Abstract
Fever is associated with increased survival during acute infection, although its mechanism of action is largely unknown. This study found evidence of an unexpectedly integrated mechanism by which fever-range temperatures stimulate lymphocyte homing to secondary lymphoid tissues by increasing L-selectin and α4β7 integrin–dependent adhesive interactions between circulating lymphocytes and specialized high endothelial venules (HEV). Exposure of splenic lymphocytes in vivo to fever-like whole-body hyperthermia (WBH; 39.8 ± 0.2°C for 6 hours) stimulated both L-selectin and α4β7 integrin–dependent adhesion of lymphocytes to HEV under shear conditions in lymph nodes and Peyer patches. The adhesiveness of HEV ligands for L-selectin and α4β7 integrin (ie, peripheral lymph node addressin and mucosal addressin cell adhesion molecule-1) also increased during WBH or febrile responses associated with lipopolysaccharide-induced or turpentine-induced inflammation. Similar increases in HEV adhesion occurred during hyperthermia treatment of lymph node and Peyer patch organ cultures in vitro, indicating that the local lymphoid tissue microenvironment is sufficient for the hyperthermia response. In contrast, WBH did not augment adhesion in squamous endothelium of nonlymphoid tissues. Analysis of homing of α4β7hi L-selectinlo murine TK1 cells and L-selectinhi α4β7 integrin-negative 300.19/L-selectin transfectant cells showed that fever-range temperatures caused a 3- to 4-fold increase in L-selectin and α4β7 integrin–dependent trafficking to secondary lymphoid tissues. Thus, enhanced lymphocyte delivery to HEV by febrile temperatures through bimodal regulation of lymphocyte and endothelial adhesion provides a novel mechanism to promote immune surveillance.
Introduction
Systemic fever and local increases in temperature at sites of inflammation are cardinal features of host responses to pathogenic stimuli. Although the highly conserved fever response is linked to increased survival,1,2 the mechanisms underlying the protective action of fever have not been fully elucidated. A central locus of control of the host immune response to foreign pathogens resides at the leukocyte-endothelial interface. To fight infections in peripheral tissues, blood-borne lymphocytes gain entry across specialized high endothelial venules (HEV) in secondary lymphoid organs (ie, lymph nodes [LN] and Peyer patches [PP]) and at extralymphoid sites of infection. Lymphocyte adhesion to HEV is initiated by the L-selectin and α4β7 integrin adhesion molecules on the microvillous processes of lymphocytes.3,4 These molecules mediate the initial attachment and slow rolling of lymphocytes on HEV counterreceptors under hemodynamic shear conditions. Subsequent G-protein–dependent chemokine activation of a β2-integrin, lymphocyte function–associated antigen 1 (LFA-1), results in firm adhesion of lymphocytes to HEV and transendothelial migration. Lymphocyte-HEV interactions in LN are initiated exclusively by L-selectin recognition of sialomucin-like receptors termed peripheral lymph node addressins (PNAd), which are identified by the MECA-79 monoclonal antibody (mAb).3-6 In PP, L-selectin and α4β7 integrins initiate lymphocyte tethering on HEV through interactions with distinct domains of MECA-367 mAb–reactive mucosal addressin cell adhesion molecule-1 (MAdCAM-1).3 7
Mammals respond to natural infection or inflammatory stimuli (eg, pyrogenic cytokines, bacterial lipopolysaccharide, and turpentine) with a mild to moderate fever (1-4°C above normal body temperature).1,2,8 Fever-range temperatures are associated with enhancement of the innate and adaptive arms of the immune response through augmentation of T-cell proliferation and cytotoxicity, bioactivity of inflammatory cytokines (eg, interferon (IFN)-α), and neutrophil motility and chemotaxis.1,2 Recent studies suggest that the highly efficient adhesion mechanism for lymphocyte recirculation can also be amplified by fever-range hyperthermia. In this regard, direct exposure of human and murine lymphocytes to long-duration, fever-range temperatures in vitro markedly stimulates both L-selectin and α4β7 integrin–dependent adhesion to HEV in frozen-section assays.9-12 In sharp contrast, fever-range temperatures did not increase LFA-1 binding activity9 or α4β7 integrin–mediated adhesion to the extracellular matrix protein fibronectin.11 Hyperthermia increases L-selectin and α4β7 integrin adhesion through the release of soluble autocrine factors, without affecting the surface density of these molecules.9-12 The 11-amino-acid carboxy-terminal cytoplasmic domain of L-selectin is required for hyperthermia-enhanced L-selectin adhesion to HEV and hyperthermia-induced stable associations with the detergent-insoluble cytoskeletal matrix.10 Thus, hyperthermia-induced anchoring of L-selectin to the structural cytoskeleton has been proposed to increase the tensile strength of L-selectin,10 allowing adherent lymphocytes to better withstand shear conditions in blood vessels. The role of febrile temperatures in regulating adhesion in HEV has not been previously investigated.
In this study, we examined the possible role of fever-like whole-body hyperthermia (WBH), which mimics the thermal element of a febrile response, in promoting homing to lymphoid tissues through the regulation of lymphocyte-endothelial adhesion in vivo. Experimental approaches that raise core body temperatures to the febrile range support investigation of direct thermal effects on adhesion, segregated from the complex neuronal, hormonal, and cytokine networks operating as a consequence of natural infection.1 2 We found that fever-range WBH induced marked changes in lymphocyte distribution that correlated with enhanced adhesion in 2 distinct cellular targets. Specifically, WBH stimulated L-selectin and α4β7 integrin function in lymphocytes as well as PNAd and MAdCAM-1 adhesion in lymphoid tissue HEV. Similar increases in lymph node HEV adhesion were observed after fever induction by bacterial lipopolysaccharide (LPS) stimulation or injection of turpentine. These results provide insight into the immunoprotective mechanisms by which febrile temperatures dynamically modulate regional recruitment of circulating lymphocytes to tissues during physiologic responses to infection and inflammation.
Materials and methods
Cells and cell lines
Human peripheral blood lymphocytes (PBL) were isolated from healthy-donor buffy-coat leukocyte concentrates (American Red Cross, Buffalo, NY) by Ficoll-Hypaque centrifugation.9,10 PBL were cultured in complete medium (RPMI 1640 medium [Gibco BRL, Grand Island, NY] with 10% fetal calf serum [Gibco BRL], 2 mMl-glutamine, 100 U/mL penicillin, and 50 μg/mL streptomycin). Stably transfected mouse pre-B 300.19 cell lines10,13 (kindly provided by G. S. Kansas, Northwestern University, Chicago, IL) that expressed either full-length human L-selectin complementary DNA (300.19/L-selectin cells) or a deletion mutant lacking the 11 carboxy-terminal residues of the cytoplasmic domain (300.19/LΔcyto cells) were maintained in complete medium containing 7 × 10−6 M β-mercaptoethanol. The mouse TK1 lymphoma cell line11,14 15 (a generous gift from E. C. Butcher, Stanford University, Stanford, CA) was maintained in complete medium containing 5.7 × 10−5 M β-mercaptoethanol. Cultured cells were exposed to hyperthermia (40°C) in a 5% carbon dioxide incubator.
Reagents and mAbs
DATK32 mAb (rat IgG2a; Pharmingen, San Diego, CA) recognizes a combinatorial epitope on the mouse α4β7 integrin heterodimer. The mAbs directed against mouse and human L-selectin (Mel-14.D54, rat immunoglobulin G2a [IgG2a]; and DREG-56, mouse IgG1, respectively), MAdCAM-1 (MECA-367, rat IgG2a), and PNAd (MECA-79, rat IgM) were from the American Type Culture Collection (Rockville, MD). Isotype-matched control antibodies were from Pharmingen. Fluorescein isothiocyanate (FITC) conjugated goat F(Ab′)2 antirat IgG was from Caltag (South San Francisco, CA).
WBH treatment and fever induction by LPS and turpentine
Female BALB/c mice (8- to 10-weeks old; Taconic Lab, Germantown, NY) were housed at a temperature of 24 ± 0.1°C. Core temperatures of sentinel mice in all experimental groups were continuously monitored using a subcutaneously implanted microchip thermotransponder (14 mm × 2.2 mm; implanted ≥ 1 week before experiments began) and a programmable data-acquisition system (Biomedic Data Systems, Maywood, NJ). The core temperature of WBH-treated mice was maintained at 39.8 ± 0.2°C by placing the animals in a 38.8°C environmental chamber (model BE5000; Memmert, East Roy, WI).16-19 In selected experiments, whole-body temperatures were maintained at 38 ± 0.3°C, 39 ± 0.4°C, or 40 ± 0.2°C by placing mice in an environmental chamber adjusted to 35 to 35.7°C, 35 to 36.5°C, and 37.6 to 38.5°C, respectively. Normothermic control mice (core temperature, 36.5 ± 0.5°C) were kept at room temperature in a darkened cabinet for the experimental period. Sterile inflammatory responses were induced by subcutaneous injection of 100 μL turpentine oil (Sigma, St Louis, MO) or saline (control) into both hind legs.8,20 LPS (10 μg/25 g body weight in 1 mL sterile phosphate-buffed saline [PBS], Escherichia coli serotype 0127:B8; Sigma) or control saline was injected intraperitoneally into mice as described previously.19
Lymphoid tissue sampling and flow cytometric analysis
Blood was aspirated from the retro-orbital venous plexus of anesthetized mice and depleted of erythrocytes by means of ammonium chloride lysis. Single-cell suspensions of spleen and peripheral lymph nodes (PLN; pooled superficial inguinal, brachial, axillary, sciatic, superficial, and deep cervical nodes) were prepared by passing tissues through a wire mesh and depleting erythrocytes by means of ammonium chloride lysis. Total numbers of lymphocytes were counted by using a hemocytometer. L-selectin and α4β7 integrin expression was determined by flow cytometry as described previously.9-11A total of 10 000 events were collected by using a FACScan flow cytometer (Becton Dickinson, Sunnyvale, CA) and analyzed with Winlist 4.0 (Verity Software House, Topsham, ME).
Frozen-section adhesion assay
Lymphocyte adhesion to HEV was evaluated in a frozen-section adhesion assay as described previously.9-11 A total of 5 × 106 cells were overlaid on 12-μm cryosections of PLN, PP, or pancreas tissues from WBH-treated or nontreated BALB/c mice. Selected lymphoid tissue specimens were blocked with mAb specific for PNAd or MAdCAM-1. Alternatively, lymphocyte samples were blocked with mAb specific for L-selectin or α4β7 integrin. The assay was performed at 4°C for 30 minutes with mechanical rotation (95-112 rpm; Labline Instrument, Melrose Park, IL). After removal of nonadherent cells, sections were fixed in 3% glutaraldehyde and stained with 0.5% toluidine and absolute ethanol. Lymphocyte adhesion was quantified using light microscopy to assess a total of 300 to 500 HEV per lymphoid tissue sample or in squamous vessels of pancreatic tissues in 10 high-powered fields per sample (equivalent area of 0.65 mm2/field).
Short-term migration assays
The 300.19/L-selectin cells and TK1 cells were cultured in vitro at 37°C or 40°C for 6 hours and then labeled with PKH-26 fluorescent dye (Sigma) for short-term homing studies as described previously.9 Internal standard cells (ie, 300.19/L-Δcyto cells or TK1 cells treated with DATK32 mAb [10 μg/mL]) were maintained at 37°C before labeling with FITC.9Approximately 3 × 107 PKH-26–labeled test cells were mixed with an equal number of FITC-labeled internal standard cells and injected into the tail vein of normothermic control BALB/c mice or WBH-treated mice. Mice were kept at room temperature during the 1-hour homing assay. Lymphoid tissues were then collected, dissociated into single-cell suspensions, resuspended in equivalent volumes of 1% formaldehyde and PBS (0.1 mL for all tissues except spleen, for which 0.3 mL was used), and mounted on glass slides. In each experiment, PKH-26–labeled and FITC-labeled cells were quantified using fluorescence microscopy in 10 randomly chosen 20 × fields (equivalent area, 0.5 mm2/field) for 2 aliquots per tissue sample.
Results
Fever-range WBH causes lymphocyte redistribution in lymphoid tissues
Direct exposure of BALB/c mice to fever-range WBH (core body temperature, 39.8 ± 0.2°C) for 6 hours dramatically altered the distribution of lymphocytes in various lymphoid tissues (Figure1). A significant decrease was observed in the total number of circulating lymphocytes, as reported previously,16 as well as in the number of L-selectin–positive and α4β7 integrin–positive lymphocytes in peripheral blood (Figure 1). A concomitant increase in lymphocyte accumulation occurred in PLN (Figure 1) as well as in PP, in which a 2.3-fold increase in the number of lymphocytes was detected (data not shown; results for PP of WBH-treated mice were significantly different from those for PP of normothermic mice [P < .02; 3 experiments]). Lymphocyte distribution in the spleen was not altered by WBH.
Fever-range WBH causes lymphocyte redistribution in lymphoid tissues.
After WBH treatment of BALB/c mice for 6 hours (core temperature, 39.8 ± 0.2°C), the total number of lymphocytes in lymphoid organs (peripheral blood [PB], PLN, and spleen) was quantified. L-selectin and α4β7 integrin expression on tissue lymphocytes was analyzed by flow cytometry. Values represent the mean ± SE of 3 independent experiments. Analysis by unpaired 2-tailed Student t test showed that the difference between the number of lymphocytes in PB and PLN of normothermic controls (37.1 ± 0.2°C) and WBH-treated mice was significant (* indicates P < .02 and **,P < .03).
Fever-range WBH causes lymphocyte redistribution in lymphoid tissues.
After WBH treatment of BALB/c mice for 6 hours (core temperature, 39.8 ± 0.2°C), the total number of lymphocytes in lymphoid organs (peripheral blood [PB], PLN, and spleen) was quantified. L-selectin and α4β7 integrin expression on tissue lymphocytes was analyzed by flow cytometry. Values represent the mean ± SE of 3 independent experiments. Analysis by unpaired 2-tailed Student t test showed that the difference between the number of lymphocytes in PB and PLN of normothermic controls (37.1 ± 0.2°C) and WBH-treated mice was significant (* indicates P < .02 and **,P < .03).
WBH stimulates L-selectin and α4β7 integrin–dependent adhesion in splenic lymphocytes
To investigate the mechanisms underlying the increase in lymphocyte accumulation in PLN and PP, we first examined the effect of WBH on the adhesion properties of murine lymphocytes (Figure2). Splenic lymphocytes were isolated from BALB/c mice treated for 6 hours with fever-range WBH (39.8 ± 0.2°C), and adhesion of those lymphocytes to PNAd or MAdCAM-1 on postcapillary HEV of frozen lymphoid tissues was assessed under mechanical shear conditions. Fever-range WBH significantly increased lymphocyte adhesion to PLN and PP HEV in comparison to adhesion of lymphocytes from normothermic mice (36.8 ± 0.2°C;P < .0001; Figure 2). Hyperthermia-induced lymphocyte adhesion to PLN HEV depended on both L-selectin and PNAd, as indicated by inhibition using function-blocking mAbs (ie, Mel-1421and MECA-79,6 respectively). The increase in lymphocyte adhesion to PP HEV observed in response to WBH was similarly shown to depend on α4β7 integrin and MAdCAM-1, on the basis of mAb blockade by the α4β7 integrin–specific mAb DATK3214 and the MAdCAM-1–specific mAb MECA-367.7 However, the surface density of both L-selectin and α4β7 integrin on splenocytes was not altered by WBH, as determined by flow cytometric analysis (data not shown). The finding that WBH regulated lymphocyte adhesion in vivo supports and extends recent observations indicating that hyperthermia acts on lymphocytes in vitro to enhance L-selectin–mediated and α4β7 integrin–mediated adhesion to HEV without affecting expression of these homing receptors.9-11
Fever-range WBH stimulates lymphocyte adhesion to PNAd and MAdCAM-1 on HEV.
Splenic lymphocytes were isolated from BALB/c mice treated for 6 hours with WBH and from normothermic mice. The cells were then tested for adhesion to PLN and PP HEV (tissues from non–heat-treated mice) in a frozen-section adhesion assay under mechanical shear conditions. The specificity of lymphocyte-HEV adhesion was determined using the indicated function-blocking mAbs. Values represent the mean ± SE of 3 independent experiments. The differences between adhesion of splenocytes from normothermic control mice and WBH-treated mice were significant (P < .0001 [*] by unpaired 2-tailed Studentt test).
Fever-range WBH stimulates lymphocyte adhesion to PNAd and MAdCAM-1 on HEV.
Splenic lymphocytes were isolated from BALB/c mice treated for 6 hours with WBH and from normothermic mice. The cells were then tested for adhesion to PLN and PP HEV (tissues from non–heat-treated mice) in a frozen-section adhesion assay under mechanical shear conditions. The specificity of lymphocyte-HEV adhesion was determined using the indicated function-blocking mAbs. Values represent the mean ± SE of 3 independent experiments. The differences between adhesion of splenocytes from normothermic control mice and WBH-treated mice were significant (P < .0001 [*] by unpaired 2-tailed Studentt test).
Fever-range WBH enhances PNAd and MAdCAM-1–dependent adhesion in HEV
To determine whether fever-like temperature also regulates adhesiveness of HEV, adhesion assays were performed using frozen lymphoid tissues from BALB/c mice treated for 6 hours with WBH. A significant increase in the number of lymphocytes bound to HEV was detected when PBL (human PBL maintained at 37°C) were overlaid on PLN sections from WBH-treated mice (P < .0008; Figure3A and 3B). Hyperthermia-induced adhesion was blocked by antihuman L-selectin–specific DREG-56 mAb22and MECA-79 mAb.6 The WBH effects were remarkably stable considering that elevated HEV adhesion was maintained during cryopreservation of PLN tissues and in assays performed at 4°C. The kinetics of the WBH response were tightly regulated. A moderate increase in HEV adhesion was detected 2 hours after WBH, whereas adhesion was augmented markedly after 6 hours of WBH (Figure 3B). Moreover, HEV adhesion in PLN returned to normal levels within 12 hours after cessation of WBH (Figure 3C). Similar increases in PLN HEV adhesion were observed in response to WBH in other murine strains (C57BL/6 and C3H) and in B-cell–deficient and T-cell–deficient severe combined immunodeficiency disease mice (S.S.E. et al, unpublished data, 2000).
Fever-range WBH augments the ability of PLN HEV to support L-selectin–dependent lymphocyte adhesion.
(A) PLN from BALB/c mice treated for 6 hours with WBH (core temperature, 39.8 ± 0.2°C) and normothermic controls (36.9 ± 0.2°C) were used in frozen-section adhesion assays. Arrows indicate HEV structures containing darkly stained adherent human PBL indicator cells; bar indicates 50 μm. (B) Quantification of human PBL adhesion to PLN HEV from normothermic mice and from mice treated for 2 or 6 hours with WBH. Before assay, PBL or PLN tissue sections were incubated for 30 minutes with the indicated function-inhibiting mAb. * indicates P < .0008; **, P < .02. (C) Treatment groups included normothermic controls, mice treated for 6 hours with WBH, and mice treated for 6 hours with WBH and then maintained at room temperature for an additional 12 hours to allow core temperatures to return to normal (37.0 ± 0.2°C). PLN were removed and used in frozen-section adhesion assays to evaluate human PBL adhesion to HEV. * indicates P < .002. (D) Adhesion of murine 300.19/L-selectin transfectants and 300.19/L-Δcyto cells was evaluated in frozen sections of LN HEV from normothermic mice and mice treated with WBH for 6 hours. * indicates P < .0007. The differences between HEV adhesion in PLN from normothermic mice and WBH-treated mice were significant by unpaired 2-tailed Student ttest.
Fever-range WBH augments the ability of PLN HEV to support L-selectin–dependent lymphocyte adhesion.
(A) PLN from BALB/c mice treated for 6 hours with WBH (core temperature, 39.8 ± 0.2°C) and normothermic controls (36.9 ± 0.2°C) were used in frozen-section adhesion assays. Arrows indicate HEV structures containing darkly stained adherent human PBL indicator cells; bar indicates 50 μm. (B) Quantification of human PBL adhesion to PLN HEV from normothermic mice and from mice treated for 2 or 6 hours with WBH. Before assay, PBL or PLN tissue sections were incubated for 30 minutes with the indicated function-inhibiting mAb. * indicates P < .0008; **, P < .02. (C) Treatment groups included normothermic controls, mice treated for 6 hours with WBH, and mice treated for 6 hours with WBH and then maintained at room temperature for an additional 12 hours to allow core temperatures to return to normal (37.0 ± 0.2°C). PLN were removed and used in frozen-section adhesion assays to evaluate human PBL adhesion to HEV. * indicates P < .002. (D) Adhesion of murine 300.19/L-selectin transfectants and 300.19/L-Δcyto cells was evaluated in frozen sections of LN HEV from normothermic mice and mice treated with WBH for 6 hours. * indicates P < .0007. The differences between HEV adhesion in PLN from normothermic mice and WBH-treated mice were significant by unpaired 2-tailed Student ttest.
To confirm the L-selectin dependence of the WBH response in PLN HEV, we examined the effect of WBH on the ability of PLN HEV to support adhesion of murine 300.19 B-cell transfectants that express full-length human L-selectin (300.19/L-selectin) or 300.19/L-Δcyto cells that express a truncated, nonfunctional form of L-selectin lacking the 11 carboxy-terminal amino acids of the cytoplasmic tail.10,13,23 L-selectin–dependent adhesion of 300.19/L-selectin cells was significantly stimulated by WBH (P < .007), whereas WBH failed to enhance binding of 300.19/L-Δcyto cells to PLN HEV (Figure 3D). Moreover, because 300.19 transfectants are LFA-1–negative,23 this experiment excluded any contributions of LFA-1 to hyperthermia-induced adhesion to HEV.
Febrile responses in mice range from 37.5 to 39.8°C after natural infections (eg, influenza and cecal infection) or stimulation by inflammatory agents (LPS and turpentine).1,2,8,20 To determine whether increased HEV adhesion occurs in a similar temperature range, mice were treated with various temperatures of WBH, and lymphocyte adhesion to lymph node HEV was examined in frozen sections (Figure 4). Significant increases in lymphocyte-HEV adhesion were observed when body temperatures were raised to 38 ± 0.3°C, 39 ± 0.4°C, and 40 ± 0.2°C. To examine the relationship between adhesion and fever-inducing inflammatory responses, the effects on L-selectin–dependent binding of lymphocytes to lymph node HEV were assessed in 2 well-characterized models of fever induction during sterile inflammatory responses.8,19 20 As shown in Figure4, a marked increase in L-selectin–dependent adhesion of lymphocytes to lymph node HEV was detected after fever induction in response to bacterial LPS, which induces systemic inflammation, or turpentine, which induces local inflamed abscesses.
Increased PLN HEV adhesion is associated with febrile temperatures after WBH treatment or inflammation induced by turpentine or LPS.
The core temperature of mice was raised to the indicated temperature by either WBH treatment for 6 hours (left panel), injection of turpentine oil ([Turp], 100 μL given subcutaneously; middle panel), or injection of LPS (10 μg/kg given intraperitoneally; right panel). Control mice were given injections of sterile saline. PLN tissues were isolated at the indicated times, and adhesion of human PBL to HEV of frozen sections was quantified under shear conditions. Before assay, selected PBL samples were pretreated with DREG-56 blocking mAb. Data are the mean ± SD results with triplicate samples; results are representative of 3 independent experiments. The differences between HEV adhesion in PLN from normothermic mice and those from mice at febrile temperatures were significant by unpaired 2-tailed Student t test. * indicates P < .0001; ▪, untreated; and ■, DREG-56 mAb treated.
Increased PLN HEV adhesion is associated with febrile temperatures after WBH treatment or inflammation induced by turpentine or LPS.
The core temperature of mice was raised to the indicated temperature by either WBH treatment for 6 hours (left panel), injection of turpentine oil ([Turp], 100 μL given subcutaneously; middle panel), or injection of LPS (10 μg/kg given intraperitoneally; right panel). Control mice were given injections of sterile saline. PLN tissues were isolated at the indicated times, and adhesion of human PBL to HEV of frozen sections was quantified under shear conditions. Before assay, selected PBL samples were pretreated with DREG-56 blocking mAb. Data are the mean ± SD results with triplicate samples; results are representative of 3 independent experiments. The differences between HEV adhesion in PLN from normothermic mice and those from mice at febrile temperatures were significant by unpaired 2-tailed Student t test. * indicates P < .0001; ▪, untreated; and ■, DREG-56 mAb treated.
Analysis of the WBH effects on PP HEV adhesion in vivo revealed that fever-range temperatures stimulated MAdCAM-1 interactions with both L-selectin and α4β7 integrin (Figure5A), representing 2 independent classes of adhesion molecules.3,4 In this regard, WBH treatment of mice increased the ability of PP HEV to support MECA-367–sensitive adhesion of L-selectin–positive and α4β7 integrin–positive human PBL and of TK1 murine T-cell lymphoma cells, which express α4β7 integrin but not functional levels of L-selectin.11 15 WBH also enhanced MAdCAM-1–dependent adhesion of L-selectin–positive 300.19 transfectant cells (Figure 5A), which do not express DATK32 mAb–reactive α4β7 integrin. The increase in adhesion observed in PP and PLN HEV in response to WBH was not associated with any apparent changes in HEV expression of MAdCAM-1 or PNAd, as determined by immunofluorescence analysis of frozen-tissue sections (S.S.E. et al, unpublished data, 2000).
Fever-range temperature stimulates MAdCAM-1–dependent adhesion in PP HEV during WBH treatment in vivo and in organ cultures in vitro.
(A,B) PP or pancreatic tissues were isolated from normothermal controls (core temperature of controls [C] was 36.8 ± 0.2°C) or mice treated 6 hours with WBH (39.7 ± 0.2°C). Tissues were cryopreserved and used in adhesion assays. (A) Selected tissue cryosections were blocked with the MAdCAM-1–specific mAb MECA-367. (B) Cells were either untreated or treated with DATK32 mAb or DREG-56 mAb. (C) Bilateral pairs of PLN were isolated from normothermic BALB/c mice, separated into 2 groups, and cultured for 6 hours in 1 mL complete medium at 37°C or 40°C in a 5% carbon dioxide incubator. PP collected from normothermic mouse pairs were cultured at 37°C and 40°C. Lymphoid tissues were then frozen and used in adhesion assays under shear conditions. To evaluate L-selectin–dependent adhesion of human PBL to PLN HEV, assays were performed without antibody or in the presence of function-blocking mAb (DREG-56 or MECA-79). To quantify α4β7 integrin/MAdCAM-1–dependent adhesion in PP organ cultures, adherence of TK1 indicator cells to HEV was evaluated without antibody or in the presence of DATK32 mAb or MECA-367 mAb. Data represent the mean ± SE values from 3 independent experiments. * indicates significant differences for the increase in HEV adhesion detected in response to hyperthermia (P < .0001 by unpaired 2-tailed Student t test).
Fever-range temperature stimulates MAdCAM-1–dependent adhesion in PP HEV during WBH treatment in vivo and in organ cultures in vitro.
(A,B) PP or pancreatic tissues were isolated from normothermal controls (core temperature of controls [C] was 36.8 ± 0.2°C) or mice treated 6 hours with WBH (39.7 ± 0.2°C). Tissues were cryopreserved and used in adhesion assays. (A) Selected tissue cryosections were blocked with the MAdCAM-1–specific mAb MECA-367. (B) Cells were either untreated or treated with DATK32 mAb or DREG-56 mAb. (C) Bilateral pairs of PLN were isolated from normothermic BALB/c mice, separated into 2 groups, and cultured for 6 hours in 1 mL complete medium at 37°C or 40°C in a 5% carbon dioxide incubator. PP collected from normothermic mouse pairs were cultured at 37°C and 40°C. Lymphoid tissues were then frozen and used in adhesion assays under shear conditions. To evaluate L-selectin–dependent adhesion of human PBL to PLN HEV, assays were performed without antibody or in the presence of function-blocking mAb (DREG-56 or MECA-79). To quantify α4β7 integrin/MAdCAM-1–dependent adhesion in PP organ cultures, adherence of TK1 indicator cells to HEV was evaluated without antibody or in the presence of DATK32 mAb or MECA-367 mAb. Data represent the mean ± SE values from 3 independent experiments. * indicates significant differences for the increase in HEV adhesion detected in response to hyperthermia (P < .0001 by unpaired 2-tailed Student t test).
In sharp contrast to the marked effects of WBH on adhesion in differentiated HEV of PLN and PP (Figure 3 and Figure 5A), WBH failed to increase adhesive interactions involving either α4β7 integrin (assessed by using normothermic TK1 cells) or L-selectin (indicated by results in normothermic 300.19/L-selectin cells) in squamous, nonspecialized blood vessels of extralymphoid tissues such as the pancreas (Figure 5B). To determine whether the lymphoid tissue microenvironment was sufficient to support HEV responses to fever-like temperature, PLN and PP organ cultures were treated in vitro with hyperthermia (40°C for 6 hours) before quantification of HEV adhesion (Figure 5C). Hyperthermia stimulated L-selectin/PNAd adhesion and α4β7 integrin/MAdCAM-1 adhesion, respectively, in HEV in PLN and PP organ cultures (P < .0001), closely paralleling the responses of these tissues during WBH (Figure 3 and Figure 5A). These data show that HEV adhesion is regulated in the local lymphoid microenvironment and does not universally require involvement of other organ systems, including the highly integrated hypothalamus-pituitary-adrenal axis, which is known to contribute to physiologic febrile responses.1 2
Effect of WBH on L-selectin and α4β7 integrin–dependent lymphocyte homing
Short-term migration studies were performed to establish that fever-range temperature controls delivery of lymphocytes to LN and PP through L-selectin and/or α4β7 integrin–dependent mechanisms. Thus, 300.19/L-selectin cells and TK1 cells were used in these in vivo homing studies to segregate contributions of L-selectin and α4β7 integrin in response to hyperthermia. Moreover, the absence of LFA-1 on 300.19/L-selectin cells23 allowed analysis of hyperthermia effects on L-selectin–mediated migration to organized lymphoid tissues without the participation of LFA-1. The distribution of adoptively transferred 300.19/L-selectin cells in PLN and PP tissues of normothermic mice was similar to that reported for lymphocytes of LFA-1–deficient mice.24 Therefore, 1 hour after injection, most 300.19/L-selectin cells were localized in the lumen of PLN and PP HEV, whereas extravasation into tissue parenchyma was more limited (ie, the proportion of 300.19/L-selectin cells within the lumen of PLN/PP HEV compared with cells within tissue parenchyma was 77% ± 1% and 23% ± 1%, respectively, with a total of 84 ± 5 cells/mm2 detected in PLN or PP tissues).
In the first series of experiments that examined hyperthermia effects on L-selectin–mediated migration, PKH-26–labeled 300.19/L-selectin cells and FITC-labeled 300.19/L-Δcyto cells were simultaneously injected intravenously into BALB/c mice that were pretreated with WBH for 6 hours. After 1 hour, lymphoid tissues were removed to quantify the relative accumulation of fluorescence-labeled cells in WBH-treated mice compared with migration of the same cell types in normothermic control mice (Figure 6). WBH caused a greater than 4-fold increase in localization of 300.19/L-selectin cells in PLN relative to results in normothermic controls. WBH also stimulated accumulation of 300.19/L-selectin cells in PP and mesenteric LN (MLN), although to a lesser extent than in PLN, possibly reflecting the requirement for α4β7 integrin for efficient homing to these tissues.3,4 25 In sharp contrast, WBH failed to augment localization of 300.19/L-Δcyto cells in PLN, MLN, or PP. The differences between localization of 300.19/L-selectin cells and 300.19/L-Δcyto cells in PLN, MLN, and PP in WBH-treated mice were significant (P < .0001), supporting the conclusion that WBH stimulates L-selectin–dependent accumulation of cells in lymphoid tissues.
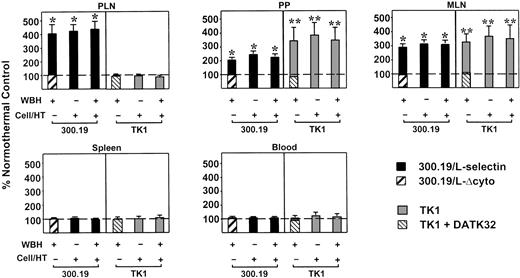
Fever-range hyperthermia stimulates L-selectin–dependent and α4β7 integrin–dependent migration of lymphocytes to LN and PP.
The 300.19/L-selectin cells and TK1 cells were cultured at 37°C or treated for 6 hours in vitro with fever-like hyperthermia (Cell/HT; 40°C), and then labeled with PKH-26 fluorescent dye. PKH-26–labeled cells were mixed with equivalent numbers of FITC-labeled internal standard cells (300.19/L-Δcyto cells or TK1 cells treated with DATK32 mAb) and injected intravenously into normothermic control mice (core temperature, 36.8 ± 0.2°C) or mice pretreated with WBH (39.7 ± 0.2°C). After 1 hour, the number of fluorescent cells in single-cell suspensions of lymphoid tissues was determined. Data represent the relative accumulation of cells in lymphoid tissues, expressed as a percentage of control cells (ie, cells of the same type maintained at 37°C) that were labeled identically and injected into normothermic mice. Therefore, 100% in the graph (broken lines) represents the behavior of cells cultured at 37°C after injection into normothermic control mice. Values are the mean ± SE of 3 independent experiments. The difference in accumulation of 300.19/L-selectin cells or TK1 cells relative to internal standard cells (300.19/L-Δcyto cells or DATK32-treated TK1 cells, respectively) was significant by unpaired 2-tailed Student ttest (* indicates P < .0001; and **,P < .005). The SEM for 300.19/L-Δcyto cells and DATK32-treated TK1 cells was less than or equal to 10% (not shown).
Fever-range hyperthermia stimulates L-selectin–dependent and α4β7 integrin–dependent migration of lymphocytes to LN and PP.
The 300.19/L-selectin cells and TK1 cells were cultured at 37°C or treated for 6 hours in vitro with fever-like hyperthermia (Cell/HT; 40°C), and then labeled with PKH-26 fluorescent dye. PKH-26–labeled cells were mixed with equivalent numbers of FITC-labeled internal standard cells (300.19/L-Δcyto cells or TK1 cells treated with DATK32 mAb) and injected intravenously into normothermic control mice (core temperature, 36.8 ± 0.2°C) or mice pretreated with WBH (39.7 ± 0.2°C). After 1 hour, the number of fluorescent cells in single-cell suspensions of lymphoid tissues was determined. Data represent the relative accumulation of cells in lymphoid tissues, expressed as a percentage of control cells (ie, cells of the same type maintained at 37°C) that were labeled identically and injected into normothermic mice. Therefore, 100% in the graph (broken lines) represents the behavior of cells cultured at 37°C after injection into normothermic control mice. Values are the mean ± SE of 3 independent experiments. The difference in accumulation of 300.19/L-selectin cells or TK1 cells relative to internal standard cells (300.19/L-Δcyto cells or DATK32-treated TK1 cells, respectively) was significant by unpaired 2-tailed Student ttest (* indicates P < .0001; and **,P < .005). The SEM for 300.19/L-Δcyto cells and DATK32-treated TK1 cells was less than or equal to 10% (not shown).
Hyperthermia pretreatment of 300.19/L-selectin cells in vitro before injection into normothermic mice also markedly increased the level of accumulation in PLN, MLN, and PP compared directly with homing of 300.19/L-selectin cells maintained at 37°C (P < .0001; Figure 6). However, combined hyperthermia treatment of cells and WBH-treatment of mice did not result in additive or synergistic effects on L-selectin–dependent accumulation in secondary lymphoid tissues. These data suggest that hyperthermia responses in lymphocytes and HEV functionally converge on a common adhesion mechanism. A significant increase in 300.19/L-selectin cell migration to PLN and PP (3-fold and 1.75-fold, respectively) was also observed after only 2 hours of stimulation by WBH or in vitro hyperthermia treatment of 300.19/L-selectin cells (data not shown).
Similar regulation of α4β7 integrin–dependent homing of TK1 cells to PP and MLN was observed in response to WBH or direct hyperthermia treatment of TK1 cells (Figure 6). Specifically, 6 hours of WBH treatment of mice or hyperthermia pretreatment of TK1 cells independently resulted in a greater than 3-fold increase in homing to PP and MLN. Hyperthermia did not augment TK1 cell homing to PLN, consistent with previous findings indicating that α4β7 integrin does not normally participate in lymphocyte homing to PLN.3,24,25 The increase in TK1 homing to PP and MLN was significant when compared directly with localization of DATK32 mAb–treated TK1 cells (P < .005), confirming the role of α4β7 integrin in directing lymphocyte homing to lymphoid tissues in response to hyperthermia. Again, combined WBH and hyperthermia treatment of TK1 cells in vitro did not further augment α4β7 integrin–dependent migration to PP or MLN. In agreement with evidence that L-selectin and α4β7 integrin do not contribute to lymphocyte localization in spleen,3,25 neither WBH nor hyperthermia treatment in vitro influenced the distribution of 300.19/L-selectin cells or TK1 cells in this tissue (Figure 6). Furthermore, fever-like hyperthermia did not alter the proportion of labeled cells in peripheral blood, despite increased migration of fluorescent cells to LN and PP. These observations likely reflect the relatively small percentage (0.5%-2%) of total cells injected intravenously that traffic to LN and PP during 1-hour short-term homing studies25 (S.S.E. et al, unpublished data, 1996).
Discussion
This study provides the first evidence of an active role for fever-like temperatures in amplifying lymphocyte delivery to secondary lymphoid tissues in vivo through the regulation of lymphocyte adhesion to HEV. Unexpectedly, fever-like hyperthermia affected adhesion in 2 distinct cellular targets. Hyperthermia stimulated lymphocyte adhesion mediated by L-selectin and α4β7 integrin, as well as HEV adhesion dependent on PNAd and MAdCAM-1. Notably, hyperthermia regulation of lymphocyte-HEV interactions, like the endogenous fever response,1,2 is evolutionarily conserved in species that diverged more than 180 million years ago (ie, mouse and human).26 The 3- to 4-fold increase in lymphocyte migration to LN and PP that occurred in response to hyperthermia potentially represents a profound increase in the opportunity for naive lymphocytes to encounter foreign pathogens during immune surveillance of secondary lymphoid tissues. Emerging evidence that subsets of memory cells share recirculation routes of naive lymphocytes through common expression of adhesion molecules and chemokine receptors (ie, L-selectin, α4β7 integrin, and the CCR7 chemokine receptor3,4 27) also raises the possibility that the thermal element of fever promotes delivery of both naive lymphocytes and antigen-primed memory subsets to LN and PP.
Hyperthermia exerts tight control on lymphocyte-endothelial adhesion. A marked increase in L-selectin–dependent and α4β7 integrin–dependent lymphocyte-endothelial adhesion and homing to LN and PP was detected within 2 to 6 hours of stimulation by hyperthermia. Importantly, lymphocyte delivery to HEV in response to fever-range temperatures temporally precedes the 8- to 24-hour interval during which antigen-specific T cells initially become activated in secondary lymphoid tissues during a primary immune response.28 The effects of hyperthermia on adhesion are also tightly regulated with respect to the endothelial target. Robust increases in adhesion were observed in cuboidal, differentiated HEV of LN and PP but not in squamous, less-differentiated endothelium of nonlymphoid tissues (ie, pancreas). Moreover, the specificity of HEV adhesion was not compromised during the hyperthermia response. Thus, WBH selectively amplified L-selectin–dependent but not α4β7 integrin–dependent delivery of cells to PLN HEV. Preliminary studies have suggested that WBH also increases adhesion of HEV-like vessels in inflamed lesions.29 Selective regulation of adhesion in differentiated HEV but not squamous endothelium by fever temperatures would serve to focus the immune response to lymphoid tissues and sites of infection while preventing an unproductive exodus of lymphocytes to other tissues during a physiologic febrile episode.
Evidence that in vitro hyperthermia treatment stimulates L-selectin–dependent and α4β7 integrin–dependent adhesion in lymphocytes and in HEV of lymphoid tissue organ cultures indicates that the hypothalamus-pituitary-adrenal axis is not pivotal for this response. Although the molecular mechanisms underlying hyperthermia-induced adhesion are not known, it is probable that local cytokine networks are involved. In this regard, hyperthermia promotes the release or bioactivity of several proinflammatory cytokines, including IFN-α, tumor necrosis factor α (TNF-α), interleukin (IL) 6, and IL-1β.1,2,19 These cytokines were found to enhance L-selectin and α4β7 integrin adhesion in vitro12 (S.S.E. et al, manuscript in preparation), directly paralleling the hyperthermia effects. Moreover, fever induction in response to LPS or turpentine is associated with high systemic levels of IL-6, TNF-α, and IL-1β,1,2,8,19,20which could be mechanistically related to the marked increase in lymphocyte-HEV adhesion observed in the current study. Fever-range temperatures stimulate L-selectin and α4β7 integrin adhesion in lymphocytes through the release of soluble autocrine factors, without increasing the surface density of these molecules (reported here and previously9-12). These findings suggest that hyperthermia enhances the affinity or avidity of selected lymphocyte adhesion molecules, possibly by altering interactions with the nonionic detergent-insoluble cytoskeletal matrix10 or the overall organization of the spectrin-actin–based cytoskeleton, as observed previously in response to fever-range hyperthermia.17,18 30
It remains to be determined whether increases in HEV adhesion reflect direct effects of hyperthermia on endothelial cells or indirect effects on stromal cells (eg, monocytes, fibroblasts, and dendritic cells) in the microenvironment of organized secondary lymphoid tissues. Studies are in progress to determine whether the remarkably stable changes in lymphocyte-HEV adhesion observed here can be explained by biochemical modifications (eg, altered sulfation or sialylation of PNAd or MAdCAM-1), conformational changes, increased clustering, or changes in the lateral mobility of adhesion molecules in lymphocyte or endothelial plasma membranes.3-5 Collectively, our findings provide new evidence for a unifying mechanism by which febrile temperatures dynamically modulate regional recruitment of circulating lymphocytes to tissues during physiologic responses to infection and inflammation.
We thank E. C. Butcher and G. S. Kansas for sharing the TK1 and 300.19 L-selectin cell lines, respectively; C. C. Stewart for advice on flow cytometry; M. McGarry and J. Lau for assistance in turpentine inflammatory studies; R. A. Bruce, C.-Y. Tsao, and A. Sumlin for technical support; and J. D. Black for critical review of the manuscript.
Supported by grants from the National Institutes of Health (CA79765 [S.S.E.], CA71599 [E.A.R.], and P30 CA16056-21), the Department of Defense (DAMD17-8-8311 [S.S.E.]), the Roswell Park Alliance Foundation (S.S.E. and E.A.R.), and the Cancer Research Institute (J.R.O.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Sharon S. Evans, Department of Immunology, Roswell Park Cancer Institute, Carlton and Elm Streets, Buffalo, NY 14263.

![Fig. 1. Fever-range WBH causes lymphocyte redistribution in lymphoid tissues. / After WBH treatment of BALB/c mice for 6 hours (core temperature, 39.8 ± 0.2°C), the total number of lymphocytes in lymphoid organs (peripheral blood [PB], PLN, and spleen) was quantified. L-selectin and α4β7 integrin expression on tissue lymphocytes was analyzed by flow cytometry. Values represent the mean ± SE of 3 independent experiments. Analysis by unpaired 2-tailed Student t test showed that the difference between the number of lymphocytes in PB and PLN of normothermic controls (37.1 ± 0.2°C) and WBH-treated mice was significant (* indicates P < .02 and **,P < .03).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/9/10.1182_blood.v97.9.2727/6/m_h80910963001.jpeg?Expires=1769085417&Signature=ObYed1IXaIj~6PRPoCFfqVrNUjDwIH4m6mYxXizYVvaR4KgC3j~SHSV-JIAW5cHSnctrqsWxjGD7MpCdX-taRw8sElveFG7BktIyCgxjI3w3bb49QNIGZax1gEgUNjL7CopOliZYZppiHpAawerFOn2iFI8vSHxohcE0qxAu239bMqyz6H~ToIs8fnJ-fdvb1NfBOQI7FEtSP-g3u3YlVt2t7I0WyV3ppk-03hXQA5gxqrkhXnpaE6wkKberZghPoMC39FBH-TcSyAGV5-eUQ4xxlnfzvA8Y8mFRv91ecZV25N49BxwBahDNZWRvtVQlluXZVK3t1E~AvPhfpAzmEw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Fever-range WBH stimulates lymphocyte adhesion to PNAd and MAdCAM-1 on HEV. / Splenic lymphocytes were isolated from BALB/c mice treated for 6 hours with WBH and from normothermic mice. The cells were then tested for adhesion to PLN and PP HEV (tissues from non–heat-treated mice) in a frozen-section adhesion assay under mechanical shear conditions. The specificity of lymphocyte-HEV adhesion was determined using the indicated function-blocking mAbs. Values represent the mean ± SE of 3 independent experiments. The differences between adhesion of splenocytes from normothermic control mice and WBH-treated mice were significant (P < .0001 [*] by unpaired 2-tailed Studentt test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/9/10.1182_blood.v97.9.2727/6/m_h80910963002.jpeg?Expires=1769085417&Signature=xmFpczCemnVcbY8LNNGhSFSUfceG3Ij0hOVhSgq4iuMnQ5Ny543Q94toV8Vkxqv1eb3WvLOjm8ieHcHfL1TBXH5alKseYuKJCGew8l2qh2NPIvYcIU~yaNDo1USGgff8l9Jt~awJgrZDc7NWCt3nVNbwVqstgiAn3sLVMAt~eeviT1B5Q-tiZ7CyezTawqBro0Jj~8EYKY4tGMAs7iVXYhoHeuLEjv59x1MDRmaojI9c3ULzZYxyVpBcpjAnis2LME9LMI2SL~V2a4yNS-Zu2~0KlJExxMqjtnWcNOKXAaY4u53d9pjhzqBjTyhn-jt-zwHyZuTf6jP~sAe-fqfa0w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 4. Increased PLN HEV adhesion is associated with febrile temperatures after WBH treatment or inflammation induced by turpentine or LPS. / The core temperature of mice was raised to the indicated temperature by either WBH treatment for 6 hours (left panel), injection of turpentine oil ([Turp], 100 μL given subcutaneously; middle panel), or injection of LPS (10 μg/kg given intraperitoneally; right panel). Control mice were given injections of sterile saline. PLN tissues were isolated at the indicated times, and adhesion of human PBL to HEV of frozen sections was quantified under shear conditions. Before assay, selected PBL samples were pretreated with DREG-56 blocking mAb. Data are the mean ± SD results with triplicate samples; results are representative of 3 independent experiments. The differences between HEV adhesion in PLN from normothermic mice and those from mice at febrile temperatures were significant by unpaired 2-tailed Student t test. * indicates P < .0001; ▪, untreated; and ■, DREG-56 mAb treated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/9/10.1182_blood.v97.9.2727/6/m_h80910963004.jpeg?Expires=1769085417&Signature=yu8deOFXdfLSTXlLGZ7PkAHw~tt9Awcma0G4WwKDxkAOnFVKYYg6wrfaqPcDWh4LUdNjqXPqgCnOrwA1UnKV81xyv9M364wGHsT-1lwyyIfY-ssVf5Ybw~FGEsju3ZyobplutXoe8ng3qNHG9G7N~ZK5DFbx~VJ9Tkgaw0yELjwKfRpM0HkuMtR~7vcE0vJ8Jzlu~Bsd6yKC3bd45j2U4VP~6HDlDhF16pf1aYB4FKvibCplFyJyJ2Y-B-mIdvMHZzypPGCbLUiVygf~W6Bk6~XLDvrlLYqedpM7iTImKzM4whz1XjtXjUrsX0CEVyuWory3rFBItzRs-McvKU-hTA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Fever-range temperature stimulates MAdCAM-1–dependent adhesion in PP HEV during WBH treatment in vivo and in organ cultures in vitro. / (A,B) PP or pancreatic tissues were isolated from normothermal controls (core temperature of controls [C] was 36.8 ± 0.2°C) or mice treated 6 hours with WBH (39.7 ± 0.2°C). Tissues were cryopreserved and used in adhesion assays. (A) Selected tissue cryosections were blocked with the MAdCAM-1–specific mAb MECA-367. (B) Cells were either untreated or treated with DATK32 mAb or DREG-56 mAb. (C) Bilateral pairs of PLN were isolated from normothermic BALB/c mice, separated into 2 groups, and cultured for 6 hours in 1 mL complete medium at 37°C or 40°C in a 5% carbon dioxide incubator. PP collected from normothermic mouse pairs were cultured at 37°C and 40°C. Lymphoid tissues were then frozen and used in adhesion assays under shear conditions. To evaluate L-selectin–dependent adhesion of human PBL to PLN HEV, assays were performed without antibody or in the presence of function-blocking mAb (DREG-56 or MECA-79). To quantify α4β7 integrin/MAdCAM-1–dependent adhesion in PP organ cultures, adherence of TK1 indicator cells to HEV was evaluated without antibody or in the presence of DATK32 mAb or MECA-367 mAb. Data represent the mean ± SE values from 3 independent experiments. * indicates significant differences for the increase in HEV adhesion detected in response to hyperthermia (P < .0001 by unpaired 2-tailed Student t test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/9/10.1182_blood.v97.9.2727/6/m_h80910963005.jpeg?Expires=1769085417&Signature=xDWlEFfYb38551tyh6LglNFnGNmqsc~d6jK0a5rKJRCQ8RuxohgY2kjQRmjDOASu7hP6ZMERqfZKYK4KCyo3uePvlsVHrppilX-Kj2TTE56FXSonUb3tc~CqD8XQTF0FLvHanU-k7yjy9RvSbb36baPBQOPZOPq~cjSLNaYg3LCreahMHpH0GrQLr77XfYMtVO7feTqn1gNkezgzokS1slY6ex4oO26ddZEOJYFu1KWtTZBskGxVJz1~40JAocuVzOsw5y7hmmrDGdl4GqlRxK-o-~QPI16NTAZF1rlhmmnY8IBoTFn87J4cAMSdv1JE2bFQJtfDmQSXoF6dxRwKYg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal