Abstract
Exploration of the immunomodulatory activities of the multifunctional cytokine interleukin-11 (IL-11) has prompted several therapeutic applications. The immunomodulatory effects of IL-11 on human antigen-presenting cells and on T cells were investigated. IL-11 inhibited IL-12 production by activated CD14+ monocytes, but not by mature dendritic cells (DCs) stimulated via CD40 ligation. Moreover, IL-11 did not affect either DC maturation, as demonstrated by phenotypic analysis and evaluation of cytokine production, or DC generation from progenitor cells in the presence of specific growth factors. Molecular analysis demonstrated the expression of IL-11 receptor messenger RNA in highly purified CD14+ monocytes, CD19+ B cells, CD8+, and CD4+T cells, and CD4+CD45RA+ naive T lymphocytes. In keeping with this finding, IL-11 directly prevented Th1 polarization of highly purified CD4+CD45RA+naive T cells stimulated with anti-CD3/CD28 antibodies, as demonstrated by significant increases of IL-4 and IL-5, by significantly decreased interferon-γ production and by flow cytometry intracellular staining of cytokines. Coincubation of naive T cells with DCs, the most potent stimulators of Th1 differentiation, did not revert IL-11–mediated Th2 polarization. Furthermore, parallel experiments demonstrated that the activity of IL-11 was comparable with that induced by IL-4, the most effective Th2-polarizing cytokine. Taken together, these findings show that IL-11 inhibits Th1 polarization by exerting a direct effect on human T lymphocytes and by reducing IL-12 production by macrophages. Conversely, IL-11 does not exert any activity on DCs. This suggests that IL-11 could have therapeutic potential for diseases where Th1 responses play a dominant pathogenic role.
Introduction
Interleukin (IL)-11 is a multifunctional cytokine that was originally isolated from the primate stromal cell line, PU-34, and later from the human MRC 5 cell line. Biologic characterization has shown diverse effects on a variety of hematopoietic and nonhematopoietic cell types (reviewed in reference 1). Due to its ability to stimulate megakaryopoiesis and thrombopoiesis, IL-11 has been approved in the United States for the treatment of chemotherapy-induced thrombocytopenia. In addition to stimulating hematopoietic progenitor cells,2 IL-11 exerts a strong anti-inflammatory activity in vitro and in vivo. By inhibiting nuclear translocation of nuclear factor-kB (NF-kB),3 IL-11 reduces production by macrophages of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), IL-1β, IL-6, and IL-12.4-6 Therapeutic applications of this effect in multiple animal models of inflammatory disorders have reduced signs of disease.4,5,7-9 In a well-characterized murine model of graft-versus-host disease (GVHD) directed at major histocompatibility complex (MHC) and minor antigens, IL-11 strongly inhibited GVHD and enhanced recipient survival.10 These effects were due to protection of the small bowel from irradiation and GVHD toxicity, suppression of TNF-α, and polarization of donor T cells to a Th2 response with decreased interferon-γ (IFN-γ) and augmented IL-4 production.10 Interestingly, a significant reduction of CD4-dependent GVHD was not associated with impairment of the cytolytic function and graft-versus-leukemia (GVL) effect of CD8+ T cells.11 Thus, a short course of IL-11 treatment before allogeneic stem cell transplantation has been proposed to help separate the GVL effect from GVHD.11 Recently, IL-11 therapy has been administered to patients with psoriasis in a phase I clinical dose-escalation trial.12 The treatment led to down-regulation of type I cytokines in psoriatic lesions and reduction of keratinocyte proliferation and cutaneous inflammation.
Taken together, these studies have revealed that IL-11 exerts a series of important immunomodulatory effects that can be applied in various therapeutic contexts. However, the issue as to whether T-cell polarization is solely due to IL-11–mediated suppression of IL-12 or whether IL-11 has additional direct effects on CD4+CD45RA+ naive T cells has yet to be addressed. The most potent antigen-presenting cells (APCs) involved in the stimulation of naive CD4+ cells are dendritic cells (DCs), which induce type I responses by their ability to produce IL-12 on maturation.13 In this study, we investigated the immunomodulatory effects of IL-11 on monocytes, human monocyte-derived DCs (Mo-DCs), and T cells. Our results demonstrate that IL-11 regulates immune responses by at least 2 potent mechanisms of action: it inhibits IL-12 production by monocytes and exerts a direct effect on CD4+ Th cell polarization.
Materials and methods
Cell sample collection and processing
Buffy coats were obtained from healthy adults during the preparation of transfusion products. Mononuclear cells (MNCs) were obtained by gradient centrifugation (Lymphoprep; 1.077 g/mL; Nycomed Pharma, Oslo, Norway). Light-density cells were washed twice in phosphate-buffered saline (PBS) with 1% bovine serum albumin (BSA; Sigma Chemical, St Louis, MO) and CD4+, CD8+, CD19+, or CD14+ cells were highly purified from the MNC fraction by MiniMacs high-gradient magnetic separation column (Myltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions.14 Flow cytometric reanalysis of purified cell fractions was performed on a gated population set on scatter properties by using FACScan equipment (Becton Dickinson, Mountain View, CA), as earlier described.14 15 A minimum of 10 000 events was collected in list mode on FACScan software. The purity of enriched populations was always more than 90% of the total cell yield.
Generation of DCs in liquid culture
The Mo-DCs were generated from peripheral blood (PB) CD14+ cells as previously described14 with minor modifications. Briefly, 1 × 106 purified CD14+ cells were cultured for 5 to 7 days in 1 mL RPMI 1640 supplemented with 10% fetal calf serum (FCS; Sera Lab, Crawley Down, Sussex, United Kingdom), antibiotics, l-glutamine, 50 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; Sandoz, Basel, Switzerland) and 800 U/mL IL-4 (Schering Plough, Kenilworth, NJ). When indicated, appropriate stimuli, such as 25 ng/mL TNF-α (Innogenetics, Zwijndrecht, Belgium), 1μg/mL soluble trimeric CD40L (Immunex, Seattle, WA), 1 μg/mL lipopolysaccharide (LPS; Sigma), were added to the culture for an additional 24 to 36 hours to induce terminal maturation of DCs.14 In selected experiments, IL-11 (50 U/mL; Endogen, Woburn, MA) was added either from the beginning of the culture or during maturation. Cultures were maintained at 37°C in 5% CO2 by replacing culture medium and cytokines at day +3. The concentration of IL-11 was chosen based on a dose-response curve (0.1-1000 U/mL) to determine optimal proliferative effects on T cells (data not shown). The generation of functionally active DCs was assessed by phase-contrast microscopy, immunophenotyping, evaluation of cytokine secretion, and mixed lymphocyte reactions (MLR).
Immunophenotype studies
Dual-color immunofluorescence was performed using the following panel of monoclonal antibodies (MoAbs)14 15: phycoerythrin (PE)– or fluorescein isothiocyanate (FITC)–conjugated antihuman CD1a (Pharmingen, San Diego, CA); FITC-conjugated antihuman CD86 (Pharmingen); FITC-BB1/B7 (anti-CD 80; Becton Dickinson); FITC-antihuman HLA-DR (Becton Dickinson); FITC or PE Leu-M3 (anti-CD 14; Becton Dickinson); FITC–Leu-4 (anti-CD3; Becton Dickinson); FITC–Leu-18 (anti-CD45RA; Becton Dickinson); PE-antihuman CD83 (Immunotech, Marseille, France); FITC–antihuman CD40 (Pharmingen); FITC–antihuman Leu-3a (anti-CD4; Becton Dickinson). Negative controls were isotype-matched irrelevant MoAbs (Pharmingen and Becton Dickinson). Cells were incubated in the dark for 30 minutes at 4°C in PBS-1% BSA. After washing, cells were resuspended in PBS and 1% paraformaldehyde and analyzed as reported above.
In vitro priming of CD4+CD45RA+ T cells
Naive T cells were highly purified from the MNC fraction by MiniMacs high-gradient magnetic separation column (MultiSort isolation kit; Myltenyi Biotec) according to the manufacturer's instructions. Flow cytometric reanalysis of purified cell fractions was performed as reported above. The purity of the enriched populations was always more than 90%. CD4+CD45RA+ T cells were then resuspended in RPMI 1640 containing 5% human AB serum and stimulated with allogeneic mature Mo-DCs (DC/T ratio = 1:10) or with plate-coated anti-CD3 and soluble anti-CD28 MoAbs (both at 1 μg, Immunotech and Pharmingen, respectively) in the presence or absence of recombinant human IL-11 (50 U/mL) or IL-4 (R&D Systems, Minneapolis, MN; 100 U/mL). In neutralization experiments, specific anti–IL-11 antibodies (5 μg/mL; Endogen), anti–IL-4 (10 μg/mL; Endogen), or matched-isotype control Ig were added to the culture. After 6 days of culture, T cells were transferred to uncoated plates in medium containing 30 U/mL recombinant human IL-2 (Proleukin, Chiron, Emeryville, CA). After 7 to 8 days of IL-2 expansion, T cells were treated for cytokine secretion (see below) and for intracellular cytokine staining. Briefly, T lymphocytes were counted and reactivated with IS Cyto Activation Kit, containing phorbol-12-myristate-13-acetate (PMA), ionomycin, and monensin (Immune Source, Los Altos, CA). After 6 hours of incubation at 37°C, cells were washed, fixed in fixation buffer (Immune Source) and permeabilized with permeabilization/wash buffer (Immune Source), following the manufacturer's instructions. T cells were then stained with anti–IL-4–PE and anti–IFN-γ–FITC antibodies (Immune Source) and finally analyzed with a flow cytometer.
Assessment of cytokine production
To assess the effects of IL-11 on DC cytokine production during maturation, immature Mo-DCs were plated at 5 × 105/mL in RPMI 1640-10% FCS in the presence of 2.5 × 105irradiated (30 Gy) murine L cells stably transfected with CD40L or untransfected, which served as internal control14 with or without IL-11. Supernatants were collected after 40 hours of coculture and assayed, in serial dilutions, in duplicate, for IL-12 (p75), IL-10, TNF-α, and IL-1 (concentration using an enzyme-linked immunosorbent assay [ELISA] kit; R & D Systems). CD14+ cells were stimulated for 16 hours with IFN-γ (1000 U/mL) and incubated with LPS (1 μg/mL; Sigma) in the presence and absence of IL-11 (50 U/mL). After 24 hours, cells were collected and supernatants were assayed for IL-12 p75 concentration. IL-11 concentration in the supernatant of DCs was also determined by an ELISA kit (R & D Systems).
To determine T cell polarization on IL-11 stimulation, CD4+CD45RA+ naive T lymphocytes were coincubated with mature Mo-DCs or grown in presence of anti-CD3 and anti-CD28 MoAbs (see above). At the end of culture, T cells were seeded in 24-well culture plates at a cell density of 1 × 106/mL in the presence of PMA (10−7 M; Sigma) and ionomycin (1 μg/mL; Sigma). After 48 hours, IL-4 and IFN-γ were assayed in the supernatant by a quantitative flow cytometry–based immunoassay (Multi Flow-IFA kit; Bioergonomics, St Paul, MN) following the manufacturer's instructions or by ELISA using matched pairs of MoAbs (Pharmingen). In selected experiments, IL-5 was measured using the Opteia kit (Pharmingen). Fluorescence intensity was evaluated with an EPICS XL flow cytometer (Coulter, Hialeah, FL).
RNA purification
Total cellular RNA was extracted using a modification of the guanidinium-cesium chloride centrifugation technique.16 To avoid a potential genomic DNA contamination of purified RNA, all the samples were digested with RQ1-DNAse for 30 minutes at 37°C by adding 10 U RQ1 (Promega), 40 U RNAsin (Promega), 1 × RQ1 buffer to a final volume of 200 μL. RNA concentration was then evaluated by reading the OD at 260 nm and by loading the samples on a 1% denaturing agarose gel. These quantitative controls are crucial to reverse transcribe the same amount of RNA from the different samples studied.
Oligonucleotide primers and probes and complementary DNA fragment labeling
Oligonucleotide primers and probes were synthesized with an automated solid-phase DNA synthesizer (Applied Biosystems, Foster City, CA, Model 381A) with the standard fosforamidites chemistry and purified by several extractions with NH4OH, incubated at 56°C for 16 hours and ethanol precipitated or purified by polyacrylamide gel electrophoresis (PAGE). All the synthesized oligomers were previously compared with the gene bank DNAsis (Hitachi, Brisbaine, CA) to avoid homologies with other gene sequences. Furthermore, we synthesized oligonucleotide primers from separate exons for excluding a potential genomic DNA contamination of the RNA samples. The sequences of IL-11 receptor (IL-11R) primer and probe used in this study are 5′-TGGTGTCTGCCTCCTCCCCCTGCC-3′ for the direct primer (DP) and 5′-TGTAGGAGTGAGGTAGCGGGTGG-3′ for the reverse probe (RP).
Oligonucleotide probes and complementary DNA (cDNA) fragments labeling were performed as already reported.16
Reverse transcription–polymerase chain reaction and Southern blotting
The reverse transcription–polymerase chain reaction (RT-PCR) reactions were carried out as previously described.16Briefly, 1 μg total RNA extracted from each sample was reverse transcribed using 400 U M-MLV reverse transcriptase (Gibco, BRL, Gaithersburg, MD) and 1 μg OligoT 15 primer (Boehringer Mannheim, Milan, Italy) for 1 hour at 42°C in 1 × RT buffer in a total volume of 30 μL. The cDNA was then heated at 95°C for 3 minutes and stored at 4°C. The cDNA (1 μL) was subsequently amplified, adding 2.5 U Taq polymerase (Promega), 0.5 μg specific DP and RP in a total volume of 50 μL, in 10 mM Tris 8.3, 50 mM KCl, 1.5 mM MgCl2, 200 μM dNTPS. The amplification was carried out performing 50 cycles using the following conditions: denaturation at 45 seconds starting from the second cycle; annealing at 70°C for 2 minutes; extension at 72°C for 3 minutes. Ten microliters of each sample was then denatured in 0.2 N NaOH, 0.4 M NaCl for 45 minutes, neutralized in 25 mM phosphate buffer, pH 6.5, for 45 minutes and then transferred by electroblotting on a positively charged nylon membrane. Hybridization of the blots with oligonucleotide-labeled probes was then performed. For each experiment the same amount of cDNA was amplified. To check the amount of cDNA in each sample, the β2-microglobulin gene was amplified by PCR with 35 cycles. A sample without RNA template was used as negative control to exclude amplified cDNA contamination. In each experiment, the gel after ethidium bromide staining and the corresponding autoradiogram after hybridization were examined. The specificity of the amplification was confirmed by hybridization with an oligonucleotide probe.
Statistical analysis
The results are expressed as the mean ± SD. Where indicated, statistical analysis was performed by means of the nonparametric paired Wilcoxon rank-sum test.
Results
IL-11 does not affect DC generation and maturation
Interleukin-11 regulates macrophage effector function by inhibiting the production of several cytokines.4-6Therefore, we evaluated the possibility that IL-11 could affect other types of APCs such as Mo-DCs. To this end, highly purified circulating CD14+ monocytes were used to generate DCs. In these experiments, 6 to 7 days of culture in presence of GM-CSF and IL-4 induced a cell population with the typical phenotype of immature Mo-DCs: CD1a+, HLA-DR+, CD80+/−, CD86+, CD83− (Figure1A). Addition of IL-11 from either the beginning of the culture (data not shown) or during the maturation induced by exposure to LPS, CD40L, or TNF-γ never modified the phenotypic profile of mature DCs, as demonstrated by the up-regulation of CD83, the down-regulation of CD1a, and the coexpression of costimulatory molecules (Figure 1B). Functionally, DCs generated with or without IL-11 showed the same potency in stimulating allogeneic lymphocytes and autologous lymphocytes coincubated in the presence of soluble proteins such as keyhole lympet hemocyanin or tetanus toxoid (data not shown).
IL-11 does not affect DC maturation mediated by CD40L, TNF-α, and LPS.
DCs cultured for 6 to 7 days with GM-CSF and IL-4 (A) were further incubated with maturation stimuli in the presence and absence of IL-11 (B). Overlay diagrams show the expression of the relevant antigens versus negative controls. Addition of CD40L, TNF-α, or LPS to immature DCs induced a phenotypic pattern typical of terminally differentiated elements: CD1a−, HLA-DR+, CD80+, CD86+, and CD83+. Addition of IL-11 (gray line) did not modify the DC profile as regards mean fluorescence intensity (MFI) or the percentage of positive cells.
IL-11 does not affect DC maturation mediated by CD40L, TNF-α, and LPS.
DCs cultured for 6 to 7 days with GM-CSF and IL-4 (A) were further incubated with maturation stimuli in the presence and absence of IL-11 (B). Overlay diagrams show the expression of the relevant antigens versus negative controls. Addition of CD40L, TNF-α, or LPS to immature DCs induced a phenotypic pattern typical of terminally differentiated elements: CD1a−, HLA-DR+, CD80+, CD86+, and CD83+. Addition of IL-11 (gray line) did not modify the DC profile as regards mean fluorescence intensity (MFI) or the percentage of positive cells.
IL-11 inhibits IL-12 production by activated monocytes but not DCs
To assess the capacity of IL-11 to modulate DC IL-12 p75 production, we induced maturation of DCs by incubating them with the murine fibroblasts L-cell line stably transfected with CD40L. For comparison, we evaluated IL-12 production of CD14+monocytes primed with IFN-γ and then exposed to LPS for 24 hours (Figure 2). As expected, CD40L proved to be an optimal inducer of IL-12 secretion by DCs. However, whereas IL-11 inhibited production of IL-12 by activated monocytes (P < .05), it did not exert any effect on DCs (Figure2A). Even in the presence of other maturation stimuli, such as LPS, IL-11 exerted no significant effect on IL-12 production (data not shown). We then evaluated the capacity of IL-11 to influence production of TNF-α, IL-10, and IL-1β by CD40L-stimulated Mo-DCs. As with IL-12, secretion of these cytokines was never significantly affected by IL-11 (Figure 2B). Taken together, these results demonstrate that IL-11 exerts a potent immunomodulatory effect on monocytes, but not on Mo-DCs.
IL-11 inhibits cytokine production by activated monocytes but not by DCs.
DCs were matured with murine L cells stably transfected with CD40L in the presence (▪) or absence (■) of IL-11. Supernatants were collected after 40 hours of coculture and assayed for IL-12 (p75) (A), IL10, TNF-α, and IL-1β concentration (B). CD14+ cells were stimulated for 16 hours with IFN-γ and then incubated with LPS. After 24 hours, cells were collected and supernatants were assayed for IL-12 (A). IL-11 reduced IL-12 production by activated monocytes from a mean value of 780 pg/mL to 100 pg/mL (P < .05). No effect was seen on DCs as regards IL-12 (A), IL10, TNF-α, or IL-1β secretion (B).
IL-11 inhibits cytokine production by activated monocytes but not by DCs.
DCs were matured with murine L cells stably transfected with CD40L in the presence (▪) or absence (■) of IL-11. Supernatants were collected after 40 hours of coculture and assayed for IL-12 (p75) (A), IL10, TNF-α, and IL-1β concentration (B). CD14+ cells were stimulated for 16 hours with IFN-γ and then incubated with LPS. After 24 hours, cells were collected and supernatants were assayed for IL-12 (A). IL-11 reduced IL-12 production by activated monocytes from a mean value of 780 pg/mL to 100 pg/mL (P < .05). No effect was seen on DCs as regards IL-12 (A), IL10, TNF-α, or IL-1β secretion (B).
IL-11 prevents type-1 cytokine response via a direct effect on CD4+CD45RA+ naive T cells
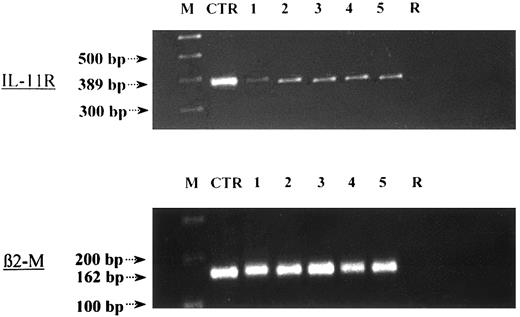
We first investigated the expression of IL-11R on a broad spectrum of cell subsets. As shown in Figure 3, CD19+, CD8+, CD4+, and CD4+CD45RA+ cells expressed IL-11R messenger RNA (mRNA). We then investigated the effects of IL-11 on the development of CD4+CD45RA+ naive T cells. Highly purified lymphocytes were stimulated with anti-CD3 and anti-CD28 MoAbs in the presence of IL-11 or IL-4, expanded with IL-2, and then reactivated with PMA plus ionomycin prior to the final assessment of their intracellular cytokine content. T cells that were not restimulated at day 6 with IL-2 did not show production of any of the cytokines assessed (data not shown).
IL-11R is expressed on the cell membrane of monocytes, T cells, and B cells.
IL-11R mRNA expression was found in highly purified CD19+(1), CD4+ (2), CD4+CD45RA+ (3), CD8+ (4), and CD14+ (5) cells by RT-PCR. IL-11R mRNA was detected at the expected site (389 bp). As a positive control (CTR), we used RNA obtained from the M07 megakaryoblastic cell line, which is known to express IL-11R. To exclude amplified cDNA contamination, a sample without RNA template served as negative control (R).
IL-11R is expressed on the cell membrane of monocytes, T cells, and B cells.
IL-11R mRNA expression was found in highly purified CD19+(1), CD4+ (2), CD4+CD45RA+ (3), CD8+ (4), and CD14+ (5) cells by RT-PCR. IL-11R mRNA was detected at the expected site (389 bp). As a positive control (CTR), we used RNA obtained from the M07 megakaryoblastic cell line, which is known to express IL-11R. To exclude amplified cDNA contamination, a sample without RNA template served as negative control (R).
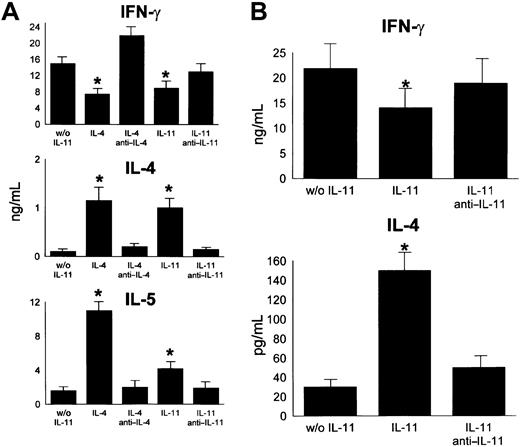
As reported in the representative example in Figure4, IL-11 was able to prevent Th1 polarization by increasing the percentage of IL-4+ cells (from 2% ± 2% to 12% ± 4%; n = 3; P < .05) and by reducing the percentage of IFN-γ–producing cells (from 30% ± 3% to 21% ± 5%; n = 3; P < .05). This effect was completely reversed by addition of an anti–IL-11 Ab. Interestingly, the immunomodulatory activity of IL-11 on naive T cells was similar to that obtained with IL-4, which is the most effective Th2- polarizing cytokine. Under IL-4 stimulation, IL-4+ and IL-4/IFN-γ–producing cells increased to mean values of 6% ± 2% and 12% ± 4%, respectively. In parallel, IFN-γ+cells decreased to 22% ± 5% (P < .05). Furthermore, measurement of IL-4, IFN-γ, and IL-5 concentrations in the culture supernatants confirmed that, as with IL-4, when naive T lymphocytes were stimulated with IL-11, they produced significantly less IFN-γ and IL-4 and IL-5 (Figure 5A). In particular, IFN-γ production was reduced by IL-11 and IL-4 from 15 ± 2.5 ng/mL to 8.8 ± 2 ng/mL and 7.2 ± 1.5 ng/mL, respectively (both P < .05). Concurrently, incubation with IL-11 and IL-4 increased IL-4 secretion from 0.1 ± 0.05 ng/mL to 1 ± 0.2 ng/mL and 1.15 ± 0.3 ng/mL, respectively, and augmented IL-5 secretion from 1.6 ± 0.03 ng/mL to 4.2 ± 0.3 ng/mL and 11 ± 1 ng/mL, respectively (all P < .02).
IL-11 directs Th2 polarization by increasing IL-4 and decreasing IFN-γ+ cells.
Highly purified CD4+CD45RA+ naive T cells were stimulated with plate-coated anti-CD3 and soluble anti-CD28 MoAbs in the presence or the absence of IL-11 or IL-4. In neutralization experiments, specific anti–IL-11 or anti–IL-4 antibodies or isotype controls were added to the culture. After 6 days of culture, T cells were transferred to uncoated plates and expanded with recombinant human IL-2 for 7 to 8 days. T cells were then stimulated with PMA and ionomycin and stained for intracellular cytokine content. IL-4+ cells were increased by the addition of IL-11 from a mean value of 2% to 12% (n = 3; P < .05), whereas IFN-γ+ cells diminished from 30% to 21% (n = 3;P < .05). Addition of anti-IL-11 antibodies reversed the results. When IL-11 was replaced by IL-4, a similar pattern of Th2 polarization was observed. An experiment representative of 3 is shown here.
IL-11 directs Th2 polarization by increasing IL-4 and decreasing IFN-γ+ cells.
Highly purified CD4+CD45RA+ naive T cells were stimulated with plate-coated anti-CD3 and soluble anti-CD28 MoAbs in the presence or the absence of IL-11 or IL-4. In neutralization experiments, specific anti–IL-11 or anti–IL-4 antibodies or isotype controls were added to the culture. After 6 days of culture, T cells were transferred to uncoated plates and expanded with recombinant human IL-2 for 7 to 8 days. T cells were then stimulated with PMA and ionomycin and stained for intracellular cytokine content. IL-4+ cells were increased by the addition of IL-11 from a mean value of 2% to 12% (n = 3; P < .05), whereas IFN-γ+ cells diminished from 30% to 21% (n = 3;P < .05). Addition of anti-IL-11 antibodies reversed the results. When IL-11 was replaced by IL-4, a similar pattern of Th2 polarization was observed. An experiment representative of 3 is shown here.
CD4+CD45RA+ naive T cells secrete Th2-type cytokines on stimulation with IL-11.
Highly purified naive T cells were stimulated with plate-coated anti-CD3 and soluble anti-CD28 MoAbs (A) (n = 2) or allogeneic mature Mo-DCs (B) (n = 3) in the presence or absence (w/o) of IL-11 (A,B) or IL-4 (A). In neutralization experiments, specific anti–IL-11 and anti–IL-4 antibodies or isotype controls were added to the culture. IL-4 and IFN-γ were assayed in the supernatant by ELISA (A) or by a quantitative flow cytometry–based immunoassay (B). IL-5 was measured using the Opteia kit. Consistent with the cytokine intracellular staining data, IL-11 and IL-4 significantly reduced IFN-γ production by naive T-cells stimulated with anti-CD3 and anti-CD28 MoAbs alone (w/o IL-11) (P < .05), whereas it increased IL-4 and IL-5 secretion (P < .02). Addition of anti–IL-11 or anti–IL-4 antibodies reversed the results, which did not significantly differ from control samples. Addition of DCs (B), did not change the pattern of type-2 cytokine production following incubation with IL-11, as can be seen from the decrease in IFN-γ production (P < .05 with respect to controls) and increase in IL-4 (P < .03). Again, anti–IL-11 or anti–IL-4 antibodies reversed the results. *P < .05.
CD4+CD45RA+ naive T cells secrete Th2-type cytokines on stimulation with IL-11.
Highly purified naive T cells were stimulated with plate-coated anti-CD3 and soluble anti-CD28 MoAbs (A) (n = 2) or allogeneic mature Mo-DCs (B) (n = 3) in the presence or absence (w/o) of IL-11 (A,B) or IL-4 (A). In neutralization experiments, specific anti–IL-11 and anti–IL-4 antibodies or isotype controls were added to the culture. IL-4 and IFN-γ were assayed in the supernatant by ELISA (A) or by a quantitative flow cytometry–based immunoassay (B). IL-5 was measured using the Opteia kit. Consistent with the cytokine intracellular staining data, IL-11 and IL-4 significantly reduced IFN-γ production by naive T-cells stimulated with anti-CD3 and anti-CD28 MoAbs alone (w/o IL-11) (P < .05), whereas it increased IL-4 and IL-5 secretion (P < .02). Addition of anti–IL-11 or anti–IL-4 antibodies reversed the results, which did not significantly differ from control samples. Addition of DCs (B), did not change the pattern of type-2 cytokine production following incubation with IL-11, as can be seen from the decrease in IFN-γ production (P < .05 with respect to controls) and increase in IL-4 (P < .03). Again, anti–IL-11 or anti–IL-4 antibodies reversed the results. *P < .05.
The same pattern of type-2 cytokine production and secretion was found when CD4+CD45RA+ cells were stimulated with TNF-α–matured DCs in the presence of IL-11 (Figure 5B). IFN-γ decreased from 21.3 ± 4.5 ng/mL to 14.1 ± 3.5 ng/mL (P < .05), whereas IL-4 production increased from 29 ± 9 pg/mL to 143 ± 20 pg/mL (P < .03) Addition of anti–IL-11 or anti–IL-4 antibodies reversed the results. The endogenous production of IL-11 by DCs was evaluated in control cultures. The amount of IL-11 found in the supernatant was minimal (50 ± 6 pg/mL) and biologically inactive.
Taken together, these results demonstrate that IL-11 directly affects T cells by driving the polarization of naive CD4+ lymphocytes to Th2 cells, and that this effect is similar to that exerted by IL-4. Moreover, IL-11 maintained the Th2-polarizing effect even when T cells were incubated with DCs, which provide a highly potent stimulus to Th1 cell differentiation.
Discussion
In T-cell polarization, naive CD4+ cells differentiate into either Th1 or Th2 effector cells. Th1 cells mainly produce IL-2 and IFN-γ to induce cell-mediated inflammatory reactions, whereas Th2 cells predominantly secrete IL-4, IL-5, IL-10, and IL-13, which are involved in B-cell activation and antibody production.17Differentiation into the Th1 or Th2 phenotype depends on stimulation by IL-12 and IL-4, respectively.18,19 IL-12 is secreted mainly by macrophages and by DCs that have been stimulated by pathogens and CD4+ cells themselves via CD40-CD40L interaction.13 IL-4 is produced by CD4+ cells, and during T-cell activation its concentration increases until it reaches the threshold necessary to induce Th2 response.17Suppression of IL-12 production or direct stimulation of IL-4 both result in Th2 polarization.20
Interleukin 11 is an IL-6–like cytokine.1 IL-11 and IL-6 both share the gp 130 molecule in their receptor complexes. IL-11 has demonstrated an anti-inflammatory activity by inhibiting proinflammatory cytokine gene expression in macrophages.4-6 In mice, administration of IL-11 during allogeneic bone marrow transplantation results in polarization of the T-cell response to Th2-type cells production, with correspondingly reduced IFN-γ and increased IL-4 serum levels.10 These effects appear to be associated with the suppression of IL-12 secreted by activated macrophages. In humans, IL-11 therapy down-regulated type I cytokines in psoriasis lesions.12
No data are currently available on the possible effects of IL-11 on human DCs, which are the most potent IL-12–mediated inducers of Th1 immune responses. Moreover, the hypothesis that IL-11R is expressed on T cells and that IL-11 can therefore exert a direct effect on T cells and NK cells, the main producers of IFN-γ, has yet to be tested. This study was designed to investigate these 2 points.
Earlier studies have shown that IL-11 inhibits IL-12 production by activated human macrophages.6 Although confirming this finding, the results of the present study indicate that unlike IL-6, which inhibits DCs differentiation from CD34+cells,21 IL-11 plays no part in the generation or functioning of DCs. We came to this conclusion after studying the development of Mo-DCs from CD14+ monocytes in the presence and absence of IL-11, as well as DC terminal maturation and cytokine production. It should be pointed out that Mo-DCs were cultured in the presence of FCS, the addition of which might have already matured the Mo-DCs before the addition of IL-11, rendering them less sensitive to its effect. However, the phenotypic profile of the Mo-DCs prior to the addition of IL-11 and maturation stimuli (Figure 1A) was typical of immature DCs, with high CD1a and low or absent CD83 expression.
On the other hand, our study also provides the first demonstration that IL-11 does indeed induce T-cell polarization by exerting a direct effect on CD4+ naive T lymphocytes that bear IL-11R. We found that IL-11 inhibited IFN-γ–producing Th1 cells while increasing IL-4+ and IL-5+ Th2 cells. These results were derived from both intracellular cytokine staining and assessment of cytokine release in the culture supernatant. To demonstrate that the effects of IL-11 were directed at T cells, activation of CD4+CD45RA+ cells was obtained by immobilized anti-CD3 and soluble anti-CD28 MoAbs in absence of DCs. The specificity of the activity of IL-11 was demonstrated by blocking experiments using anti–IL-11 antibodies. We also found that the ability of IL-11 to polarize CD4+ T cells to the Th2 phenotype was not reversed by coincubation with DCs, which provide the most potent stimulus for Th1 differentiation. Interestingly, we found that the modulatory effects of IL-11 were similar to those of IL-4, which is the most effective cytokine for the production of Th2 cells. It should be noted that in our experiments the polarizing effect of IL-11 was partial rather than complete, because the incubated T cells continued to produce a residual amount of IFN-γ. In comparison with other cytokines, IL-11 differs in its activity from IL-10, which inhibits Th1 responses by preventing DC maturation and activation and by reducing IL-12 expression by macrophages and DCs,22 and also from IL-6, which causes differentiation of Th2 cells by up-regulating IL-4 production by T lymphocytes.20 However, despite its different mechanism of action, like IL-6 and IL-10, IL-11 may play a physiologic role in the regulation of immune response by affecting the proinflammatory and anti-inflammatory cytokine balance.
The finding that IL-11 is a potent anti-inflammatory and Th2-polarizing cytokine underscores the clinical potential of IL-11 in Th1-predominant inflammatory diseases such as autoimmune disorders and GVHD.10-12 Administration of IL-11 might also help to avoid rejection of solid organ transplants. The notion that IL-11 exerts its modulatory effects on naive T cells suggests that its administration may minimize impairment of amnestic immune responses.
We are grateful to Robin M. T. Cooke for editing.
Supported by Italian Association for the Research against Cancer (AIRC), Milan, Italy, and the Istituto Superiore di Sanità (AIDS project) CNR (N. 00.00118-ST97), and MURST EX-40%.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Roberto M. Lemoli, Institute of Hematology and Medical Oncology “Seràgnoli” Via Massarenti, 9 Bologna, Italy; e-mail: rmlemoli@med.unibo.it.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal