes in the dark. After the reaction was stopped with 50 μL of 3 M H2SO4, the plates were read at 450 nm. G-CSF concentrations were calculated from the standard curve using log-log linear regression.
Neutralization of IL-17 and G-CSF function in vivo
To block IL-17 function, we performed adenovirus-mediated cytokine delivery of the cDNA encoding for soluble murine IL-17 receptor (AdIL-17R), delivered intravenously (5 × 109PFU/mouse). Expression of smIL-17R serves as a decoy, binding IL-17 and preventing it from reaching its cellular receptor. Control mice were injected with adenovirus encoding enhanced green fluorescent protein (AdEGFP) intravenously (5 × 109PFU/mouse). Blood samples were collected for systemic leukocyte counts and serum samples on days 0, 3, 5, and 10. For in vivo G-CSF neutralization studies, mice were injected with 1 mg anti–G-CSF antibody or 1 mg preimmune (IgG) serum. Blood counts and serum samples were collected on days 0, 1, 6, and 10.
Statistical analysis
Systemic leukocyte counts, serum G-CSF levels, and plasma IL-17 levels were compared using one-way analysis of variance and the Tukey or Dunn method multiple-comparison test by SigmaStat 2.03 (SPSS, Chicago, IL).
Results
Distribution of CD18+/+ and CD18−/−neutrophils in chimeric mice
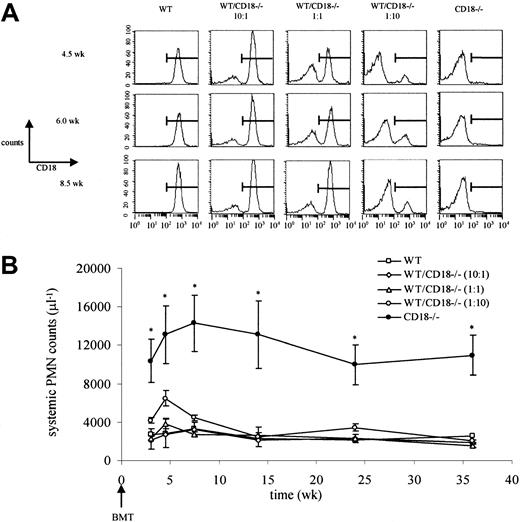
To investigate the mechanism(s) underlying the high neutrophil levels in leukocyte adhesion molecule–deficient mice, we first generated chimeric mice reconstituted with varying ratios of CD18+/+ and CD18−/− bone marrow. This resulted in ratios of CD18+/+ and CD18−/−neutrophils in the circulation that corresponded to the ratios at which bone marrow cells were transplanted (wild-type/CD18−/− at 10:1, 1:1, and 1:10) (Figure 1A). We hypothesized that neutrophils derived from CD18−/− bone marrow cells would show high counts if the neutrophilia was accumulation dependent because these cells, but not the CD18+/+ neutrophils, would remain in the circulation. Remarkably, despite the presence of CD18−/−neutrophils in the circulation (approximately 10%, 50%, or 90% of systemic neutrophils in 10:1, 1:1, or 1:10 reconstituted mice, respectively; Figure 1A), neutrophil counts were not significantly different from those in mice reconstituted with 100% CD18+/+ bone marrow cells (Figure 1B). The presence of only 10% CD18+/+ neutrophils was sufficient to prevent the severe neutrophilia seen in mice reconstituted with CD18−/− bone marrow cells (Figure 1B) or in CD18−/− mice.8 These data indicate that the neutrophilia is not driven by the accumulation of neutrophils in the circulation and suggest that the high neutrophil levels result from altered hematopoiesis that may be due to defective neutrophil trafficking.
Neutrophilia in CD18−/− mice is not caused by neutrophil accumulation in the systemic circulation.
(A) Lethally irradiated wild-type (WT) mice underwent transplantation with wild-type, CD18−/−, or a 10:1, 1:1, or 1:10 mixture of bone marrow. Relative amounts of CD18+/+ and CD18−/− neutrophils in the systemic microcirculation were followed by flow cytometry. The percentages of CD18−/−neutrophils in the circulation at 4.5, 6.0, or 8.5 weeks corresponded to the relative concentrations of CD18−/− bone marrow that was transplanted. (B) Severe neutrophilia in CD18−/−mice was restored to normal by CD18+/+ neutrophils. *Significantly different from all other groups (P < .05). Data are expressed as mean ± SEM (n = 3). BMT indicates bone marrow transplantation; PMN, polymorphonuclear neutrophils.
Neutrophilia in CD18−/− mice is not caused by neutrophil accumulation in the systemic circulation.
(A) Lethally irradiated wild-type (WT) mice underwent transplantation with wild-type, CD18−/−, or a 10:1, 1:1, or 1:10 mixture of bone marrow. Relative amounts of CD18+/+ and CD18−/− neutrophils in the systemic microcirculation were followed by flow cytometry. The percentages of CD18−/−neutrophils in the circulation at 4.5, 6.0, or 8.5 weeks corresponded to the relative concentrations of CD18−/− bone marrow that was transplanted. (B) Severe neutrophilia in CD18−/−mice was restored to normal by CD18+/+ neutrophils. *Significantly different from all other groups (P < .05). Data are expressed as mean ± SEM (n = 3). BMT indicates bone marrow transplantation; PMN, polymorphonuclear neutrophils.
Increased G-CSF and IL-17 serum levels in adhesion molecule–deficient mice
We next tested whether neutrophilia in adhesion molecule–deficient mice may be caused by a common mechanism that increases hematopoiesis. On the basis of previous data showing the necessity of G-CSF for granulopoiesis21 and the induction of granulopoiesis in vivo through IL-17 administration,26we investigated the role of these prohematopoietic cytokines in CD18−/− mice. Serum G-CSF and plasma IL-17 levels were significantly elevated (P < .05) in CD18−/−mice (514 ± 109 pg/mL and 159 ± 28 pg/mL, respectively) compared with wild-type mice (12 ± 2 pg/mL and 20 ± 6 pg/mL, respectively) (Figure 2A,D). Therefore, G-CSF and IL-17 levels were examined in mice lacking single or multiple leukocyte adhesion molecules, including E-selectin (E−/−), E- and P-selectin (EP−/−), E- and P-selectin and ICAM-1 (EPI−/−), CD18 integrins (CD18−/−), CD18 and E-selectin (CD18−/−E−/−), and CD18 and P-selectin (CD18−/−P−/−).
G-CSF and IL-17 levels are elevated in leukocyte adhesion molecule–deficient mice.
Severely neutrophilic CD18−/− mice showed significantly elevated levels of (A) G-CSF and (D) IL-17 compared with wild-type mice (P < .05). G-CSF and IL-17 levels in E−/−(open circles), EP−/− (closed squares), EPI−/− (open squares), CD18−/− (open triangles), CD18−/−E−/− (closed triangles), and CD18−/−P−/− (open diamonds) mice were elevated in proportion to the level of neutrophilia in these mice (B,E). C57BL/6 wild-type mice are indicated by closed circles. Data are expressed as mean ± SEM (n = 4-14). Elevated (C) G-CSF and (F) IL-17 levels in individual leukocyte adhesion molecule–deficient mice correlated with circulating neutrophil levels (r = 0.73 and r = 0.62, respectively). The correlation between G-CSF or IL-17 levels and neutrophil counts regardless of genotype suggests a common mechanism of altered hematopoiesis resulting from impaired leukocyte trafficking.
G-CSF and IL-17 levels are elevated in leukocyte adhesion molecule–deficient mice.
Severely neutrophilic CD18−/− mice showed significantly elevated levels of (A) G-CSF and (D) IL-17 compared with wild-type mice (P < .05). G-CSF and IL-17 levels in E−/−(open circles), EP−/− (closed squares), EPI−/− (open squares), CD18−/− (open triangles), CD18−/−E−/− (closed triangles), and CD18−/−P−/− (open diamonds) mice were elevated in proportion to the level of neutrophilia in these mice (B,E). C57BL/6 wild-type mice are indicated by closed circles. Data are expressed as mean ± SEM (n = 4-14). Elevated (C) G-CSF and (F) IL-17 levels in individual leukocyte adhesion molecule–deficient mice correlated with circulating neutrophil levels (r = 0.73 and r = 0.62, respectively). The correlation between G-CSF or IL-17 levels and neutrophil counts regardless of genotype suggests a common mechanism of altered hematopoiesis resulting from impaired leukocyte trafficking.
E−/− single mutant mice showed a small increase in serum G-CSF levels (33 ± 4 pg/mL) compared with wild-type mice (12 ± 2 pg/mL) (Figure 2B). Serum G-CSF levels were significantly elevated in EP−/− (345 ± 51 pg/mL), EPI−/−(161 ± 42 pg/mL), CD18−/− (514 ± 109 pg/mL), CD18−/−E−/− (302 ± 83 pg/mL), and CD18−/−P−/− mice (208 ± 28 pg/mL), corresponding to the levels of neutrophilia in these mice (Figure 2B). In individual mice, serum G-CSF levels correlated with circulating neutrophil levels regardless of the underlying mutation (Figure 2C). IL-17 plasma levels were also significantly elevated in EP−/− (33 ± 11 pg/mL), EPI−/−(111 ± 21 pg/mL), CD18−/− (159 ± 28 pg/mL), CD18−/−E−/− (76 ± 16 pg/mL), and CD18−/−P−/− mice (219 ± 27 pg/mL) (Figure 2E). Plasma IL-17 levels correlated with circulating neutrophil levels in individual leukocyte adhesion molecule–deficient mice (Figure 2F).
Neutralization of IL-17 or G-CSF in vivo
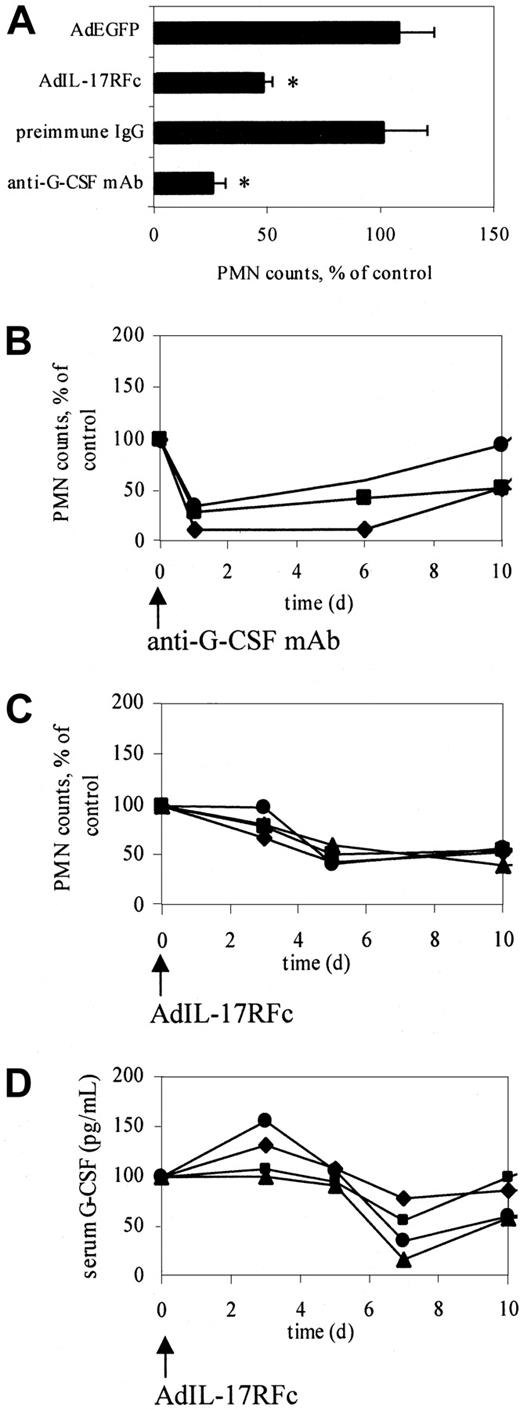
On the basis of these data, we hypothesized that IL-17 may increase neutrophil counts in adhesion molecule–deficient mice by stimulating G-CSF release. To directly test this hypothesis, we blocked IL-17 function in CD18−/−E−/− mice, which have high neutrophil counts and high levels of IL-17 (Figure 2). CD18−/−E−/− mice were injected intravenously with 5 × 109 PFU AdIL-17R (IL-17 receptor) or a control virus (AdEGFP). Neutrophil counts showed a peak reduction (approximately 50%, P < .05) 5 days after AdIL-17R administration (Figure 3A), but not after AdEGFP administration (Figure 3A). Neutrophil counts in individual mice administered AdIL-17R began to decrease after day 3 and remained significantly reduced through day 10 (Figure 3C). The delayed reduction in neutrophil counts with AdIL-17R treatment is probably caused by delayed protein expression in this adenoviral system. Blocking IL-17 function reduced the serum G-CSF levels in CD18−/−E−/− mice by 53%. G-CSF levels began to decrease after day 3 and reached a minimum on day 7 (Figure 3D).
Neutralization of G-CSF or IL-17 significantly decreases circulating neutrophil counts in CD18−/−E−/− mice.
(A) Peak response of neutralizing IL-17 or G-CSF function. Neutralizing IL-17 function using AdIL-17R decreased neutrophil counts in CD18−/−E−/− mice (n = 4) by approximately 50% on day 5, showing that IL-17 participates in regulating granulopoiesis. Anti–G-CSF antibody reduced neutrophil counts in CD18−/−E−/− mice (n = 3) by approximately 70% on day 1, indicating that G-CSF is the major regulator of neutrophils. *Significantly different from control group (P < .05). Data are expressed as mean ± SEM. (B) Time course of normalized neutrophil counts in individual mice treated with anti–G-CSF antibody. (C) Time course of normalized neutrophil counts in individual mice treated with AdIL-17RFc. (D) Time course of serum G-CSF levels in individual mice treated with AdIL-17RFc. Neutrophil counts and serum G-CSF levels were normalized to day 0 (100%). Different symbols represent difference mice.
Neutralization of G-CSF or IL-17 significantly decreases circulating neutrophil counts in CD18−/−E−/− mice.
(A) Peak response of neutralizing IL-17 or G-CSF function. Neutralizing IL-17 function using AdIL-17R decreased neutrophil counts in CD18−/−E−/− mice (n = 4) by approximately 50% on day 5, showing that IL-17 participates in regulating granulopoiesis. Anti–G-CSF antibody reduced neutrophil counts in CD18−/−E−/− mice (n = 3) by approximately 70% on day 1, indicating that G-CSF is the major regulator of neutrophils. *Significantly different from control group (P < .05). Data are expressed as mean ± SEM. (B) Time course of normalized neutrophil counts in individual mice treated with anti–G-CSF antibody. (C) Time course of normalized neutrophil counts in individual mice treated with AdIL-17RFc. (D) Time course of serum G-CSF levels in individual mice treated with AdIL-17RFc. Neutrophil counts and serum G-CSF levels were normalized to day 0 (100%). Different symbols represent difference mice.
Similarly, CD18−/−E−/− mice injected with a neutralizing anti–G-CSF antibody (1 mg/mouse), but not a control antibody, showed a maximum reduction (approximately 70%,P < .01) in neutrophil counts on day 1 (Figure3A) because of the immediate effects of circulating antibody. Neutrophil counts in individual mice treated with an anti–G-CSF antibody remained significantly reduced through day 6 (Figure 3B). Despite the reduction in neutrophil counts in mice treated with anti–G-CSF, plasma IL-17 levels remained elevated in these mice (112.3 ± 47.9 pg/mL before and 167.5 ± 53.8 pg/mL 3 days after anti–G-CSF administration). This suggests that G-CSF is downstream from IL-17 in a regulatory loop controlling neutrophil counts in adhesion molecule–deficient mice.
Discussion
Previous data17,18 have suggested that neutrophilia in adhesion molecule–deficient mice possibly results from passive accumulation of neutrophils in the systemic circulation. This theory is refuted by normal neutrophil counts in chimeric mice reconstituted with mixtures of CD18−/− and CD18+/+ bone marrow, showing that only 10% CD18+/+ neutrophils in the systemic circulation are sufficient to maintain neutrophil homeostasis. These data are consistent with a recent study showing that lethally irradiated wild-type mice reconstituted with a 1:1 mixture of wild-type and CD18−/− fetal liver cells did not develop neutrophilia as seen in mice reconstituted with CD18−/−fetal liver cells alone.14 This study also showed that neither the circulating life span nor the bone marrow transit time of CD18−/− neutrophils was increased compared with wild-type neutrophils,14 further supporting the conclusion that the neutrophilia is not the result of passive accumulation of these cells in the vascular compartment.
Instead, neutrophilia in leukocyte adhesion molecule–deficient mice appears to result from increased hematopoiesis through an altered regulatory feedback loop. CD18-null mice showed a 20-fold increase in IL-3 and IL-6 levels compared with wild-type mice.8Similarly, EP−/− mice had 40-fold increased IL-3 levels and 5-fold increased GM-CSF levels compared with wild-type mice.9 IL-3 and GM-CSF stimulate granulopoiesis, but no direct causality or correlation with circulating neutrophil levels was shown. In the present study, we not only demonstrate a significant correlation between G-CSF or IL-17 and neutrophil counts, but also show that blocking these cytokines normalizes neutrophilia.
G-CSF is a potent stimulator of granulopoiesis16; however, G-CSF has been investigated so far in only one adhesion molecule–deficient mouse, the CD18−/−E−/−mouse.12 Here, we show a significant correlation between neutrophil levels and serum G-CSF levels in 6 different mutants and wild-type mice. Within each genotype, G-CSF levels significantly correlated with neutrophil counts. For example, CD18−/−mice with relatively low neutrophil counts have almost normal G-CSF levels (Figure 2A),12 whereas higher neutrophil counts are associated with high levels of G-CSF. Anti–G-CSF antibody drastically reduced neutrophil levels (approximately 70%) in severely neutrophilic CD18−/−E−/− mice, indicating that G-CSF is a key mediator of neutrophil proliferation in these mice. This is consistent with findings in G-CSF−/− and G-CSFR−/− mice, which have severely reduced neutrophil counts.21 33
Many cytokines have been shown to stimulate granulopoiesis, including GM-CSF, stem cell factor, IL-3, IL-6, IL-11, and flt3/flk2 ligand, most with efficiencies less than that of G-CSF.34-44 These cytokines act synergistically with G-CSF to stimulate maximum granulopoiesis.34-40 45-47 It is possible that G-CSF–independent granulopoiesis pathways are up-regulated in leukocyte adhesion molecule–deficient mice to stimulate granulopoiesis in the absence of G-CSF, which is suggested by the incomplete effect of blocking G-CSF.
IL-17 levels significantly correlated with neutrophil levels in all genotypes. Blocking IL-17 function reduced neutrophil numbers by approximately 50% in CD18−/−E−/− mice and was associated with reduced G-CSF. Our data are the first to show IL-17 as a mediator of neutrophilia in leukocyte adhesion molecule–deficient mice. Reduced G-CSF levels after blocking IL-17 but unaltered IL-17 levels after blocking G-CSF show that IL-17 acts upstream of G-CSF. Numerous cytokines, including IL-1, GM-CSF, IL-3, IFN-γ, IL-4, and TNF-α, can stimulate G-CSF release and possibly regulate granulopoiesis independently of IL-17.19,20 48
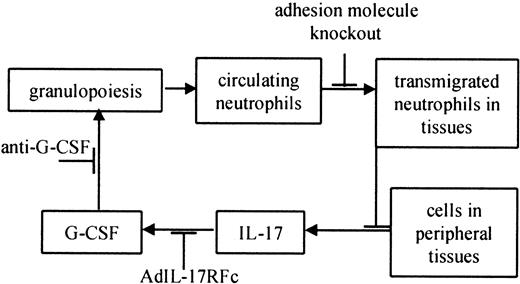
The loss of important leukocyte adhesion molecules critically impairs the host defense. It is possible that neutrophilia partially results from enhanced (or “emergency”) hematopoiesis in response to subclinical normal flora infections. Uncleared subclinical infections might constantly signal for more neutrophils. Both CD18−/− and EP−/− mice show severe phenotypes, including ulcerative dermatitis that begins at approximately 3 to 5 months of age.8,10 Although we cannot formally rule out a role for subclinical infection, 3 lines of evidence suggest that neutrophilia in these mice is not driven by infection. First, EPI−/− mice also have severely elevated neutrophil levels, but these mice do not develop skin lesions and have normal life spans. Neutrophil levels are also 10- to 20-fold elevated in EP−/− mice without obvious signs of infection10 (S.B.F., unpublished observations, May 2000). Second, Frenette et al9 showed that neonatal EP−/− mice (younger than 18 hours) had significantly elevated neutrophil levels compared with wild-type mice, presumably before infections could cause neutrophilia. Third, maintaining EP−/− mice on antibiotic water does not eliminate neutrophilia (S.B.F., unpublished observations, May 2000). Finally, bacterial and fungal cultures of EP−/− and CD18−/−E−/− mice have been repeatedly negative in the lungs, blood, spleen, liver, and lymph nodes.9,10,12 On the basis of our data and these findings, we propose that defective neutrophil trafficking in leukocyte adhesion molecule–deficient mice impairs a regulatory feedback loop maintaining neutrophil homeostasis (Figure 4). It appears that decreased neutrophil levels in peripheral tissues result in increased production of hematopoietic cytokines, including IL-17, by unidentified cells in these tissues. IL-17 and possibly other cytokines stimulate G-CSF to increase neutrophil production and maturation (Figure 4).49 Our data suggest that hematopoietic cytokine production is regulated by transmigrated neutrophils. The precise mechanism and site of this regulatory step remain to be determined.
Feedback loop controlling normal neutrophil homeostasis in leukocyte adhesion molecule–deficient mice.
Increased granulopoiesis increases circulating neutrophils. Some of these neutrophils migrate into peripheral tissues, including the skin, gut, and mucous membranes.49 A defect in neutrophil trafficking breaks the feedback loop, which is restored by adding transmigration-competent neutrophils (this study). Cells in the tissues produce IL-17,23 which causes the release of G-CSF,23 which in turn increases granulopoiesis.16 19 Other stimuli are likely to influence neutrophil homeostasis at each step of the feedback loop (not shown).
Feedback loop controlling normal neutrophil homeostasis in leukocyte adhesion molecule–deficient mice.
Increased granulopoiesis increases circulating neutrophils. Some of these neutrophils migrate into peripheral tissues, including the skin, gut, and mucous membranes.49 A defect in neutrophil trafficking breaks the feedback loop, which is restored by adding transmigration-competent neutrophils (this study). Cells in the tissues produce IL-17,23 which causes the release of G-CSF,23 which in turn increases granulopoiesis.16 19 Other stimuli are likely to influence neutrophil homeostasis at each step of the feedback loop (not shown).
Our findings establish that restoring physiologic neutrophil functions, such as trafficking, in adhesion molecule–deficient mice prevents the development of neutrophilia. Disrupting the feedback loop at 3 different checkpoints shows that the transmigration–IL-17–G-CSF axis appears to be the major regulating mechanism of neutrophil homeostasis in leukocyte adhesion molecule–deficient mice in vivo. Additional modifiers and parallel pathways are likely to participate in regulating neutrophil numbers.
We thank Drs A. L. Beaudet (Baylor College of Medicine, Houston, TX) and D. C. Bullard (University of Alabama at Birmingham) for E−/−, EP−/−, EPI−/−, and CD18−/− mice. We thank Dr Blake Roessler for assistance with the in vitro adenoviral recombination of the AdIL-17R vector. We also thank Mike Kavanagh for technical assistance.
Supported by National Institutes of Health grants R01-HL54136 to K.L.; NRSA-HL10447 to S.B.F.; R01-CA81125-01 to P.S.S.; R29-AA10384, R01-HL61271, and R01-HL62052 to J.K.K.; and AA09803 to G.J.B.; and by Leukemia Society of America Translational Research Award 6191 to P.O.S.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Klaus Ley, Department of Biomedical Engineering, University of Virginia, Health Sciences Center Box 800759, Charlottesville, VA 22908; e-mail: klausley@virginia.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal