Abstract
In human leukocyte antigen haplotype–mismatched transplantation, extensive T-cell depletion prevents graft-versus-host disease (GVHD) but delays immune recovery. Granulocyte colony-stimulating factor (G-CSF) is given to donors to mobilize stem cells and to recipients to ensure engraftment. Studies have shown that G-CSF promotes T-helper (Th)-2 immune deviation which, unlike Th1 responses, does not protect against intracellular pathogens and fungi. The effect of administration of G-CSF to recipients of mismatched hematopoietic transplants with respect to transplantation outcome and functional immune recovery was investigated. In 43 patients with acute leukemia who received G-CSF after transplantation, the engraftment rate was 95%. However, the patients had a long-lasting type 2 immune reactivity, ie, Th2-inducing dendritic cells not producing interleukin 12 (IL-12) and high frequencies of IL-4– and IL-10–producing CD4+ cells not expressing the IL-12 receptor β2 chain. Similar immune reactivity patterns were observed on exposure of donor cells to G-CSF. Elimination of postgrafting administration of G-CSF in a subsequent series of 36 patients with acute leukemia, while not adversely affecting engraftment rate (93%), resulted in the anticipated appearance of IL-12–producing dendritic cells (1-3 months after transplantation versus > 12 months in transplant recipients given G-CSF), of CD4+ cells of a mixed Th0/Th1 phenotype, and of antifungal T-cell reactivity in vitro. Moreover, CD4+ cell counts increased in significantly less time. Finally, elimination of G-CSF–mediated immune suppression did not significantly increase the incidence of GVHD (< 15%). Thus, this study found that administration of G-CSF to recipients of T-cell–depleted hematopoietic transplants was associated with abnormal antigen-presenting cell functions and T-cell reactivity. Elimination of postgrafting administration of G-CSF prevented immune dysregulation and accelerated functional immune recovery.
Introduction
In fully human leukocyte antigen (HLA) haplotype–mismatched transplantation, extensive T-cell depletion is the obligatory choice because it prevents severe graft-versus-host disease (GVHD)1 without the need for postgrafting immune suppression. Administration of high doses of T-cell–depleted peripheral blood stem cells (PBSCs), obtained after mobilization with granulocyte colony-stimulating factor (G-CSF), has made HLA haplotype–mismatched transplantation a clinical reality for the more than 40% of patients who do not have a matched donor.2-4 With engraftment rates higher than 95% and GVHD rates lower than 10%, a 25% probability of event-free survival at 2.5 years was achieved in patients at very high risk of leukemia relapse and transplantation-related mortality.3 Moreover, the HLA incompatibility triggers specific donor-versus-recipient natural killer cell alloreactions that have antileukemic potential without causing GVHD.5 However, immune recovery is particularly slow and associated with a 40% infection-related mortality rate (due mostly to fungal infections and to which previous chemotherapy and colonization by infectious agents undoubtedly make a contribution).3Similar patterns of immune reconstitution and relatively high infection-related mortality rates are common in other T-cell–depleted transplantations, such as those using matched T-cell–depleted transplants from unrelated donors.6
In adults, because of declining thymic function, immune recovery originates from expansion of the mature T cells infused with the graft, with de novo production of naive T cells occurring months later.7-11 In unmanipulated transplants, peripheral T-cell expansion is antagonized by the immune suppressive therapy for GVHD prophylaxis.12-14 In addition, tissue damage caused by conditioning regimens prevents T-cell homing to peripheral lymphoid tissues,15,16 where generation and maintenance of T-cell memory take place.17 In T-cell–depleted transplantation, the absence of postgrafting immune suppressive treatments allows undisturbed homeostatic expansion of the few T cells in the graft. In this case, however, immune recovery is hindered by the paucity of the starting T-cell population. As a consequence of the combination of perturbed homing to peripheral lymphoid tissues and the nonphysiologic high number of cell divisions required for T-cell regrowth, immune abnormalities characterize posttransplantation immune recovery.18-24
G-CSF is currently used not only to mobilize peripheral blood CD34+ cells in donors but also to treat recipients in the early posttransplantation phase to ensure engraftment.2,3Several studies in animals and human volunteers showed that G-CSF decreases production of inflammatory cytokines,25increases production of interleukin 10 (IL-10),26 and promotes mobilization of T-helper (Th)-2–inducing dendritic cells (DCs)27 and Th2 immune deviation.28-30 Thus, a byproduct of G-CSF could be an immune suppressive action on the mobilized cells, which is generally considered an advantage in non-T-cell–depleted PBSC transplantation because it may attenuate the risk of acute GVHD. However, in mismatched transplants, the T-cell depletion that prevents acute and chronic GVHD also causes prolonged immune suppression. In this setting, administration of G-CSF after transplantation might not be required to prevent graft-versus-host reactivity and could adversely affect recovery of antinfection T-cell reactivity.
To understand whether postgrafting administration of G-CSF in recipients of HLA haplotype–mismatched hematopoietic transplants hinders immune recovery, we here analyzed the recovery of immune reactivity with respect to antigen-presenting cell (APC) and Th-cell functions in patients who were treated with G-CSF and patients who were not given the agent. We also examined whether immune reactivity of donor cells was altered by in vitro or in vivo exposure to G-CSF. We found that postgrafting administration of G-CSF to transplant recipients prevented recovery of immune responses by affecting both APC and Th-cell functions. In addition, G-CSF adversely affected immune reactivity of donor cells. Moreover, elimination of postgrafting administration of G-CSF to transplant recipients prevented immune dysregulation and accelerated functional immune recovery.
Patients and methods
Transplantations
Data on posttransplantation immune functions were collected from 43 patients whose clinical outcome data were reported in 19983 and from 36 patients in a series of transplantations done between January 1999 and July 2000 (Table1). Donors, who were either parents, siblings, or children of the recipients, were assessed for HLA compatibility by serologic typing. All pairs of donors and recipients were identical for only one HLA haplotype (haploidentical) and incompatible at the HLA-A, HLA-B, HLA-C, and DR loci of the unshared haplotype. Patients in the 2 series did not differ with respect to diagnosis and poor-risk factors for leukemia relapse. In both series, patients underwent conditioning with total-body irradiation followed by administration of thiotepa, rabbit antithymocyte globulins (ATG; Fresenius, Bad Homburg, Germany), and fludarabine as described previously.3 The ATG used in the 2 protocols were of equal potency, as they had a cytotoxicity titer of 1:512. Donor G-CSF–mobilized peripheral blood cells, collected by leukapheresis, were T-cell depleted by sheep red blood cell rosetting plus positive selection of CD34+ cells with Ceprate SC columns (Cell Pro, Bothell, WA) in the first series3 and by using a magnetically activated cell-sorter (CliniMACS; Miltenyi Biotec, Bergisch Gladbach, Germany) in the second. The inoculum used in the 2 series contained approximately the same number of CD34+, CD3+, CD4+, and CD8+ cells but differed with respect to CD14+ cell contamination, which was higher in the first series (Table 1). No postgrafting immune suppressive treatment was given for GVHD prophylaxis. All patients in the first series received G-CSF in a dosage of 5 μg/kg of body weight during the first 20 days after grafting. Patients in the second series were not given G-CSF at any time.
Clinical details, graft composition, and outcomes in leukocyte antigen haplotype–mismatched transplant recipients receiving and not receiving granulocyte colony-stimulating factor (G-CSF) after transplantation
| Characteristic . | G-CSF* (n = 43; 1995-1998)3 . | No G-CSF (n = 36; 1999-2000) . |
|---|---|---|
| Mean (range) age, y | 22 (4-53) | 30 (11-60) |
| Disease | ||
| Acute myeloblastic leukemia | 20 | 20 |
| Acute lymphoblastic leukemia | 23 | 16 |
| Status at transplantation | ||
| Poor-risk complete remission | 7 | 7 |
| Beyond second complete remission | 21 | 15 |
| Chemoresistant relapse | 15 | 14 |
| Graft processing | E-ros + Ceprate | MACS |
| Graft composition | ||
| Mean (range) CD34+ × 106/kg | 10.8 (3.1-27.5) | 12.1 (5.1-25.4) |
| Mean (range) CD3+ × 104/kg† | 2.0 (0.1-4.2) | 1.1 (0.1-3) |
| Mean (range) percentage of CD14+cells | 11.8 (0.8-51.8) | < 2 |
| No. (%) of primary engraftments | 41 (95.3) | 34 (93) |
| Neutrophils > 0.5 × 109/L (day after transplantation) | 9 | 13 |
| Platelets > 50 × 109/L (day after transplantation) | 18 | 17 |
| No. (%) of deaths during remission | 15/43 (35) | 9/36 (25)‡ |
| No. (%) of relapses | 15/43 (35) | 9/36 (25)‡ |
| No. (%) of cases of acute GVHD | 0 | 4 |
| Characteristic . | G-CSF* (n = 43; 1995-1998)3 . | No G-CSF (n = 36; 1999-2000) . |
|---|---|---|
| Mean (range) age, y | 22 (4-53) | 30 (11-60) |
| Disease | ||
| Acute myeloblastic leukemia | 20 | 20 |
| Acute lymphoblastic leukemia | 23 | 16 |
| Status at transplantation | ||
| Poor-risk complete remission | 7 | 7 |
| Beyond second complete remission | 21 | 15 |
| Chemoresistant relapse | 15 | 14 |
| Graft processing | E-ros + Ceprate | MACS |
| Graft composition | ||
| Mean (range) CD34+ × 106/kg | 10.8 (3.1-27.5) | 12.1 (5.1-25.4) |
| Mean (range) CD3+ × 104/kg† | 2.0 (0.1-4.2) | 1.1 (0.1-3) |
| Mean (range) percentage of CD14+cells | 11.8 (0.8-51.8) | < 2 |
| No. (%) of primary engraftments | 41 (95.3) | 34 (93) |
| Neutrophils > 0.5 × 109/L (day after transplantation) | 9 | 13 |
| Platelets > 50 × 109/L (day after transplantation) | 18 | 17 |
| No. (%) of deaths during remission | 15/43 (35) | 9/36 (25)‡ |
| No. (%) of relapses | 15/43 (35) | 9/36 (25)‡ |
| No. (%) of cases of acute GVHD | 0 | 4 |
E-ros + Ceprate indicates sheep red blood cell rosetting plus positive selection of CD34+ cells with use of Ceprate SC columns; MACS, magnetically activated cell sorter; and GVHD, graft-versus-host disease.
G-CSF was given in a dosage of 5 μg/kg of body weight daily for the first 20 days after transplantation.
CD3+ T cells contained the same proportion (50%-70%) of CD4+ cells in the two series.
There were no significant differences between patients receiving G-CSF and those not receiving G-CSF (P = .34) as determined 270 days after transplantation.
Assessment of chimerism
Starting on day 12 after transplantation, chimerism of peripheral blood and bone marrow cells was determined by a bimonthly assessment using polymerase chain reaction (PCR) amplification of a panel of variable-number tandem-repeat regions with different DNA polymorphism patterns in donor and recipient cells.3 All postengraftment blood samples used in the studies described here showed 100% donor chimerism.
Assessment of immunologic variables
Immunologic variables were monitored in patients who received G-CSF after transplantation, in patients who did not receive G-CSF, and in donor cells after in vitro and in vivo exposure to G-CSF by using the following techniques.
Flow cytometry.
Indirect immunofluorescence using primary monoclonal antibodies (BD Pharmingen, San Diego, CA) plus secondary fluorochrome-conjugated goat antimouse Ig antibodies (Southern Biotechnology Associates, Birmingham, AL) and flow cytometry (FACSCalibur; Becton Dickinson, San Jose, CA) was used to determine the CD3, CD4, and CD8 phenotypes of freshly isolated and cultured T cells from transplant recipients and their donors and the CD1, HLA class II, and CD86 phenotype of DC preparations.
T-cell clones.
T-cell clones were obtained by limiting dilution of peripheral blood mononuclear cells (PBMC) separated by the Ficoll-Hypaque density-gradient method in the presence of 1% (vol/vol) phytohemagglutinin (PHA; Difco, Detroit, MI), 100 U/mL recombinant IL-2, and irradiated feeder cells, as described previously.19-21 CD3+/CD4+ clones were identified using immunofluorescence and were activated with PHA for 4 to 24 hours before assessment of cytokine production, cytokine gene expression, and cytokine receptor gene expression.
T-cell responses against fungi.
PBMC or T cells purified from PBMC by negative immunomagnetic selection with anti-CD14, anti-CD20, and anti-CD56 antibodies (BD Pharmingen) were cultured at a concentration of 1 × 106/mL or under limiting-dilution conditions19-21 in the presence of irradiated (20 Gy [2000 rad]) 1 × 106/mL autologous PBMC previously pulsed for 4 hours with Candida albicans or heat-inactivated Aspergillus fumigatus31,32 at a cell to fungus ratio of 1:1 in RPMI-1640 medium containing 10% human serum. Exogenous IL-2 (100 U/mL; Chiron, Amsterdam, Holland) was added after 14 days. Growing cultures were identified microscopically and split as necessary with medium containing IL-2. Specificity was assessed by measuring tritium-thymidine incorporation of resting cultures (24 hours in the absence of IL-2) restimulated with fungus-pulsed autologous APCs for 2 days. Control cultures grown in the absence of fungi did not show proliferation on microscopical examination or tritium-thymidine uptake assessment. Cells growing under these conditions (ie, IL-2 starvation for the first 14 days to ensure specificity) invariably displayed a CD4+ phenotype. Frequencies of proliferating cultures were calculated as described previously.19-21 Culture supernatants were assessed for cytokine content.
Type 1 versus type 2 functional identification.
IL-12 production and gene expression in DCs, IL-4 and IL-10 production and gene expression, and expression of the IL-12 receptor β2 subunit (IL-12Rβ2) gene in CD4+ clones and PBMC were used to identify type 1 versus type 2 reactivity.33
Monocyte and DC preparation and activation
Monocytes were isolated on a MiniMACS device (Miltenyi Biotec) by means of positive immunomagnetic selection with microbeads coated with anti-CD14 antibody. Lipopolysaccharide (LPS) was used to activate monocytes to produce IL-12. DCs were obtained by culturing monocytes in the presence of granulocyte-monocyte colony-stimulating factor and IL-4 according to the protocol described by Sallusto and Lanzavecchia.34 A trimeric human CD40 ligand–leucine-zipper fusion protein (Immunex, Seattle, WA) was used at a concentration of 1000 ng/mL for 24 hours to trigger IL-12 production in DC preparations.35
Reverse transcriptase–polymerase chain reaction
RNA was extracted in Trizol reagent (Gibco BRL, Grand Island, NY) from 2 × 106 cells according to the manufacturer's instructions. Reverse transcriptase–polymerase chain reaction (RT-PCR) was performed (35-45 cycles) on a PCR Express thermal cycler (Hybaid, Ashford, United Kingdom) using Amplitaq Gold polymerase (PerkinElmer, Branchburg, NJ). Primer pairs, their target DNAs, annealing temperatures, and PCR-product lengths are shown in Table2. The β-actin primers were used as a control for both reverse transcription and the PCR reaction itself and also for comparing the amount of products from samples obtained with the same primers. PCR products were resolved by 1.6% agarose gel electrophoresis and visualized by using ethidium bromide staining and UV light exposure.
Primers, conditions, and products of cytokine and cytokine receptor RT-PCR
| Target cDNA . | Forward primer . | Reverse primer . | T (°C) . | Product (bp) . |
|---|---|---|---|---|
| IL-4 | GTTCTTCCTGCTAGCATGTGC | CATGATCGTCTTTAGCCTTTCC | 57 | 400 |
| IL-10 | ATCTCCGAGATGCCTTCAGCAG | GCATTCTTCACCTGCTCCACG | 56 | 307 |
| IL-12p40 | AAGATGTGTCACCAGCAGTTGG | CGCAGAATGTCAGGGAGAAGT | 57 | 825 |
| IL-12Rβ2 | TTGGAGTGAATCATTGAGAGCAC | TTCTCTGAAATCAGAGCAGACAC | 56 | 546 |
| β-actin | GTGATGGTGGGCATGGGTC | CCGTGGCCATCTCTTGCTC | 58 | 561 |
| Target cDNA . | Forward primer . | Reverse primer . | T (°C) . | Product (bp) . |
|---|---|---|---|---|
| IL-4 | GTTCTTCCTGCTAGCATGTGC | CATGATCGTCTTTAGCCTTTCC | 57 | 400 |
| IL-10 | ATCTCCGAGATGCCTTCAGCAG | GCATTCTTCACCTGCTCCACG | 56 | 307 |
| IL-12p40 | AAGATGTGTCACCAGCAGTTGG | CGCAGAATGTCAGGGAGAAGT | 57 | 825 |
| IL-12Rβ2 | TTGGAGTGAATCATTGAGAGCAC | TTCTCTGAAATCAGAGCAGACAC | 56 | 546 |
| β-actin | GTGATGGTGGGCATGGGTC | CCGTGGCCATCTCTTGCTC | 58 | 561 |
RT-PCR indicates reverse transcriptase–polymerase chain reaction; cDNA, complementary DNA; T, annealing temperature; bp, base pairs; IL, interleukin; and R, receptor.
Cytokine assays
IL-4, IL-10, interferon γ (IFN-γ), and IL-12p70 production was quantified in culture supernatants by enzyme-linked immunosorbent assays (ELISAs; Euroclone, Paignton-Devon, United Kingdom).
Statistical analysis
Differences between data sets were evaluated using the 2-tailed Student t test.
Results
Transplantation outcome and immunologic variables in patients receiving G-CSF treatment and those not receiving G-CSF treatment after transplantation
Transplantation outcome.
The engraftment rate did not differ significantly between patients treated with G-CSF and those not given the agent. Forty-one of the 43 patients treated with G-CSF had primary engraftment, and neither acute nor chronic GVHD developed in any of them3 (Table 1). Similarly, 34 of the 36 patients not treated with G-CSF had primary engraftment. There was a difference between the 2 groups in the time required to reach a neutrophil count of 0.5 × 109/L (500/mm3), which was delayed from day 9 to day 13 in patients not given G-CSF. The infection-related mortality rate in patients not given G-CSF was 25%, whereas that in patients who received G-CSF was 35%. Four cases of acute GVHD cases were observed (Table 1).
T-cell responses.
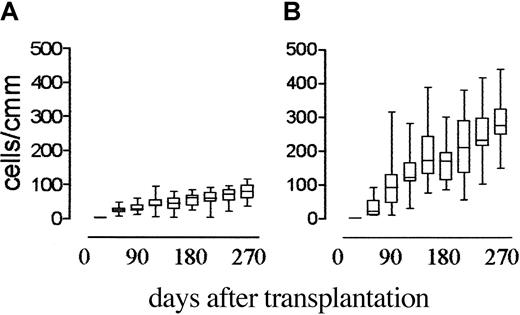
As previously shown,3 T-cell recovery in transplant recipients who received G-CSF after transplantation was delayed. CD4+ T-cell counts were 0.05 × 109/L (50/mm3) 180 days after transplantation, still below 0.1 × 109/L (100/mm3) at 270 days (Figure 1), and below 0.2 × 109/L (200/mm3) at 16 months (data not shown). In patients not treated with G-CSF, CD4+cell counts were higher than 0.1 × 109/L (100/mm3) 60 days after transplantation and higher than 0.3 × 109/L (300/mm3) at 180 days (Figure 1) and were therefore significantly higher than those in patients given G-CSF (P < .0001) at all time points from day 60 onward.
Effect of postgrafting administration of G-CSF on CD4+ cell recovery in HLA-mismatched recipients of hematopoietic transplants.
Shown are the time kinetics of CD4+ T-cell recovery in patients who received G-CSF (panel A) compared with those who did not (panel B). CD4+ cell counts in patients who did not receive G-CSF were significantly higher than those in patients who had (P < .0001) at all time points from day 60 after transplantation onward.
Effect of postgrafting administration of G-CSF on CD4+ cell recovery in HLA-mismatched recipients of hematopoietic transplants.
Shown are the time kinetics of CD4+ T-cell recovery in patients who received G-CSF (panel A) compared with those who did not (panel B). CD4+ cell counts in patients who did not receive G-CSF were significantly higher than those in patients who had (P < .0001) at all time points from day 60 after transplantation onward.
Because of the use of different graft-processing devices, the 2 protocols differed not only with respect to administration of G-CSF to the recipient after transplantation but also in the number of donor G-CSF–primed monocytes infused with the transplant (Table 1). The transfer of G-CSF–primed monocytes might have affected immune recovery. Lack of contaminating monocytes was a constant feature of the stem cell–selection procedures done with the CliniMACS device used in the second series of patients (those not given G-CSF). In contrast, the Cell Pro purification system used in the first series (patients given G-CSF) left behind relatively high numbers of monocytes in the graft. The contamination varied from a maximum proportion of 51.8% to a minimum of 0.8% among the different preparations. Thus, in the latter series, we identified 8 patients who received an inoculum that contained less than 2% monocytes and another 8 patients who received an inoculum that contained more than 40% monocytes. In spite of such a wide difference in the content of G-CSF–primed monocytes in the transplant, no differences were detected in the degree of postgrafting CD4+ T-cell lymphocytopenia in the 2 groups of patients; in particular, a lower proportion of G-CSF–primed monocytes in the graft was not associated with improved immune recovery (0.048 ± 0.018 × 109/L versus 0.056 ± 0.015 × 109/L CD4+ cells [48 ± 18/mm3 versus 56 ± 15/mm3] 6 months after grafting). Therefore, the amount of G-CSF–primed monocytes infused with the transplant did not appear to be essential for postgrafting T-cell recovery.
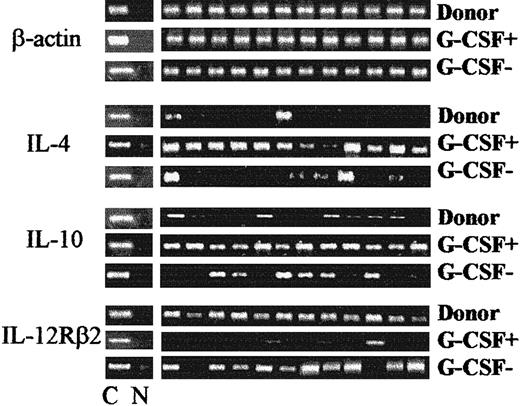
We next analyzed the pattern of Th1 versus Th2 immune reactivity during lymphocyte recovery by evaluating IL-4, IL-10, and IL-12Rβ2 gene expression with RT-PCR. Figure2 shows results of representative analyses of PHA-derived CD4+ clones from patients treated with G-CSF and those not treated with G-CSF between 2 and 6 months after grafting. In patients treated with G-CSF, the presence of messenger RNA (mRNA) for IL-4 was detected in more than 90% of clones, compared with less than 20% of donor clones (P < .05), and mRNA for IL-10 was present in more than 90% of clones, compared with about 35% of donor clones (P < .05). Moreover, IL-12Rβ2 mRNA was not detected in CD4+ clones but was observed in 100% of control clones. An opposite pattern of Th reactivity was observed in patients who did not receive G-CSF: few CD4+ clones showed IL-4 and IL-10 messages and virtually all showed IL-12Rβ2 mRNA. Failure to express IL-12Rβ2 persisted for up to 6 months in patients treated with G-CSF, whereas IL-12Rβ2 expression was detected in patients not given G-CSF as early as 2 months after transplantation (Figure 3). IFN-γ mRNA was detected in virtually all CD4+ clones, irrespective of G-CSF treatment (data not shown).
Effect of postgrafting administration of G-CSF on Th-cell functional phenotype in HLA-mismatched recipients of hematopoietic transplants.
Shown are IL-4, IL-10, and IL-12Rβ2–chain gene expression by PHA-derived CD4+ clones from patients treated with G-CSF (G-CSF+) compared with patients not treated with G-CSF (G-CSF−). Shown are RT-PCR analyses from one donor, one patient treated with G-CSF, and one patient not treated with G-CSF (both 3 months postgrafting); these are representative of analyses done in 10 donors and in all patients between 2 and 6 months postgrafting. C indicates β-actin–specific, cytokine-specific, or cytokine receptor–specific control; and N, no DNA added to the amplification mix during PCR.
Effect of postgrafting administration of G-CSF on Th-cell functional phenotype in HLA-mismatched recipients of hematopoietic transplants.
Shown are IL-4, IL-10, and IL-12Rβ2–chain gene expression by PHA-derived CD4+ clones from patients treated with G-CSF (G-CSF+) compared with patients not treated with G-CSF (G-CSF−). Shown are RT-PCR analyses from one donor, one patient treated with G-CSF, and one patient not treated with G-CSF (both 3 months postgrafting); these are representative of analyses done in 10 donors and in all patients between 2 and 6 months postgrafting. C indicates β-actin–specific, cytokine-specific, or cytokine receptor–specific control; and N, no DNA added to the amplification mix during PCR.
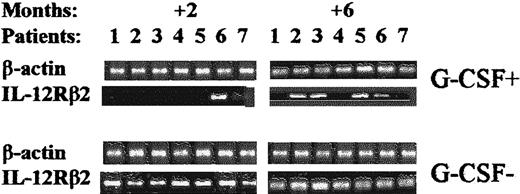
Effect of postgrafting administration of G-CSF on the kinetics of recovery of IL-12Rβ2–chain gene expression in HLA-mismatched recipients of hematopoietic transplants.
Shown are the time kinetics of IL-12Rβ2 gene expression in PBMC samples from patients treated with G-CSF (G-CSF+) compared with patients not treated with G-CSF (G-CSF−). Representative RT-PCR analyses done 2 and 6 months postgrafting are depicted. In contrast to the findings in patients treated with G-CSF, IL-12Rβ2mRNA was detected 2 months after transplantation in all patients not given G-CSF.
Effect of postgrafting administration of G-CSF on the kinetics of recovery of IL-12Rβ2–chain gene expression in HLA-mismatched recipients of hematopoietic transplants.
Shown are the time kinetics of IL-12Rβ2 gene expression in PBMC samples from patients treated with G-CSF (G-CSF+) compared with patients not treated with G-CSF (G-CSF−). Representative RT-PCR analyses done 2 and 6 months postgrafting are depicted. In contrast to the findings in patients treated with G-CSF, IL-12Rβ2mRNA was detected 2 months after transplantation in all patients not given G-CSF.
The recovery of Aspergillus-specific andCandida-specific T-cell responsiveness was assessed by limiting-dilution analyses of the frequencies of proliferating fungus-specific CD4+ cells. T-cell reactivity to fungi was first detected 18 to 24 months after transplantation in patients treated with G-CSF. Elimination of G-CSF treatment accelerated recovery of antifungal reactivity, which was already detectable by 9 to 12 months, and promoted an increase in the frequencies of fungus-specific precursors. Subsequent assessments showed persistence of and a slow increase in the frequencies of responding cells in both series (Figure4). Therefore, recovery of CD4+ cell numbers, Th0 and Th1 function, and antifungal responsiveness were accelerated in patients not treated with G-CSF.
Effect of postgrafting administration of G-CSF on recovery of antifungal T-cell responses in HLA-mismatched recipients of hematopoietic transplants.
Shown is a frequency analysis of C albicans–specific CD4+ cells in G-CSF–treated patients (A) and patients not treated with G-CSF (B) and of A fumigatus–specific CD4+ cells in G-CSF–treated patients (C) and patients not given the agent (D), as a function of time after grafting. The plots indicate the frequency of pathogen-specific T cells (y axis) as a function of time (x axis) after transplantation in G-CSF–treated patients (G-CSF+) and patients not treated with G-CSF (G-CSF−). C albicans–specific andA fumigatus–specific clonable CD4+ cells were determined by limiting-dilution asessments done monthly after transplantation. Fungus-specific T cells appeared earlier and at a higher frequency in patients not treated with G-CSF.
Effect of postgrafting administration of G-CSF on recovery of antifungal T-cell responses in HLA-mismatched recipients of hematopoietic transplants.
Shown is a frequency analysis of C albicans–specific CD4+ cells in G-CSF–treated patients (A) and patients not treated with G-CSF (B) and of A fumigatus–specific CD4+ cells in G-CSF–treated patients (C) and patients not given the agent (D), as a function of time after grafting. The plots indicate the frequency of pathogen-specific T cells (y axis) as a function of time (x axis) after transplantation in G-CSF–treated patients (G-CSF+) and patients not treated with G-CSF (G-CSF−). C albicans–specific andA fumigatus–specific clonable CD4+ cells were determined by limiting-dilution asessments done monthly after transplantation. Fungus-specific T cells appeared earlier and at a higher frequency in patients not treated with G-CSF.
Production of IL-12 by APCs.
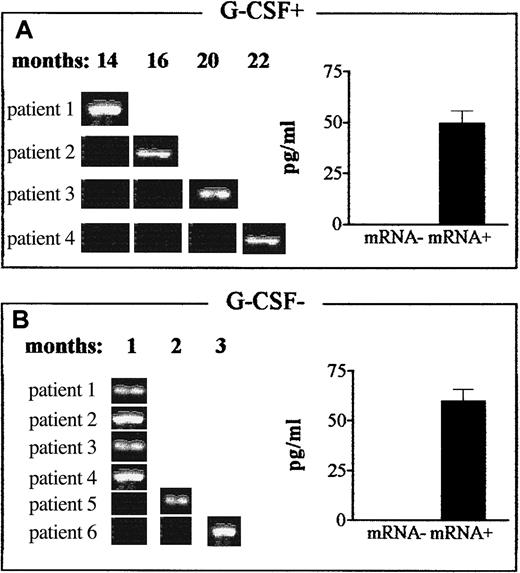
The amount of IL-12 produced by DCs dictates the quality of the effector Th-cell response.33,36 High levels of IL-12 promote Th1 responses, whereas low levels are associated with Th2 induction. To determine whether monocyte-derived DCs had Th2-inducing features, we measured IL-12–inducible p40 subunit mRNA and IL-12p70 protein production33 after activation by CD40 ligand.35 In patients who received G-CSF after grafting, a long-lasting (> 1 year) inability of DCs to produce IL-12 at the protein and message levels was observed. Identical results were obtained with freshly isolated, LPS-activated monocytes (data not shown). In most patients who were not given G-CSF, IL-12 production by DCs was already restored by 1 month after transplantation (Figure5). Eventually, all patients tested in both series had DCs capable of producing IL-12. Therefore, APCs from patients who did not receive G-CSF did not show Th2-inducing features.
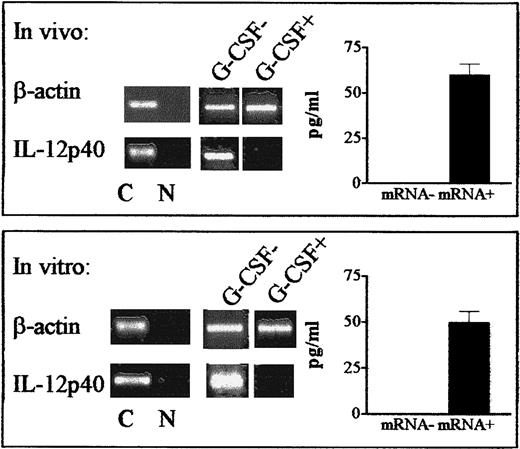
Effect of postgrafting administration of G-CSF on production of IL-12 by DCs in HLA-mismatched recipients of hematopoietic transplants.
Expression of the gene for the inducible IL-12p40 subunit and production of the bioactive IL-12p70 protein were determined in DCs from G-CSF–treated patients (G-CSF+, panel A) and patients not given G-CSF (G-CSF−, panel B). In patients who received G-CSF postgrafting, DCs expressing the IL-12p40 gene were first detected 14 to 22 months after transplantation, whereas they were first detected 1 to 3 months in patients not given G-CSF (RT-PCR results, left side of each panel). Patients were chosen randomly for this analysis at the time of transplantation, and data are representative of more extensive analyses in patients in both series. ELISA analyses (right side of each panel) confirmed that IL-12p40 gene expression (mRNA+) correlated with actual production of bioactive IL-12 in each treatment protocol, ie, in G-CSF–treated patients (G-CSF+, panel A) and patients not given G-CSF (G-CSF−, panel B). The ELISA results are mean ± SE values for IL-12 production by IL-12p40 mRNA+ samples in the 2 series.
Effect of postgrafting administration of G-CSF on production of IL-12 by DCs in HLA-mismatched recipients of hematopoietic transplants.
Expression of the gene for the inducible IL-12p40 subunit and production of the bioactive IL-12p70 protein were determined in DCs from G-CSF–treated patients (G-CSF+, panel A) and patients not given G-CSF (G-CSF−, panel B). In patients who received G-CSF postgrafting, DCs expressing the IL-12p40 gene were first detected 14 to 22 months after transplantation, whereas they were first detected 1 to 3 months in patients not given G-CSF (RT-PCR results, left side of each panel). Patients were chosen randomly for this analysis at the time of transplantation, and data are representative of more extensive analyses in patients in both series. ELISA analyses (right side of each panel) confirmed that IL-12p40 gene expression (mRNA+) correlated with actual production of bioactive IL-12 in each treatment protocol, ie, in G-CSF–treated patients (G-CSF+, panel A) and patients not given G-CSF (G-CSF−, panel B). The ELISA results are mean ± SE values for IL-12 production by IL-12p40 mRNA+ samples in the 2 series.
Immune suppressive effects of G-CSF on normal cells
Production of IL-12 by APCs.
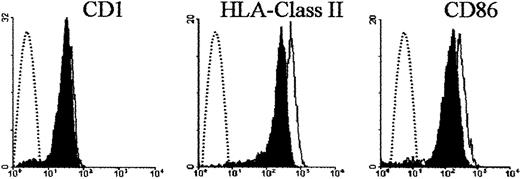
The dramatic loss of the ability of DCs to produce IL-12 in patients who received G-CSF after transplantation prompted us to investigate whether G-CSF affected production of IL-12 by DCs from donors. Monocytes isolated from donors given G-CSF produced less IL-12 than control monocytes on LPS stimulation (data not shown). More important, DCs differentiated from monocytes treated with G-CSF in vivo did not express IL-12p40 mRNA or produce IL-12p70 protein in response to CD40 ligand (Figure 6). To determine whether this effect could be reproduced by in vitro exposure of donor cells to G-CSF, monocytes were incubated in the presence of G-CSF for 24 hours, washed, and subjected to standard DC differentiation conditions.34 Strikingly, DCs from G-CSF–treated monocytes did not express detectable IL-12p40 mRNA or produce IL-12p70 protein (Figure 6), whereas control DCs did. G-CSF did not have such an effect when it was added to fully differentiated DCs (data not shown). G-CSF treatment did not significantly interfere with expression of CD1 by DCs, and HLA class II and CD86 expression was only minimally decreased (Figure 7).
Effect of G-CSF on production of IL-12 by DCs from donors.
Expression of the gene for the inducible IL-12p40 subunit and production of the bioactive IL-12p70 protein were determined in DCs differentiated from monocytes from healthy donors. Top panels (in vivo) show results when monocytes from G-CSF–treated donors were subjected to standard DC differentiation conditions and activated with CD40 ligand. Unlike control DCs (G-CSF−), DCs differentiated from monocytes treated in vivo with G-CSF– did not express the IL-12p40 gene (shown is one representative RT-PCR assay, left side). Bottom panels (in vitro) show results when normal monocytes were incubated in the presence of G-CSF for 24 hours, washed, and subjected to standard DC differentiation conditions. DCs differentiated from G-CSF–treated monocytes did not express the IL-12p40 gene (left side). ELISA analyses (right side of each panel) confirmed that IL-12p40 gene expression (mRNA+) correlated with actual production of bioactive IL-12. Values are mean ± SE levels of IL-12 protein production by all IL-12p40 mRNA+ samples in the in vivo (top) and in vitro (bottom) G-CSF treatment protocols. C indicates β-actin–specific or IL-12p40–specific control; and N, no DNA added to the amplification mix during PCR.
Effect of G-CSF on production of IL-12 by DCs from donors.
Expression of the gene for the inducible IL-12p40 subunit and production of the bioactive IL-12p70 protein were determined in DCs differentiated from monocytes from healthy donors. Top panels (in vivo) show results when monocytes from G-CSF–treated donors were subjected to standard DC differentiation conditions and activated with CD40 ligand. Unlike control DCs (G-CSF−), DCs differentiated from monocytes treated in vivo with G-CSF– did not express the IL-12p40 gene (shown is one representative RT-PCR assay, left side). Bottom panels (in vitro) show results when normal monocytes were incubated in the presence of G-CSF for 24 hours, washed, and subjected to standard DC differentiation conditions. DCs differentiated from G-CSF–treated monocytes did not express the IL-12p40 gene (left side). ELISA analyses (right side of each panel) confirmed that IL-12p40 gene expression (mRNA+) correlated with actual production of bioactive IL-12. Values are mean ± SE levels of IL-12 protein production by all IL-12p40 mRNA+ samples in the in vivo (top) and in vitro (bottom) G-CSF treatment protocols. C indicates β-actin–specific or IL-12p40–specific control; and N, no DNA added to the amplification mix during PCR.
Effect of G-CSF on donor DC phenotype.
Shown is the surface expression of CD1, HLA class II, and CD86 molecules on DCs differentiated from donor monocytes exposed to G-CSF. Donor monocytes were incubated in the presence of G-CSF for 24 hours, washed, subjected to standard DC differentiation conditions, and phenotyped by immunofluorescence. Shaded areas indicate DCs differentiated from G-CSF–treated monocytes; solid lines, control DCs; and broken lines, negative controls.
Effect of G-CSF on donor DC phenotype.
Shown is the surface expression of CD1, HLA class II, and CD86 molecules on DCs differentiated from donor monocytes exposed to G-CSF. Donor monocytes were incubated in the presence of G-CSF for 24 hours, washed, subjected to standard DC differentiation conditions, and phenotyped by immunofluorescence. Shaded areas indicate DCs differentiated from G-CSF–treated monocytes; solid lines, control DCs; and broken lines, negative controls.
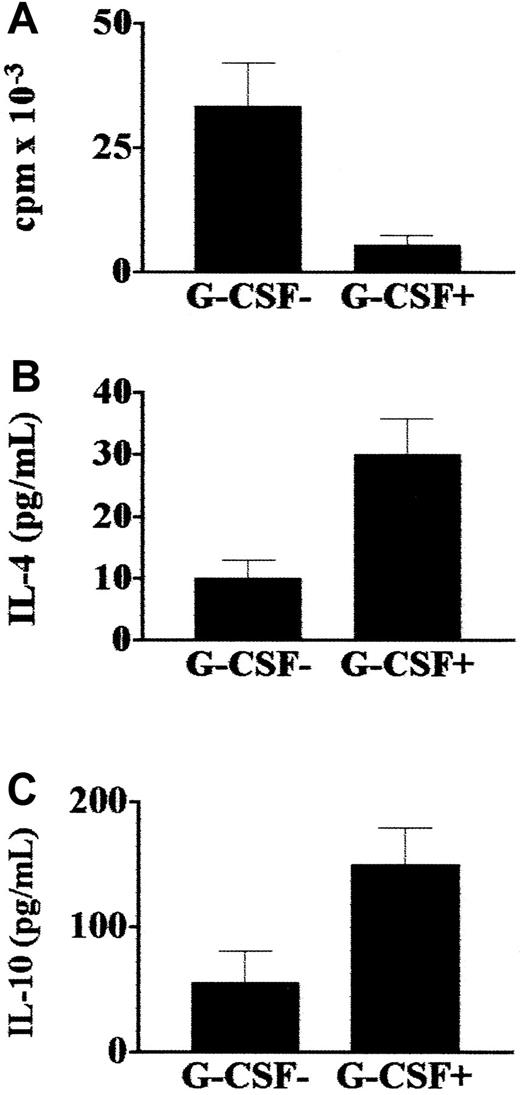
T-cell responses.
The effects of administration of G-CSF on donor T-cell effector functions were evaluated. Compared with responses in the same donors before G-CSF was given, T cells from donors who received G-CSF had a reduction of more than 90% in their proliferative responses to pathogens such as C albicans (Figure8, panel A). These cultures showed a type 2 immune deviation, as they produced several-fold more IL-4 and IL-10 than cultures of cells from the same donors before administration of G-CSF (Figure 8, panels B and C).
Effect of G-CSF on donor T-cell responses to fungi.
Proliferative activity and cytokine production by C albicans–specific T cells isolated from healthy donors before (G-CSF−) and after (G-CSF+) stem cell mobilization with G-CSF. T cells from donors treated with G-CSF had reduced proliferative responses toC albicans (A) and produced more IL-4 (B) and IL-10 (C) than cells from untreated donors. Values are means ± SE.
Effect of G-CSF on donor T-cell responses to fungi.
Proliferative activity and cytokine production by C albicans–specific T cells isolated from healthy donors before (G-CSF−) and after (G-CSF+) stem cell mobilization with G-CSF. T cells from donors treated with G-CSF had reduced proliferative responses toC albicans (A) and produced more IL-4 (B) and IL-10 (C) than cells from untreated donors. Values are means ± SE.
Discussion
Administration of high doses of T-cell–depleted PBSCs has allowed successful transplantation from fully HLA haplotype–mismatched family members to treat high-risk patients with leukemia who do not have a matched donor.2-4 In the current study, we found that postgrafting administration of G-CSF resulted in impaired production of IL-12 by DCs and in delayed recovery of T cells with functional Th1 reactivity. Indeed, a long-lasting Th2 phenotype was observed. These immune abnormalities persisted for a long time. Exposure of donor cells to G-CSF in vivo or in vitro induced similar dysfunctional immune reactivity, suggesting that G-CSF was probably responsible for the abnormalities observed in patients. Elimination of postgrafting administration of G-CSF did not significantly modify the engraftment rate (93%), but it favorably affected APC and T-cell functional recovery.
In patients who received G-CSF, Th1-cell recovery was delayed to the benefit of a type 2 immune reactivity, as indicated by the increased production of IL-4 and IL-10 and lack of IL-12 receptor expression by CD4+ T cells and the impaired production of IL-12 by DCs. Experimental studies using murine models of fungal infection found that Th-cell reactivity plays a central role in regulating immune responses to pathogens, with Th1 reactivity being responsible for resistance and Th2 reactivity being associated with susceptibility.37-39Nonprotective Th2 responses are triggered by APCs whose ability to produce IL-12 has been down-regulated.33,36 Indeed, a high level of production of IL-12 by APCs is a key factor in the initiation of protective Th1 immunity against fungi, bacteria, and viruses.33,36 We observed that for more than 1 year after transplantation, monocytes and, more important, monocyte-derived DCs did not express the gene for the inducible IL-12p40 subunit or produce bioactive IL-12. The implications of this finding as a major risk factor for infection are illustrated by genetic IL-12 and IL-12 receptor defects in primary immune deficiencies characterized by a dramatic loss of immunity to intracellular bacteria.40
It may be argued that the delay in immune recovery observed in patients who received G-CSF after transplantation was due to the transfer of G-CSF–primed functionally dysregulated monocytes to the recipient resulting from differences in graft processing (Table 1). However, because no improvement was observed in CD4+ cell recovery after grafting in patients treated with G-CSF, regardless of the number of contaminating monocytes in the graft, it appears that the infusion of G-CSF–primed monocytes with the graft did not adversely affect immune recovery.
We therefore wondered whether posttransplantation administration of G-CSF to ensure engraftment could hinder the few T cells in the graft from undergoing homeostatic expansion in the lymphopenic host. Several immunoregulatory activities have been ascribed to G-CSF, and there are some divergent data on cellular expression of G-CSF receptors. A direct G-CSF effect on T cells and expression of G-CSF receptors on T cells were reported.30 However, in another study, monocytes but not T cells were found to express functional G-CSF receptors,41 a finding in line with the observation that G-CSF down-regulates inflammatory cytokines25,41 and increases IL-10 production in monocytes.26
An analysis of the relation between the G-CSF–mediated effects on APCs and those on T cells was beyond the scope of this study. However, if G-CSF made a major contribution to the immune deficiency observed in our patients, the functional abnormalities we noted should have been reproduced in donor cells. Short-term treatment of monocytes with G-CSF in vitro and administration of G-CSF in vivo to donors resulted in total abrogation of the ability of monocyte-derived DCs to produce IL-12. The shut off of production of IL-12 by DCs is of particular physiologic relevance, because this will directly affect antigen presentation in vivo. Indeed, we found that administration of G-CSF to healthy donors markedly reduced their ex vivo T-cell responses to fungi and conferred a nonprotective Th2 phenotype. The IL-12 blockage exerted by G-CSF on donor APCs and its inhibitory and Th2-inducing effects on immune responses to fungi raised the concern that G-CSF might have been responsible for the failure of APCs to produce IL-12 and for the slow recovery of protective Th1 responses in our patients.
Thus, beginning in January 1999, we stopped administering G-CSF to recipients of T-cell–depleted mismatched hematopoietic transplants. Patients in this new series had the same poor-risk prognostic factors as those in our previously described series (Table 1). Without administration of G-CSF, neutrophil recovery was delayed by 96 hours, but the overall engraftment rate was not significantly affected, as it remained above 90% constantly. Moreover, the elimination of posttransplantation G-CSF–mediated immune suppression did not significantly increase the incidence of GVHD, as only 4 cases of the disease occurred.
The improvement in immune variables was striking. Production of IL-12 by DCs was promptly restored in most cases by 1 month after transplantation (versus > 12 months in recipients given G-CSF), and the CD4+ cell count reached 0.1 × 109/L (100/mm3) at 1 to 2 months (versus about 12 months) postgrafting and 0.3 × 109/L (300/mm3) at 5 to 6 months (versus > 18 months). CD4+ T cells showed a mixed Th0/Th1 pattern, and T-cell responses to pathogens such asC albicans and A fumigatus appeared earlier and at higher frequencies in patients not treated with G-CSF than in those given the agent. Emerging experimental and clinical evidence42-44 clearly indicates that development of an appropriate Th response is instrumental in the mobilization and activation of antifungal effector phagocytes. In this regard, our findings suggest that treatment with G-CSF may negatively affect overall antifungal resistance in patients who have undergone transplantation. It has already been documented that G-CSF does not increase neutrophil killing of fungal blastospores.45
It may seem perplexing that in patients, in contrast to donors, short-term administration of G-CSF during the first 20 days after grafting (at a time when T cells are not even detectable in the circulation) exerted such long-lasting effects and that elimination of G-CSF was associated with such rapid, long-lasting improvements in immune function. It is likely that in donors, unlike patients, fully differentiated functional DCs and T cells, already formed in great numbers at the time of administration of G-CSF, accounted for the difference. Indeed, the efficiency of G-CSF treatment in blocking production of IL-12 by donor monocyte-derived DCs indicates that monocytes are very susceptible to G-CSF, the effects of which are retained stably by DC progeny. In patients with advanced-stage leukemia, few host APCs and T cells are expected to survive the conditioning regimen, and most postgrafting DCs are generated de novo from the transplanted CD34+ stem cells. Consequently, administration of G-CSF to transplant recipients might program precursors to mature into non-IL-12–producing DCs. This in turn could induce a type 2 immune deviation in the few transplanted T cells.33,36 Thus, administration of G-CSF after transplantation may initiate a vicious cycle. First, it promotes generation of IL-12–deficient APCs, which induce Th2 immune responses. Th2 cells produce IL-4 and IL-10, which are recognized down-regulatory factors for IL-12 production in APCs,33 and thus perpetuate the IL-12 deficiency over time. Indeed, IL-10 is known to inhibit both the ability to produce IL-12 and the stimulatory capacity of DCs, thereby giving rise to tolerogenic DCs46 with a residual Th2-driving function.47 Moreover, because of the presence of T-cell lymphocytopenia, DC maturation may have been impaired by the relative unavailability of T-cell–dependent, CD40 ligand–mediated stimulatory signals.35 In conclusion, the current study provides the first evidence that administration of G-CSF to recipients of T-cell–depleted transplants is associated with dramatic immune suppressive effects on APC functions and T-cell responses and that elimination of G-CSF administration to such recipients corrects many aspects of the postgrafting immune deficiency syndrome without significantly affecting engraftment or incidence of GVHD.
The implications of this study are not limited to HLA haplotype–mismatched transplantation. Our observations may apply to any form of transplantation that relies on few input T cells for immune recovery and is associated with slow immune recovery and relatively high infection rates—for example, matched unrelated-donor, T-cell–depleted transplantation.6 An improvement in immune recovery after eliminating posttransplantation administration of G-CSF can reasonably be expected when T-cell depletion is the only GVHD prophylaxis, because the immune suppressive effects of drugs such as steroids, methotrexate, or cyclosporine would annul the benefits of dropping G-CSF from the posttransplantation treatment regimen.
The infection-related mortality rate in patients not treated with G-CSF was 25%, whereas it was 35% in patients given G-CSF. This difference was not statistically significant in this study, but longer-term evaluations of infection-related mortality and morbidity in more patients are needed to determine significance and demonstrate whether better protection from pathogens was provided. We hope that the current observations will open the door to safer and broader applications of haploidentical hematopoietic transplantation and eventually result in improved overall survival. The improved immune recovery may facilitate the success of therapeutic strategies aimed at further enhancing protection against pathogens, such as the infusion of donor T cells with reduced alloreactive potential48 and the redirection of Th reactivity by cytokine antagonists.49
We thank Geraldine Anne Boyd for editorial assistance and Antonella Santucci for statistical analysis.
Supported by a grant from Associazione Italiana per la Ricerca sul Cancro. I.V. is the recipient of a fellowship from Fondazione Italiana per la Ricerca sul Cancro.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Andrea Velardi, Sezione di Ematologia e Immunologia Clinica, Università di Perugia, Policlinico Monteluce, 06122-Perugia, Italy; e-mail: velardi@unipg.it.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal