Abstract
Although protein tyrosine phosphatase (PTP) inhibitors used in combination with other stimuli can induce interleukin 2 (IL-2) production in T cells, a direct implication of nuclear factor of activated T cells (NFAT) has not yet been demonstrated. This study reports that exposure of leukemic T cells and human peripheral blood mononuclear cells to bis-peroxovanadium (bpV) PTP inhibitors markedly induce activation and nuclear translocation of NFAT. NFAT activation by bpV was inhibited by the immunosuppressive drugs FK506 and cyclosporin A, as well as by a specific peptide inhibitor of NFAT activation. Mobility shift assays showed specific induction of the NFAT1 member by bpV molecules. The bpV-mediated NFAT activation was observed to be important for the up-regulation of the human immunodeficiency virus 1 (HIV-1) long terminal repeat (LTR) and the IL-2 promoter; NFAT1 was demonstrated to be particularly important in bpV-dependent positive action on HIV-1 LTR transcription. The active participation of p56lck, ZAP-70, p21ras, and calcium in the bpV-mediated signaling cascade leading to NFAT activation was confirmed, using deficient cell lines and dominant-negative mutants. Finally, overexpression of wild-type SHP-1 resulted in a greatly diminished activation of NFAT by bpV, suggesting an involvement of SHP-1 in the regulation of NFAT activation. These data were confirmed by constitutive NFAT translocation observed in Jurkat cells stably expressing a dominant-negative version of SHP-1. The study proposes that PTP activity attenuates constitutive kinase activities that otherwise would lead to constant NFAT activation and that this activation is participating in HIV-1 LTR stimulation by PTP inhibition.
Introduction
T-cell activation results in the induction of several genes that play crucial roles for the subsequent functions that these cells accomplish in the immune system. The induction of different types of transcription factors is responsible for the transcriptional activation of these immune response genes. Paradoxically, these same factors positively modulate human immunodeficiency virus type-1 (HIV-1) replication, the causal agent of acquired immune deficiency syndrome (AIDS).1 In fact, HIV-1 transcription is very intimately linked to T-cell activation, due to the overlapping of the signal transduction requirement between T-cell lymphokine gene expression and HIV-1 long terminal repeat (LTR) transactivation.2,3 This is consequential to the HIV-1 LTR architecture sharing many different motifs found in regulatory regions of genes induced following T-cell activation.4 One of the factors binding to these motifs is the well-known nuclear factor-kappa B (NF-κB). NF-κB–binding sites have been identified in the locus of several T-cell–specific genes.5 The NF-κB (Rel) family is made of several members that either form homodimers or heterodimers. Some of these dimers are actually involved in higher order transcriptional complexes.6 7
The nuclear factor of activated T cells (NFAT) is another family of Rel-related transcription factors known to be activated early in time following T-cell activation. Several NFAT family members are present in human T cells, such as NFAT1, NFAT2, and NFAT4.8 NFAT factors are generally sequestered in the cytoplasm and translocated to the nucleus on an increase in intracellular calcium content.9 Modulation of intracellular calcium triggers conformational changes in calmodulin and increases its binding to the calcineurin serine/threonine phosphatase, leading in turn to its activation. The ensuing NFAT dephosphorylation by calcineurin renders the nuclear-localizing sequence accessible, allowing nuclear translocation.9 This mechanistic description of the activation steps of NFAT has also been well established by the demonstration that immunosuppressor drugs cyclosporin A (CsA) and FK506 both inhibited calcineurin phosphatase activity.10,11 NFAT often associates with the newly synthesized AP-1 complex, thereby acquiring high transactivating potential on binding to its consensus sequence 5′-(T/A)GGAAA(A/N)(A/T/C)-3′.9 Certain NFAT-binding sites suggested to be cooperative with AP-1 are known to resemble NF-κB–binding sites and include the CD28RE element located in promoters of cytokine genes.12,13 In addition to interleukin 2 (IL-2), numerous T-cell–expressed genes are regulated by the NFAT transcription factors, and their transcriptional regulation is consequently sensitive to either CsA or FK506.9
Activation of T cells is a very complex process that involves cell-to-cell interactions of several cell surface molecules. Although multimerization of the T-cell receptor (TCR) induces a cascade of intracellular events, optimal T-cell activation requires signaling through other co-receptors.14 Several second messengers modulate signals induced from the membrane to the nucleus, but one common theme is an increase in intracellular tyrosine phosphorylation levels.15 Different studies have shown the importance of this increase in phosphotyrosine levels, which initially depends on 2 specific protein tyrosine kinases (PTKs), p56lck and p59fyn.16,17 Another important PTK involved in the TCR-initiated cascade includes ZAP-70.18 A balance between PTK and protein tyrosine phosphatase (PTP) activities controls intracellular tyrosine phosphorylation levels.19PTPs are therefore very important modulators of the T-cell activation cascade generally being considered as inhibitors of T-cell activation.20 This has been clearly indicated by studies of the PTP SHP-1.21 In fact, inhibition of PTP in the T cell induces cellular activation and IL-2 production.22,23PTPs also play an important role in the activation of the NF-κB and AP-1 factors in T cells.24,25 We have previously shown that treatment with the potent bis-peroxovanadium (bpV) PTP inhibitor resulted in the activation of the HIV-1 LTR in T cells partly through NF-κB.26 However, this work also demonstrated the implication of a NF-κB–independent pathway.
We were thus interested in defining the cellular factor responsible for the NF-κB–independent activation of the HIV-1 LTR by bpV molecules. Our initial efforts were focused on NFAT, given its newly suggested implication in HIV-1 replication.27 28 In this study, we show that bpV compounds strongly activate the NFAT transcription factor in both T-cell lines and peripheral blood mononuclear cells (PBMCs) and that this factor is important in HIV-1 and IL-2 gene regulation by PTPs. PTP activity in human T cells is thus a down-regulator of NFAT activation.
Materials and methods
Cell lines
Jurkat (clone E6-1)29 and Jurkat-derived JCaM-TAg, J.RT3, and P116 cells lines were used in this study. JCaM-TAg30 (Dr A. Weiss, Howard Hughes Medical Center, San Francisco, CA), J.RT3,31,32 and P11633 (Dr Robert T. Abraham, The Mayo Clinic, Rochester, MN) are deficient in p56lck, TCR, and ZAP-70/Syk expression, respectively. 1G5 cells (AIDS Research and Reference Reagent Program, NIAID, National Institutes of Health, Rockville, MD) were derived from Jurkat and stably express the luciferase gene under the control of the HIV-1SF2 LTR.34 Dr Richard S. Lewis (Stanford University School of Medicine, Stanford, CA) provided Jurkat derivatives deficient in capacitative calcium entry.35 A stable Jurkat T-cell clone (J.SHP-1C/S), expressing a dominant version of SHP-1, was obtained from Dr Matthew L. Thomas (Washington University School of Medicine, St Louis, MO).36 All of these cell lines were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS; Hyclone Laboratories, Logan, UT), glutamine (2 mM), penicillin G (100 U/mL), and streptomycin (100 μg/mL). The human 293T cell line was grown in complemented Dulbecco modified Eagle medium. G418 (Gibco BRL, Burlington, ON, Canada) and Hygromycin B (Calbiochem, La Jolla, CA) were added to the appropriate concentrations when needed.
Preparation of PBMCs
PBMCs from healthy donors were isolated by Ficoll-Hypaque density gradient centrifugation. PBMCs were then either used directly for stimulation (hereafter called “fresh”) or cultured in RPMI medium containing 20% FBS in the presence of 3 μg/mL phytohemagglutinin (PHA-P; Sigma, St Louis, MO) and 30 U/mL recombinant human IL-2 (rhIL-2) for 3 days and then rested for 3 days in complete RPMI medium (hereafter called “starved”) prior to activation.
Plasmids and antibodies
The plasmids pLTR-LUC (wild-type LTR) and pmκBLTR-LUC (NF-κB–mutated LTR) were provided by Dr K. L. Calame (Columbia University, New York, NY).37 The pκB-TATA-LUC plasmid (Dr W. C. Greene, The J. Gladstone Institutes, San Francisco, CA) contains the HIV-1 κB enhancer region and a TATA box upstream of the luciferase reporter gene.38 pIL-2-LUC and pNFAT-LUC contain the complete 320-base pair (bp) IL-2 promoter and the minimal IL-2 promoter with 3 tandem copies of the NFAT1-binding site, respectively (Dr G. Crabtree, Stanford University Medical School).39 The pLTR/N17 plasmid coding for the dominant-negative mutant p21rasN17 under the control of murine leukemia virus LTR was obtained from Dr John Telford (Immunobiological Research Institute, Siena, Italy).40 The human p56lck-encoding pEFneoLckWT and the empty pEFneo vectors41 were obtained from Dr Clement Couture (Lady Davis Institute, Montreal, Canada). pcDNA3-dnNFAT expresses a dominant-negative NFAT mutant (Dr Roger J. Davis, University of Massachusetts Medical School, Worcester, MA).42 The NFAT-inhibiting pVIVIT-GFP expression vector and the empty vector43 were supplied by Dr Anjana Rao (Harvard Medical School, Boston, MA). The VIVIT-GFP fragment was excised from this vector and cloned in the pRc/β-actin vector with HindIII/NotI restriction sites (pRc/β-actin VIVIT-GFP). Vectors encoding for wild-type or dominant-negative (C453S) SHP-1 plus the vector pSFFVneo were provided by Dr Matthew L. Thomas (Washington University School of Medicine).36 Vectors encoding for wild-type and kinase-dead ZAP-70, plus the empty vector,44 were obtained from Dr Arthur Weiss (Howard Hughes Medical Center). The pREP4-NFAT1 and pREP4-NFAT2 expression vectors along with the pREP4 vector were supplied by Dr Tim Hoey (Tularik, San Francisco, CA). Rabbit antisera specific for NFAT1, NFAT4, or all NFAT members (panNFAT)8were obtained from Dr Nancy Rice (National Cancer Institute, Frederick, MD). Anti-NFAT2 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies from the anti-CD3 OKT3 hybridoma were purified with mAbTrap protein G affinity columns (Pharmacia LKB Biotechnology AB, Uppsala, Sweden). Purified anti-CD28 antibodies (clone 9.3) were given by Dr Jeffrey A. Ledbetter (Bristol-Myers Squibb, Seattle, WA).45
Transfections and reporter gene assays
Transient transfections of T-cell lines using the DEAE-Dextran method were performed as previously described.26Electroporation of PBMCs was conducted according to the protocol of Hughes and Pober.46 Stably transfected Jurkat cells were obtained by electroporation. Briefly, 107 cells were transfected in 400 μL of medium with 20 μg pNFAT-LUC or pIL-2-LUC (250 V and 960 μF). After 24 hours, cells were diluted at 5 × 104 cells/mL in the presence of 1 mg/mL G418. Pooled G418-resistant cells were identified as either J-NFAT-LUC or J–IL-2–LUC. Transient transfection of 293T cells were performed by a calcium phosphate transfection protocol. Stably or transiently transfected cells were next seeded at a density of 105cells/well in 96-well plates and left unstimulated or treated for 8 hours (unless specified) with bpV[HOpic], bpV[bipy], bpV[pic], PHA-P, phorbol 12-myristate 13-acetate (PMA; Sigma), ionomycin (Iono; Calbiochem), anti-CD3 antibody (clone OKT3), or anti-CD28 antibody (clone 9.3) in a final volume of 200 μL. For some experiments, prior to activation, cells were pretreated with FK506 (Fujisawa, Osaka, Japan) or CsA (Sigma) for 15 minutes at 37°C or with sodium salicylate (NaSal; Laboratories Mat, Beauport, QC, Canada) for 1 hour at 37°C. Cell viability was estimated by the (3-(4,5 dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay.47 Luciferase activity was determined with a Dynex 96-well plate luminometer device (Chantilly, VA).26
IL-2 production
Following a 24-hour stimulation, supernatants from J–IL-2–LUC cells were removed, and quantification of secreted IL-2 was carried out, using a commercial cytokine-matched antibody pair enzyme-linked immunosorbent assay (ELISA) kit (Endogen, Woburn, MA).
Preparation of nuclear extracts and electrophoretic mobility shift assay
Jurkat cells or PBMCs were left untreated or incubated for 1 hour at 37°C with bpV[pic] or PMA/Iono. Cells were next washed with ice-cold PBS, and nuclear extracts were prepared according to the microscale preparation protocol.48 Electrophoretic mobility shift assay (EMSA) was performed as previously described,26 using the following double-stranded oligonucleotides: the consensus NFAT-binding site from the murine IL-2 promoter (5′-TCGAGCCCAAAGAGGAAAATTTGTTTCATG-3′), the consensus NF-κB–binding site (5′-ATGTGAGGGGACTTTCCCAGGC-3′), the wild-type HIV-1 enhancer (5′-CAAGGGACTTTCCGCTGGGGACTTTCCAGGG-3′), and the κB–mutated HIV-1 enhancer (5′-CAACTCACTTTCCGCTGCTCACTTTCCAGGG-3′). Supershift assays were performed by preincubation of nuclear extracts with 1 μL antibody for 30 minutes on ice.
Results
bpV induction of wild-type and NF-κB–mutated HIV-1 LTR is FK-506/CsA sensitive
Because NFAT has been shown to be important in the regulation of HIV-1 replication,27,28 we tested whether FK506 and CsA, 2 known inhibitors of calcineurin and NFAT activation, could block bpV-mediated activation of the HIV-1 LTR.26 The 1G5 T-cell line containing integrated HIV-1 LTR-LUC constructs was used. In agreement with our previous findings,26 the addition of 3 different bpV molecules (ie, bpV[bipy], bpV[HO pic], and bpV[pic]) strongly induced HIV-1 LTR activity (49.8-, 49.4-, and 48.5-fold increases compared to a 4.5-fold increase with PHA) (Figure1A). NaSal, a known inhibitor of NF-κB translocation49 led to a partial loss of the bpV-dependent induction of HIV-1 LTR-driven luciferase activity (Figure 1A). Because PHA acts mainly on NFAT,27 NaSal did not affect PHA-mediated LTR activation; however, FK506 and CsA both inhibited this induction of the LTR. Surprisingly, FK506 and CsA also blocked bpV-mediated induction of HIV-1 LTR activity. Furthermore, when either FK506 or CsA was added in combination with NaSal, greater inhibition of HIV-1 LTR activity was apparent in bpV-treated cells. No detectable toxicity was measured on cell incubation with any combination of these inhibitors (data not shown). Inability to completely inhibit bpV-mediated HIV-1 LTR activation by the different combinations is probably due to residual NF-κB activity. The bpV molecule bpV[pic] was used for subsequent experiments, being representative of the other bpV compounds.
bpV-mediated induction of HIV-1 LTR is sensitive to NaSal, FK506, and CsA.
(A) 1G5 cells were either left untreated (■) or pretreated with NaSal (2.5 mM) (░), FK506 (10 ng/mL) (▪), CsA (100 ng/mL) (▥), or a combination of NaSal with either FK506 (▤) or CsA ( ) for 1 hour at 37°C. Following treatment, cells were either left untreated or treated for 8 hours with the following agents: PHA (3 μg/mL), bpV[bipy], bpV[HO pic], or bpV[pic] (10 μM). Jurkat cells were transiently transfected by the DEAE-Dextran protocol with 15 μg of either pκB-TATA-LUC (B) or pmκBLTR-LUC (C). After 24 hours, transfected cells were either left untreated (■) or were pretreated with FK506 (10 ng/mL) (▪) for 1 hour at 37°C. Following treatment, cells were stimulated for 8 hours with the following agents: PHA (3 μg/mL), PHA (3 μg/mL)/PMA (20 ng/mL), or bpV[pic] (10 μM). Cells were then lysed and evaluated for luciferase activity as described in “Materials and methods.” Values are the mean of 3 different measured samples ± SD. This is representative of 3 independent experiments.
) for 1 hour at 37°C. Following treatment, cells were either left untreated or treated for 8 hours with the following agents: PHA (3 μg/mL), bpV[bipy], bpV[HO pic], or bpV[pic] (10 μM). Jurkat cells were transiently transfected by the DEAE-Dextran protocol with 15 μg of either pκB-TATA-LUC (B) or pmκBLTR-LUC (C). After 24 hours, transfected cells were either left untreated (■) or were pretreated with FK506 (10 ng/mL) (▪) for 1 hour at 37°C. Following treatment, cells were stimulated for 8 hours with the following agents: PHA (3 μg/mL), PHA (3 μg/mL)/PMA (20 ng/mL), or bpV[pic] (10 μM). Cells were then lysed and evaluated for luciferase activity as described in “Materials and methods.” Values are the mean of 3 different measured samples ± SD. This is representative of 3 independent experiments.
bpV-mediated induction of HIV-1 LTR is sensitive to NaSal, FK506, and CsA.
(A) 1G5 cells were either left untreated (■) or pretreated with NaSal (2.5 mM) (░), FK506 (10 ng/mL) (▪), CsA (100 ng/mL) (▥), or a combination of NaSal with either FK506 (▤) or CsA ( ) for 1 hour at 37°C. Following treatment, cells were either left untreated or treated for 8 hours with the following agents: PHA (3 μg/mL), bpV[bipy], bpV[HO pic], or bpV[pic] (10 μM). Jurkat cells were transiently transfected by the DEAE-Dextran protocol with 15 μg of either pκB-TATA-LUC (B) or pmκBLTR-LUC (C). After 24 hours, transfected cells were either left untreated (■) or were pretreated with FK506 (10 ng/mL) (▪) for 1 hour at 37°C. Following treatment, cells were stimulated for 8 hours with the following agents: PHA (3 μg/mL), PHA (3 μg/mL)/PMA (20 ng/mL), or bpV[pic] (10 μM). Cells were then lysed and evaluated for luciferase activity as described in “Materials and methods.” Values are the mean of 3 different measured samples ± SD. This is representative of 3 independent experiments.
) for 1 hour at 37°C. Following treatment, cells were either left untreated or treated for 8 hours with the following agents: PHA (3 μg/mL), bpV[bipy], bpV[HO pic], or bpV[pic] (10 μM). Jurkat cells were transiently transfected by the DEAE-Dextran protocol with 15 μg of either pκB-TATA-LUC (B) or pmκBLTR-LUC (C). After 24 hours, transfected cells were either left untreated (■) or were pretreated with FK506 (10 ng/mL) (▪) for 1 hour at 37°C. Following treatment, cells were stimulated for 8 hours with the following agents: PHA (3 μg/mL), PHA (3 μg/mL)/PMA (20 ng/mL), or bpV[pic] (10 μM). Cells were then lysed and evaluated for luciferase activity as described in “Materials and methods.” Values are the mean of 3 different measured samples ± SD. This is representative of 3 independent experiments.
Jurkat cells were tested for a bpV[pic] response with the pκB–TATA–LUC vector containing the NFAT- and NF-κB–targeted HIV-1 enhancer region (−105/−70). In transfected Jurkat cells, significant induction of luciferase activity was seen with bpV[pic], which was FK506 sensitive (Figure 1B). PHA and PHA/PMA induction of luciferase activity were also diminished following FK506 treatment, the latter being less inhibited likely due to strong NF-κB activation by PMA (Figure 1B). Similar inhibitions by CsA for all tested activators were observed (data not shown). To demonstrate that this FK506/CsA inhibition was independent of NF-κB, Jurkat cells were transfected with the NF-κB–mutated pmκBLTR-LUC construct. Induction of luciferase activity on the addition of either PHA, PHA/PMA, or bpV[pic] was again observed, although a lesser difference in terms of HIV-1 LTR activation was noticed between PHA and PHA/PMA. Luciferase activity in these transfected Jurkat cells pretreated with FK506 was only weakly induced by the above agents (Figure 1C). These results further suggested that the NF-κB–independent signaling pathway activated by bpV[pic] was FK506/CsA sensitive and involved the −105/−70 HIV-1 enhancer region.
NFAT-dependent luciferase expression is markedly stimulated by the bpV compound
Because NFAT was the obvious candidate in bpV[pic] activation, transfections were performed in Jurkat cells with pNFAT-LUC containing 3 tandem repeats of the human IL-2–derived NFAT-binding site, the human IL-2 minimal promoter, and the luciferase reporter gene. A strong induction of NFAT-dependent luciferase activity was mediated by the PHA/PMA combination (Figure 2A). Interestingly, a more potent induction was observed on bpV[pic] stimulation of transfected Jurkat cells. Time kinetic analyses showed a transient increase in luciferase activity that peaked from 6 to 8 hours for PHA/PMA (53.5-fold and 61.1-fold increase), whereas maximal activation was reached during the 8- to 12-hour period for bpV[pic] (92.2-fold and 73.2-fold increase). For the subsequent experiments, the 8-hour time frame was chosen for treatment of transfected cell lines. Similar analyses were performed with freshly isolated PBMCs electroporated with pNFAT-LUC and showed bpV[pic]-mediated NFAT activation (albeit at a lower extent than in PMA/Iono-treated PBMCs) (Figure 2B). In addition, time kinetics were different in PBMCs in comparison to Jurkat cells, showing a much slower and sustained induction.
NFAT-driven luciferase gene expression is induced by bpV molecules and is FK506 sensitive.
(A) Jurkat cells were transiently transfected by the DEAE-Dextran protocol with 15 μg pNFAT-LUC. Following a 24-hour incubation period, cells were either left untreated (▪) or stimulated with PHA (3 μg/mL)/PMA (20 ng/mL) (■) or bpV[pic] (10 μM) (●) and lysed after 4, 6, 8, 12, and 24 hours of stimulation. (B) Ficoll Hypaque–isolated PBMCs were first treated with PHA-L for 20 hours and then electroporated in the presence of 15 μg of pNFAT-LUC. Following a 2-hour incubation period, cells were stimulated with bpV[pic] (10 μM) (⋄) or PMA (20 ng/mL)/Iono (1 μM) (■) for 8 hours. (C) Jurkat cells stably transfected with pNFAT-LUC were either left untreated (■) or pretreated with FK506 (10 ng/mL) (▪). After 15 minutes, cells were stimulated for 8 hours with PHA (3 μg/mL), PMA (20 ng/mL), PHA (3 μg/mL)/PMA (20 ng/mL), bpV[pic] (10 μM), or bpV[pic] (10 μM)/PMA (20 ng/mL). (D) Jurkat cells were transiently transfected by the DEAE-Dextran protocol with 15 μg of pNFAT-LUC plus 15 μg of either the control vector pEGFP (■ or the NFAT-inhibitor expression vector pVIVIT-GFP (▪). Following a 24-hour incubation period, cells were left untreated or were stimulated with either PHA (3 μg/mL), PMA (20 ng/mL)/Iono (1 μM), or bpV[pic] (10 μM) for 8 hours. Cells were lysed and evaluated for luciferase activity. Values are the mean of 3 different measured samples ± SD. This is representative of 2 independent experiments.
NFAT-driven luciferase gene expression is induced by bpV molecules and is FK506 sensitive.
(A) Jurkat cells were transiently transfected by the DEAE-Dextran protocol with 15 μg pNFAT-LUC. Following a 24-hour incubation period, cells were either left untreated (▪) or stimulated with PHA (3 μg/mL)/PMA (20 ng/mL) (■) or bpV[pic] (10 μM) (●) and lysed after 4, 6, 8, 12, and 24 hours of stimulation. (B) Ficoll Hypaque–isolated PBMCs were first treated with PHA-L for 20 hours and then electroporated in the presence of 15 μg of pNFAT-LUC. Following a 2-hour incubation period, cells were stimulated with bpV[pic] (10 μM) (⋄) or PMA (20 ng/mL)/Iono (1 μM) (■) for 8 hours. (C) Jurkat cells stably transfected with pNFAT-LUC were either left untreated (■) or pretreated with FK506 (10 ng/mL) (▪). After 15 minutes, cells were stimulated for 8 hours with PHA (3 μg/mL), PMA (20 ng/mL), PHA (3 μg/mL)/PMA (20 ng/mL), bpV[pic] (10 μM), or bpV[pic] (10 μM)/PMA (20 ng/mL). (D) Jurkat cells were transiently transfected by the DEAE-Dextran protocol with 15 μg of pNFAT-LUC plus 15 μg of either the control vector pEGFP (■ or the NFAT-inhibitor expression vector pVIVIT-GFP (▪). Following a 24-hour incubation period, cells were left untreated or were stimulated with either PHA (3 μg/mL), PMA (20 ng/mL)/Iono (1 μM), or bpV[pic] (10 μM) for 8 hours. Cells were lysed and evaluated for luciferase activity. Values are the mean of 3 different measured samples ± SD. This is representative of 2 independent experiments.
Stably pNFAT-LUC–transfected Jurkat cells (J–NFAT–LUC) were also tested with the various NFAT inducers with or without FK506 pretreatment. J-NFAT-LUC cells were highly responsive to PHA in the absence or presence of PMA (48.9- and 72.1-fold, respectively), whereas PMA alone had no effect (Figure 2C). An important induction of luciferase activity in J-NFAT-LUC cells was observed with bpV[pic] (113.3-fold) and was further enhanced in the presence of PMA (208.1-fold). After pretreatment with subcytotoxic FK506 concentrations, NFAT activation was abrogated for all tested agents, including bpV[pic]. Finally, to confirm NFAT activation by bpV[pic], Jurkat cells were transfected with pNFAT-LUC and a vector that encodes a GFP protein fused to VIVIT, a highly specific NFAT-inhibiting peptide.43 The expression of this NFAT inhibitor caused a strong inhibition of NFAT activation by bpV[pic] and the other agents (Figure 2D). Similar inhibitions of NFAT activation were also obtained when the dominant-negative mutant dnNFAT42 was instead transfected in Jurkat cells (data not shown). These results hence demonstrated for the first time that PTP inhibitors directly activate NFAT in a more pronounced fashion in comparison to frequently used NFAT activators.
Nuclear translocation of NFAT by bpV[pic]
Nuclear translocation of NFAT induced by bpV[pic] was evaluated by EMSA. A strong NFAT-specific signal was obtained with PMA/Iono-treated nuclear extracts (Figure3A, lane 2). bpV[pic] also led to the induction of a strong signal, which was competed by increasing concentrations of unlabeled NFAT oligonucleotide (compare lane 3 with lanes 4-6) but not by excess of unlabeled NF-κB oligonucleotide (lane 7). For identification of this signal, bpV-treated Jurkat nuclear extracts were preincubated with NFAT member-specific antibodies. The signal induced by bpV[pic] was completely abrogated by the addition of anti-NFAT1 antibodies with a concomitant supershift (Figure 3B, lanes 2 and 3). No such effect could be observed with anti-NFAT2 or anti-NFAT4 antibodies (lanes 4 and 5). The presence of a weak supershift in anti-NFAT2–incubated nuclear extracts might, however, be reminiscent of a minimal representation of this member in the shifted complex. The fast migrating band in these EMSA experiments likely represented degraded NFAT.
bpV-mediated nuclear translocation of NFAT.
(A) Jurkat cells were either left untreated or stimulated with PMA (20 ng/mL)/Iono (1 μM) (P/I), or bpV[pic] (10 μM) for 1 hour. Nuclear extracts were then prepared as described in “Materials and methods.” Nuclear extracts from bpV-stimulated Jurkat cells (10 μg) were incubated with γ32P-labeled murine IL-2–derived NFAT-binding site for 20 minutes in the absence (lane 3) or presence of 10-, 100-, or 200-fold excess of unlabeled NFAT oligonucleotide (lanes 4, 5, and 6, respectively) or 100-fold excess of unlabeled NF-κB oligonucleotide (lane 7). Labeled NFAT oligonucleotides were incubated with nuclear extracts from unstimulated Jurkat cells as a negative control (lane 1) or from PMA/Iono-stimulated Jurkat cells as a positive control (lane 2). Samples were then migrated on a native polyacrylamide gel, dried, and exposed on Kodak X-OMAT film. (B) Nuclear extracts from bpV-stimulated Jurkat cells were either left untreated (lane 2) or preincubated with a panel of polyclonal antisera to NFAT1 (lane 3), NFAT2 (lane 4), or NFAT4 (lane 5) for 30 minutes on ice. Samples were then incubated for 20 minutes with the labeled NFAT oligonucleotide and migrated on a native polyacrylamide gel to be subsequently exposed on Kodak X-OMAT film. Extracts from unstimulated Jurkat cells were used as a negative control (lane 1). (C) Nuclear extracts from bpV-treated starved PBMCs (lanes 2-7) or bpV-treated fresh PBMCs (lanes 9-14) were preincubated with antisera such as preimmune normal rabbit serum (NRS) (lanes 3 and 10), NFAT1 (lanes 4 and 11), NFAT2 (lanes 5 and 12), NFAT4 (lanes 6 and 13), and panNFAT (lanes 7 and 14) and then incubated with the NFAT-labeled probe. Lanes 1 and 8 represent nuclear extracts from untreated starved and fresh PBMCs, respectively. Samples were then migrated on a native polyacrylamide gel, dried, and exposed on Kodak X-OMAT film. Free probe, specific complex and supershifted complexes (SS) are indicated on the left side of each panel.
bpV-mediated nuclear translocation of NFAT.
(A) Jurkat cells were either left untreated or stimulated with PMA (20 ng/mL)/Iono (1 μM) (P/I), or bpV[pic] (10 μM) for 1 hour. Nuclear extracts were then prepared as described in “Materials and methods.” Nuclear extracts from bpV-stimulated Jurkat cells (10 μg) were incubated with γ32P-labeled murine IL-2–derived NFAT-binding site for 20 minutes in the absence (lane 3) or presence of 10-, 100-, or 200-fold excess of unlabeled NFAT oligonucleotide (lanes 4, 5, and 6, respectively) or 100-fold excess of unlabeled NF-κB oligonucleotide (lane 7). Labeled NFAT oligonucleotides were incubated with nuclear extracts from unstimulated Jurkat cells as a negative control (lane 1) or from PMA/Iono-stimulated Jurkat cells as a positive control (lane 2). Samples were then migrated on a native polyacrylamide gel, dried, and exposed on Kodak X-OMAT film. (B) Nuclear extracts from bpV-stimulated Jurkat cells were either left untreated (lane 2) or preincubated with a panel of polyclonal antisera to NFAT1 (lane 3), NFAT2 (lane 4), or NFAT4 (lane 5) for 30 minutes on ice. Samples were then incubated for 20 minutes with the labeled NFAT oligonucleotide and migrated on a native polyacrylamide gel to be subsequently exposed on Kodak X-OMAT film. Extracts from unstimulated Jurkat cells were used as a negative control (lane 1). (C) Nuclear extracts from bpV-treated starved PBMCs (lanes 2-7) or bpV-treated fresh PBMCs (lanes 9-14) were preincubated with antisera such as preimmune normal rabbit serum (NRS) (lanes 3 and 10), NFAT1 (lanes 4 and 11), NFAT2 (lanes 5 and 12), NFAT4 (lanes 6 and 13), and panNFAT (lanes 7 and 14) and then incubated with the NFAT-labeled probe. Lanes 1 and 8 represent nuclear extracts from untreated starved and fresh PBMCs, respectively. Samples were then migrated on a native polyacrylamide gel, dried, and exposed on Kodak X-OMAT film. Free probe, specific complex and supershifted complexes (SS) are indicated on the left side of each panel.
EMSAs were also performed with freshly isolated PBMCs or PHA/IL-2–stimulated PBMCs rested for 3 days before bpV treatment. Treatment of starved or fresh PBMCs with bpV[pic] resulted in nuclear translocation of NFAT (Figure 3C, lanes 2 and 9). Specificity of the signal was demonstrated through competition experiments (data not shown). Supershift analyses again indicated specific supershifting with anti-NFAT1 and panNFAT (recognizing all NFAT members) sera in both PBMC nuclear extracts (Figure 3C, lanes 4, 7, 11, and 14). The remaining fast migrating band observed in lane 4 might be reminiscent of bpV[pic] induction of another NFAT member that would be induced in PBMCs, but its identification has yet to be determined. Overall, these experiments suggested that bpV[pic] activates NFAT through its nuclear translocation in both Jurkat and PBMCs and that most of these translocated factors consist of NFAT1.
NFAT is important for HIV-1 LTR activation by bpV[pic]
Because NFAT might be an important factor in bpV[pic]-mediated HIV-1 LTR activation (Figure 1), this assumption was directly tested with the VIVIT NFAT inhibitor. However, to avoid a potential quenching effect on the activation of the HIV-1 LTR from the NF-κB–binding sites present in the cytomegalovirus promoter of the original plasmid, the VIVIT-GFP fragment was cloned upstream of the β-actin promoter (pRc/β-actin VIVIT-GFP). In fact, the addition of pRc/β-actin had little effect on the activation of the HIV-1 LTR by bpV compound when compared to Jurkat cells transfected with pLTR-LUC alone (26-fold compared to 24-fold) (data not shown). Jurkat cells were thus transfected with pLTR-LUC along with the empty pRc/β-actin or pRc/β-actin VIVIT-GFP vectors. Data showed that the NF-κB–activating PMA agent was not affected by the addition of the VIVIT-GFP expression vector, whereas this vector led to a near 60% decrease in luciferase activity in both bpV[pic]- and PHA-stimulated cells (Figure 4A).
Importance of NFAT in HIV-1 LTR regulation by bpV[pic].
(A) Jurkat cells were transiently transfected by the DEAE-Dextran protocol with 15 μg pLTR-LUC plus 15 μg of either the control vector pRc/β-actin (■) or the pRc/β-actin VIVIT-GFP vector (▪). Following a 24-hour incubation period, cells were either left untreated or stimulated with PHA (3 μg/mL), PMA (20 ng/mL), or bpV[pic] (10 μM) and lysed after 8 hours of stimulation. Luciferase activity was evaluated as the mean of 3 different measured samples and is represented as fold increase over untreated samples. This is representative of 3 independent experiments. (B) Nuclear extracts from bpV-treated Jurkat cells were incubated with the HIV-1 enhancer probe and competed with either 100-fold cold excess of NFAT oligonucleotide (lane 2), NF-κB oligonucleotide (lane 3), wild-type HIV-1 enhancer oligonucleotide (lane 4), or HIV-1 enhancer oligonucleotide mutated at the NF-κB–binding sites (lane 5). Samples were then migrated on a native polyacrylamide gel, dried, and exposed on Kodak X-OMAT film. Free probe and specific complexes are indicated on the left side of the panel. (C) 293T cells were transiently transfected by the calcium phosphate protocol with 5 μg pLTR-LUC plus 5 μg of either pREP4, pREP4-NFAT1, or pREP4-NFAT2. Cells were washed 16 hours after transfection and incubated for another 24 hours in fresh medium before lysis. Luciferase activity was evaluated as the mean of 3 different measured samples. This is representative of 3 independent experiments.
Importance of NFAT in HIV-1 LTR regulation by bpV[pic].
(A) Jurkat cells were transiently transfected by the DEAE-Dextran protocol with 15 μg pLTR-LUC plus 15 μg of either the control vector pRc/β-actin (■) or the pRc/β-actin VIVIT-GFP vector (▪). Following a 24-hour incubation period, cells were either left untreated or stimulated with PHA (3 μg/mL), PMA (20 ng/mL), or bpV[pic] (10 μM) and lysed after 8 hours of stimulation. Luciferase activity was evaluated as the mean of 3 different measured samples and is represented as fold increase over untreated samples. This is representative of 3 independent experiments. (B) Nuclear extracts from bpV-treated Jurkat cells were incubated with the HIV-1 enhancer probe and competed with either 100-fold cold excess of NFAT oligonucleotide (lane 2), NF-κB oligonucleotide (lane 3), wild-type HIV-1 enhancer oligonucleotide (lane 4), or HIV-1 enhancer oligonucleotide mutated at the NF-κB–binding sites (lane 5). Samples were then migrated on a native polyacrylamide gel, dried, and exposed on Kodak X-OMAT film. Free probe and specific complexes are indicated on the left side of the panel. (C) 293T cells were transiently transfected by the calcium phosphate protocol with 5 μg pLTR-LUC plus 5 μg of either pREP4, pREP4-NFAT1, or pREP4-NFAT2. Cells were washed 16 hours after transfection and incubated for another 24 hours in fresh medium before lysis. Luciferase activity was evaluated as the mean of 3 different measured samples. This is representative of 3 independent experiments.
To corroborate these results, EMSA analyses were performed, using an HIV-1 enhancer probe. A specific broad signal was observed with extracts from bpV[pic]-stimulated Jurkat cells (Figure 4B, lane 1). Because it seemed possible that this signal resulted from superimposition of NFAT and NF-κB complexes, competitions with excess of NF-κB or NFAT oligonucleotide were thus performed. As presented in lanes 2 and 3, respectively, competition with the NFAT oligonucleotide led to the isolation of the lower part of the signal, and competition with the NF-κB oligonucleotide resulted in isolation of the upper part (ie, the presumed NFAT complex). Supershift assays revealed that indeed the upper signal reacted with panNFAT antibodies (data not shown). When competition was performed with the unlabeled HIV-1 enhancer region, both signals were outcompeted (lane 4), whereas, as expected, only the slow migrating band was competed with cold excess of the NF-κB–mutated HIV-1 enhancer oligonucleotide (lane 5). To identify the activated NFAT member bound to the HIV-1 enhancer region, supershift experiments were performed and indicated that this bpV-induced complex was again largely represented by the NFAT1 member (data not shown).
Because this NFAT member has previously been shown to act negatively on HIV-1 LTR activity,50 NFAT1 was directly tested for its LTR-activating potential by co-transfecting 293T cells with pLTR-LUC and NFAT1 or NFAT2 expression vectors (Figure 4C). LTR activity was increased by both NFAT1 and NFAT2, and, importantly, this induction was more pronounced in NFAT1-transfected cells (10.3- versus 3-fold). These data hence suggested that NFAT1 expression can up-regulate HIV-1 LTR activity and is important in the induction of the HIV-1 LTR by our PTP inhibitor.
bpV molecules in combination with other agents activate transcription of the IL-2 promoter and IL-2 production. The pervanadate PTP inhibitor has previously been shown to up-regulate the expression of IL-2 in the presence of other activators.22-24 51Because IL-2 transcription is dependent on NFAT-binding sites, we wanted to determine whether bpV[pic] could equally induce IL-2 gene expression in an NFAT-dependent manner. As demonstrated in Figure5A, PHA/PMA treatment of pIL-2–LUC–transfected Jurkat cells revealed a maximal 3-fold induction of luciferase activity. Importantly, bpV[pic] in combination with PMA could equally activate IL-2 promoter-dependent luciferase activity (5.4-fold). Neither PHA, PMA, nor bpV[pic] alone resulted in IL-2 promoter activation in this transient transfection system (data not shown). The kinetic of luciferase induction observed with bpV[pic]/PMA was fairly similar to PHA/PMA, except that maximal luciferase induction reached its plateau between 8 and 12 hours. In stably pIL-2–LUC–transfected Jurkat cells (J–IL-2–LUC), PHA/PMA- and bpV[pic]/PMA-mediated activation of the IL-2 promoter were both FK506 sensitive (Figure 5B).
Activation of the complete IL-2 promoter by bpV compounds.
(A) Jurkat cells were transiently transfected by the DEAE-Dextran protocol with 15 μg pIL-2–LUC plasmid. After a 24-hour incubation, cells were either left untreated (▪) or were stimulated with PHA (3 μg/mL)/PMA (20 ng/mL) (■) or bpV[pic] (10 μM)/PMA (20 ng/mL) (●) for various times (0, 4, 6, 8, 12, and 24 hours), lysed, and monitored for luciferase activity. (B) J–IL-2–LUC cells were either left untreated (■) or pretreated for 15 minutes with FK506 (10 ng/ml) (▪) before being left untreated or stimulated with PHA (3 μg/mL)/PMA (20 ng/mL) and bpV[pic] (10 μM)/PMA (20 ng/mL). Cells were lysed 8 hours after stimulation, and luciferase activity was monitored. (C) J–IL-2–LUC cells were treated with various activators (PHA, 3 μg/mL; bpV[pic], 10 μM; Iono, 1 μM; PMA, 20 ng/mL; anti-CD3 antibody, 3 μg/mL; anti-CD28 antibody, 1 μg/mL) and lysed after 8 hours to be monitored for luciferase activity. (D) J–IL-2–LUC cells were treated with the same activators as in panel C. Cell-free supernatants were collected 24 hours after stimulation, and IL-2 was quantified through ELISA as described in “Materials and methods.” Luciferase activity was monitored as described in “Materials and methods.” Results for all panels are the means ± SD for triplicate samples and are representative of 2 independent experiments.
Activation of the complete IL-2 promoter by bpV compounds.
(A) Jurkat cells were transiently transfected by the DEAE-Dextran protocol with 15 μg pIL-2–LUC plasmid. After a 24-hour incubation, cells were either left untreated (▪) or were stimulated with PHA (3 μg/mL)/PMA (20 ng/mL) (■) or bpV[pic] (10 μM)/PMA (20 ng/mL) (●) for various times (0, 4, 6, 8, 12, and 24 hours), lysed, and monitored for luciferase activity. (B) J–IL-2–LUC cells were either left untreated (■) or pretreated for 15 minutes with FK506 (10 ng/ml) (▪) before being left untreated or stimulated with PHA (3 μg/mL)/PMA (20 ng/mL) and bpV[pic] (10 μM)/PMA (20 ng/mL). Cells were lysed 8 hours after stimulation, and luciferase activity was monitored. (C) J–IL-2–LUC cells were treated with various activators (PHA, 3 μg/mL; bpV[pic], 10 μM; Iono, 1 μM; PMA, 20 ng/mL; anti-CD3 antibody, 3 μg/mL; anti-CD28 antibody, 1 μg/mL) and lysed after 8 hours to be monitored for luciferase activity. (D) J–IL-2–LUC cells were treated with the same activators as in panel C. Cell-free supernatants were collected 24 hours after stimulation, and IL-2 was quantified through ELISA as described in “Materials and methods.” Luciferase activity was monitored as described in “Materials and methods.” Results for all panels are the means ± SD for triplicate samples and are representative of 2 independent experiments.
Various combinations of activators were next tested on J–IL-2–LUC cells. As described elsewhere52 and in agreement with our above results, PHA, Iono, or PMA alone did not result in any activation of the IL-2 promoter, whereas bpV[pic] had a modest effect (data not shown). However, when combining bpV[pic] with these agents, activation of the IL-2 promoter was observed. With the bpV[pic]/PHA and bpV[pic]/Iono combination, activation of the IL-2 promoter was demonstrated (21- and 49-fold increases, respectively) (Figure 5C). Stronger promoter activation (143-fold) was achieved when combining bpV[pic] and PMA. However, the strongest increase was obtained when combining bpV[pic] to PMA/Iono (220-fold increase). As previously observed in Jurkat cells,53 simultaneous stimulation through TCR (anti-CD3 antibody) and CD28 resulted in a modest increase in IL-2 promoter activity, whereas stimulation through each of the receptors alone showed no promoter modulation (data not shown). However, addition of bpV[pic] to either anti-CD3, anti-CD28 antibodies, or both strongly stimulated IL-2 promoter–driven luciferase activity (36-, 119-, and 156-fold activation, respectively). IL-2 production in the supernatant of these stimulated J–IL-2–LUC cells was also measured and fairly reflected IL-2 promoter activity (Figure 5D). Indeed, when IL-2 production was plotted against luciferase activity, a strong correlation was obtained (data not shown). Our results therefore demonstrated the induction of IL-2 expression and secretion by bpV molecules when used in combination with a variety of agents, including antigen-mimicking agents (anti-CD3/anti-CD28).
NFAT activation by bpV[pic] is dependent on TCR-proximal signaling events
We next wanted to characterize the signaling pathway activated by the bpV compounds leading to NFAT activation. The implication of the PTKs p56lck and ZAP-70 in bpV-induced NFAT activation was examined. When transfected with the pNFAT-LUC plasmid, p56lck-deficient JCaM-TAg cells were unresponsive to most tested activators, including bpV[pic] (Figure6A). To demonstrate that this lack of bpV-mediated NFAT induction was p56lckdependent, we co-transfected JCaM-TAg cells with pNFAT-LUC and the p56lck-encoding vector pEFneoLckWT. PHA/PMA-induced NFAT activation was then restored in JCaM-TAg cells, consistent with the p56lck-dependent nature of TCR signaling. This same p56lck dependency was observed for bpV[pic]-induced luciferase activity in JCaM-TAg. The ZAP-70–deficient P116 cell line was then similarly investigated. On transfection with the pNFAT-LUC reporter vector plus a control vector, no NFAT activation was observed for most of the tested stimuli (Figure6B). However, when a wild-type ZAP-70 expression vector was added, response to these stimuli, including bpV[pic], was restored. ZAP-70 kinase activity was required for bpV-dependent signaling to be conveyed, as no NFAT activation was restored in P116 cells transfected with an expression vector encoding kinase-inactive ZAP-70 (Figure 6B). PMA/Iono was always a potent activator of NFAT in all these tested cell lines, and no important differences following transfections of the different vectors were apparent (Figure 6A,B).
bpV[pic]-mediated activation of NFAT is dependent on TCR-proximal events.
(A) JCaM-TAg cells (p56lck-negative) were transiently transfected by the DEAE-Dextran protocol with 15 μg of the pNFAT-LUC plasmid plus 30 μg of either the control vector pEFneo (■) or the p56lck-encoding vector pEFneoLckWT (■). After a 24-hour incubation, cells were treated or not with PHA (3 μg/mL)/PMA (20 ng/mL), bpV[pic] (10 μM), or PMA (20 ng/mL)/Iono (1 μM). Cells were lysed 8 hours after stimulation and measured for luciferase activity. (B) P116 cells (ZAP-70-deficient) were transiently transfected by the DEAE-Dextran protocol with 15 μg of the pNFAT-LUC plasmid plus 15 μg of either the empty vector (■), a ZAP-70 WT (▨) or ZAP-70 kinase-dead expression vector (▪). At 24 hours after transfection, cells were treated or not with PHA (3 μg/mL), PHA (3 μg/mL)/PMA (20 ng/mL), PMA (20 ng/mL)/Iono (1 μM), or bpV[pic] (10 μM) for 8 hours and lysed. Luciferase activity was monitored as described in the “Materials and methods” section. Results are the means ± SD for triplicate samples and are representative of 2 independent experiments.
bpV[pic]-mediated activation of NFAT is dependent on TCR-proximal events.
(A) JCaM-TAg cells (p56lck-negative) were transiently transfected by the DEAE-Dextran protocol with 15 μg of the pNFAT-LUC plasmid plus 30 μg of either the control vector pEFneo (■) or the p56lck-encoding vector pEFneoLckWT (■). After a 24-hour incubation, cells were treated or not with PHA (3 μg/mL)/PMA (20 ng/mL), bpV[pic] (10 μM), or PMA (20 ng/mL)/Iono (1 μM). Cells were lysed 8 hours after stimulation and measured for luciferase activity. (B) P116 cells (ZAP-70-deficient) were transiently transfected by the DEAE-Dextran protocol with 15 μg of the pNFAT-LUC plasmid plus 15 μg of either the empty vector (■), a ZAP-70 WT (▨) or ZAP-70 kinase-dead expression vector (▪). At 24 hours after transfection, cells were treated or not with PHA (3 μg/mL), PHA (3 μg/mL)/PMA (20 ng/mL), PMA (20 ng/mL)/Iono (1 μM), or bpV[pic] (10 μM) for 8 hours and lysed. Luciferase activity was monitored as described in the “Materials and methods” section. Results are the means ± SD for triplicate samples and are representative of 2 independent experiments.
More downstream effectors of NFAT activation were also evaluated for their importance in bpV[pic]-induced cascade. Intracellular calcium, which is known to be crucial for NFAT activation was investigated through the use of Jurkat-derived cell lines defective for capacitative calcium entry. After transfection with pNFAT-LUC, compared with Jurkat cells, these defective cells were not as responsive to the NFAT activators PMA/Iono, PHA, and bpV[pic] (data not shown). Furthermore, similar to the TCR-mediated cascade, the bpV[pic]-activated pathway also involved a functional p21ras protein as assessed by the use of the p21rasN17dominant-negative mutant (data not shown).
These results demonstrated that several effectors involved in TCR-dependent signaling were similarly required in the cascade initiated by bpV compounds culminating in the activation of NFAT.
Possible involvement of SHP-1 in NFAT activation by bpV[pic]
Because of its previously reported negative regulatory role in proximal TCR-mediated biochemical events,36,54 we reasoned that SHP-1 might be the bpV-targeted PTP. We further assumed that overexpression of wild-type SHP-1 should down-modulate bpV-dependent activation of NFAT. We tested this hypothesis by co-transfecting Jurkat cells with the pNFAT-LUC plasmid plus increasing doses of wild-type or dominant-negative SHP-1-encoding vectors. As shown in Figure7A, NFAT activation by several different agents, including bpV[pic], was strongly reduced following overexpression of wild-type SHP-1. However, overexpression of the SHP-1 mutant resulted, at the lowest quantity of plasmid, in small increases of NFAT activation (Figure 7B). To corroborate these results, nuclear extracts were prepared from the Jurkat-derived J.SHP-1C/S cell line stably transfected with a vector expressing a dominant-negative form of the SHP-1 protein (termed SHP-1C/S).36 EMSA analysis showed a NFAT-specific signal with nuclear extracts from untreated J.SHP-1C/S cells, which was competed with cold NFAT oligonucleotide (Figure 7C, compare lanes 5 and 6). No such complex was apparent in untreated Jurkat cells (lane 1). Furthermore, when PMA/Iono stimulation was performed, the NFAT-specific signal was stronger in J.SHP-1C/S than in Jurkat cells (compare lanes 3 and 7). These results suggested that the bpV compound might target SHP-1 and that this PTP would act as a regulator of NFAT activity.
Overexpression of wild-type SHP-1 negatively modulates the bpV-mediated signal transduction pathway leading to NFAT activation.
Jurkat cells were transiently transfected by the DEAE-Dextran protocol with 15 μg pNFAT-LUC and increasing quantities (0 μg, ■; 7.5 μg, ░; and 15 μg, ▪) of a vector encoding for the wild-type (A) or a dominant-negative version (B) of SHP-1. The total amount of DNA for each transfection was kept constant by the addition of filler DNA. Following a 24-hour incubation period, cells were left untreated or stimulated with either PHA (3 μg/mL), PHA (3 μg/mL)/PMA (20 ng/mL), or bpV[pic] (10 μM) for 8 hours. Cells were then lysed, and luciferase activity was monitored as described in “Materials and methods.” Results are the means ± SD for triplicate samples and are representative of 3 independent experiments. (C) Jurkat (lanes 1-4) or J.SHP-1C/S (lanes 5-8) cells were either left untreated (lanes 1-2 and 5-6) or stimulated with PMA (20 ng/mL)/Iono (1 μM) (lanes 3-4 and 7-8) for 1 hour. Nuclear extracts were then prepared as described in “Materials and methods,” and 10 μg was incubated with the γ32P-labeled NFAT probe for 20 minutes in the absence (lane 1, 3, 5, and 7) or presence of 100-fold excess of unlabeled NFAT oligonucleotide (lanes 2, 4, 6, and 8). Samples were then migrated on a native polyacrylamide gel, dried, and exposed on Kodak X-OMAT film. Free probe and specific complexes are indicated on the left side of the panel.
Overexpression of wild-type SHP-1 negatively modulates the bpV-mediated signal transduction pathway leading to NFAT activation.
Jurkat cells were transiently transfected by the DEAE-Dextran protocol with 15 μg pNFAT-LUC and increasing quantities (0 μg, ■; 7.5 μg, ░; and 15 μg, ▪) of a vector encoding for the wild-type (A) or a dominant-negative version (B) of SHP-1. The total amount of DNA for each transfection was kept constant by the addition of filler DNA. Following a 24-hour incubation period, cells were left untreated or stimulated with either PHA (3 μg/mL), PHA (3 μg/mL)/PMA (20 ng/mL), or bpV[pic] (10 μM) for 8 hours. Cells were then lysed, and luciferase activity was monitored as described in “Materials and methods.” Results are the means ± SD for triplicate samples and are representative of 3 independent experiments. (C) Jurkat (lanes 1-4) or J.SHP-1C/S (lanes 5-8) cells were either left untreated (lanes 1-2 and 5-6) or stimulated with PMA (20 ng/mL)/Iono (1 μM) (lanes 3-4 and 7-8) for 1 hour. Nuclear extracts were then prepared as described in “Materials and methods,” and 10 μg was incubated with the γ32P-labeled NFAT probe for 20 minutes in the absence (lane 1, 3, 5, and 7) or presence of 100-fold excess of unlabeled NFAT oligonucleotide (lanes 2, 4, 6, and 8). Samples were then migrated on a native polyacrylamide gel, dried, and exposed on Kodak X-OMAT film. Free probe and specific complexes are indicated on the left side of the panel.
Discussion
The activation of NF-κB, AP-1, and signal transducer and activator of transcription by PTP inhibitors has been reported earlier.24-26,55 56 Because we have previously demonstrated that bpV compounds modulate the HIV-1 LTR by both NF-κB–dependent and –independent signaling pathways, we were interested in identifying the other transcription factor(s) acting on the HIV-1 LTR in conjunction with NF-κB. We demonstrate herein that the inhibition of PTPs by bpV[pic] results in the activation of the NFAT transcription factor.
The immunosuppressors FK506 and CsA were initially observed to diminish bpV-mediated activation of the HIV-1 LTR or the isolated enhancer region. Because these calcineurin inhibitors have been shown to also affect NF-κB activation in certain cascades,57-59 we have tested their inhibitory potential on a NF-κB–mutated HIV-1 LTR-LUC construct and have demonstrated that bpV activation of this LTR was equally inhibited. The activation of the NFAT factor by the bpV compound was, however, directly tested in pNFAT-LUC–transfected Jurkat cells and PBMCs, which were highly responsive to bpV[pic]. In Jurkat cells, specificity of this bpV-dependent NFAT activation was confirmed through the use of the NFAT inhibitor VIVIT-GFP and FK506.
We have also determined that bpV compounds led to NFAT translocation. This was observed with nuclear extracts from bpV-stimulated Jurkat and PBMCs. A more detailed analysis revealed that NFAT1 was highly prevalent in these extracts. This is consistent with earlier demonstration of the dominance of NFAT1 in the DNA-binding activity of nuclear extracts from activated peripheral blood T lymphocytes.8 Through specific competition experiments and supershift assays, we have further shown that there was an important induction of an HIV-1 enhancer-bound NFAT complex by bpV compounds and that again NFAT1 was abundant in this complex (data not shown). This latter result is unexpected, as this same NFAT member has been proposed to negatively regulate the HIV-1 enhancer.50 However, the results obtained in pLTR-LUC–transfected Jurkat cells with the VIVIT-GFP inhibitor strongly suggest a positive contribution of NFAT1 in the activation of HIV-1 LTR by bpV compounds. We have also shown that NFAT1 could positively modulate HIV-1 LTR activity in 293T cells. Differences in experimental settings could account for this existing discrepancy between our result and the results of Macian and Rao.50 In fact, most of the experiments performed in their study were focused on the use of a reporter vector containing one NF-κB repeat. To attain a synergy between NFAT and NF-κB,27 the 2 repeats located in the HIV-1 enhancer might be required for both factors to bind and cooperate. Such a synergistic interaction between these 2 factors has been observed in the γ-interferon promoter.60 These results would thus argue that, in bpV[pic]-stimulated Jurkat cells, NFAT1 might be acting positively on HIV-1 gene expression.
Our results have also demonstrated that bpV led to the activation of the IL-2 promoter and an increase in secreted IL-2 levels when added with PMA, Iono, anti-CD3, anti-CD28 antibodies, and PHA. Similar observations have already been described with pervanadate.22,24,51 The activation of the IL-2 promoter by bpV[pic] was FK506 sensitive, which further suggests the implication of NFAT in IL-2 expression in Jurkat cells as it has recently been demonstrated by Chow et al.42 In fact, using their dominant-negative mutant of NFAT, our results indicated that bpV-mediated IL-2 promoter induction was similarly sensitive to its expression (data not shown). Hence, our results suggest that the multifactorial requirement for IL-2 expression is in part greatly helped by the bpV compound through concomitant activation of the NF-κB, NFAT, and AP-1 transcription factors.
Implication of both ZAP-70 and p56lck PTKs in this bpV-activated signaling cascade has been determined. This suggests that the point of entry of the PTP inhibitors is membrane proximal and likely results from the usual signaling pathway induced by TCR multimerization. This assumption is further supported by previous studies that show pervanadate-induced tyrosine phosphorylation of several membrane-associated signal transducers, including p56lck and ZAP-70.24,61 However, the importance of the TCR in the bpV-mediated induction of NFAT is not clear, as TCR-negative J.RT3 cells demonstrated reproducible levels of bpV-induced NFAT activation (data not shown). Intracytoplasmic association of p56lck with different cell surface molecules makes their possible implication an interesting alternative.62,63 Preliminary results also suggest that CD45, the required PTP for TCR-dependent signaling events, is not essential for NFAT activation by bpV compounds (data not shown). CD45 is thus an unlikely target for bpV molecules in NFAT activation, which comes in contrast with its previously reported implication in the induction of NF-κB by pervanadate.24
The observed role of p21ras in the bpV-mediated activation of NFAT is not unexpected since several examples in the literature have shown the importance of the Ras signaling pathway in NFAT transcriptional activation.64,65 This is thought to occur by the activation of the AP-1 factor, which would cooperatively act with NFAT.9 AP-1 activation by pervanadate, another PTP inhibitor, has already been described,24 and, based on preliminary results, AP-1 can be equally activated by bpV molecules in T cells (data not shown). The implication of calcium in the process leading to NFAT activation by bpV compounds was also expected, considering its crucial importance in the activation process of NFAT and also given the previously reported induction of intracellular calcium mobilization by PTP inhibitors.22,24 51
Studies have reported that treatment with pervanadate activates second messengers involved in T-cell activation (eg, p56lck, p59fyn, PLCγ1, and calcium influx).23,66 Herein, we further demonstrate that blocking of PTP activity results in direct NFAT activation. PTP activity might then be attenuating a constitutive kinase activity that otherwise would lead to a constant NFAT activation, a hypothesis that would closely parallel results obtained on the Jak1 signaling pathway.56 Interestingly, SHP-1 has been reported to decrease kinase activity or tyrosine phosphorylation of several substrates of interest to our work, including CD3ζ, PLCγ1, p56lck, p59fyn, ZAP-70, and Syk.21,36,67-75 In fact, our results have demonstrated that SHP-1 greatly inhibited the effect of bpV on NFAT activation. In addition, a Jurkat cell line stably expressing a dominant version of SHP-1 had a constitutive nuclearly localized NFAT signal. These results are not in agreement with the study by Jin et al61 that has indicated that SHP-1 might not be the pervanadate-targeted PTP restraining the activity of TCR-proximal kinases. However, discrepancy between their results and ours might be explained by the use of different PTP inhibitors and cell types. Moreover, Jin et al61 have focused on tyrosine phosphorylation events, whereas our group has evaluated NFAT activation.
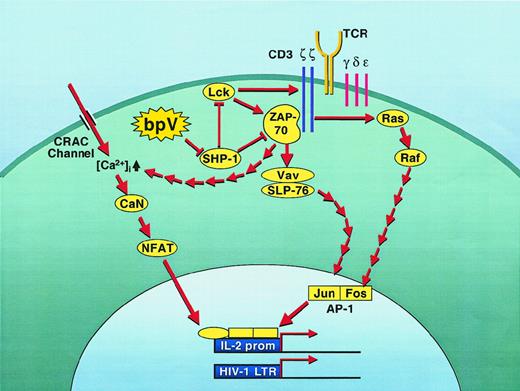
On the basis of our results, we propose a model (Figure8) in which bpV would initiate a cascade through the targeting of SHP-1. The ensuing activation of the PTKs p56lck and ZAP-70 would then lead to activation of AP-1 and permit intracellular calcium release and entry via the CRAC (Ca2+ release-activated Ca2+) pumps. This pathway might also be the cascade leading to the activation of NF-κB by bpVs that we and others have initially shown.26,55,76 However, preliminary data from our group have demonstrated that certain differences (including membrane-proximal events) are clearly apparent between the NFAT and NF-κB induction processes by bpVs (data not shown). This proposed model is consistent with what has been described in the SHP-1–defective motheaten (me/me) mouse model. Indeed, IL-2 production in these mice requires a lower threshold and is more extensive following antigenic stimulation, a phenomenon that has been attributed to higher p56lck and p59fyn PTK activities.69,77 78 Hence, the action of the bpV compounds might mimic a deficiency in SHP-1, leading to higher levels of IL-2 production by lowering the needed threshold following T-cell activation. Concomitant activation of other cellular factors is, however, needed for full production of IL-2, as bpV compounds were not sufficient to induce IL-2 production by themselves nor is IL-2 production constitutive in T cells from motheaten mice.
Proposed model for the regulation of NFAT activity by SHP-1.
Inhibition of constitutive SHP-1 activity by bpV molecules triggers a series of biochemical events characteristic of the early steps of TCR signaling. As such, the inhibition of SHP-1 leads to the initiation of a cascade through the sudden activation of ZAP-70 and p56lck, 2 well-known substrates of SHP-1. From these activated PTKs, a typical TCR-like downstream cascade of events would then induce NFAT-dependent transcriptional activity through the activation of both the Vav/SLP76 and Ras/Raf pathways, which culminate in activation of the AP-1 factor. In parallel, capacitance calcium entry would also be turned on through both the CRAC ion pump and the ZAP-70–dependent intracellular calcium release. The concomitant activation of both NFAT and AP-1 would then lead to the transcriptional induction of the IL-2 gene as well as the HIV-1 LTR (although this latter probably does not necessitate the presence of AP-1).
Proposed model for the regulation of NFAT activity by SHP-1.
Inhibition of constitutive SHP-1 activity by bpV molecules triggers a series of biochemical events characteristic of the early steps of TCR signaling. As such, the inhibition of SHP-1 leads to the initiation of a cascade through the sudden activation of ZAP-70 and p56lck, 2 well-known substrates of SHP-1. From these activated PTKs, a typical TCR-like downstream cascade of events would then induce NFAT-dependent transcriptional activity through the activation of both the Vav/SLP76 and Ras/Raf pathways, which culminate in activation of the AP-1 factor. In parallel, capacitance calcium entry would also be turned on through both the CRAC ion pump and the ZAP-70–dependent intracellular calcium release. The concomitant activation of both NFAT and AP-1 would then lead to the transcriptional induction of the IL-2 gene as well as the HIV-1 LTR (although this latter probably does not necessitate the presence of AP-1).
The demonstration that bpV molecules can activate IL-2 gene expression and NF-κB and NFAT, 2 transcription factors crucial in the regulation of the immune system, renders these compounds attractive for the restoration of normal immune functions in immunosuppressed individuals. These characteristics are thus interesting features of bpV compounds for potential use in new therapies. In addition, their efficiency in activating HIV-1 transcription could also be used for purging therapies in HIV-1–infected individuals, thereby allowing to clear out the reservoirs harboring latently infected CD4+ T cells. We are currently defining other intracellular second messengers participating in bpV-mediated NFAT activation. These studies will help decipher the differences between TCR- and bpV-mediated T-cell signaling and will permit elaboration on new pathways leading to NFAT activation.
Supported by grant HOP-15575 from the Canadian Institutes of Health Research HIV/AIDS Research Program (M.J.T.), by a Doctoral Fellowship from the Medical Research Council (MRC) of Canada (J.-F.F.), and by a Scholarship Award (Junior 1) from the Fonds de la Recherche en Santé du Québec (FRSQ) (B.B.). G.A.R. is the recipient of a PhD Fellowship from the FRSQ/Fonds pour la Formation de Chercheurs et l'Aide à la Recherche, and M.J.T. is the recipient of a Canada Research Chair in Human Immuno-Retrovirology.
J.-F.F. and B.B. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michel J. Tremblay, Laboratoire d'Immuno-Rétrovirologie Humaine, Centre de Recherche en Infectiologie, RC709, Centre Hospitalier Universitaire de Québec, Pavillon CHUL, 2705 boul. Laurier, Ste-Foy, QC, Canada G1V 4G2; e-mail:michel.j.tremblay@crchul.ulaval.ca.

![Fig. 1. bpV-mediated induction of HIV-1 LTR is sensitive to NaSal, FK506, and CsA. / (A) 1G5 cells were either left untreated (■) or pretreated with NaSal (2.5 mM) (░), FK506 (10 ng/mL) (▪), CsA (100 ng/mL) (▥), or a combination of NaSal with either FK506 (▤) or CsA () for 1 hour at 37°C. Following treatment, cells were either left untreated or treated for 8 hours with the following agents: PHA (3 μg/mL), bpV[bipy], bpV[HO pic], or bpV[pic] (10 μM). Jurkat cells were transiently transfected by the DEAE-Dextran protocol with 15 μg of either pκB-TATA-LUC (B) or pmκBLTR-LUC (C). After 24 hours, transfected cells were either left untreated (■) or were pretreated with FK506 (10 ng/mL) (▪) for 1 hour at 37°C. Following treatment, cells were stimulated for 8 hours with the following agents: PHA (3 μg/mL), PHA (3 μg/mL)/PMA (20 ng/mL), or bpV[pic] (10 μM). Cells were then lysed and evaluated for luciferase activity as described in “Materials and methods.” Values are the mean of 3 different measured samples ± SD. This is representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/8/10.1182_blood.v97.8.2390/5/m_h80810920001.jpeg?Expires=1765973667&Signature=xsjJ3ZUDwUdmWs33iSJJxDmDoYdv-8WKjVBnlsyQveiWPwBMgDrQK88fNejtRWZHNlBS8fLvF-miJTw-lty9C5keQHixZ57F057T-2cT8EHuL5NvfGw5MTCsrJCilnvBtlk5m-lojAVMcoEJe~S3N3it4qVNWH8RDDEkhfnJNv~qrb36i1t0kJLiez1snDSYypqElLzp7w8J9W3OznADELwdL~uBZgn7bSQPXhd-~lbOmdmooiLLl~kvfuEoZVBw1dncyRLkr5Wa~KK58Tgvt2wT7VoBn8hjOklbT1LKQpSz7HOWjFB-uYWiC0rtdNlUUB06bDZSNDa2yBx3kreZrg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. NFAT-driven luciferase gene expression is induced by bpV molecules and is FK506 sensitive. / (A) Jurkat cells were transiently transfected by the DEAE-Dextran protocol with 15 μg pNFAT-LUC. Following a 24-hour incubation period, cells were either left untreated (▪) or stimulated with PHA (3 μg/mL)/PMA (20 ng/mL) (■) or bpV[pic] (10 μM) (●) and lysed after 4, 6, 8, 12, and 24 hours of stimulation. (B) Ficoll Hypaque–isolated PBMCs were first treated with PHA-L for 20 hours and then electroporated in the presence of 15 μg of pNFAT-LUC. Following a 2-hour incubation period, cells were stimulated with bpV[pic] (10 μM) (⋄) or PMA (20 ng/mL)/Iono (1 μM) (■) for 8 hours. (C) Jurkat cells stably transfected with pNFAT-LUC were either left untreated (■) or pretreated with FK506 (10 ng/mL) (▪). After 15 minutes, cells were stimulated for 8 hours with PHA (3 μg/mL), PMA (20 ng/mL), PHA (3 μg/mL)/PMA (20 ng/mL), bpV[pic] (10 μM), or bpV[pic] (10 μM)/PMA (20 ng/mL). (D) Jurkat cells were transiently transfected by the DEAE-Dextran protocol with 15 μg of pNFAT-LUC plus 15 μg of either the control vector pEGFP (■ or the NFAT-inhibitor expression vector pVIVIT-GFP (▪). Following a 24-hour incubation period, cells were left untreated or were stimulated with either PHA (3 μg/mL), PMA (20 ng/mL)/Iono (1 μM), or bpV[pic] (10 μM) for 8 hours. Cells were lysed and evaluated for luciferase activity. Values are the mean of 3 different measured samples ± SD. This is representative of 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/8/10.1182_blood.v97.8.2390/5/m_h80810920002.jpeg?Expires=1765973667&Signature=PKOHoW05HbEg540J9aj7zKRIzgGWBP3mAz4iQ6wcmslJ1HamfXR6XNdEk6ympzv-YTHdVvWLVm0szawS5978nhLxJylfA5HshsA32G8Hyz-Qc4i4f7rCroG1rYvKU34gu2B-KldCcWtds74a8MQVMvShZs4feH9mTmhnMJSqbGISgK8izaAr02Cg5TAdDbuDf1IbHdSqPm8GddY6Mly24LNweO5~M3zNyWiIXOFim8SlatIF8fwyJ~2nbjW4y9rTC0bVnwMBdoiZV0Eo6nTnaL2e~waMFW-kMwOlmY3hOYtIuHrnVcgoJKzRjxox~QtKuu~lzHW8KMIlk5HRoHSesA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. bpV-mediated nuclear translocation of NFAT. / (A) Jurkat cells were either left untreated or stimulated with PMA (20 ng/mL)/Iono (1 μM) (P/I), or bpV[pic] (10 μM) for 1 hour. Nuclear extracts were then prepared as described in “Materials and methods.” Nuclear extracts from bpV-stimulated Jurkat cells (10 μg) were incubated with γ32P-labeled murine IL-2–derived NFAT-binding site for 20 minutes in the absence (lane 3) or presence of 10-, 100-, or 200-fold excess of unlabeled NFAT oligonucleotide (lanes 4, 5, and 6, respectively) or 100-fold excess of unlabeled NF-κB oligonucleotide (lane 7). Labeled NFAT oligonucleotides were incubated with nuclear extracts from unstimulated Jurkat cells as a negative control (lane 1) or from PMA/Iono-stimulated Jurkat cells as a positive control (lane 2). Samples were then migrated on a native polyacrylamide gel, dried, and exposed on Kodak X-OMAT film. (B) Nuclear extracts from bpV-stimulated Jurkat cells were either left untreated (lane 2) or preincubated with a panel of polyclonal antisera to NFAT1 (lane 3), NFAT2 (lane 4), or NFAT4 (lane 5) for 30 minutes on ice. Samples were then incubated for 20 minutes with the labeled NFAT oligonucleotide and migrated on a native polyacrylamide gel to be subsequently exposed on Kodak X-OMAT film. Extracts from unstimulated Jurkat cells were used as a negative control (lane 1). (C) Nuclear extracts from bpV-treated starved PBMCs (lanes 2-7) or bpV-treated fresh PBMCs (lanes 9-14) were preincubated with antisera such as preimmune normal rabbit serum (NRS) (lanes 3 and 10), NFAT1 (lanes 4 and 11), NFAT2 (lanes 5 and 12), NFAT4 (lanes 6 and 13), and panNFAT (lanes 7 and 14) and then incubated with the NFAT-labeled probe. Lanes 1 and 8 represent nuclear extracts from untreated starved and fresh PBMCs, respectively. Samples were then migrated on a native polyacrylamide gel, dried, and exposed on Kodak X-OMAT film. Free probe, specific complex and supershifted complexes (SS) are indicated on the left side of each panel.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/8/10.1182_blood.v97.8.2390/5/m_h80810920003.jpeg?Expires=1765973667&Signature=QhZbXHoD5iwZOzqx0vZYJzAhEe1aMsW03YpOsVUK6IXqR-U1lRH-MJpfdHqi6Bh0OPN91yvaLb3eWL~d2pqtv8vxjulIf7N89O1qdi4SQTZ~LEh22~fSjTfkIfP38RNR8Ak0XA7XYWAXS37T2XG54G0yRdhDUL0emU2Y9i7i80V4oO2s-doRb-pw0sgDuLaSmtodMcLQgKRnOw9ngBHLoJAwjLXpX4IMEgWm5EI1bQbx~GZkQHTj5hwqV2vMVIKfwkOjZZUmQLDWeGkfYGJINNW3lbb1BXz6nl4kBCa0iJaXH~ZVbsZqqkh-ASkyvkbup37sveN0vdRfeh9OTxKo2Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Importance of NFAT in HIV-1 LTR regulation by bpV[pic]. / (A) Jurkat cells were transiently transfected by the DEAE-Dextran protocol with 15 μg pLTR-LUC plus 15 μg of either the control vector pRc/β-actin (■) or the pRc/β-actin VIVIT-GFP vector (▪). Following a 24-hour incubation period, cells were either left untreated or stimulated with PHA (3 μg/mL), PMA (20 ng/mL), or bpV[pic] (10 μM) and lysed after 8 hours of stimulation. Luciferase activity was evaluated as the mean of 3 different measured samples and is represented as fold increase over untreated samples. This is representative of 3 independent experiments. (B) Nuclear extracts from bpV-treated Jurkat cells were incubated with the HIV-1 enhancer probe and competed with either 100-fold cold excess of NFAT oligonucleotide (lane 2), NF-κB oligonucleotide (lane 3), wild-type HIV-1 enhancer oligonucleotide (lane 4), or HIV-1 enhancer oligonucleotide mutated at the NF-κB–binding sites (lane 5). Samples were then migrated on a native polyacrylamide gel, dried, and exposed on Kodak X-OMAT film. Free probe and specific complexes are indicated on the left side of the panel. (C) 293T cells were transiently transfected by the calcium phosphate protocol with 5 μg pLTR-LUC plus 5 μg of either pREP4, pREP4-NFAT1, or pREP4-NFAT2. Cells were washed 16 hours after transfection and incubated for another 24 hours in fresh medium before lysis. Luciferase activity was evaluated as the mean of 3 different measured samples. This is representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/8/10.1182_blood.v97.8.2390/5/m_h80810920004.jpeg?Expires=1765973667&Signature=kdaHsElLtLRZui4wo2olDAIYUSu6dfmUyv9kRiP0g6BA~2ldAe98Tj8gYzK5JnUAEsBPrXgdqrs9f2C0Clt3g2w1e1-cukRjskAs~KPSgywVgLEJWpEdPQ~al6bnH3hBswQxtLyZOW~iKgVdNftTqRtPHdBPYA~PZHQ7oyZTgaWutyBK0drRWDbkVBOjuGG5aelk4Z5c4R1BS3-vkAukyoHNT~PyIB4HWA8j2rMIE3BGF~hg~k5pjlDCoOZaC2x8y96PpN7z0yLkOvWIKnTnboT-cOfXuDPRyWt8uc3yk~5CUMuT6rql1YqmDpgpwAuMHieN3APMzrNpdKwEn8CVKA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Activation of the complete IL-2 promoter by bpV compounds. / (A) Jurkat cells were transiently transfected by the DEAE-Dextran protocol with 15 μg pIL-2–LUC plasmid. After a 24-hour incubation, cells were either left untreated (▪) or were stimulated with PHA (3 μg/mL)/PMA (20 ng/mL) (■) or bpV[pic] (10 μM)/PMA (20 ng/mL) (●) for various times (0, 4, 6, 8, 12, and 24 hours), lysed, and monitored for luciferase activity. (B) J–IL-2–LUC cells were either left untreated (■) or pretreated for 15 minutes with FK506 (10 ng/ml) (▪) before being left untreated or stimulated with PHA (3 μg/mL)/PMA (20 ng/mL) and bpV[pic] (10 μM)/PMA (20 ng/mL). Cells were lysed 8 hours after stimulation, and luciferase activity was monitored. (C) J–IL-2–LUC cells were treated with various activators (PHA, 3 μg/mL; bpV[pic], 10 μM; Iono, 1 μM; PMA, 20 ng/mL; anti-CD3 antibody, 3 μg/mL; anti-CD28 antibody, 1 μg/mL) and lysed after 8 hours to be monitored for luciferase activity. (D) J–IL-2–LUC cells were treated with the same activators as in panel C. Cell-free supernatants were collected 24 hours after stimulation, and IL-2 was quantified through ELISA as described in “Materials and methods.” Luciferase activity was monitored as described in “Materials and methods.” Results for all panels are the means ± SD for triplicate samples and are representative of 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/8/10.1182_blood.v97.8.2390/5/m_h80810920005.jpeg?Expires=1765973667&Signature=dgdTnaXGxUnMCYVvT8JQ1U9Z9Ar1qFbe~UspMYOhnFxoe9oV1Mx8xPTws1KJd1fcxhTMLRaJQNSZPQA3YELlWfZcmFj~BGEFx-XsFwVb8ciPif61DdkhmdFNxyOJ4wI0VApYzb3khuGgCnwIebM34LTl2Q9uEZTQC4v62CTHIY6R~jU3k8qnNKofFcL-m45eEJXgA4DYKrj3hnVNuxehHGuAMAfpNd1Do-NIv9iBm-4xffkOEYVwTans6QkiUPBB8v0SOJJszCCEsbYA8ixkHYbZlieENST35-Da0S8Ok9F9Y2834rgHID1K2oPsP8hxom~a--K1qCW2JOuJi9W9TQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. bpV[pic]-mediated activation of NFAT is dependent on TCR-proximal events. / (A) JCaM-TAg cells (p56lck-negative) were transiently transfected by the DEAE-Dextran protocol with 15 μg of the pNFAT-LUC plasmid plus 30 μg of either the control vector pEFneo (■) or the p56lck-encoding vector pEFneoLckWT (■). After a 24-hour incubation, cells were treated or not with PHA (3 μg/mL)/PMA (20 ng/mL), bpV[pic] (10 μM), or PMA (20 ng/mL)/Iono (1 μM). Cells were lysed 8 hours after stimulation and measured for luciferase activity. (B) P116 cells (ZAP-70-deficient) were transiently transfected by the DEAE-Dextran protocol with 15 μg of the pNFAT-LUC plasmid plus 15 μg of either the empty vector (■), a ZAP-70 WT (▨) or ZAP-70 kinase-dead expression vector (▪). At 24 hours after transfection, cells were treated or not with PHA (3 μg/mL), PHA (3 μg/mL)/PMA (20 ng/mL), PMA (20 ng/mL)/Iono (1 μM), or bpV[pic] (10 μM) for 8 hours and lysed. Luciferase activity was monitored as described in the “Materials and methods” section. Results are the means ± SD for triplicate samples and are representative of 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/8/10.1182_blood.v97.8.2390/5/m_h80810920006.jpeg?Expires=1765973667&Signature=joAT1GFPhNzfi3jqOcKvqpz-EJki2rcbfAvhht-XApupd8NDC~hxOZ~QZlrK3hbnh-aKrzFz0oUG5wDlhhZfCRJVdoolB8ZU3WozWI6Hj4rD0INOvBjKB4c8ku~K29wDp4DN2zg8eMCyqFcwGqgKXV11gmzBMICLSh~Mdh3lbAwm9zCRFsu6Ls5-q-G05~5Qk9JJciRjoJA06eBJFLGAQY4XcSUKekTBCsEpEldvkSdFySk6h8itAfvEg1GzZQdfybD5ves6bkSvYO-6SmSxC-gUvTD0m9lU6fhVvPqGb5zMs61mLYgwyt9aTPUByt3Rf90kxutlF06gEtqZRKF0wQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Overexpression of wild-type SHP-1 negatively modulates the bpV-mediated signal transduction pathway leading to NFAT activation. / Jurkat cells were transiently transfected by the DEAE-Dextran protocol with 15 μg pNFAT-LUC and increasing quantities (0 μg, ■; 7.5 μg, ░; and 15 μg, ▪) of a vector encoding for the wild-type (A) or a dominant-negative version (B) of SHP-1. The total amount of DNA for each transfection was kept constant by the addition of filler DNA. Following a 24-hour incubation period, cells were left untreated or stimulated with either PHA (3 μg/mL), PHA (3 μg/mL)/PMA (20 ng/mL), or bpV[pic] (10 μM) for 8 hours. Cells were then lysed, and luciferase activity was monitored as described in “Materials and methods.” Results are the means ± SD for triplicate samples and are representative of 3 independent experiments. (C) Jurkat (lanes 1-4) or J.SHP-1C/S (lanes 5-8) cells were either left untreated (lanes 1-2 and 5-6) or stimulated with PMA (20 ng/mL)/Iono (1 μM) (lanes 3-4 and 7-8) for 1 hour. Nuclear extracts were then prepared as described in “Materials and methods,” and 10 μg was incubated with the γ32P-labeled NFAT probe for 20 minutes in the absence (lane 1, 3, 5, and 7) or presence of 100-fold excess of unlabeled NFAT oligonucleotide (lanes 2, 4, 6, and 8). Samples were then migrated on a native polyacrylamide gel, dried, and exposed on Kodak X-OMAT film. Free probe and specific complexes are indicated on the left side of the panel.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/8/10.1182_blood.v97.8.2390/5/m_h80810920007.jpeg?Expires=1765973667&Signature=yCEVRUMOQVOu7Y8kMCvuLsga4J8RfGU5IlkPskqSg5lV8bDpzBTldwfU8pPMPrEQtfeytIQdDmL-fxcRHnAraGjTLX~oLxx3oy88LMBCY0~ccfbQteuTPyq1Yy3CjguQWdAe4fpbW3fJ6~AF9y3KqFM3jKhHGWS9vAAR8HIZUE8UjXGueLUOyp6oruShM8~901afynJZ9RslSal4itnThBC3Flr~M395CgGRr2fpW014WUU1m1pC~drf8O9KnpjoliE7-0TfnYq2AgLPotpXvkbkzNOy-4yCRhEeiL~HCgOxbtuyyuIh78nBjgMbAq~9YB~dHvidoqQKorXZe3dqXA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal