Abstract
Lymphomas were studied in kindreds with autoimmune lymphoproliferative syndrome (ALPS; Canale-Smith syndrome), a disorder of lymphocyte homeostasis usually associated with germline Fas mutations. Fas (CD95/APO-1) is a cell surface receptor that initiates programmed cell death, or apoptosis, of activated lymphocytes. Lymphoma phenotype was determined by immunohistochemistry, frequency of CD3+CD4−CD8− T-cell–receptor α/β cells by flow cytometry, nucleotide sequences of the gene encoding Fas (APT1, TNFRSF6), and the percentage of lymphocytes undergoing apoptosis in vitro. Of 223 members of 39 families, 130 individuals possessed heterozygous germline Fas mutations. Eleven B-cell and T-cell lymphomas of diverse types developed in 10 individuals with mutations in 8 families, up to 48 years after lymphoproliferation was first documented. Their risk of non-Hodgkin and Hodgkin lymphomas, respectively, was 14 and 51 times greater than expected (each P < .001). Investigation of these 10 patients and their relatives with Fas mutations revealed that all had defective lymphocyte apoptosis and most had other features of ALPS. The tumor cells retained the heterozygous Fas mutations found in the peripheral blood and manifested defective Fas-mediated killing. These data implicate a role for Fas-mediated apoptosis in preventing B-cell and T-cell lymphomas. Inherited defects in receptor-mediated lymphocyte apoptosis represent a newly appreciated risk factor for lymphomas.
Introduction
Chromosomal translocations leading to the dysregulation of cell division and differentiation contribute to lymphomagenesis.1-3 Another genetic mechanism that contributes to lymphoma development is the acquired failure of lymphocytes to be susceptible to programmed cell death, or apoptosis. Some lymphoma cells overexpress Bcl-2, a protein that inhibits apoptosis.4-6 Each of these predisposing molecular and cytogenetic defects arise within individual somatic cells, but it is unclear whether they relate to the hereditary risk for lymphoma.
The recent recognition of the autoimmune lymphoproliferative syndrome (ALPS; also called the Canale-Smith syndrome) afforded us the opportunity to explore its cause, inherited defects in genes that promote lymphocyte apoptosis, as an additional contributor to lymphoma development.7-18 ALPS is characterized by chronic lymphadenopathy and splenomegaly of early onset, autoimmune phenomena, and expanded populations of T-cell receptor α/β (TCRα/β) CD3+CD4−CD8−, or double-negative, T cells (DNTCs). Among over 70 reported kindreds with ALPS,7-16 most are cases of ALPS type Ia that are associated with mutations in the APT1 (TNFRSF6) gene encoding Fas (CD95/Apo-1). Patients with mutations in theFas ligand gene (TNFSF6)16 possess ALPS type Ib. Cases with mutations in caspase 10 are termed ALPS type II,17 and those without defined mutations are termed ALPS type III.11 18
Fas is a transmembrane receptor in the tumor necrosis factor receptor (TNFR) gene family. B- and T- lymphocyte apoptosis is initiated by binding of the Fas ligand to Fas.19 Fas transduces the death signal through its cytoplasmic “death domain,” the binding site for proteins that activate cysteine proteases (caspases) that mediate the apoptosis cascade.20-22 The prominent lymphoid abnormalities in ALPS in the absence of significant pathology in other organ systems suggest that the Fas-Fas ligand apoptosis pathway has 2 primary roles: the elimination of unneeded and potentially deleterious lymphocytes, and the killing of virus-infected cells.19 We now ask whether Fas-mediated apoptosis plays a role in the prevention of lymphoid malignancy.
Patients, materials, and methods
Study subjects
One hundred members of 26 families studied at the National Institutes of Health (NIH) were found to have deleterious Fas mutations: 6 members of 5 of these families developed B-cell lymphomas (Figure 1 and Table1). In the course of this work, we learned of 4 more lymphomas among 30 individuals with Fas mutations in 13 additional families including 2 cases in Germany23 and 2 individuals studied in New York.
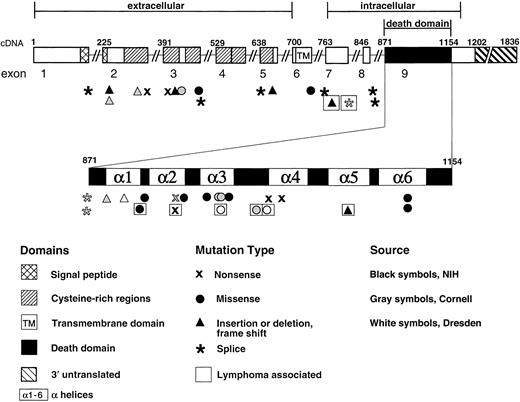
Structure of the TNFRSF6 (APT1) gene encoding the Fas protein, showing locations of the mutations associated with ALPS and lymphoma.
Exon 9 is expanded to show α-helical regions of the intracellular death domain.67 Mutations are newly described or previously reported from NIH (indicated by black symbols),8,11,15,44 Cornell University, New York (indicated by gray symbols),10,26 or Dresden, Germany (indicated by white symbols).30 Mutations seen in individuals with lymphoma are indicated by boxed symbols.
Structure of the TNFRSF6 (APT1) gene encoding the Fas protein, showing locations of the mutations associated with ALPS and lymphoma.
Exon 9 is expanded to show α-helical regions of the intracellular death domain.67 Mutations are newly described or previously reported from NIH (indicated by black symbols),8,11,15,44 Cornell University, New York (indicated by gray symbols),10,26 or Dresden, Germany (indicated by white symbols).30 Mutations seen in individuals with lymphoma are indicated by boxed symbols.
Clinical and laboratory profiles of lymphoma patients with germline Fas mutation
| Study center . | Patient . | Sex . | Age* . | Adenopathy . | Splenomegaly . | Autoimmune disease . | DNTCs (%)† . | Cells killed (%)‡ . | ALPS1-153 . | Yr from onset1-155 . | Lymphoma . | Fas mutation . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anemia . | Thrombocytopenia . | Neutropenia . | Autoantibodies . | ||||||||||||
| Bethesda | 3-II-1 | Male | 38 | + | + | + | − | − | + | 6.1 | 32 | + | 22 | NLP Hodgkin disease | 915A→C |
| Bethesda | 3-II-3 | Male | 27 | + | + | + | − | − | ND | ND | ND | − | 21 | T-cell–rich B lymphoma | 915A→C |
| Bethesda | 26-II-4 | Male | 54 | + | + | + | + | − | + | 0.7 | 3 | − | 48 | Burkitt lymphoma | 973A→T |
| Bethesda | 30-II-7 | Female | 30 | + | + | + | + | − | − | 5.9 | 6 | + | 15 | Burkitt lymphoma | 1074delT |
| Bethesda | 45-III-2 | Male | 12 | + | + | + | + | − | + | 11.7 | 12 | + | 6 | Hodgkin disease | 779del11 |
| Bethesda | 55-II-1 | Male | 19 | + | + | − | + | − | + | 9.7 | 1 | + | 15 | Follicular B lymphoma | 942C→T |
| New York | P9-II-4 | Male | 30 | + | + | + | − | − | ND | ND | 29 | − | 21 | Hodgkin disease | IVS7(+2)T→A |
| New York | P10-I-1 | Female | 38 | + | + | − | + | − | + | 18.0 | 5 | + | 0 | Hodgkin disease | 1003C→T |
| Germany | G3-II-8 | Male | 31 | + | − | − | − | − | ND | 4.0 | < 2 | − | 0 | Hodgkin disease; marginal zone B-cell lymphoma | 1009A→G |
| Germany | G3-III-4 | Female | 7 | + | + | − | + | − | + | 75 | 12 | + | 2 | T-cell lymphoma | 1009A→G |
| Study center . | Patient . | Sex . | Age* . | Adenopathy . | Splenomegaly . | Autoimmune disease . | DNTCs (%)† . | Cells killed (%)‡ . | ALPS1-153 . | Yr from onset1-155 . | Lymphoma . | Fas mutation . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anemia . | Thrombocytopenia . | Neutropenia . | Autoantibodies . | ||||||||||||
| Bethesda | 3-II-1 | Male | 38 | + | + | + | − | − | + | 6.1 | 32 | + | 22 | NLP Hodgkin disease | 915A→C |
| Bethesda | 3-II-3 | Male | 27 | + | + | + | − | − | ND | ND | ND | − | 21 | T-cell–rich B lymphoma | 915A→C |
| Bethesda | 26-II-4 | Male | 54 | + | + | + | + | − | + | 0.7 | 3 | − | 48 | Burkitt lymphoma | 973A→T |
| Bethesda | 30-II-7 | Female | 30 | + | + | + | + | − | − | 5.9 | 6 | + | 15 | Burkitt lymphoma | 1074delT |
| Bethesda | 45-III-2 | Male | 12 | + | + | + | + | − | + | 11.7 | 12 | + | 6 | Hodgkin disease | 779del11 |
| Bethesda | 55-II-1 | Male | 19 | + | + | − | + | − | + | 9.7 | 1 | + | 15 | Follicular B lymphoma | 942C→T |
| New York | P9-II-4 | Male | 30 | + | + | + | − | − | ND | ND | 29 | − | 21 | Hodgkin disease | IVS7(+2)T→A |
| New York | P10-I-1 | Female | 38 | + | + | − | + | − | + | 18.0 | 5 | + | 0 | Hodgkin disease | 1003C→T |
| Germany | G3-II-8 | Male | 31 | + | − | − | − | − | ND | 4.0 | < 2 | − | 0 | Hodgkin disease; marginal zone B-cell lymphoma | 1009A→G |
| Germany | G3-III-4 | Female | 7 | + | + | − | + | − | + | 75 | 12 | + | 2 | T-cell lymphoma | 1009A→G |
Presence (+), or absence or lack of confirmation (−) of clinical finding.
ND indicates not done; NLP Hodgkin disease, nodular lymphocyte-predominant Hodgkin disease.
When last studied, or died.
Percent of TCR α/βCD4−CD8− T cells. The laboratory normal value is < 1.0%.
Percent of cells killed after treatment with an anti-Fas MoAb. The NIH laboratory normal value is 52% ± 12% (mean ± SD).
The case does (+) or does not (−) meet the ALPS case definition. Immunophenotyping could not be done to confirm an elevated percentage of DNTCs in subjects who had died.
Time in years from manifesting first features of ALPS until lymphoma diagnosis.
All individuals with ALPS-like features within these families with Fas mutations and their available relatives in each of the study centers were enrolled after written informed consent in institutional review board–approved research protocols. All amenable subjects were examined and bled for extensive studies. In all, 223 persons in 39 families were evaluated.
Case definition
Autoimmune lymphoproliferative syndrome was defined as chronic, nonmalignant lymphadenopathy and/or splenomegaly, defective lymphocyte apoptosis in vitro, and 1% or more DNTCs.
Flow cytometry analysis
Immunohistochemical analysis
Detection of Fas gene mutation
Cell culture and stimulation
The PBMCs were separated from heparinized venous blood or buffy coat fractions by Ficoll-Hypaque (Pharmacia Fine Chemicals, Piscataway, NJ) density gradients. PBMCs at 106/mL were activated with 10 μg/mL phytohemagglutinin (PHA) in RPMI 1640 with 10% fetal calf serum, 2 mM glutamine, and penicillin-streptomycin. Cultures were maintained in recombinant human interleukin 2 (IL-2) (100-200 IU/mL) (Midwest Medical, Bridgeton, MO) with refeeding every 48 to 72 hours.
Transformation of immortalized Epstein-Barr virus cell lines
The Epstein-Barr virus (EBV)-transformed B-cell lines were prepared and cultured by standard methods.27
Induction of apoptosis
Statistical methods
For the analysis of TCR α/β DNTC results, median values for the percentage of total T-cell number were compared by the Wilcoxon 2-sample test. The same method was applied to cell death induced by anti-Fas MoAb in both T and B cells. All statistical tests were 2-tailed.
For estimation of the risk of developing lymphoma, patient years were calculated using the age at the most recent evaluation or when lymphoma was diagnosed. The Poisson distribution was used to determine the probabilities of observing 5 or more non-Hodgkin or Hodgkin lymphoma cases, given the expected numbers (9.4 and 2.6 cases/100 000, respectively) reported in the SEER Cancer Statistics Review for U.S. men and women aged less than 65 years.28 With a median age of 27.8 years (range 1-86 years) and only 5 study individuals with Fas mutations over age 65, the SEER data for the population under age 65 years most closely described the distribution of ages in this study. The 95% confidence interval for the observed to expected ratio was determined, as described.29
Results
Case histories of lymphoma patients
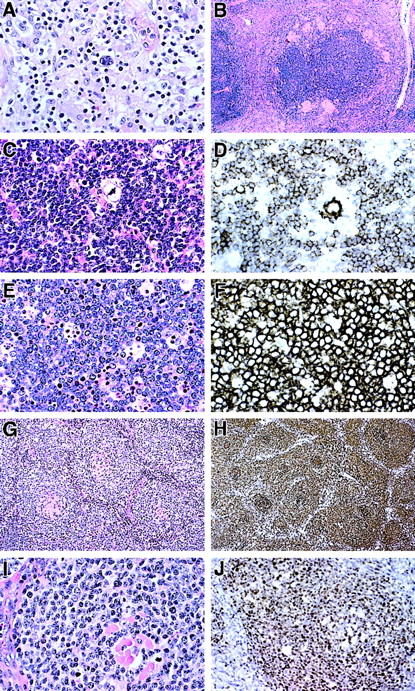
In NIH family 3 with a Fas alteration (915A→C; T225P; Figure 1) subject 3-II-3 had anemia, cervical adenopathy, and a palpable spleen at age 4 years and underwent 2 nondiagnostic lymph node biopsies before a third at age 25 suggested Hodgkin disease. Chemotherapy and radiation therapy were instituted for stage IIIA disease; however, he died of extensive disease. The pathologic specimens were reviewed (by E.S.J.) and reclassified as histiocyte-rich, T-cell–rich large B-cell lymphoma that was negative for EBV by in situ hybridization (Figure2A).30 31 The lymphoma cells were CD20+ but negative for CD30 and CD15 in a background of T cells and histiocytes.
Histopathology and immunopathology of lymphomas in ALPS patients.
(A) T-cell and histiocyte-rich B-cell lymphoma diagnosed in patient 3-II-3. Large polylobated tumor cells, which stained for CD20, are seen in a background of T lymphocytes and histiocytes. (B-D) Nodular lymphocyte-predominant Hodgkin disease diagnosed in patient 3-II-1. (B) A rim of epithelioid granulomas surrounds a large nodule containing neoplastic cells. (C) A single polylobated L&H cell is identified in a background of small lymphocytes. (D) A CD20+ L&H cell is rosetted by a rim of CD20− T cells. Numerous CD20+ small B cells are present in the background. (E) Burkitt lymphoma presenting as an omental mass in patient 26-II-4. Tumor cells are moderate in size with clumped chromatin and basophilic nucleoli. A prominent starry sky is present. (F) Tumor cells uniformly express CD20. The growth fraction was 100% with MIB-1 (Ki-67) staining. Identical histologic and immunophenotypic features were seen in lymphoma involving small bowel in patient 30-III-2. (G) An unusual form of follicular lymphoma was diagnosed in patient 55-II-1. Neoplastic cells surround hyalinized and possibly regressed follicular remnants. (H) CD20 immunohistochemistry emphasized follicular pattern. (I) The neoplastic cells are moderate in size with clumped chromatin. (J) A follicle-center origin for tumor cells is supported by Bcl-6 immunohistochemistry, which selectively stains the neoplastic cells within the follicles. Original magnification (A), ×400; (B), ×60; (C), ×400; (D), ×400; (E), ×400; (F), ×400; (G), ×200; (H), ×200; (I), ×400; and (J), ×250.
Histopathology and immunopathology of lymphomas in ALPS patients.
(A) T-cell and histiocyte-rich B-cell lymphoma diagnosed in patient 3-II-3. Large polylobated tumor cells, which stained for CD20, are seen in a background of T lymphocytes and histiocytes. (B-D) Nodular lymphocyte-predominant Hodgkin disease diagnosed in patient 3-II-1. (B) A rim of epithelioid granulomas surrounds a large nodule containing neoplastic cells. (C) A single polylobated L&H cell is identified in a background of small lymphocytes. (D) A CD20+ L&H cell is rosetted by a rim of CD20− T cells. Numerous CD20+ small B cells are present in the background. (E) Burkitt lymphoma presenting as an omental mass in patient 26-II-4. Tumor cells are moderate in size with clumped chromatin and basophilic nucleoli. A prominent starry sky is present. (F) Tumor cells uniformly express CD20. The growth fraction was 100% with MIB-1 (Ki-67) staining. Identical histologic and immunophenotypic features were seen in lymphoma involving small bowel in patient 30-III-2. (G) An unusual form of follicular lymphoma was diagnosed in patient 55-II-1. Neoplastic cells surround hyalinized and possibly regressed follicular remnants. (H) CD20 immunohistochemistry emphasized follicular pattern. (I) The neoplastic cells are moderate in size with clumped chromatin. (J) A follicle-center origin for tumor cells is supported by Bcl-6 immunohistochemistry, which selectively stains the neoplastic cells within the follicles. Original magnification (A), ×400; (B), ×60; (C), ×400; (D), ×400; (E), ×400; (F), ×400; (G), ×200; (H), ×200; (I), ×400; and (J), ×250.
This individual's brother, subject 3-II-1, had documented cervical and axillary lymphadenopathy by age 3 years. Six biopsies revealed reactive hyperplasia before age 15, when he developed Coombs-positive hemolytic anemia, hepatosplenomegaly, and thrombocytopenia. Splenectomy was performed at age 18. At age 25, further biopsy showed lymphoma (Figure 2B-D), confirmed as an EBV-negative nodular lymphocyte-predominant Hodgkin disease on review. He received combination chemotherapy for stage IIIA disease; however, he relapsed in 1986 and again in 1992. On re-evaluation in 1995, he was free of tumor and asymptomatic, although diffuse peripheral lymphadenopathy persisted.
Study of NIH family 26 with a heterozygous Fas alteration (973A→T; D244V; Figure 1) identified subject 26-II-4, who presented at age 2 with adenopathy and splenomegaly and was diagnosed with acute leukemia. Splenectomy was performed at age 3, and idiopathic thrombocytopenic purpura occurred at age 23. At age 50, Burkitt lymphoma was recognized in an omental mass and confirmed as an EBV-positive B-cell lymphoma (Figure 2E); the tumor remitted with chemotherapy. At age 54, he developed Coombs-positive hemolysis.
Studies of NIH family 30 with a Fas alteration (1074delT; T227fs [frame-shift]; Figure 1) identified female subject 30-II-7with anemia, adenopathy, and splenomegaly at age 5 weeks, splenectomy and lymph node biopsy at age 7, followed by autoimmune hemolytic anemia and idiopathic thrombocytopenic purpura (ITP). At age 17, a new abdominal mass represented EBV-negative Burkitt lymphoma (Figure 2E,F). She was treated with cyclophosphamide, vincristine, and prednisone. At age 31, she remains free of lymphoma.
NIH patient 45-III-2 is a 12-year-old boy who presented with adenopathy, splenomegaly, anemia, and ITP at ages 5 and 7, which led to splenectomy and lymph node biopsy that showed changes typical of ALPS (Table 1).32 At age 10, his Fas mutation (779def11; K181fs [frameshift]; Figure 1) was found. At 11, he lost weight and experienced marked enlargement of lymph nodes in the neck and chest; a biopsy demonstrated Hodgkin disease, EBV-positive, mixed cellularity subtype. He is currently completing multidrug chemotherapy.
NIH subject 55-II-1 is a 19-year-old man (Table 1). He presented with splenomegaly at age 2, underwent splenectomy at age 4, and experienced chronic adenopathy and multiple episodes of thrombocytopenia treated with glucocorticosteroids or intravenous immunoglobulin. A lymph node biopsy at age 4 was nondiagnostic, but 3 biopsies for enlarging axillary, submandibular, and inguinal nodes at age 17 demonstrated a nonclassifiable B-cell lymphoma with atypical follicular proliferation and chromosomal rearrangements (Figure 2G-J). The axillary lymph node was effaced by an atypical follicular proliferation. The neoplastic cells in it were positive for CD20, CD19, CD79a, and Bcl-6 but negative for CD10 and bcl-2, suggestive of a follicle center origin (Bcl-6+) but unusual for follicular lymphoma (Bcl-2−, CD10−).33 The cells were negative for surface immunoglobulin and for EBV. Stains for CD5, cyclin D1, CD23, and CD11c were negative. Rearrangements of theIg and TCR-γ genes were examined by polymerase chain reaction (PCR) and showed no clonal bands.34 The cells were negative by PCR for translocations involving the Ig heavy chain gene and the Bcl-2 major breakpoint region and the Bcl-1 major translocation cluster. The submandibular and inguinal lymph nodes showed abnormal follicular B-cell proliferation consistent with lymphoma. The cells were surface Ig−, but dim monoclonal cytoplasmic κ light chain expression was detected by flow cytometry. Two of the node biopsies showed identical clonal cytogenetic abnormalities with unbalanced translocations involving multiple chromosomes. His peripheral blood showed a heterozygous Fas mutation (942C→T; R234X; Figure 1). Four cycles of rituximab yielded partial response. With further nodal enlargement, additional biopsies revealed a more aggressive, Bcl-2+ follicular lymphoma that remitted with multidrug chemotherapy.
In New York family 9 with Fas mutations (1003C→T; T254I; Figure 1), subject P9-II-4 manifested adenopathy, splenomegaly, and autoimmune hemolysis at age 5. Over the next decade, he underwent a splenectomy and several nondiagnostic lymph node biopsies. At age 26, he developed night sweats, and further biopsy revealed Hodgkin disease, mixed cellularity type. His maternal aunt (P9-I-5) had adenopathy since infancy and died at age 36 from a T-cell–rich large B-cell lymphoma. Fas sequencing could not be conducted on her available tissue sections, and her case is not included in the analyses below.
In New York family P10 with a Fas mutation (IVS7(+2)T6A; P201fs; Figure1), subject P10-I-1 is a 38-year-old woman with Hodgkin disease, nodular sclerosis type, stage IA, at age 5, treated successfully with local irradiation. Three years later, she developed abdominal and inguinal adenopathy and splenomegaly. Repeat biopsies and splenectomy showed only reactive changes compatible with ALPS,32 but no lymphoma. ITP developed at age 17.
An EBV-positive T-cell lymphoma (G3-III-4) in German family 3 with a Fas mutation (1009A→G; E256G; Figure 1) was reported recently.23 Also reported was individual G3-II-8, who had Hodgkin disease, mixed cellularity subtype, at age 13.23 Seventeen years later, however, he presented with fever, wasting, and night sweats. A malignant, marginal zone non-Hodgkin B-cell lymphoma was identified. He died despite 4 cycles of chemotherapy.
No lymphomas were uncovered in the remaining 31 study families. The specific mutations associated with ALPS in all 39 families are shown in Figure 1.
Flow cytometry
Flow cytometry was performed on peripheral blood lymphocytes from 57 members of the 8 families with lymphomas. Of 36 members with Fas mutations tested, 31 possessed elevated (≥ 1%) TCR α/β DNTCs (median of 4.0%; range 0.5%-75%). All 21 healthy relatives tested without mutations had significantly lower (< 1%) DNTC percentages (P < .001).
Induction of Fas-mediated apoptosis
Fifty-four members of the lymphoma kindreds underwent apoptosis tests. Lymphocytes from 36 individuals with Fas mutations showed depressed lymphocyte apoptosis (median cell loss 5%; range 0%-32%), whereas for 18 subjects lacking Fas mutations, the median percentile of T cells undergoing apoptosis was 44% (range 14%-70%;P < .001). Fas-mediated killing of EBV-transformed B cells was studied in 11 subjects from NIH family 3. In the 4 individuals with mutations, 11% to 25% of B cells died, whereas 33% to 59% of B cells died from 7 relatives with normal Fas alleles (P = .006). Thus, Fas defects result in impaired apoptosis of both B cells and T cells, as noted previously.11
Fas-mediated killing and genetics in tumor cells
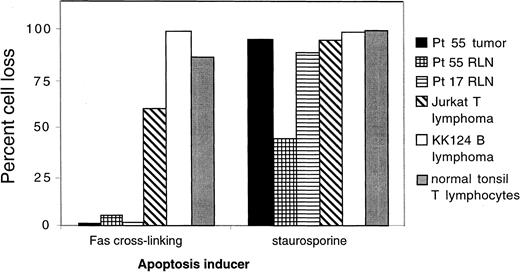
In vitro studies showed that defective apoptosis is a feature of both nonmalignant and lymphomatous cells from ALPS lymph node tissues and that the heterozygous mutation transmitted in the germline is sufficient for the development of lymphoma. Cells from a lymphomatous node of patient 55-II-1 and from a typical nonlymphomatous node from an ALPS patient32 were tested and compared with Jurkat T-cell lymphoma and KK124B Burkitt lymphoma (a gift of Dr Kishor Bhatia) cell lines as well as activated fresh normal human tonsillar lymphocytes. The cells were treated with MoAbs that trigger Fas-mediated cell death or with staurosporine, which kills cells via Fas-independent mitochondrial pathways. Using flow cytometry, it was possible to distinguish the lymphomatous cells from patient 55-II-1 as being relatively large B cells, consistent with their immunohistologic appearance (Figure 2G-I). High percentages of tonsillar lymphocytes, Jurkat cells, and KK124B cells were killed by both apoptosis inducers (Figure 3). Resting lymph node (RLN) cells from ALPS patient 17-II-1were relatively refractory to Fas-mediated death but sensitive to staurosporine, as expected.19 Both the malignant and nonmalignant lymph node B cells from patient 55-II-1 were refractory to Fas killing but sensitive to staurosporine. Thus, malignant transformation did not introduce or mitigate the inherited apoptotic defect.
Resistance of lymphoma cells to Fas-induced apoptosis.
Cell suspensions prepared from a lymphomatous lymph node from NIH ALPS proband 55 were subjected to apoptosis induction either via antibody cross-linking of Fas or by 5 μg/mL staurosporine that induces apoptosis by the mitochondrial pathway. The percentages of cells dying are compared with the results with resting lymph node (RLN) cells from patient 55, with cells from a family 17 ALPS patient, with Jurkat T-lymphoma cells, with KK124B Burkitt lymphoma cells, and with normal human tonsil cells. Flow cytometry analysis showed that lymphoma cells from patient 55 were of large size and exhibited B-cell markers and that both the lymphoma cells and the smaller resting lymphocytes expressed normal levels of the Fas receptor on the cell surface.
Resistance of lymphoma cells to Fas-induced apoptosis.
Cell suspensions prepared from a lymphomatous lymph node from NIH ALPS proband 55 were subjected to apoptosis induction either via antibody cross-linking of Fas or by 5 μg/mL staurosporine that induces apoptosis by the mitochondrial pathway. The percentages of cells dying are compared with the results with resting lymph node (RLN) cells from patient 55, with cells from a family 17 ALPS patient, with Jurkat T-lymphoma cells, with KK124B Burkitt lymphoma cells, and with normal human tonsil cells. Flow cytometry analysis showed that lymphoma cells from patient 55 were of large size and exhibited B-cell markers and that both the lymphoma cells and the smaller resting lymphocytes expressed normal levels of the Fas receptor on the cell surface.
Finally, complementary DNAs (cDNAs) prepared from malignant CD19+ B cells sorted from a node from this patient and nonmalignant cells of a lymph node from patient 31-III-1were sequenced. Multiple cDNA clones prepared from proband 55 retained the 942C→T mutation seen in his peripheral blood and no other Fas mutations, whereas the majority of clones revealed wild-type Fas sequences. From proband 31, similar numbers of clones were mutant and wild type. Thus, the tumor in patient 55, like the reactive node of patient 31, showed only heterozygous Fas mutations.
Risk of lymphoma development
The ratio of observed cases of non-Hodgkin lymphoma (5 cases/3774 patient years, not counting the second lymphoma in subject G3-II-8) among the 130 individuals with mutations to the expected rate among the general population less than 65 years of age (9.4 cases/100 000 patient years)28 was 14 (95% confidence interval [CI] 5- to 33-fold), a significant increase (P < .001). The ratio of observed to expected cases of Hodgkin lymphoma among the general population (5 cases/3774 patient years versus 2.6/100 000 patient years)28 was 51 (95% CI 17- to 119-fold; P < .001).
Discussion
We examined the possibility that germline Fas mutations and the concomitant defect in lymphocyte apoptosis are hereditary predisposing factors to lymphoma. One hundred thirty members of 39 kindreds that possess inherited Fas mutations were studied; lymphomas cosegregated with Fas mutations in 10 individuals in 8 of the families. These 8 families included 75 members of whom 43, including all 10 individuals with lymphoma, exhibited some or all of the manifestations of ALPS, such as lymphoproliferation, especially of DNTCs, and autoimmunity. The risk of Hodgkin and non-Hodgkin lymphoma in individuals with inherited Fas mutations in our study is 51 and 14 times greater than expected, respectively. We found that both the nonmalignant and malignant lymphocytes from the lymphoma subjects exhibited defective Fas-mediated killing, but they did not have a general apoptosis defect, because the cells responded normally to other apoptosis inducers (Figure3).
A significant feature of lymphoma in ALPS is its diversity.1 The 5 cases of Hodgkin disease were of various types including nodular sclerosing, nodular-lymphocyte predominant, and mixed cellularity. The others represented several histologic types of lymphoma, including a T-cell type, a histiocyte-rich T-cell–rich large B-cell type, a follicular B-cell type, a marginal zone B-cell type, and 2 cases of Burkitt lymphoma. The preponderance of B-cell malignancies is notable given the fact that apoptosis in both T and B cells is abnormal in ALPS and that the major nonmalignant cell types that expand in ALPS are DNTCs, which are believed to be mature T cells that have lost expression of their CD4 and CD8 coreceptors.8,11,19Therefore, Fas defects do not appear to shift the distribution between T and B lymphoid malignancies. Given that our study group is still small, we cautiously infer that Fas apoptosis deficiency affects a general protective mechanism against B-cell malignancy rather than one that controls a particular B-cell subtype. Such a conclusion would be consistent with evidence that Fas governs the fate of developing B cells in the bone marrow as well as mature B cells in the peripheral immune organs.39
The involvement of Fas mutations in lymphoma is distinct from other apoptosis defects that have been postulated to play a role in lymphoma. Previously, genetic alterations in Bcl-2–related molecules were associated with lymphoid malignancy40-42; however, Bcl-2 protects against an apoptosis pathway emanating from the mitochondrion that was not defective in the lymphoma sample from patient 55 (Figure3).19 By contrast, Fas is a cell surface receptor that promotes apoptosis in response to a specific inducer, Fas ligand, to govern lymphocyte homeostasis during immune responses.19The lymphomas we observed are all associated with alterations in the intracytoplasmic region of the Fas receptor, termed the “death domain.” Individuals with mutations that affect the death domain have the greatest defect in Fas-mediated apoptosis and the greatest genetic penetrance and severity of clinical symptoms.43 44 Therefore, the propensity to develop B lymphomas relates to the severity of apoptosis defect engendered by specific lesions in the Fas gene, implying that the specific apoptotic function of Fas is of primary importance in protecting against lymphoma.
Our findings are in accord with prior data associating defects in Fas function with lymphoid malignancy. Several studies revealed underexpression of Fas in lymphoma45-50 and multiple myeloma,51-54 and somatic chromosomal abnormalities in non-Hodgkin lymphoma in the region of chromosome 10 encoding Fas.55-61 Moreover, somatic Fas mutations have been reported in multiple myeloma, adult T-cell leukemia, childhood T-lineage acute lymphoblastic leukemia,35-37 and in 9 of 150 non-Hodgkin lymphomas.38 Among these 9 cases, 3 were heterozygous and involved the Fas death domain, including 1 with the identical mutation seen in our patient 26-II-4. Three others involved mutations outside the death domain in one allele but deletion of the second Fas allele.38 We demonstrated heterozygosity of the Fas mutation in tumor cells from one of our cases (55-II-1); however, the cumulative evidence indicating that the mutations in all of our patients are dominant-negative implies that all tumors that arose in them involved heterozygous Fas mutations.
In our cases, the average age of ALPS onset was 5 years, whereas the average age of lymphoma diagnosis was 28. The cases where somatic alterations in Fas were described in lymphomas more typically arose later in life.35-38 The lag between the manifestations of ALPS and lymphoma could represent time for other genetic or environmental lymphomagenic factors to operate on the substrate of defective apoptosis. This conclusion is supported by studies of mice defective in Fas apoptosis that show lymphoid malignancies arising in conjunction with expression of the L-myc oncogene or as a consequence of aging.7 62-65
The mechanism by which Fas defects in ALPS predispose to lymphomas might involve several components. The most obvious possibility is that a general expansion of the lymphoid pool provides a larger target cell population for other transforming events. This does not seem to fully explain our observations, because the greatest expansions are in T cells, yet we observe B-cell malignancies. Nonetheless, the absolute increases in B cells, which are stimulated by the expanded T helper 1 cells, and marked elevations in IL-10 in ALPS could be important for the increased lymphoma incidence.66 Other, more specific mechanisms could also participate. Defects in the cytolytic pathway for killing virally infected cells mediated by Fas could allow EBV or other oncogenic viruses to persist. A similar apoptotic clearance mechanism may operate on transformed B cells. Because Fas ligand is expressed by T cells and not B cells, T cells could eliminate Fas-sensitive malignant cells. The study of lymphoma in ALPS provides a genetic model of germline Fas deficiency that will allow further examination of these issues.
We thank Drs John Bohnsack, Howard Britton, Sandra Buys, Brian Corden, Anthony Infante, Donald Mahoney, Annelise Sitarz, and David Virshup for referring patients for study; the participating families themselves for their cooperation; Ms Jean Whitehouse, Ms Romy Lehmann, and Ms Margaret R. Brown for flow cytometry analysis of peripheral blood; Lindsay A. Middleton, for ascertainment of family data; Dr Maryalice Stetler-Stevenson for flow cytometry analysis of lymph nodes; Drs Lixin Zheng, Gretchen Gibney, Michelle Johnson, and Richard Siegel for assistance with apoptosis assays; Drs Mark Raffeld and Lynn Sorbara for molecular studies; Drs Diane Arthur and Linda Cooley for cytogenetic studies; Dr Douglas Kingma for EBV in situ hybridizations; Uri Lopatin for additional statistical help; Drs Margaret Tucker and Joachim Roesler for helpful discussions; Dr G. Koehler for reviewing selected histologic sections; Drs Louis Staudt and Ian Magrath for critical reviews of the manuscript; and Ms Brenda Rae Marshall for editorial assistance.
The contribution of K.B.E. to this work was supported, in part, by grants from the National Institutes of Health (AR4548I), and the fellowship of C.B. was supported by the Cure for Lymphoma Foundation. A.M.J.P. was supported by the Deutsche Forschungsgememeinschaft (DFG 609/1-1). J.W. was supported by an Arthritis Foundation fellowship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Note added in proof
Drs Howard Britton and Thomas S. DeNapoli informed us of the recent development of Hodgkin disease, mixed cellularity type, in subject 26-IV-5, a 6-year-old boy previously described with ALPS since age 1 (reference 15).
Author notes
Stephen E. Straus, LCI, NIAID, 31 Center Dr, Rm 5B-37, National Institutes of Health, Bethesda, MD 20892; e-mail:sstraus@nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal