Abstract
CD28 is the major costimulatory molecule on T cells. CD28 activation, in conjunction with T-cell receptor engagement, up-regulates transcription of several cytokines, including interleukin-2 (IL-2), through transcriptional activation of the RE/AP composite element. Although CD28 is not normally expressed on B cells or plasma cells, more than 90% of extramedullary myelomas (a late stage B-cell neoplasm) express CD28. The functional significance of this is unknown. The results of this study demonstrate that CD28 stimulates transcriptional activation of RE/AP-based reporters in B cells and myeloma cells. However, CD28 stimulation does not up-regulate IL-2 production in myeloma cell lines, demonstrating that the IL-2 promoter may not be a relevant RE/AP-containing target of CD28 in myelomas. Instead, an RE/AP composite element has been identified within the promoter of the IL-8 gene, a chemokine that promotes angiogenesis. Furthermore, stimulation of endogenous CD28 expressed by 3 myeloma cell lines increased IL-8 production. Therefore, the study demonstrates that CD28 is functional in myelomas to up-regulate transcription of endogenous genes, includingIL-8. The proposal is made that aberrant expression of CD28 may play a role in the progression of multiple myeloma.
Introduction
Myelomas are monoclonal B-cell malignancies, arising from a late stage differentiated B cell, and share many characteristics with immunoglobulin-secreting plasma cells (reviewed in references 1 and 2). Multiple myeloma accounts for approximately 1% of annual deaths from cancer each year.1 Myelomas are initially limited to the bone marrow, most likely due to a growth requirement for interleukin-6 (IL-6), which is produced by bone marrow stromal cells (BMSC)1 in patients with myeloma.3-5 Direct interaction between BMSC and myeloma cells induces IL-6 secretion by BMSC.6 In addition to IL-6, BMSC from myeloma patients also aberrantly secrete the chemokine IL-8,7 although the function of IL-8 secreted by BMSC on myelomas is not known. IL-8 belongs to the family of ELR-CXC chemokines, which have angiogenic activity.8 9 It is possible that the aberrant production of IL-8 by the BMSC of patients with myeloma does not have a direct effect on myeloma growth and survival per se, but may instead function to promote angiogenesis. However, in later stages of the disease, myeloma cells can be found outside of the bone marrow (extramedullary growth), suggesting that they no longer require cytokines/chemokines produced by the BMSC. Extramedullary myelomas, therefore, must either produce their own cytokines/chemokines or become independent of them for growth and survival.
Interestingly, a majority of myeloma samples from patients with advanced disease (as defined by a relapse after initial treatment, failure of initial treatment, or extramedullary growth) express the cell surface protein CD28, whereas CD28 is not expressed on normal B cells or plasma cells.10,11 At initial diagnosis, CD28 can be expressed on myeloma cells in some patients; however, more than 90% of extramedullary myelomas (which by definition have metastasized from the bone marrow and can grow into palpable masses in soft tissues) express CD28.12,13 Thus, expression of CD28 by myelomas correlates with metastasis, rather than the initial tumorigenesis. Because myelomas develop independently of CD28 expression, CD28 may not function by directly stimulating growth of myeloma cells, but rather may be involved in promoting metastasis. CD28 has also been found on the surface of almost all human myeloma cell lines.10-15CD28 is the only cell surface marker identified to date that correlates with an advanced state of multiple myeloma in humans. CD28 is not normally expressed in B cells,10,11 but is found on T cells where it plays an important role in T-cell activation.16 17
Stimulation of T cells through the antigen receptor is insufficient for T-cell activation. A second, costimulatory signal is also required, which is primarily produced by engagement of CD28 with its ligands CD80 or CD86 on the surface of antigen-presenting cells (APCs).18,19 CD28 costimulation leads to increased proliferation and up-regulation of several genes, includingIL-2, by both an increase in their transcription and an increase in their messenger RNA (mRNA) stability.20-22 Transcriptional activation of theIL-2 gene requires the RE/AP composite element, which is located approximately 160 nucleotides upstream of the transcription start site.23 RE/AP contains both a nuclear factor (NF)-κB site, which was originally identified as the CD28 response element within the IL-2 promoter, and the adjacent AP-1 site.24-26 Mutation of either site abrogates activation.24 CD28 alone cannot activate RE/AP—an additional signal is required, which can be supplied by either T-cell receptor (TCR) engagement, phorbol ester (PMA), or the combination of phorbol ester and calcium ionophore (PMA and ionomycin).
Because CD28 expression correlates with advanced disease in myeloma, signal transduction through CD28 may play a role in disease progression. It has been demonstrated that stimulation of CD28 in myeloma cell lines induced association of the cytoplasmic tail of CD28 with the p85 subunit of phosphatidyl inositol 3-kinase (PI3K).27 The functional role of PI3K in CD28 activation in T cells is controversial. Mutation of the PI3K binding site in CD28 had minimal effect on the ability of CD28 to up-regulate IL-2 production or transcription in one cell line,28,29 but eliminated CD28 function in another.30 Recent studies have suggested a possible role for Akt, a serine/threonine kinase, which is regulated by the products of PI3K in mediating NF-κB activation by CD28.31 Nevertheless, a clear pathway from CD28 to the activation of the RE/AP transcriptional complex is not completely defined.
Here, we provide evidence that CD28 is able to couple to downstream signaling pathways that up-regulate gene expression in myelomas. CD28 is shown to up-regulate transcription of the RE/AP composite element from the IL-2 promoter in 2 different myeloma cell lines that express CD28 endogenously. However, CD28 stimulation does not up-regulate IL-2 production in myeloma cell lines, demonstrating that the IL-2 promoter may not be a relevant RE/AP-containing target of CD28 in myelomas. Interestingly, we found that there is a sequence similar to RE/AP within the IL-8 promoter. This RE/AP-like sequence within the IL-8 promoter functions as a true composite element, because both the RE (NF-κB/CD28RE) and AP (AP-1) sites are required for CD28 up-regulation of either a multimerized IL-8 RE/AP reporter construct or the intact IL-8 promoter. Moreover, stimulation of endogenous CD28 expressed by 3 human myeloma cell lines markedly increased IL-8 production. Therefore, we have demonstrated that CD28 is functional in myelomas to up-regulate transcription of endogenous genes, including IL-8. We propose that the aberrant expression of CD28 by myeloma cells may contribute to multiple myeloma progression and metastasis through gene up-regulation, which in a subset of myeloma cells may include IL-8.
Materials and methods
Plasmids
The IL-2 promoter construct was a gift from Gerry Crabtree (Stanford University, Palo Alto, CA).32 The wild-type IL-8 promoter luciferase construct and the NF-κB mutated IL-8 promoter luciferase construct was a gift from Ken LeClair (Aquila Biopharmaceuticals, Framingham, MA). Mutations of the adjacent AP-1 site within the IL-8 promoter luciferase reporter were performed by polymerase chain reaction (PCR) mutagenesis using the following oligonucleotides (mutated nucleotides are in boldface):
5′ AAATCGTGGAATTTCCTCTACAAATATGAAAA 3′
3′ TTTAGCACCTTAAAGGAGATGTTTATACTTTT 5′
The choice of mutated nucleotides was based on the mutagenesis of the IL-2 RE/AP reporter construct, which eliminated activation when either the NF-κB or AP-1 sites were mutated.24 The IL-2 RE/AP reporter was generated by inserting 4 copies of RE/AP oligonucleotides into the SalI site in pΔODLO as previously described.24The nuclear factor of activated T cells (NFAT) reporter, as well as the CD8/28 and CD8T expression constructs, were previously described.33 34 The IL-8 RE/AP reporter constructs were generated by inserting 4 copies of the following oligonucleotides into the SalI site in pΔODLO (mutated nucleotides are in boldface):
IL-8 RE/AP: 5′ TCGAGAAATCGTGGAATTTCCTCTGACATAATG- AAAAG 3′
3′ CTTTAGCACCTTAAAGGAGACTGTATTACTTTCCAGCT 5′
IL-8 m/AP:5′ TCGAGAAATCGTGGACCTCGATCTGACATAATG- AAAAG 3′
3′ CTTTAGCACCTGGAGCTAGACTGTATTACTTTCCAGCT 5′
IL-8 RE/m:5′ TCGAGAAATCGTGGAATTTCCTCTACAAATATGA- AAAG 3′
3′ CTTTAGCACCTTAAAGGAGATGTTTATACTTTCCAGCT 5′
IL-8 m/m: 5′ TCGAGAAATCGTGGACCTCGATCTACAAATATGAAAAG 3′
3′ CTTTAGCACCTGGAGCTAGATGTTTATACTTTCCAGCT 5′
These mutations were based on the original mutations generated in the IL-2 RE/AP sequence that abolished activation.24 The reporter constructs were verified by sequencing.
Cell lines
The Bal17 cell line was a gift from Tony DeFranco (University of California, San Francisco, CA). The DT40 cell line was a gift from Tomohiro Kurosaki (Kansai Medical University, Moriguchi, Japan). The H929 human myeloma cell line was a gift from W. Michael Kuehl (National Institutes of Health, Bethesda, MD). The SK-MM-1 human myeloma cell line was a gift from Alan Houghton (Memorial Sloan-Kettering Cancer Center, New York, NY). All other human and murine myeloma cell lines were purchased from American Tissue Culture Collection (ATCC; Bethesda, MD). The CD28+ human myeloma cell RPMI8226 was described elsewhere.10 13
Transfections
Stimulations and luciferase assays
Twenty to 28 hours after transfections, 105 live cells were stimulated as described in the figure legend. For activation of RE/AP reporter constructs in B-cell lines and myeloma cell lines (Figures 1, 2, and 4), cells were stimulated for 6 hours as used previously in T-cell lines.24 Longer time points in B-cell lines and myeloma cell lines do not increase RE/AP activation (data not shown). PMA and ionomycin were purchased from Calbiochem (San Diego, CA), and used at a concentration as described in the figure legends for each cell line. C305 is a monoclonal antibody (mAb) against the Jurkat clonotypic TCR and was used at a 1:1000 dilution of miniperm material (Heraeus, Newtown, CT). Antihuman CD28 15E8 antibodies were purchased from Caltag (Burlingame, CA), and used at a final concentration of 1 μg/mL. Antimouse CD28 ascites 37.51 was a gift from Jim Allison (University of California, Berkeley, CA) and used at a final concentration of 1:1000. As an antibody control, MOPC315 (mouse immunoglobulin G1 (IgG1), isotype matched to anti–human CD28 mAb 15E8) was purchased from Cappel (Costa Mesa, CA) and used at a final concentration of 1 μg/mL. CD8 stimulation was performed using 1:1000 dilution of OKT8 ascites, which was generated from a hybridoma purchased from ATCC. FK506 (tacrolimus, purchased from the University of California, San Francisco, pharmacy) was used at a final concentration of 100 ng/mL, and added 15 minutes prior to stimulation. Luciferase assays were performed as previously described.35
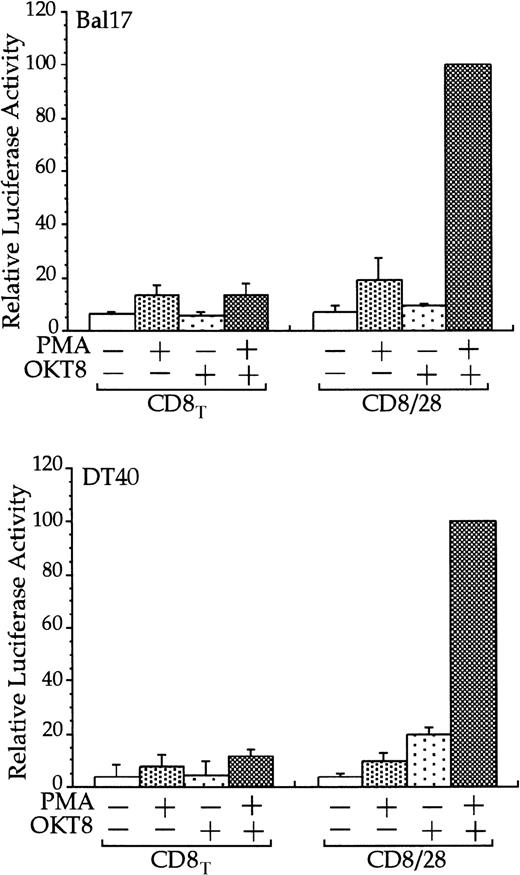
The cytoplasmic tail of CD28 up-regulates transcription of an RE/AP- based reporter in B cells.
The Bal17 and DT40 B cell lines were transfected with the IL-2 RE/AP reporter and an expression plasmid containing a chimeric molecule containing the extracellular and transmembrane regions of CD8 and the intracellular tail of CD28 (CD8/28) or the extracellular and transmembrane regions of CD8 alone (CD8T). The following day, the 105 live cells were unstimulated or stimulated with PMA (1 ng/mL for DT40 cells and 25 ng/mL for Bal17) and anti-CD8 antibody OKT8 at a 1:1000 dilution of ascites for 6 hours as denoted in the figure. Luciferase activity was standardized to the activation of PMA and OKT8 in CD8/28 transfected cells (n = 100). The actual average luciferase counts for PMA/OKT8 stimulation of RE/AP in CD8/28-transfected cells were 8270 in Bal17 and 80 604 in DT40. The results shown are the average of 3 independent transfections. Error bars reflect the SD from the mean.
The cytoplasmic tail of CD28 up-regulates transcription of an RE/AP- based reporter in B cells.
The Bal17 and DT40 B cell lines were transfected with the IL-2 RE/AP reporter and an expression plasmid containing a chimeric molecule containing the extracellular and transmembrane regions of CD8 and the intracellular tail of CD28 (CD8/28) or the extracellular and transmembrane regions of CD8 alone (CD8T). The following day, the 105 live cells were unstimulated or stimulated with PMA (1 ng/mL for DT40 cells and 25 ng/mL for Bal17) and anti-CD8 antibody OKT8 at a 1:1000 dilution of ascites for 6 hours as denoted in the figure. Luciferase activity was standardized to the activation of PMA and OKT8 in CD8/28 transfected cells (n = 100). The actual average luciferase counts for PMA/OKT8 stimulation of RE/AP in CD8/28-transfected cells were 8270 in Bal17 and 80 604 in DT40. The results shown are the average of 3 independent transfections. Error bars reflect the SD from the mean.
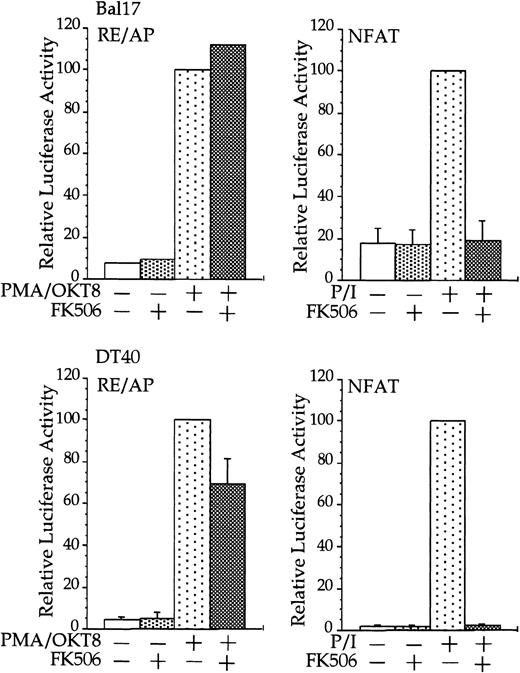
CD28 activation of RE/AP is resistant to FK506 in B cells.
Transfections and stimulations were performed as in Figure 1, with the expression and reporter constructs as denoted in the figure. For RE/AP and CD8/28 cotransfections, the results were standardized to the activation of PMA/OKT8 (n = 100); the actual average luciferase counts were 6613 for Bal17 and 73 938 for DT40. For NFAT transfections, the results were standardized to the activation of PMA/ionomycin; the actual average luciferase counts were 2297 for Bal17 and 70 289 for DT40. FK506 was added to some samples at a final concentration of 100 ng/mL before stimulation. For samples transfected with the NFAT reporter, ionomycin was added at a final concentration of 1 μM. The results shown are the average of 3 independent transfections. Error bars reflect the SD from the mean.
CD28 activation of RE/AP is resistant to FK506 in B cells.
Transfections and stimulations were performed as in Figure 1, with the expression and reporter constructs as denoted in the figure. For RE/AP and CD8/28 cotransfections, the results were standardized to the activation of PMA/OKT8 (n = 100); the actual average luciferase counts were 6613 for Bal17 and 73 938 for DT40. For NFAT transfections, the results were standardized to the activation of PMA/ionomycin; the actual average luciferase counts were 2297 for Bal17 and 70 289 for DT40. FK506 was added to some samples at a final concentration of 100 ng/mL before stimulation. For samples transfected with the NFAT reporter, ionomycin was added at a final concentration of 1 μM. The results shown are the average of 3 independent transfections. Error bars reflect the SD from the mean.
FACS analysis
For FACS analysis of murine myeloma cell lines, phycoerythrin-conjugated antimurine CD28 (BD-Pharmingen, San Diego, CA) and phycoerythrin-conjugated mouse-Ig G1 (BD-Pharmingen) were used as per the manufacturer's instructions.
IL-8 enzyme-linked immunosorbent assay
A matched antibody pair for performing human IL-8 enzyme-linked immunosorbent assays (ELISAs) was purchased from Pierce-Endogen (Woburn, MA). ELISAs were performed using 50 μL supernatant from stimulated cell cultures as per the manufacturer's instructions.
Results
CD28 activates an RE/AP reporter in B cells
Stimulation of CD28, in conjunction with TCR engagement, leads to the expression of several genes, including the IL-2 gene, through an increase in transcription as well as an increase in mRNA stability.20-22 Transcriptional up-regulation of theIL-2 gene by CD28 occurs through the RE/AP composite element within the IL-2 promoter.24-26 If CD28 is functional in multiple myeloma cells, then CD28 might be expected to up-regulate transcription of an RE/AP reporter in B-lineage cells. Bal17 murine and DT40 chicken B cells were transfected with a chimeric molecule containing the extracellular and transmembrane regions of CD8 and the cytoplasmic tail of CD28. A chimeric molecule was used to avoid any interaction and subsequent constitutive activation that could occur between CD28 and its ligands CD80 and CD86, which are normally expressed on B cells. As shown in Figure1, CD8/CD28 chimera stimulation, in conjunction with PMA, activated transcription from the RE/AP reporter in both B-cell lines. This is consistent with CD28 stimulation in T cells—CD28 alone is insufficient to activate RE/AP transcription and requires a second signal such as TCR stimulation or PMA. This up-regulation was dependent on the cytoplasmic tail of CD28, because a truncation of CD8 lacking the CD28 cytoplasmic tail did not activate RE/AP in either the Bal17 or DT40 cell lines (Figure 1). In addition, activation of RE/AP by PMA and CD28 was resistant to FK506 treatment, demonstrating that CD28 signaling in B cells, as in T cells, is resistant to FK506 (Figure 2). This is in marked contrast to responses seen with an NFAT transcriptional reporter. FK506 inhibited activation of an NFAT reporter in these B cells after stimulation with PMA and ionomycin (Figure 2). RE/AP is a composite element composed of NF-κB and an adjacent AP-1 site; both sites are required for activation in T cells.24 Similarly, both sites were also required for RE/AP activation in Bal17 and DT40 (data not shown). Therefore, CD28 is functional to up-regulate transcription in B-lineage cells and CD28-mediated activation of RE/AP in B- and T-cell lines is similar because (1) a second signal is required, (2) the cytoplasmic tail of CD28 is required, (3) activation by CD28 is resistant to FK506, and (4) both the NF-κB and AP-1 sites of RE/AP are required for activation.
CD28 is expressed on murine myeloma cell lines
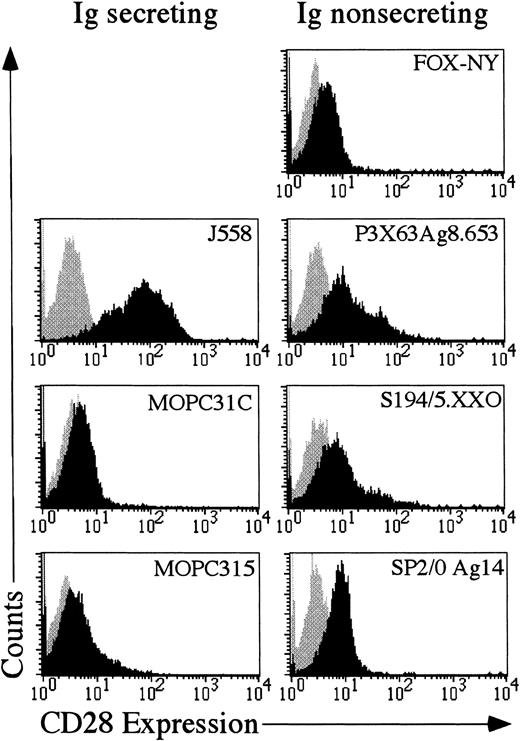
If aberrant expression of CD28 functions in the progression of multiple myeloma in humans, then it might be expected that murine myelomas would also express CD28. Seven different murine myeloma cells were examined for expression of CD28. As shown in Figure3, 4 of the 7 cell lines, J558, SP2/0 Ag14, P3X63Ag8.653 and S194/5.XXO had substantial amounts of CD28 on the cell surface. The other 3 cells lines were only slightly positive for CD28. Therefore, CD28 may play a role in multiple myeloma, because as previously shown in human myelomas12 13 and as shown here, at least some mouse myelomas aberrantly express CD28.
Expression of CD28 in murine myeloma cell lines.
Each myeloma cell line was stained with either mouse IgG1-phycoerythrin (Becton Dickinson) or antimurine CD28-phycoerythrin (Pharmingen). The gray shaded area reflects the histogram of cells stained with mouse IgG1-phycoerythrin. The black shaded area reflects the histogram of cells stained with anti-CD28 phycoerythrin.
Expression of CD28 in murine myeloma cell lines.
Each myeloma cell line was stained with either mouse IgG1-phycoerythrin (Becton Dickinson) or antimurine CD28-phycoerythrin (Pharmingen). The gray shaded area reflects the histogram of cells stained with mouse IgG1-phycoerythrin. The black shaded area reflects the histogram of cells stained with anti-CD28 phycoerythrin.
Endogenous CD28 activates RE/AP transcription in myeloma cell lines
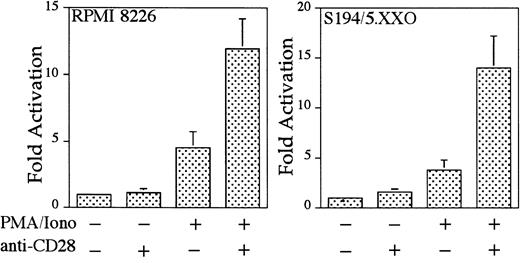
To examine whether CD28 functions in myeloma cells to up-regulate transcription, 2 CD28-expressing myeloma cell lines (human myeloma cell line RPMI 822611 12 and murine myeloma cell line S194/5.XXO) were transfected with an IL-2–derived RE/AP luciferase reporter construct and examined for endogenous CD28-mediated RE/AP activation. As shown in Figure 4, CD28 engagement alone did not activate RE/AP, but CD28 could further activate RE/AP transcription in the presence of PMA and ionomycin in both myeloma cell lines. These increases, although not as large as those seen in T cells, were significant (with P < .01 for both cell lines). Therefore, endogenous CD28 expressed in human and mouse myeloma cell lines is capable of up-regulating transcription through an RE/AP element derived from the IL-2gene.
Endogenous CD28 up-regulates transcription of an RE/AP reporter in myeloma cell lines.
The human myeloma cell line RPMI8226 and murine myeloma cell line S194/5.XXO were transfected with an RE/AP reporter. The following day, the 105 live cells were unstimulated or stimulated with PMA/ionomycin (25 ng/mL and 1 mM, respectively) and anti-CD28 (Caltag) for 6 hours as denoted in the figure. RPMI 8226 cells were stimulated with antihuman CD28 (Caltag) at 1 μg/mL. S194/5.XXO cells were stimulated with a 1:1000 dilution of antimurine CD28 ascites. Luciferase activity was standardized to the unstimulated control for each cell line (n = 1). The actual average counts for background RE/AP luciferase activity were 649 in RPMI 8226 and 402 in S194/5.XXO. The results shown are the average of 4 independent transfections. Error bars reflect the SD from the mean.
Endogenous CD28 up-regulates transcription of an RE/AP reporter in myeloma cell lines.
The human myeloma cell line RPMI8226 and murine myeloma cell line S194/5.XXO were transfected with an RE/AP reporter. The following day, the 105 live cells were unstimulated or stimulated with PMA/ionomycin (25 ng/mL and 1 mM, respectively) and anti-CD28 (Caltag) for 6 hours as denoted in the figure. RPMI 8226 cells were stimulated with antihuman CD28 (Caltag) at 1 μg/mL. S194/5.XXO cells were stimulated with a 1:1000 dilution of antimurine CD28 ascites. Luciferase activity was standardized to the unstimulated control for each cell line (n = 1). The actual average counts for background RE/AP luciferase activity were 649 in RPMI 8226 and 402 in S194/5.XXO. The results shown are the average of 4 independent transfections. Error bars reflect the SD from the mean.
CD28 stimulation up-regulates IL-8 production in a myeloma cell line
Because CD28 is capable of activating transcription in B cells and myeloma cells, and the expression of CD28 correlates well with extramedullary myelomas that have metastasized from the bone marrow, CD28 stimulation leading to gene expression may play a functional role in multiple myeloma progression. However, although CD28 stimulation in myeloma cells up-regulates transcription of the RE/AP element from the IL-2 promoter, it is unlikely that IL-2 is the target of CD28 in myeloma cells because CD28 stimulation failed to up-regulate IL-2 production in all myeloma cell lines we examined (n = 4, data not shown). It is possible that other genes with RE/AP elements within their promoters are the relevant targets of CD28 in myeloma.
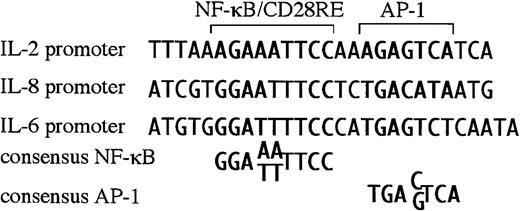
Newly diagnosed multiple myeloma is restricted to the bone marrow, most likely due to a requirement for cytokines/chemokines produced by the BMSC for growth and survival. For myelomas to metastasize and grow outside of the bone marrow, these extramedullary myelomas must have become independent of BMSC factors, or they must produce their own. BMSC from myeloma patients aberrantly produce IL-6 and IL-8.7 Interestingly, both the IL-6 and IL-8 promoters are responsive to CD28 in T cells,37 38 dependent on NF-κB sites within their respective promoters. On closer examination, both promoters were found have RE/AP-like sequences, an NF-κB site juxtaposed to an AP-1 site (Figure5). Because the IL-6 and IL-8 promoters contain RE/AP-like elements, perhaps these genes are relevant targets of CD28 in multiple myeloma progression.
There is a sequence similar to RE/AP in the IL-6 and IL-8 promoter.
The RE/AP sequence from the IL-2 promoter is compared with a similar sequence in the IL-6 promoter and the IL-8 promoter, as well as consensus NF-κB and AP-1 sites.
There is a sequence similar to RE/AP in the IL-6 and IL-8 promoter.
The RE/AP sequence from the IL-2 promoter is compared with a similar sequence in the IL-6 promoter and the IL-8 promoter, as well as consensus NF-κB and AP-1 sites.
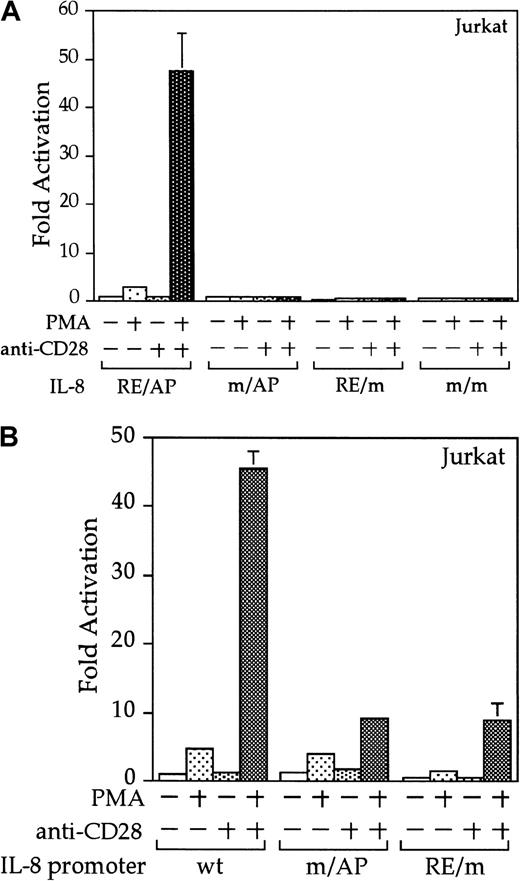
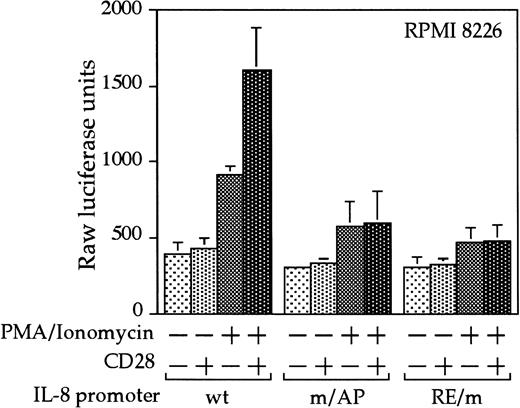
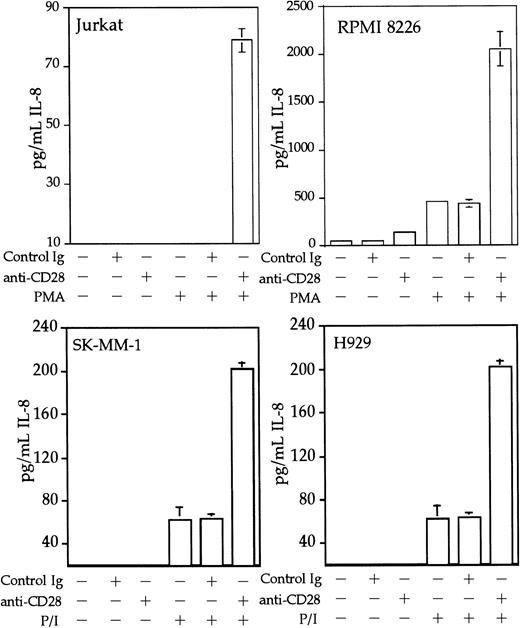
Interleukin 8 is a member of the ELR-CXC chemokines with angiogenic activity, whose promoter has been demonstrated in T cells to be up-regulated by CD28.38 We tested whether this sequence from the IL-8 promoter functions as a composite transcriptional element similar to IL-2 RE/AP. A reporter construct containing multimerized copies of the IL-8 RE/AP element behaved similarly to an IL-2 RE/AP reporter with respect to requiring 2 signals for activation in T cells (Figure 6A and data not shown). Mutagenesis of the IL-8 RE/AP reporter, as well as mutagenesis of the intact IL-8 promoter, demonstrate that CD28 responsiveness of the IL-8 promoter is due to a true RE/AP composite element, because both the RE (NF-κB/CD28RE) and AP (AP-1) sites are required for full activation (Figure 6A,B). Therefore, perhaps one RE/AP-containing target of CD28 in myeloma cells is IL-8. As shown in Figure7, CD28 stimulation increased transcription of the intact IL-8 promoter in RPMI 8226 myeloma cells (P < .02 for anti-CD28/PMA/ionomycin as compared to PMA/ionomycin alone). This up-regulation was absolutely dependent on the RE/AP site, because mutation of either the RE or AP sites abrogated activation (Figure 7). Not only was an effect of CD28 stimulation observed on the IL-8 promoter reporter construct, but CD28 (but not an isotype-matched control antibody) could up-regulate IL-8 production in the 3 human myeloma cell lines, RPMI 8226, SK-MM-1, and H929. Similar synergy is observed in Jurkat T cells (Figure8). Therefore, for the first time, we have demonstrated that stimulation of endogenous CD28 expressed by myeloma cells is capable of up-regulating an endogenous gene,IL-8.
The IL-8 promoter contains a true RE/AP composite element.
(A) Jurkat T cells were transfected with reporters containing 4 copies of the wild-type (wt) IL-8 RE/AP element, or constructs containing mutations at the NF-κB site, AP-1 site, or both. The following day, the 105 live cells were unstimulated or stimulated with 5 ng/mL PMA and 1 μg/mL anti-CD28 (Caltag) for 6 hours as denoted in the figure. Luciferase activity was standardized to the unstimulated control (n = 1). The actual average counts for background IL-8 RE/AP luciferase activity were 1020. The results shown are the average of 3 independent transfections. Error bars reflect the SD. (B) Jurkat cells were transfected as above, except that reporter constructs containing either the wild-type IL-8 promoter or ones containing mutations at either the NF-κB site or AP-1 sites within the IL-8 RE/AP sites were used. Luciferase activity was standardized to the unstimulated control (n = 1). The actual average counts for background IL-8 promoter luciferase activity were 2256. The results shown are the average of 3 independent transfections. Error bars reflect the SD.
The IL-8 promoter contains a true RE/AP composite element.
(A) Jurkat T cells were transfected with reporters containing 4 copies of the wild-type (wt) IL-8 RE/AP element, or constructs containing mutations at the NF-κB site, AP-1 site, or both. The following day, the 105 live cells were unstimulated or stimulated with 5 ng/mL PMA and 1 μg/mL anti-CD28 (Caltag) for 6 hours as denoted in the figure. Luciferase activity was standardized to the unstimulated control (n = 1). The actual average counts for background IL-8 RE/AP luciferase activity were 1020. The results shown are the average of 3 independent transfections. Error bars reflect the SD. (B) Jurkat cells were transfected as above, except that reporter constructs containing either the wild-type IL-8 promoter or ones containing mutations at either the NF-κB site or AP-1 sites within the IL-8 RE/AP sites were used. Luciferase activity was standardized to the unstimulated control (n = 1). The actual average counts for background IL-8 promoter luciferase activity were 2256. The results shown are the average of 3 independent transfections. Error bars reflect the SD.
CD28 stimulation leads to up-regulation of the IL-8 promoter in myeloma cells.
RPMI 8226 myeloma cells were transfected as in Figure 3, with either a wild-type IL-8 promoter or ones containing mutations at either the NF-κB site or AP-1 site within the IL-8 RE/AP sites. Cells were stimulated for 18 hours. Raw luciferase units were used without standardization. The results shown are the average of 3 independent transfections. Error bars reflect the SD.
CD28 stimulation leads to up-regulation of the IL-8 promoter in myeloma cells.
RPMI 8226 myeloma cells were transfected as in Figure 3, with either a wild-type IL-8 promoter or ones containing mutations at either the NF-κB site or AP-1 site within the IL-8 RE/AP sites. Cells were stimulated for 18 hours. Raw luciferase units were used without standardization. The results shown are the average of 3 independent transfections. Error bars reflect the SD.
CD28 stimulation enhances IL-8 production by myeloma cells.
Jurkat T cells, RPMI 8226, SK-MM-1, or H929 human myeloma cells were stimulated with PMA, 25 ng/mL for Jurkat, SK-MM-1, and H929, 1 ng/mL for RPMI 8226, 1 μM ionomycin, 1 μg/mL anti-CD28, and/or 1 μg/mL isotype-matched control antibody MOPC195 as indicated. The cells were used at a concentration of 1 × 106/mL. After 18 hours, the supernatants were tested for IL-8 by ELISA, using the matched antibody pair and reagents from Pierce-Endogen as per the manufacturer's instructions. Within each experiment, the stimulations were performed in triplicate. The results shown are the average of 3 independent experiments. Error bars reflect the SD.
CD28 stimulation enhances IL-8 production by myeloma cells.
Jurkat T cells, RPMI 8226, SK-MM-1, or H929 human myeloma cells were stimulated with PMA, 25 ng/mL for Jurkat, SK-MM-1, and H929, 1 ng/mL for RPMI 8226, 1 μM ionomycin, 1 μg/mL anti-CD28, and/or 1 μg/mL isotype-matched control antibody MOPC195 as indicated. The cells were used at a concentration of 1 × 106/mL. After 18 hours, the supernatants were tested for IL-8 by ELISA, using the matched antibody pair and reagents from Pierce-Endogen as per the manufacturer's instructions. Within each experiment, the stimulations were performed in triplicate. The results shown are the average of 3 independent experiments. Error bars reflect the SD.
Discussion
We provide the first evidence that CD28 expressed on myeloma cells is functional to up-regulate gene transcription, using an RE/AP reporter. The activation of the RE/AP reporter by CD28 in 2 B-cell lines, DT40 and Bal17, establishes that CD28 can mediate signals in B-cell lineage cells to up-regulate promoters containing this composite element. Moreover, the signals induced are similar to those previously characterized for the activation of RE/AP in T cells because CD28-mediated up-regulation (1) required its cytoplasmic tail, (2) required a second signal, (3) was resistant to FK506, and (4) required both the NF-κB and AP-1 sites of the RE/AP reporter. These observations are directly relevant for one class of B lineage cells that have the unusual phenotype of CD28 expression. Moreover, endogenous CD28 expressed on myeloma cells up-regulated RE/AP transcription. We have identified an RE/AP composite element in the IL-8 promoter and demonstrated that this RE/AP site is critical for CD28-mediated up-regulation of the IL-8 promoter in both the Jurkat T-cell line and, importantly, in the RPMI 8226 myeloma cell line. In addition, CD28 stimulation significantly increased IL-8 protein secretion in 3 myeloma cell lines (RPMI 8226, SK-MM-1, and H929).
Interleukin 8 is an ELR-containing CXC chemokine with angiogenic activity. The best correlation with CD28 expression on myelomas is with metastasis and growth of myelomas outside the bone marrow (extramedullary myelomas). The initial growth and development of myelomas can occur independently of CD28, because CD28 is not commonly found on myelomas from patients at diagnosis.12,13Although extramedullary myelomas are not particularly vascularized, there must be some angiogenesis for the growth and survival of extramedullary myelomas that can form palpable masses a few centimeters in diameter. Perhaps CD28 functions in extramedullary myelomas to induce tumor-promoting angiogenic factors. Because IL-8 has angiogenic activity, and the aberrant expression of CD28 by human myelomas correlates well with metastasis, CD28 may play a functional role in the progression of multiple myeloma. Indeed, the expression of IL-8 has been shown to regulate tumor metastasis in 2 other human cancers, melanoma and prostate cancer, using animal models.39,40Therefore, it is possible that IL-8 secretion induced through CD28 functions in metastasis, which is the reason more than 90% of metastasized, extramedullary myelomas express CD28.12 It is interesting to note that the ligands for CD28, CD80 and CD86, can be found on the surface of myeloma cells12,15 41 and are normally expressed by B cells. Therefore, CD28 expressed by multiple myeloma cells could potentially be constitutively stimulated, by CD80 or CD86 or both expressed on neighboring myeloma cells, or potentially even on the same cell.
Although we have demonstrated that stimulation of endogenous CD28 can up-regulate IL-8 production by myeloma cells, it does not preclude the involvement of additional targets of CD28 in myeloma cells or show that IL-8 is the critical gene up-regulated by CD28 that leads to multiple myeloma metastasis. Also, IL-8 may be the relevant target of CD28 in a subset, but not all, myelomas. For example, IL-6 may be a relevant target in others. CD28 up-regulates the IL-6 promoter in T cells.38 We were unable to demonstrate up-regulation of IL-6 in myeloma cell lines (n = 15, data not shown), or that stimulation or inhibition of CD28 could enhance or slow the growth of myeloma cell lines (n = 5, data not shown). Nevertheless, it is still possible that this may occur in a different subset of myelomas in vivo. However, because we have demonstrated that CD28 is capable of up-regulating endogenous genes in myelomas, future work will focus on whether myelomas freshly isolated from patient samples up-regulate IL-8 in response to CD28 stimulation, whether IL-8 can promote myeloma metastasis and whether any other CD28-responsive genes may also be up-regulated in myelomas.
Our data also suggest new forms of therapy for advanced multiple myeloma, based on CD28 expression and function in extramedullary disease. Although newly diagnosed multiple myelomas respond to current chemotherapy, a relapse in which the cells are resistant to intervention almost always occurs, with fatal consequences. It is possible that the aberrant expression of CD28 plays a causative role in multiple myeloma disease progression. We have demonstrated that endogenous CD28 expressed by myeloma cells is capable of up-regulating transcription of an RE/AP reporter, and that CD28 stimulation up-regulates production of the RE/AP-containing gene, IL-8. If CD28 plays a functional role in multiple myeloma disease progression, then inhibition of CD28 function may slow the progress of disease.
We thank Drs. Michael Shapiro, Jeanne Baker, and Tim Finco for critical reading of the manuscript. V.S.S. is a Special Fellow of the Leukemia and Lymphoma Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Arthur Weiss, Box 0795, Rm U330, University of California, San Francisco, San Francisco, CA 94143; e-mail:aweiss@medicine.ucsf.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal