Abstract
The migration capability of dendritic cells (DCs) is regulated by their response to factors, namely chemokines, that characterize maturation stage and shape their functional activities. This study examines the morphology, expression of chemokines/chemokine receptors, and migration properties of DCs generated after treatment of monocytes with type I interferon (IFN) and granulocyte-macrophage colony-stimulating factor (GM-CSF) (IFN-DCs). IFN-DCs showed phenotypical and morphologic features undetectable in DCs generated in the presence of interleukin 4 (IL-4) and GM-CSF, such as expression of CD83 and CD25 and the presence of CD44+, highly polarized, thin, and long dendrites. IFN-DCs markedly migrated in response to β-chemokines (especially MIP-1β) and expressed the Th-1 chemokine IP-10. Notably, IFN-DCs showed an up-regulation of CCR7 as well as of its natural ligand MIP-3β, characteristics typical of mature DCs. Of interest, IFN-DCs exhibited a marked chemotactic response to MIP-3β in vitro and strong migratory behavior in severe combined immunodeficient (SCID) mice. In SCID mice reconstituted with human peripheral blood leukocytes, IFN-DCs induced a potent primary human antibody response and IFN-γ production, indicative of a Th-1 immune response. These results define the highly specialized maturation state of IFN-DCs and point out the existence of a “natural alliance” between type I IFN and monocyte/DC development, instrumental for ensuring an efficient connection between innate and adaptive immunity.
Introduction
Dendritic cells (DCs) are the most potent antigen-presenting cells playing a pivotal role in the induction of the immune response.1-3 DCs are located in peripheral tissues, in sites where they can optimally survey for incoming pathogens. The interaction of DCs with pathogens leads to migration to secondary lymphoid organs where they initiate a specific immune response. Notably, the migration capability of DCs is strictly regulated by their response to soluble factors, namely chemokines4 5 that characterize maturation stage and shape functional activities of DCs.
Chemokines represent a family of 8- to 10-kDa secreted proteins capable of regulating migration and activation not only of leukocytes, including DCs, but also of stromal cells.6,7 It is well documented that migration of DCs is tightly regulated as a function of maturation.8-12 In particular, immature DCs respond to many CC and CXC chemokines, such as MIP-1α, MIP-1β, RANTES, and MIP-3α, whereas mature DCs have lost their responsiveness to most of these chemokines, as a result of down-regulation of receptor expression or activity. However, mature DCs have been reported to respond to MIP-3β/ELC and 6Ckine/SLC as a consequence of an up-regulation of their receptor (CCR7). Of interest, studies in knock-out mice for CCR7 have shown the crucial importance of the CCR7/MIP-3β interaction for the generation of a primary immune response.13 All this emphasizes the essential role of certain chemokines/chemokine receptors and DC migration properties in the generation of the immune response.
DCs are derived from hematopoietic progenitor cells,2,3and distinct subtypes of human circulating DCs have been detected in the blood.14 15 However, the mechanisms regulating generation, functions, and survival of blood-circulating DCs in response to infections are largely unknown. The rapid generation of active DCs endowed with potent migratory capabilities would be advantageous for a prompt immune response to incoming pathogens.
Blood monocytes are highly versatile cells playing crucial roles in the maintenance of immune homeostasis. These cells circulate in the bloodstream, transmigrate through vascular endothelium, and localize in peripheral and mucosal tissues, where they differentiate into different cell types.16,17 Monocyte-derived mature DCs are currently generated in vitro by 2 sequential treatments,18 leading first to the so-called “immature DCs,” after exposure for several days to both granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin 4 (IL-4), and then to mature DCs, after a subsequent addition of stimuli such as lipopolysaccharide (LPS), CD40L, or virus infection. However, the in vivo relevance of monocyte differentiation into DCs remains unclear, especially because exposure of monocytes to IL-4 can hardly mimic the cytokine milieu likely to be present under in vivo conditions at the infection site.
Although results indicate that DC maturation can occur directly from monocytes during transendothelial migration,19 the natural factors potentially involved in physiologic events of DC maturation from monocytes have remained largely unknown.
Type I interferons (IFNs) are cytokines expressed at basal levels under physiologic conditions, whose production is highly enhanced during infections.20 Data have shown that type I IFNs act as important signals not only for the regulation of innate immunity21 but also for the induction of a potentially protective T-cell response in both mouse models22-25 and humans.26-28 A new interest in the role of type I IFNs as a possible bridge system linking innate and adaptive immunity has stemmed from the identification of the so-called “natural IFN-producing cells” (a rare cell population in the blood capable of producing 200 to 1000 times more IFN than other blood cells after microbial challenge) as CD4+CD11c− type 2 DC precursors (pDC2s),29 also defined as plasmacytoid DCs (PDCs).30 Recently, it has been shown that PDCs can drive a potent Th-1 polarization after activation with influenza virus and CD40L by mechanisms mediated by endogenous type I IFN and IL-12,31 thus challenging the view of PDCs as Th-2–polarizing DC precursors.29
Data from some laboratories have shown that type I IFN can act as an important signal for differentiation and maturation of DCs.32-34 In particular, we have reported that type I IFN promotes a rapid differentiation of GM-CSF–treated human monocytes into DCs endowed with potent functional activities both in vitro and in vivo.34
In this study, we describe the typical features of the DCs generated after a short-term exposure of GM-CSF–treated human monocytes to type I IFN (cells named as IFN-DCs) as compared with DCs generated in the presence of IL-4 (IL-4-DCs), with a special attention to the expression of chemokines/chemokine receptors and migration capability in response to chemotactic factors and in severe combined immunodeficient (SCID) mice.
We found that IFN-DCs exhibit a wide spectrum of features with characteristics typical of mature DCs and endowed with a strong migratory response to specific chemokines as well as with potent functional activities in vivo. These highly active DCs, obtained after a single-step cytokine treatment of freshly isolated monocytes, may represent the natural key crucial player in the generation of a prompt immune response to infections.
Materials and methods
Cell separation and culture
Peripheral blood mononuclear cells were obtained from heparinized blood of healthy donors by Ficoll density gradient centrifugation (Seromed, Berlin, Germany). Monocytes were obtained by standard Percoll density gradient centrifugation. Monocytes were further enriched by depleting contaminating cells by using negative immunoselection by microbeads conjugated to a monoclonal anti-hapten antibody directed to a cocktail of hapten-conjugated CD3, CD7, CD19, CD45RA, and CD56 antibodies (MACS Cell Isolation Kits; Miltenyi Biotec, Germany). After this procedure, the resulting cell population was represented by more than 98% CD14+monocytes, as assessed by flow cytometry. Blood-derived monocytes were plated at the concentration of 1 to 2 × 106 cells/mL in RPMI 1640 (Gibco BRL, Gaithersburg, MD) supplemented with 10% LPS-free fetal calf serum. Cultures were added with GM-CSF (500 U/mL) and either IL-4 (25 ng/mL; specific activity, 1 × 107 IU/mg) (R&D Systems, Minneapolis, MN) or consensus IFN-α, a synthetic type I IFN35 kindly provided by Amgen (USA) at the concentration of 1000 U/mL. After 3 days of culture, nonadherent and loosely adherent cells were collected and used for subsequent analysis. At day 3, the recovery of DCs with respect to the total number of cultured monocytes was approximately 50% to 60%. In all the experiments, cell viability was 96% or greater as evaluated by the trypan blue exclusion method. All reagents were tested for the absence of detectable levels of LPS by Limulus Amaebocyte lysate assay (Bio-Whittaker).
Immunophenotypic analysis
Cells were washed and resuspended in phosphate-buffered saline containing 1% human serum and incubated with a series of fluorochrome-conjugated monoclonal antibodies (mAbs) to human antigens for 30 minutes at 4°C. The following mAbs were used for immunofluorescent staining: anti-CD14, -CD80, -CD23, -CD25, (Becton Dickinson, San Jose, CA), -CD83, -CD86, -CD123, -CCR5, and -CXCR4 (Pharmingen, San Diego, CA). Cells were analyzed by flow cytometry. Data were collected and analyzed by using a FACSort (Becton Dickinson) flow cytometer. Data analysis was performed by CellQuest software (Becton Dickinson). Cells were electronically gated according to light scatter properties to exclude cell debris and contaminating lymphocytes.
Immunocytochemistry
IFN-DCs and IL-4-DCs were spun onto glass slides (Shandon, Cheshire, United Kingdom) at the concentration of 104cells/mL, fixed with methanol (70%) for 10 minutes at 4°C, and stained by antibodies to CD44 (DAKO, Denmark), using the peroxidase-antiperoxidase (DAKO) method. Cells were counterstained with Mayer haematoxilyn.36
Scanning electron microscopy
IFN-DCs and IL4-DCs were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) at room temperature for 20 minutes. Following fixation in 1% OsO4 for 30 minutes, cells were dehydrated through graded ethanols, critical point dried in CO2, and gold coated by sputtering. The samples were examined with a Cambridge 360 scanning electron microscopy.
Chemotaxis assay
Cell migration was performed in 24-well Transwell cell culture chamber (Costar, Corning, NY) as described previously.37 38 Briefly, 5 × 105 cells cultured in complete medium with either IFN/GM-CSF or IL-4/GM-CSF for 3 days were loaded in the upper chamber compartment. RANTES, MIP1α, MIP1β (500 ng/mL) (R&D System), MIP3α, and MIP3β (100 ng/mL) (Peprotech, Rocky Hill, NJ) were diluted in serum-free medium and added to the lower compartment. After 2 hours of incubation at 37°C, the cells that migrated through the 8 μm-pore size polycarbonate filters in the lower compartment were collected and counted. Each assay was performed in triplicate. The lower compartment of control chambers contained medium alone. In some experiments, the DCs collected from the lower compartment were stained with an anti-CD83 mAb and analyzed by flow cytometry.
Reverse transcriptase–polymerase chain reaction
The messenger RNA (mRNA) from DCs was extracted by RNAzol B and processed as previously described.34 Reverse transcriptase–polymerase chain reaction (RT-PCR) analysis was used to amplify CCR6 (5′-GGAGAAGCCTGAGGACTTGTA, 3′-ATTTCAGCGATGTTTTCGACT), CCR7 (5′-TCCTTCTCATCAGCAAGCTGTC, 3′-GAGGCAGCCCAGGTCCTTGAAG), MIP3β (5′-CACCCTCCATGGCCCTGCTACT, 3′-TAACTGCTGCGGCGCTTCATCT), DC-CK1 (5′-ACAAAGAGCTCTGCTGCCTC, 3′-CCCACTTCTTATTGGGGTCA), TARC (5′-CCTCCTCCTGGGGGCTTCTCTG, 3′-GACTTTAATCTGGGCCCTTTGTGC), IP-10 (5′-TGATTTGCTGCCTTATCTTTCTGA, 3′-CAGCCTCTGTGTGGTCCATCCTTG), MDC (5′-CAGCCTGACAAATCACAGTG, 3′-CTGGATGACACTGAGCTGG), and IL-15 (5′-CTCGTCTAGAGCCAACTGGGTGAATGTAAT-AAG, 3′-TACTTACTCGAGGAATCAATTGCAATCAAGAAGTG).
Complementary DNA was amplified for 25 to 30 cycles by using the following conditions: 94°C for 40 seconds, 62°C for 40 seconds, and 72°C for 40 seconds. To amplify MIP3β mRNA, the annealing temperature was 58°C. β-Actin primers were used to normalize the levels of human RNA in all the samples.
In vivo studies in SCID mice
CB17 SCID/SCID female mice (Harlan, Nossan, Italy) were used at 4 weeks of age and kept under specific pathogen-free conditions. SCID mice were housed in microisolator cages, and all food, water, and bedding were autoclaved prior to use.
In vivo migration of DCs after injection into SCID mice.
Migration of DCs after injection into SCID mice was evaluated as follows. Briefly, 2 × 106 DCs were injected intravenously into SCID mice. After 4 hours, mice were killed, and skin and spleen were collected. DNA was extracted, and the presence of human sequences was determined by DNA-PCR by using specific primers for the HLA-DQα, as described elsewhere.39
Detection of human IFN-γ and antibodies to the HIV-1 gp120/160 and p24 proteins in hu-PBL-SCID mice immunized with DCs.
Hu-PBLs were obtained from the peripheral blood of healthy donors. All donors were screened for HIV-1 and hepatitis prior to donation. The hu-PBLs were obtained by Ficoll-Paque density gradient centrifugation. Cells (20 × 106) were resuspended in 0.5 mL RPMI 1640 medium and injected intraperitoneally into the recipient mice. At days 0 and 7, mice were injected intraperitoneally with 1.5 × 106 autologous DCs, pulsed 2 hours at 37°C with chemically inactivated HIV-1 (40 ng of p24) before injection. HIV-1 was inactivated by aldrithiol (AT)-2 treatment as described elsewhere.40 At days 14 and 21, mice were given a boost dose of AT-2–inactivated HIV-1. At day 28, mice were killed, and sera from hu-PBL-SCID mice were assayed for the presence of human anti-HIV antibodies. Individual sera from hu-PBL-SCID mice injected with HIV-1–pulsed DCs were assayed by Western blot (Cambridge Biotech, Rockville, MD). Briefly, individual nitrocellulose strips were incubated overnight with different mouse serum samples (diluted 1:20) or with a human positive control serum (diluted 1:1000). Visualization of the human immunoglobulins specifically bound to HIV-1 proteins was obtained by incubation with substrate chromogen after the addition of biotin-conjugated goat antihuman immunoglobulin G (IgG) and streptavitin-conjugated horseradish peroxidase. Western blot strips were examined by densitometry, using the Quantity One 4.2.1 program (Bio-Rad) to detect the intensity of serum antibody reactivity toward the HIV-1 gp120/160 and p24 antigens. The mean values detected in 3 sera from control nonimmunized hu-PBL-SCID mice were used as the cut-off to determine the specific antibody reactivity in the serum from immunized chimeras.
Peritoneal washings from individual mice were collected and analyzed for the presence of human IFN-γ by enzyme-linked immunosorbent assay (R&D Systems). Analysis was performed in triplicate, and laboratory standards were included. Assay sensitivity was 3 pg/mL.
Results
Specific phenotype and morphology of IFN-DCs
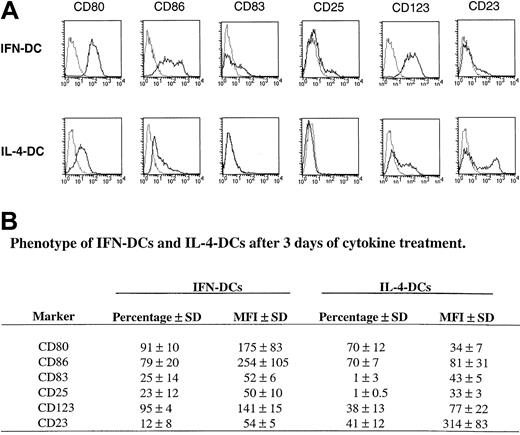
Figure 1A,B illustrates the typical phenotypic characteristics of the IFN-DCs as compared with IL-4-DCs at 3 days of culture. IFN-DCs were characterized by a higher expression of the costimulatory molecules CD80 and CD86 as compared with IL-4-DCs. The DC maturation marker CD83 was expressed by a remarkable percentage of the IFN-DCs, whereas it was undetectable in IL-4-DCs. Notably, IFN-DCs expressed high levels of the lymphoid DC marker CD123 (IL-3Rα), which was poorly detected in IL-4-DCs, and moderate levels of CD25, not detectable in IL-4-DCs. In IFN-DCs, CD25 expression occurred only in CD83+ CD14− cells expressing high levels of CD86 (data not shown). Finally, IFN-DCs exhibited a marked reduction in the expression of CD23 (FcεRII), which was consistently expressed in IL-4-DCs. These results show that a 3-day exposure of freshly isolated GM-CSF–treated monocytes to type I IFN, instead of IL-4, results in the generation of a characteristic type of partially mature DCs, as evidenced by a consistent expression of CD83 and CD25, characterized by a differential expression of certain membrane antigens (CD123 and CD23) with respect to IL-4-DCs.
Immunophenotypic pattern of IFN-DCs as compared with IL-4-DCs.
Highly purified CD14+ monocytes were cultured with GM-CSF and either IL-4 or type I IFN for 3 days, stained with a panel of antibodies, and analyzed by flow cytometry. (A) This panel shows the representative dot histogram FACS profiles obtained with monocytes isolated from 1 of 4 donors, whose DC cultures were tested on day 3. Relative profiles obtained with isotype-matched control antibodies (dotted lines) are shown. (B) This panel reports the mean percentages of positive cells and of mean fluorescence intensity (MFI) obtained in 4 experiments performed with monocytes from different donors.
Immunophenotypic pattern of IFN-DCs as compared with IL-4-DCs.
Highly purified CD14+ monocytes were cultured with GM-CSF and either IL-4 or type I IFN for 3 days, stained with a panel of antibodies, and analyzed by flow cytometry. (A) This panel shows the representative dot histogram FACS profiles obtained with monocytes isolated from 1 of 4 donors, whose DC cultures were tested on day 3. Relative profiles obtained with isotype-matched control antibodies (dotted lines) are shown. (B) This panel reports the mean percentages of positive cells and of mean fluorescence intensity (MFI) obtained in 4 experiments performed with monocytes from different donors.
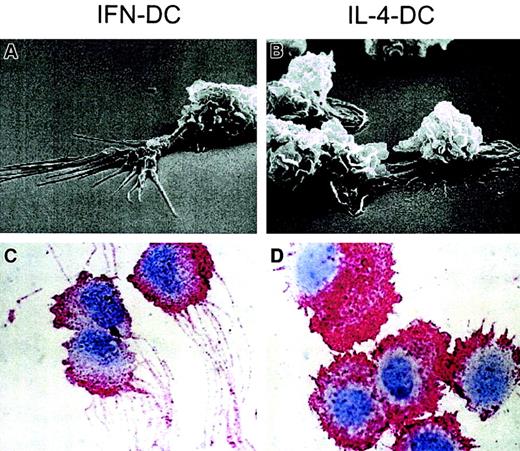
Figure 2A,B shows the typical morphologic differences between the 2 cell types at 3 days of culture, as revealed by scanning electron microscopy. IFN exposure led to the formation of typical dendriticlike protrusions, generally longer than cell body and often ramified to form a sort of brush border at the protrusion periphery. By contrast, squat and randomly distributed cell protrusions were observed in IL-4-DCs, which maintained a substrate-associated polarity showing evident adhesion plaques. IFN-DCs often developed axonlike protruding structures leading to cell-to-cell dot contact regions and indicating a cell-cell-associated polarity (data not shown). Immunocytochemical analysis by using anti-CD44 antibodies was also performed (Figure 2C,D), because preliminary experiments had revealed that such staining specifically outlined dendrite structures. Clear-cut differences were observed in comparing IFN-DCs versus IL-4-DCs. In particular, a remarkably higher number of CD44-stained dendrites were observed in IFN-DCs as compared with IL-4-DCs. The dendrites of IFN-DCs were mostly thin, long (up to 21-30 μ in length), and highly polarized (Figure 2C). On the contrary, the typical CD44+-stained morphology of IL-4-DCs was that of a larger cell with squat and short dendrites, resembling ruffles of different size (Figure 2D). IL-4-DCs did not show the unidirectional orientation typical of IFN-DCs.
Morphologic features of IFN-DCs.
Scanning electron microscopy analysis of IFN-DCs (A) showed a number of typical dendriticlike protrusions, not detected in IL-4-DCs (B), that were well adhering to the substrate. Immunocytochemistry for CD44 in IFN-DCs (C) and IL-4-DCs (D) (peroxidase-antiperoxidase method and hematoxylin counterstaining; magnification ×1500). Note the thin and long dendrites of IFN-DCs as compared with the squat dendrites of the IL-4-DCs. The CD44 staining is typically localized on dendrites, nicely outlining them.
Morphologic features of IFN-DCs.
Scanning electron microscopy analysis of IFN-DCs (A) showed a number of typical dendriticlike protrusions, not detected in IL-4-DCs (B), that were well adhering to the substrate. Immunocytochemistry for CD44 in IFN-DCs (C) and IL-4-DCs (D) (peroxidase-antiperoxidase method and hematoxylin counterstaining; magnification ×1500). Note the thin and long dendrites of IFN-DCs as compared with the squat dendrites of the IL-4-DCs. The CD44 staining is typically localized on dendrites, nicely outlining them.
Response to β-chemokines and expression of Th-1 type chemokines in IFN-DCs
The morphologic differences described above could reflect a different migration attitude of the 2 cell populations. We then investigated the levels of expression of chemokines/chemokine receptors in both IFN-DCs and IL-4-DCs. In fact, the migration and function of DCs is strictly regulated by their response to chemokines as well as by the expression of DC-derived chemokines, whose production can markedly shape their functional activities.4 5
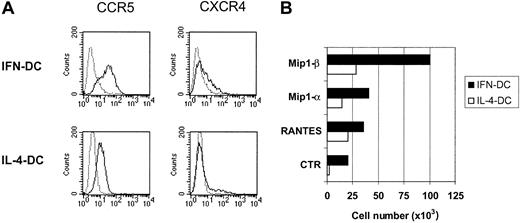
In a first set of experiments, we measured the levels of expression of the β-chemokine receptor CCR5 and the α-chemokine receptor CXCR4 in both DC types. As shown in Figure3A, CCR5 was expressed at higher levels on the cell membrane of IFN-DCs as compared with IL-4-DCs, whereas no difference in CXCR4 expression was appreciable. This higher expression of CCR5 was associated with a stronger chemotactic response of IFN-DCs to the β-chemokines RANTES, MIP-1α, and, especially, MIP-1β, as revealed by measuring the migration capability of DCs using 2 compartment systems with chemokine-containing medium (Figure 3B).
CCR5 expression and migratory response to β-chemokines by IFN-DCs.
(A) Dot histogram FACS profiles showing CCR5 and CXCR4 staining of IFN-DCs and IL-4-DCs. (B) DCs (5 × 105), treated for 3 days with the indicated cytokines, were resuspended in complete medium and seeded in the upper compartments of 8-μm pore size filter Transwell chambers, while 0.5 μg/mL of the relevant chemokine in serum-free medium was added to the lower compartments. The lower wells of control chambers contained medium alone. After 2 hours of incubation, the cells that migrated to the lower compartment were counted. Assays were performed in triplicate. SDs did not exceed 15%. Results of one representative experiment of 4 are shown.
CCR5 expression and migratory response to β-chemokines by IFN-DCs.
(A) Dot histogram FACS profiles showing CCR5 and CXCR4 staining of IFN-DCs and IL-4-DCs. (B) DCs (5 × 105), treated for 3 days with the indicated cytokines, were resuspended in complete medium and seeded in the upper compartments of 8-μm pore size filter Transwell chambers, while 0.5 μg/mL of the relevant chemokine in serum-free medium was added to the lower compartments. The lower wells of control chambers contained medium alone. After 2 hours of incubation, the cells that migrated to the lower compartment were counted. Assays were performed in triplicate. SDs did not exceed 15%. Results of one representative experiment of 4 are shown.
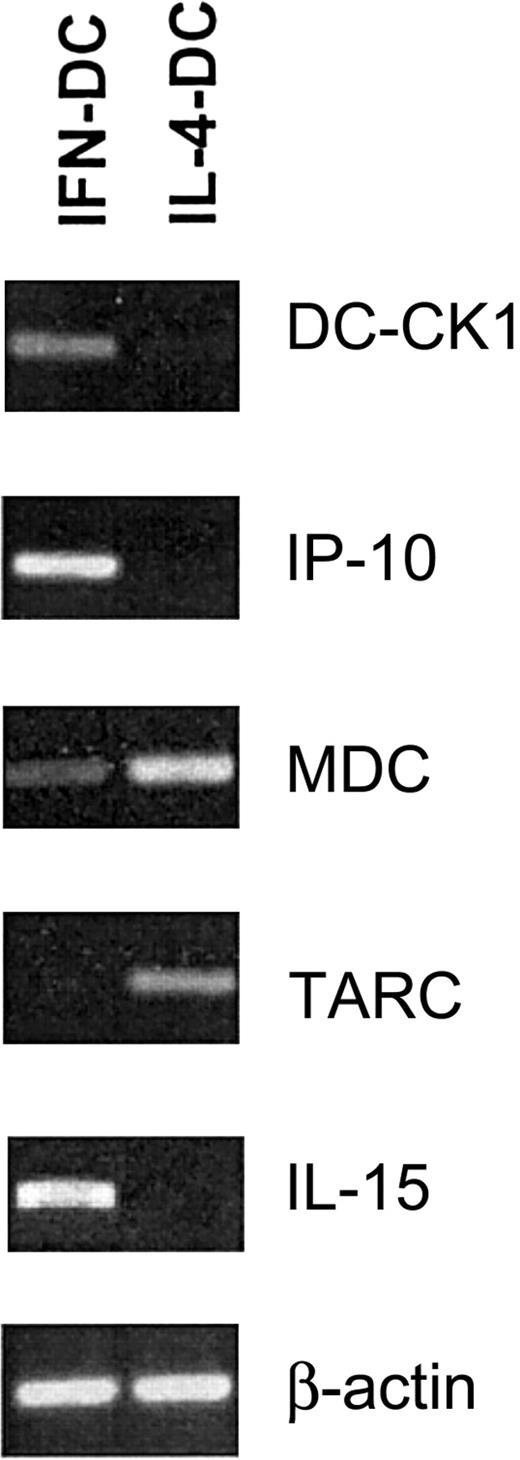
We then analyzed the expression of several chemokines in both DC types (Figure 4). DC-CK1, a chemokine highly expressed in human DCs,41 was markedly expressed in IFN-DCs. IP-10, a chemokine specific for memory Th1 lymphocyte,42,43 was expressed at higher levels in IFN-DCs than in IL-4-DCs. In contrast, the expression of MDC and TARC, chemokines specifically recruiting Th2 lymphocytes,44 was higher in IL-4-DCs than in IFN-DCs. Consistent with a previous report,34 IL-15 transcript was specifically detected in IFN-DCs and not in IL-4-DCs.
Chemokine expression in IFN-DCs versus IL-4-DCs.
RT-PCR analysis showing expression of Th-1 and Th-2 chemokines in DC generated after a 3-day treatment of monocytes with either IFN/GM-CSF or IL-4/GM-CSF. Data are representative of 3 experiments performed with cells from different donors.
Chemokine expression in IFN-DCs versus IL-4-DCs.
RT-PCR analysis showing expression of Th-1 and Th-2 chemokines in DC generated after a 3-day treatment of monocytes with either IFN/GM-CSF or IL-4/GM-CSF. Data are representative of 3 experiments performed with cells from different donors.
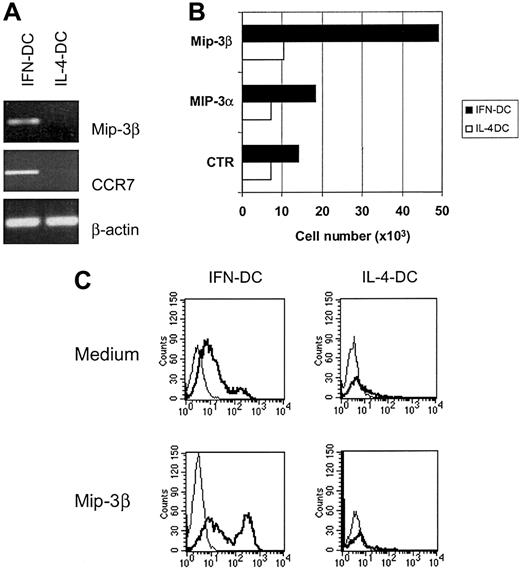
IFN-DCs express CCR7 as well as MIP-3β and CD83+cells rapidly migrate in response to exogenous MIP-3β
Mature DCs respond to MIP-3β/ELC and 6Ckine/SLC as a consequence of an up-regulation of their receptor CCR7, and studies in knock-out mice for CCR7 have shown the crucial importance of the CCR7/MIP-3β interaction for the generation of a primary immune response.13 Thus, we evaluated the expression of CCR7 and MIP3β in IFN-DCs as compared with IL-4-DCs. RT-PCR analysis showed that IFN-DCs exhibited marked levels of expression of both CCR7 and its natural ligand MIP-3β, which were not detectable in IL-4-DCs (Figure 5A). Of interest, when both types of DCs were tested for their capacity to migrate in response to exogenous MIP-3β, a marked chemotactic response to this chemokine was specifically observed for IFN-DCs (Figure 5B). In contrast, both IFN-DCs and IL-4-DC did not significantly migrate in response to MIP-3α (CCR6 ligand). Notably, the majority (more than 80%) of the IFN-DCs migrated in the lower compartments containing medium alone expressed the CD83 marker. This expression was even higher in IFN-DCs migrated in response to MIP3β (Figure 5C). In contrast, the few IL-4-DCs that migrated in response to the same chemokine under identical experimental conditions did not express CD83 (Figure5C).
Up-regulation of CCR7 and MIP-3β expression in IFN-DCs and CD83+ cell migration in response to exogenous MIP-3β.
(A) RT-PCR analysis showing the up-regulation of CCR7 and MIP-3β mRNAs in IFN-DCs as compared with IL-4-DCs. (B) Strong migratory capacity of IFN-DCs in response to MIP3β. Migration assays were performed in a 24-well Transwell cell culture chamber (Costar). MIP3α and MIP3β chemokines (100 ng/mL) were loaded in the lower compartment. After 2 hours of incubation, the cells that migrated in the lower compartment were collected and counted. Assays were performed in triplicate. SDs did not exceed 15%. (C) The majority of IFN-DCs migrated in response to MIP-3β expressed the CD83 marker. After migration assay, cells recovered from the lower compartment of the Transwell chambers were collected and analyzed by flow cytometry. MIP-3β-responsive cells in IFN-DCs were mostly CD83+, whereas the few IL-4-DCs migrated in response to MIP-3β did not express CD83. The addition of MIP3-β to the cell cultures did not induce up-regulation of CD83 (data not shown). The results are representative of 3 experiments performed with cells from different donors.
Up-regulation of CCR7 and MIP-3β expression in IFN-DCs and CD83+ cell migration in response to exogenous MIP-3β.
(A) RT-PCR analysis showing the up-regulation of CCR7 and MIP-3β mRNAs in IFN-DCs as compared with IL-4-DCs. (B) Strong migratory capacity of IFN-DCs in response to MIP3β. Migration assays were performed in a 24-well Transwell cell culture chamber (Costar). MIP3α and MIP3β chemokines (100 ng/mL) were loaded in the lower compartment. After 2 hours of incubation, the cells that migrated in the lower compartment were collected and counted. Assays were performed in triplicate. SDs did not exceed 15%. (C) The majority of IFN-DCs migrated in response to MIP-3β expressed the CD83 marker. After migration assay, cells recovered from the lower compartment of the Transwell chambers were collected and analyzed by flow cytometry. MIP-3β-responsive cells in IFN-DCs were mostly CD83+, whereas the few IL-4-DCs migrated in response to MIP-3β did not express CD83. The addition of MIP3-β to the cell cultures did not induce up-regulation of CD83 (data not shown). The results are representative of 3 experiments performed with cells from different donors.
IFN-DCs migrate with high efficiency in vivo and induce a potent human antibody response along with IFN-γ production in SCID mice reconstituted with human PBLs
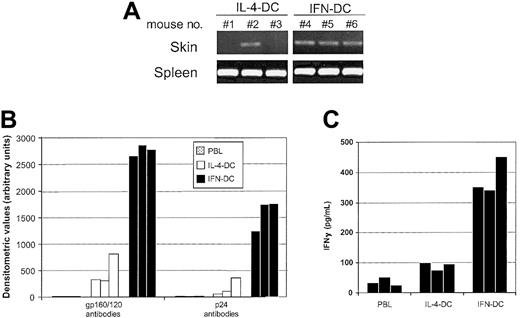
It was important to evaluate whether the strong migratory capability of IFN-DCs observed in vitro also occurred in vivo and was associated with the capacity to induce an effective primary immune response. To compare the capacity of both cell types to migrate in vivo, DCs were injected intravenously into SCID mice, and DNA-PCR analysis to detect human DNA sequences in different tissues was performed 4 hours after injection. Figure6A illustrates the representative results obtained in one of 3 experiments in which we compared the migration efficiency of IFN-DCs versus that of IL-4-DCs. All the mice injected with either IFN-DCs or IL-4-DCs showed the presence of human DNA sequences in their spleens. In contrast, approximately 30% of mice injected with IL-4-DCs showed some evidence of DC migration to the skin, whereas all the mice inoculated with IFN-DCs proved to be positive for the presence of human DNA sequences in the skin.
IFN-DCs migrate with high efficiency in vivo and induce a potent antibody response toward the HIV-1 gp120/160 and p24 antigens along with a high production of human IFN-γ in hu-PBL-SCID mice.
(A) SCID mice were injected intravenously with 2 × 106IFN-DCs or IL-4-DCs. After 4 hours, the presence of human DNA sequences in the skin and spleen from each mouse was assessed as described in “Materials and methods.” Results of one representative experiment of 3 are shown. For each experiment, there were 3 mice per group. (B) Human antibodies against the HIV-1 gp120/160 and p24 proteins in the sera from hu-PBL-SCID mice immunized on day 0 and boosted on day 7 with 1.5 × 106 autologous DCs pulsed with AT-2-inactivated HIV-1. At days 14 and 21, mice were further boosted with AT-2-inactivated virus. Antibodies to the HIV-1 gp120/160 and p24 proteins were assessed as described in “Materials and methods.” Results represent the values detected in serum from individual mice killed at day 28. There were 3 mice for each group. (C) IFN-γ production in peritoneal washings of immunized and control hu-PBL-SCID mice was evaluated by using a specific enzyme-linked immunosorbent assay, as described in “Materials and methods.” In a previous set of 3 different experiments, the levels of human IFN-γ detected in the peritoneal washings of hu-PBL-SCID mice injected with unpulsed autologous DCs, under identical conditions, were never higher than those found in the peritoneal lavages of mice only injected with hu-PBLs (data not shown).
IFN-DCs migrate with high efficiency in vivo and induce a potent antibody response toward the HIV-1 gp120/160 and p24 antigens along with a high production of human IFN-γ in hu-PBL-SCID mice.
(A) SCID mice were injected intravenously with 2 × 106IFN-DCs or IL-4-DCs. After 4 hours, the presence of human DNA sequences in the skin and spleen from each mouse was assessed as described in “Materials and methods.” Results of one representative experiment of 3 are shown. For each experiment, there were 3 mice per group. (B) Human antibodies against the HIV-1 gp120/160 and p24 proteins in the sera from hu-PBL-SCID mice immunized on day 0 and boosted on day 7 with 1.5 × 106 autologous DCs pulsed with AT-2-inactivated HIV-1. At days 14 and 21, mice were further boosted with AT-2-inactivated virus. Antibodies to the HIV-1 gp120/160 and p24 proteins were assessed as described in “Materials and methods.” Results represent the values detected in serum from individual mice killed at day 28. There were 3 mice for each group. (C) IFN-γ production in peritoneal washings of immunized and control hu-PBL-SCID mice was evaluated by using a specific enzyme-linked immunosorbent assay, as described in “Materials and methods.” In a previous set of 3 different experiments, the levels of human IFN-γ detected in the peritoneal washings of hu-PBL-SCID mice injected with unpulsed autologous DCs, under identical conditions, were never higher than those found in the peritoneal lavages of mice only injected with hu-PBLs (data not shown).
To evaluate whether the marked migration potential of IFN-DCs paralleled an enhanced immune-priming activity and a polarized immune response, we immunized SCID mice reconstituted with hu-PBL (hu-PBL-SCID mice) with autologous IFN-DCs, which had been pulsed in vitro with inactivated HIV-1 before their injection into the chimeric animals. As control groups, we used nonimmunized hu-PBL-SCID mice or chimeras immunized with virus-pulsed IL-4-DCs. Sera of hu-PBL-SCID immunized with IFN-DCs exhibited much higher levels of human antibodies against the HIV-1 gp120/gp160 and p24 proteins as compared with sera from animals immunized with IL-4-DCs (Figure 6B). Notably, considerable levels of human IFN-γ were only observed in hu-PBL-SCID mice immunized with IFN-DCs (Figure 6C), indicating that IFN-DCs can promote a strong Th-1 polarized immune response not only in vitro34but also in vivo.
Discussion
We have shown that a single-step treatment of freshly isolated monocytes with type I IFN, together with GM-CSF added as monocyte survival factor, results in the rapid generation of DCs expressing chemokines and chemokine receptors typical of mature DCs and endowed with marked migratory capabilities, along with a Th-1 polarization of the immune response in vivo. The detailed comparison of IFN-DCs versus IL-4-DCs revealed remarkable differences in terms of phenotype, morphology, expression of chemokines and chemokine receptors, migration capability, and immune priming activities in vivo. IFN-DCs exhibited a selective expression of the DC maturation markers CD83 and CD25, in association with high levels of costimulatory molecules. Morphologic studies revealed that IFN-DCs, but not IL-4-DCs, rapidly acquired typical DC features within 2 to 3 days, showing the formation of markedly oriented dendrites. Intriguingly, a polarized CD44 staining of dendrites was typical of IFN-DCs. Notably, CD44 is involved in a number of monocyte-macrophage functions,45 including transendothelial migration and cytokine production.46 On the whole, the comparative morphologic analysis suggested that IFN-DCs, but not IL-4-DCs, had rapidly acquired a highly polarized DC phenotype, suggestive of a high intrinsic migration attitude. Notably, polarization of adhesion molecules on circulating cells, such as monocytes47 or lymphocytes,48 correlates with both increased adhesion potential and migratory capacity.
Migration is essential for the pivotal role of DCs as sentinels of the immune system. Each step of DC trafficking is mostly regulated by the interaction of chemokines released by a variety of host cells with their receptors on DCs. Migration of DCs is tightly regulated as a function of maturation. Thus, immature DCs respond to inflammatory chemokines, such as MIP-1α, MIP-1β, RANTES, and MIP-3α, whereas mature DCs have generally lost their responsiveness to most of these chemokines, as a result of down-regulation of receptor expression or activity (reviewed in Dieu-Nosjean et al4 and Sozzani et al5). However, mature DCs respond to MIP-3β/ELC and 6Ckine/SLC as a consequence of an up-regulation of their specific receptor CCR7.4,5 The evaluation of the chemokine expression in IFN-DCs versus IL-4-DCs revealed major differences, potentially relevant for a differential polarization of the immune response. In particular, IFN-DCs expressed high levels of IP-10 and IL-15, whereas IL-4-DCs preferentially showed MDC and TARC expression. Trafficking of Th1 and Th2 lymphocytes can be differentially controlled through the selective expression of these factors. In fact, IP-10 is a potent chemoattractant for activated/memory T cells and natural killer cells by binding to the receptor CXCR3, and it preferentially recruits Th-1 lymphocytes.42,43 Likewise, IL-15 is involved in the survival of T cells24 and is important for a Th-1 type of immune response as well as for the induction of B-cell proliferation and differentiation.49 In contrast, MDC and TARC attract chronically activated Th-2 lymphocytes, controlling trafficking or development of Th-2 lymphocytes.44
IFN-DCs exhibited an up-regulation of CCR7 (not detectable in IL-4-DCs) along with an induced expression of its natural ligand (ie, MIP-3β). Of interest, IFN-DCs showed a specific migration response to exogenous MIP-3β, indicating that functional CCR7 receptors were expressed on these cells. Notably, the vast majority of IFN-DCs that migrated in response to MIP-3β were mature CD83+ DCs. In this regard, it is worth mentioning that the expression and response to MIP-3β are considered crucial events in the DC-mediated generation of immune response.4,5 The role of MIP-3β/CCR7 interaction is supported by the profound immune defects observed in mice either defective for MIP-3β expression50 or knock-out for the CCR7 receptor.13 Thus, it is assumed that mature DCs entering the draining lymph nodes are driven into the paracortical area mostly in response to the production of MIP-3β and/or 6Ckine by cells in the T-cell zone. Production of MIP-3β by mature CCR7+DCs9 may also contribute to the amplification of the chemotactic response. Because MIP-3β can attract both mature DCs and CCR7-expressing lymphocytes, this chemokine can play a key role in allowing antigen-loaded DCs to encounter specific T cells. Therefore, the finding that IFN-DCs express MIP-3β and functionally active CCR7 receptors is consistent with an acquired mission of mature DCs.
Comparative studies in SCID mice revealed that IFN-DCs migrated more efficiently to the skin than IL-4-DCs after injection. Likewise, IFN-DCs were highly competent in inducing a strong primary human antibody response in vivo, when antigen-pulsed DCs were inoculated into hu-PBL-SCID mice. The human antibody response toward the HIV-1 gp120/160 and p24 antigens was remarkably consistent among individual animals and impressive, when compared with the barely detected response observed in hu-PBL-SCID mice immunized with IL-4-DCs. Although further studies are required to understand the exact mechanisms underlying such remarkable in vivo activity of IFN-DCs, we envision that multiple factors up-regulated by IFN (including MIP-3β, CCR7, IP-10, and IL-15) can be involved in the induction of a primary immune response in this animal model. A differential in vivo survival of DCs after injection could also explain the enhanced capability of IFN-DCs to induce a potent primary antibody response in hu-PBL-SCID mice. Because IFN-DCs induce a marked Th-1 type of immune response not only in vitro34 but also in vivo, as revealed by the strong induction of human IFN-γ production in hu-PBL-SCID mice (Figure 6C), we may assume that these cells exhibit characteristics similar to those of the so-called “active DCs,” generated after a short-term exposure of immature IL-4-DCs to LPS stimulation, recently described by Langenkamp et al.51 Thus, we may argue that the generation of fully active monocyte-derived active DCs does not require the IL-4 step of culture (generally 6 days) and a further in vitro stimulation by potent maturation signals. Of interest Thurnher et al52recently reported that DCs generated from monocytes with IL-4/GM-CSF are impaired in certain functional activities such as migration or the ability to induce natural killer cell activation or Th-2 cell differentiation, as a direct consequence of IL-4 suppressive effects. Moreover, because IL-4 exhibits some suppressive effects on monocyte antigen expression, the addition of this cytokine at the beginning of monocyte culture may affect the physiologic response of monocytes to the IFN-induced DC generation. In this regard, it is worth mentioning that McRae et al53 have recently reported that type I IFN can inhibit differentiation of human DCs from CD14+monocytes under conditions in which monocytes had been concomitantly treated with IL-4 and GM-CSF. This conclusion was mostly inferred by the reduction of the number of DCs and by the impairment of the response to tumor necrosis factor α–induced maturation, evaluated as reduced expression of certain markers such as CD1a.53However, these results may not necessarily be in contrast with data obtained by our group34 as well as by others,32,33 showing a promoting effect of IFN on the DC differentiation/maturation of freshly isolated monocytes only exposed to GM-CSF, especially if we consider that exposure of monocytes to IL-4 can change the normal pattern of response of monocytes to IFN. Of interest, McRae et al53 refer to their monocyte-derived cells cultured with the presence of GM-CSF, IL-4, and IFN-β as DCs, because of their loss of CD14 and their expression of CD83, and conclude that this phenotype “resembles that of direct blood-derived DC.”
In conclusion, the ensemble of our results on chemokine expression and migratory and functional activities of IFN-DCs lead to a novel view on the naturally occurring DCs generated from monocytes in response to infections. Concentrations of type I IFN similar to those used in our experiments are likely to be transiently present in the microenvironment of circulating or tissue monocytes in the course of infection, leading to the rapid generation of DCs endowed with the requisites necessary for a prompt induction of an immune response. Thus, exposure of monocytes to type I IFN can represent the early mechanism involved in the maturation/induction of DC in response to virus infection and possibly to other invading pathogens or tumors. Of interest, the results on human IFN-DCs reported in this study are consistent with recent data in mice indicating that (1) type I IFN is indeed an extremely potent adjuvant when co-injected in immunocompetent animals together with a reference antigen and (2) the adjuvant activity of type I IFN can be mediated by a direct action of IFN on DCs.54 With regard to human monocyte–derived DCs, we speculate that the strict distinction between immature DCs and mature DCs may reflect more an in vitro–established definition based on the current methods for generation of immature DC by using IL-4, rather than a natural event occurring at the level of blood monocytes during infection. This concept may have implications for the therapeutic use of DCs, because we envision that the use of IFN-DCs can exhibit advantages in terms of time for generation and potential efficacy with respect to the current procedures of DC preparations for clinical use. Finally, the elucidation of this rapid pathway of DC generation from monocytes characterized by chemokine expression pattern and migratory/functional activities typical of fully active mature DCs underscores the important “natural alliance” between monocytes and type I IFNs for ensuring a prompt connection between innate and immune response against infections.
We thank M. Ferrantini, D. F. Tough, and D. E. Mosier for helpful comments and discussion, as well as Cinzia Gasparrini and Anna Ferrigno for excellent secretarial assistance.
Supported in part by European Community (EC contract no. B104-CT98-0466), the Italian Association for Cancer Research, and the “Italian Project on AIDS” (ISS-Ministry of Health) (contract no. 40D.3 2001).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Filippo Belardelli, Laboratory of Virology, Istituto Superiore di Sanità, Viale Regina Elena n 299, 00161 Rome, Italy; e-mail: belard@iss.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal