Abstract
Acute graft-versus-host disease (aGVHD), the fatal side effects of bone marrow transplantation, was shown to be accompanied by elevation of serum levels of interleukin 18 (IL-18). In this study, the mechanism underlying the accumulation of IL-18 in aGVHD in mice was investigated. Lethally irradiated recipients having transplantation with H-2 disparate donor splenocytes demonstrated aGVHD and contained markedly elevated serum levels of IL-18. In contrast, recipients having transplantation with gld/gld spleen cells, which lack functional Fas ligand (FasL), contained only normal ranges of IL-18, indicating FasL-mediated IL-18 release in aGVHD. The wild-type hosts engrafted with caspase-1–deficient cells revealed marked increases of IL-18 similar to those engrafted with wild-type cells, whereas caspase-1–deficient recipients engrafted with wild-type cells showed only a slight elevation of serum IL-18, indicating that IL-18 elevation is derived from host cells in a caspase-1–dependent manner. These results suggest FasL-mediated caspase-1–dependent IL-18 secretion in aGVHD in mice.
Introduction
Interleukin 18 (IL-18) is a cytokine with wide-ranging biologic functions that include activation not only of innate immunity, but also of acquired immunity, including both Th1 responses, particularly in collaboration with IL-12, and Th2 responses.1-5 Furthermore, IL-18 is involved in the development of cytotoxic T lymphocytes and natural killer cells.4-6 The regulatory mechanism of IL-18 secretion is distinct from that of usual secretory cytokines.4,5 IL-18 is stored as biologically inactive precursor (pro–IL-18) and is secreted after cleavage by appropriate cutting enzymes.4,5 Caspase-1 is a prerequisite for the secretion of IL-18 upon activation by certain stimuli, including lipopolysaccharide (LPS).7-10Caspase-1–like is required for the secretion of IL-18 fromPropionibacterium acnes–elicited Kupffer cells after stimulation with Fas ligand (FasL), although this study does not exclude the possible involvement of caspase-1.11 Thus, the precise regulatory mechanism of IL-18 secretion is still uncertain. Recently, we and others have shown that the serum concentration of IL-18 is elevated in patients with acute graft-versus-host disease (aGVHD).12,13 In aGVHD, the Fas/FasL system plays an essential role in the development of fatal tissue injuries.14 15 Here, we investigated the mechanism underlying the accumulation of IL-18 in a mouse model of aGVHD and found that IL-18 is secreted in a FasL-initiated, caspase-1–dependent manner.
Study design
Mice
C57BL/6 (B6) mice, B6XDBA/2 F1 (BDF1) mice, and BALB/c mice were purchased from SLC (Shizuoka, Japan). B6gld/gld mice were purchased from CLEA Japan (Osaka, Japan). Caspase-1–deficient mice and wild-type (WT) littermates in a mixture of 129 and B6 background (B6/129) were used.16Caspase-1–deficient mice were back-crossed with BALB/c mice, and F8 mice were used. All of the mice used (females, 6-10 weeks old) were kept under specific pathogen-free conditions.
Reagents
L5178Y cells transfected with mouse FasL (mFasL)11,17 and neutralizing anti–mouse FasL monoclonal antibody (mAb; MFL-1, hamster IgG)11,17 were kindly provided by Dr N. Kayagaki (Juntendo University, Tokyo, Japan).11 LPS derived from Escherichia coli055:B5 was purchased from Difco (Detroit, MI). Culture medium generally used in this study was RPMI-1640 (Wako Pure Chemical, Osaka, Japan) supplemented with 10% fetal calf serum (JRH Bioscience, Lenexa, KS), 100 U/mL penicillin, 100 μg/mL streptomycin, 50 μM 2-mercaptoethanol, and 2 mM L-glutamine (Gibco BRL, Grand Island, NY).
Induction of aGVHD
aGVHD was induced by intravenous injection of 5 × 107 viable, unfractionated donor spleen cells in lethally irradiated (9 Gy) recipients. Control mice received syngeneic (syn) spleen cells (5 × 107).
Assay for IL-18
The concentration of IL-18 was determined by an enzyme-linked immunosorbent assay (ELISA) kit (MBL, Nagoya, Japan).
Preparation of Kupffer cells
Kupffer cells were isolated from variously treated mice as shown previously.11 Kupffer cells (1 × 106/mL) were incubated with mFasL (1 × 106/mL) in the presence of 20 μg/mL anti–mouse FasL or with 1 μg/mL LPS for 24 hours.
Statistical analysis
The Student t test or the Fisher protected least significant difference test was performed.P < .05 was considered statistically significant.
Results and discussion
Elevated serum levels of IL-18 in aGVHD mice
As reported previously, levels of serum IL-18 are elevated in patients with aGVHD.12,13 This was also the case for mouse aGVHD. As shown in Figure 1A, IL-18 serum levels increased after transplantation of WT B6 spleen cells in 9 Gy–irradiated BDF1 mice (aGVHD-induced mice) and reached a plateau at day 10 (P < .0001 versus syngeneic controls). The serum levels of IL-18 correlated with the donor cell number infused, and the duration required for reaching their peaks became shortened (data not shown). Furthermore, there is a correlation between the concentration of serum IL-18 and the severity of pathologic changes in aGVHD target organs, such as spleen, liver, and intestines (H. I., unpublished data, August 2000). In contrast, IL-1β, which is processed by the same enzymes that cleave pro– IL-18,11,18,19 was not elevated in the serum of aGVHD-induced mice (data not shown). Therefore, high serum levels of IL-18 but not IL-1β seem to be an indicator of aGVHD not only in human patients,12 but also in mouse experimental models.
FasL-dependent elevation of serum IL-18 levels after induction of aGVHD.
(A) Elevation of IL-18 serum levels after induction of aGVHD. BDF1 mice were lethally irradiated and underwent transplantation with 5 × 107 spleen cells from BDF1 (Syn, ■) or WT B6 (aGVHD, ●) mice. At the indicated day, the serum was sampled for measurement of IL-18 concentration by ELISA. Data represent the mean ± SD of 5 mice in each experimental group. *P < .0001 by Fisher PLSD test. Similar results were obtained in 3 independent experiments. (B) Lack of increase in serum levels of IL-18 in the recipients engrafted with gld/gldspleen cells. Lethally irradiated BDF1 mice had transplantation with 5 × 107 spleen cells from BDF1 mice (Syn), WT B6 mice (aGVHD), or gld/gld B6 mice (gld/gld). At day 10, serum levels of IL-18 were measured by ELISA. Data represent the mean ± SD of 5 mice in each experimental group. Similar results were obtained in 3 independent experiments. (C) Fas/FasL-dependent IL-18 secretion in vitro by Kupffer cells from aGVHD hosts. Kupffer cells were prepared from BDF1 mice having transplantation with BDF1 spleen cells (Syn; ■ A,C,E,G) or WT B6 cells (aGVHD; ▪ B,D,F,H) at day 7, and were incubated with 1 μg/mL LPS or mFasL in the presence or absence of neutralizing anti–murine FasL (αFasL) mAb for 24 hours. The IL-18 concentration in each supernatant was measured by ELISA. Control hamster IgG did not down-regulate the secretion of IL-18 from Kupffer cells from hosts having transplantation with BDF1 or WT B6 cells upon stimulation with mFasL. Data are presented as mean ± SD of triplicate cultures. Similar results were obtained in 3 independent experiments. ND indicates not detectable; NS, not significant.
FasL-dependent elevation of serum IL-18 levels after induction of aGVHD.
(A) Elevation of IL-18 serum levels after induction of aGVHD. BDF1 mice were lethally irradiated and underwent transplantation with 5 × 107 spleen cells from BDF1 (Syn, ■) or WT B6 (aGVHD, ●) mice. At the indicated day, the serum was sampled for measurement of IL-18 concentration by ELISA. Data represent the mean ± SD of 5 mice in each experimental group. *P < .0001 by Fisher PLSD test. Similar results were obtained in 3 independent experiments. (B) Lack of increase in serum levels of IL-18 in the recipients engrafted with gld/gldspleen cells. Lethally irradiated BDF1 mice had transplantation with 5 × 107 spleen cells from BDF1 mice (Syn), WT B6 mice (aGVHD), or gld/gld B6 mice (gld/gld). At day 10, serum levels of IL-18 were measured by ELISA. Data represent the mean ± SD of 5 mice in each experimental group. Similar results were obtained in 3 independent experiments. (C) Fas/FasL-dependent IL-18 secretion in vitro by Kupffer cells from aGVHD hosts. Kupffer cells were prepared from BDF1 mice having transplantation with BDF1 spleen cells (Syn; ■ A,C,E,G) or WT B6 cells (aGVHD; ▪ B,D,F,H) at day 7, and were incubated with 1 μg/mL LPS or mFasL in the presence or absence of neutralizing anti–murine FasL (αFasL) mAb for 24 hours. The IL-18 concentration in each supernatant was measured by ELISA. Control hamster IgG did not down-regulate the secretion of IL-18 from Kupffer cells from hosts having transplantation with BDF1 or WT B6 cells upon stimulation with mFasL. Data are presented as mean ± SD of triplicate cultures. Similar results were obtained in 3 independent experiments. ND indicates not detectable; NS, not significant.
Fas/FasL-mediated IL-18 secretion in aGVHD
As reported previously, Fas-expressing macrophages release biologically active IL-18 upon stimulation with FasL, depending on a non–caspase-1, caspace-dependent manner.11 It has also been demonstrated that FasL-expressing cells were induced after induction of aGVHD.14 15 To investigate whether the Fas/FasL system is involved in the secretion of IL-18 in aGVHD, we transplanted splenocytes from gld/gld B6 mice, which lack functional FasL. As shown in Figure 1B, BDF1 mice having transplantation with gld/gld splenocytes did not show elevated serum levels of IL-18 compared with those engrafted with WT splenocytes (P < .01). No obvious increase in serum levels of IL-18 was observed by day 21 after transplantation withgld/gld splenocytes (data not shown). Furthermore, when 10-fold splenocytes from gld/gld B6 mice were transplanted into BDF1 mice, no significant elevation of serum IL-18 levels was observed by day 14 (data not shown). These results strongly suggest that IL-18 in aGVHD accumulates in a Fas/FasL-mediated fashion.
Next, we investigated what cell types secrete IL-18 upon stimulation with FasL. To address this, we prepared Kupffer cells (tissue macrophages in the liver) from aGVHD mice and incubated them with FasL-expressing cells in vitro. As shown in Figure 1C, Kupffer cells from aGVHD mice secreted IL-18 in response to membrane-associated FasL (column D), which was completely inhibited by neutralizing anti-FasL antibody (column F), indicating that the Kupffer cells secreted IL-18 upon stimulation with FasL. Kupffer cells acquired Fas expression after the induction of aGVHD (data not shown). Thus, the Fas/FasL system seems to play a role not only as an effector molecule involved in various tissue injuries including aGVHD,14,15,20 21 but also as a regulating factor prerequisite for the secretion of IL-18 in aGVHD.
Caspase-1–dependent IL-18 secretion after stimulation with FasL
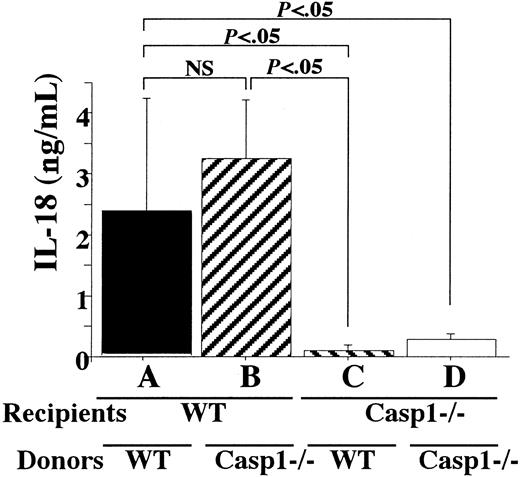
Although, as we demonstrated previously, caspase-1–deficient Kupffer cells derived from P acnes–primed mice can secrete IL-18 after stimulation with FasL,11 it is still possible that FasL stimulation might also activate caspase-1. Therefore, we investigated whether FasL-induced IL-18 accumulation in aGVHD is dependent on caspase-1. To test this possibility, we transplanted caspase-1–deficient or WT B6/129 spleen cells into caspase-1–deficient or WT BALB/c mice, respectively (Figure2). Caspase-1–deficient hosts having transplantation with caspase-1–deficient cells showed only a slight elevation in serum levels of IL-18 (Figure 2, column D), whereas WT hosts having transplantation with WT cells contained high serum levels of IL-18 (column A), indicating caspase-1–dependent IL-18 release. Furthermore, IL-18 serum levels were elevated in the WT hosts having transplantation with caspase-1–deficient donor cells (column B), whereas the inverse combination resulted in only a trace increase in the serum IL-18 concentration (column C). These data indicate that most of the elevated IL-18 in the circulation of aGVHD hosts is derived from hosts. Indeed, Kupffer cells from WT recipients engrafted with WT or caspase-1–deficient donor cells secreted IL-18 upon stimulation with mFasL, whereas those from caspase-1–deficient hosts secreted a trace amount of it, if any (data not shown). Moreover, only minor changes were observed in various target organs for aGVHD in caspase-1–deficient mice engrafted with WT or caspase-1–deficient allografts (H. I., unpublished data, December 2000). Therefore, IL-18 may be released from recipient cells in a FasL-mediated, caspase-1–dependent manner.
Caspase-1–dependent secretion of IL-18 is derived from hosts.
WT or caspase-1–deficient BALB/c (Casp1−/−) mice were lethally irradiated and underwent transplantation with WT or caspase-1–deficient B6/129 spleen cells (5 × 107). At day 5, serum was sampled and IL-18 levels were determined. Serum IL-18 in irradiated WT or caspase-1–deficient BALB/c mice having transplantation with BALB/c spleen cells at day 5 was not detectable. NS indicates not significant. Data represent the mean ± SD of 5 mice in each experimental group. Similar results were obtained in 3 independent experiments.
Caspase-1–dependent secretion of IL-18 is derived from hosts.
WT or caspase-1–deficient BALB/c (Casp1−/−) mice were lethally irradiated and underwent transplantation with WT or caspase-1–deficient B6/129 spleen cells (5 × 107). At day 5, serum was sampled and IL-18 levels were determined. Serum IL-18 in irradiated WT or caspase-1–deficient BALB/c mice having transplantation with BALB/c spleen cells at day 5 was not detectable. NS indicates not significant. Data represent the mean ± SD of 5 mice in each experimental group. Similar results were obtained in 3 independent experiments.
This is the first report that demonstrates FasL-mediated IL-18 accumulation in actually occurring diseases. As previously reported, pro–IL-18 is stored in Kupffer cells,22 and IL-18 requires cleavage for its secretion by appropriate enzymes that are distinct depending on the sorts of stimuli.7,8,11 The molecular basis of the processing of IL-18 is still unknown. A recent study demonstrated that upon stimulation with LPS, caspase-1 is activated via Toll-like receptor (TLR)-4, a signaling receptor for LPS, but independently of myeloid differentiation factor-88, an adaptor molecule for TLR-mediated signaling, leading to IL-18 secretion.22 However, we do not yet know the signaling pathway after TLR-4 activation. This is also the case for FasL-induced IL-18 secretion. Our present study suggests that FasL-mediated IL-18 processing might consist of 2 pathways. One is a caspase-1-like–dependent pathway, and the other is the caspase-1–dependent pathway shown here. We do not know the mechanism for how FasL stimulation selectively activates caspase-1 in aGVHD-induced mice or how the same stimulation preferentially activates caspase-1–like in P acnes–primed mice. Perhaps, FasL-mediated activation of different caspases may be involved in the development of distinct pathophysiologic events, such as massive liver necrosis in P acnes/soluble FasL-treated mice11 or severe bile duct destruction in aGVHD.23 Caspase-1 might be a potential therapeutic target for manipulating aGVHD. We are now investigating the pathologic role of IL-18 in aGVHD.
We thank Dr N. Kayagaki (Juntendo University, Tokyo, Japan) for providing mAb against murine FasL and mFasL, Dr K. Kuida (Vertex Pharmaceuticals, Boston, MA) for providing caspase-1–deficient mice, and Drs E. Seki and H. Nakano at Hyogo College of Medicine for the preparation of Kupffer cells. We are also grateful to Ms Asako Yamamoto for excellent technical assistance.
Supported in part by grants and Hitec Research Center Grant from the Ministry of Education, Science, and Culture, Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kenji Nakanishi, Department of Immunology and Medical Zoology, Hyogo College of Medicine, 1-1 Mukogawa-cho, Nishinomiya, Hyogo 663-8501, Japan; e-mail:nakaken@hyo-med.ac.jp.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal