Abstract
Tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), essential components in the pathogenesis of immunoinflammatory diseases, are strongly induced in monocytes by direct contact with stimulated T lymphocytes. This study demonstrates that adult human serum (HS) but not fetal calf or cord blood serum displays inhibitory activity toward the contact-mediated activation of monocytes by stimulated T cells, decreasing the production of both TNF-α and IL-1β. Fractionation of HS and N-terminal microsequencing as well as electroelution of material subjected to preparative electrophoresis revealed that apolipoprotein A-I (apo A-I), a “negative” acute-phase protein, was the inhibitory factor. Functional assays and flow cytometry analyses show that high-density lipoprotein (HDL)-associated apo A-I inhibits contact-mediated activation of monocytes by binding to stimulated T cells, thus inhibiting TNF-α and IL-1β production at both protein and messenger RNA levels. Furthermore, apo A-I inhibits monocyte inflammatory functions in peripheral blood mononuclear cells activated by either specific antigens or lectins without affecting cell proliferation. These results demonstrate a new anti-inflammatory activity of HDL-associated apo A-I that might have modulating functions in nonseptic conditions. Therefore, because HDL has been shown to bind and neutralize lipopolysaccharide, HDL appears to play an important part in modulating both acute and chronic inflammation. The novel anti-inflammatory function of apo A-I reported here might lead to new therapeutic approaches in inflammatory diseases such as rheumatoid arthritis, multiple sclerosis, systemic lupus erythematosus, and atherosclerosis.
Introduction
Tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) are strongly induced in monocytes by direct contact with stimulated T lymphocytes, both cells involved in immunoinflammatory diseases such as rheumatoid arthritis, multiple sclerosis, systemic lupus erythematosus, and atherosclerosis. The importance of TNF-α and IL-1 in chronic inflammation has been well established. Based on the premise that T lymphocytes play a pivotal role in the pathogenesis of chronic inflammatory diseases, we demonstrated that direct cell-cell contact with stimulated T lymphocytes is a major stimulus triggering the production of large amounts of TNF-α and IL-1β in monocytes.1-3 Various stimuli are able to induce T cells to activate monocytes by direct cellular contact: (1) mitogens, for example, a combination of phytohemagglutinin (PHA) and phorbol myristate acetate (PMA),1,4-6 (2) cross-linking of CD3 by immobilized anti-CD3 monoclonal antibody (mAb) with or without cross-linking of the costimulatory molecule CD28,7,8 (3) antigen-recognition on antigen-specific T-cell clones,8 and (4) cytokines.9 The identity of the ligands on plasma membrane of stimulated T cells that trigger the signaling of monocyte-macrophages as well as that of the counter-ligands on monocytes is still elusive. However, in the human system some of the signaling may be attributed to β2-integrins, CD69, CD23, CD40-CD40L and lymphocyte activation gene-3 (LAG-3).1,4,5,10-14 Membrane-associated TNF-α and IL-1 do not play a crucial part in this cellular interaction, contrasting with their significant role in activation processes induced by stimulated T cells in human fibroblasts/synoviocytes or microvascular endothelial cells.2,3,15 16
We hypothesized that, in analogy with other homeostatic systems, natural inhibitors are likely to exist that interfere with the lymphocyte/monocyte interaction. Such inhibitory activity should be present in plasma because stimulated T lymphocytes in the bloodstream of normal subjects or patients during inflammation show little evidence of contact-mediated activation of monocytes. Indeed, the occasional presence of TNF-α and IL-1β in the blood of patients is mainly the result of their overproduction at the tissue level where direct contact between monocyte-macrophages and T cells occurs. This is further confirmed by the finding that monocytes from normal subjects or patients need to be stimulated ex vivo to produce significant amounts of cytokines.17-19 Therefore, serum factors might display an inhibitory activity in the vascular compartment, whereas during the initial inflammatory events in the target tissue, monocyte activation by cellular contact might occur in the absence of such inhibitory factors. In a subsequent phase, due to the increase in vascular permeability at the inflammatory site, these factors might diffuse in the extravascular compartment and interfere with the cross-talk between cells. This could account for the fluctuations of the clinical status often observed in patients with chronic inflammation. In this study we demonstrate that human serum (HS) displays an inhibitory effect on the contact-mediated activation of monocytes. By assessing the inhibitory activity of HS fractions on TNF-α and IL-1β production induced by stimulated T cells in monocytes or monocytic cells (THP-1 cells), apolipoprotein A-I (apo A-I), a “negative” acute-phase protein,20 is identified as the inhibitory factor.
Materials and methods
Reagents
Phaseolus vulgaris leucophytohemagglutinin (PHA) (E-Y Laboratories, San Mateo, CA); phorbol myristate acetate (PMA), paraformaldehyde, phenylmethylsulfonyl fluoride (PMSF), pepstatin A, leupeptin, iodoacetamide, polymyxin B sulfate, neuraminidase, and bovine serum albumin (Sigma Chemicals, St Louis, MO); RPMI-1640, phosphate-buffered saline (PBS) without Ca++ and Mg++, fetal calf serum (FCS), penicillin, streptomycin, andl-glutamine (Gibco, Paisley, Scotland) were purchased from the designated suppliers. All other reagents were of analytical grade or better. Pooled normal HS was obtained from the Blood Transfusion Center of Geneva University Hospital.
Antibodies
Mouse mAbs against apo A-I were either purchased from Calbiochem-Novabiochem (La Jolla, CA) or isolated by affinity chromatography on protein G-Sepharose (Pharmacia, Uppsala, Sweden) from culture supernatants of hybridoma purchased from the American Tissue Culture Collection (ATCC, Rockville, MD).
T cells and preparation of T-cell plasma membranes
HUT-78, a human T cell line,21 was obtained from the ATCC. Cells were maintained in RPMI-1640 medium supplemented with 10% heat-inactivated FCS, 50 μg/mL streptomycin, 50 IU/mL penicillin, and 2 mM l-glutamine (complete RPMI medium) in 5% CO2-air humidified atmosphere at 37°C. HUT-78 cells (1 × 106 cells/mL) were stimulated for 6 hours by PHA (1 μg/mL) and PMA (5 ng/mL). Stimulated HUT-78 cells were either fixed with 1% paraformaldehyde1,4 or their plasma membranes prepared as previously described.15 T lymphocytes were obtained from buffy coats of healthy donors as previously described,1 and contained 94% to 98% CD2+, 83% to 94% CD3+, and less than 2% CD14+ as assessed by flow cytometry. T lymphocytes were stimulated for 48 hours by PHA (1 μg/mL) and PMA (5 ng/mL), washed thoroughly, and fixed with 1% paraformaldehyde as previously described.1 4Peripheral blood mononuclear cells (PBMC) were obtained from buffy coats of healthy donors by density centrifugation on Ficoll-Paques (Amersham-Pharmacia, Uppsala, Sweden).
Monocytes and monocytic cells
Protein concentration and N-terminal microsequencing
The protein concentrations were determined by the method of Bradford. The purified inhibitory fraction was subjected to 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene difluoride (PVDF) membrane, and visualized by Coomassie blue staining. The Mr = 28-kd band was excised and N-terminal sequence analysis was performed on a Procise 494-HT protein sequencer (PerkinElmer, Foster City, CA).
PBMCcultures
The PBMC were cultured in 96-well culture plates at a density of 4 × 105 cells/200 μL/well in the presence of the indicated stimulus for 48 hours (cytokine production) or 72 hours (proliferation). For proliferation, 3H-thymidine was added 24 hours before cell harvesting.
Contact-mediated activation of THP-1 cells and monocytes
The THP-1 cells (5 × 104 cells/well) or monocytes (8 × 104 cells/well) were dispensed onto 96-well culture plates (Falcon, Becton Dickinson, Plymouth, England) and activated by the indicated stimulus in a total volume of 200 μL complete RPMI medium in the presence or absence of the indicated inhibitor. After 48 hours, culture supernatants were analyzed for their contents in TNF-α and IL-1β as previously described.1 4
Isolation of serum high-density lipoproteins
by high-density ultracentrifugation
The HS lipoproteins were isolated according to Havel and colleagues.23 Briefly, to remove the chylomicrons, HS was centrifuged for 45 minutes at 20 000 rpm using a Beckman JA 20.1 rotor (Beckman Instruments, Fullerton, CA). Serum free of chylomicrons was then centrifuged for 24 hours and 37 minutes at 50 000 rpm. The upper phase containing very low-density lipoproteins (VLDL) was discarded. The lower phase was adjusted to a density of 1.063 g/mL by the addition of solid NaBr and centrifuged for 24 hours 37 minutes at 50 000 rpm. The low-density lipoproteins (LDL) were recovered in the upper phase. The lower phase was adjusted to d = 1.23 g/mL by the addition of solid NaBr and centrifuged for 60 hours 47 minutes at 45 000 rpm. High-density lipoproteins (HDL) were recovered in the upper phase while the lower phase contained remaining serum proteins. All ultracentrifugations were carried out at 4°C using a Beckman 50.2 Ti rotor (Beckman Instruments). The recovered lipoprotein and protein fractions were then dialyzed against PBS and tested for their inhibitory activity.
Flowcytometry
The HDL were labeled with fluorescein isothiocyanate (FITC-HDL) as described.24 The binding of FITC-HDL to cells was analyzed by direct flow cytometry on a flow cytometer (Epics, Coulter Electronics, Hialeah, FL) essentially as described previously.25 The mean fluorescence intensity was recorded on gating of living cells and expressed in arbitrary units in 4 decade logarithmic scale. The percentage of positive cells was based on the percentage of fluorescent events exceeding unconjugated FITC control.
HDLdelipidation
The extraction of HDL apolipoproteins was performed as described.26 Briefly, 1 volume of HDL isolated by ultracentrifugation was slowly added to 12 volumes of ice-cold methanol on constant stirring. Then 28 volumes of ice-cold diethylether was added to the solution. After 10 minutes stirring on ice, the mixture was centrifuged at 500g for 5 minutes. The protein pellet was resuspended in 40 volumes of diethylether. After 10 minutes stirring on ice, the mixture was centrifuged as above. The pellet was recovered and dried under nitrogen flux. To minimize aggregation, the delipidated HDL lipoproteins were solubilized at 2 mg/mL proteins in 0.1 M Tris-HCl pH 7.4 containing 0.1M NaCl, 1 mM NaN3, 1 mM EDTA, and 2 M guanidinium chloride and then dialyzed in PBS.
Treatmentof HDL with proteinase K
The HDL (100 μg proteins) were incubated in the presence or absence of 1 U proteinase K linked to agarose beads (Sigma Fine Chemicals, St Louis, MO) in a final volume of 200 μL PBS at 37°C for 1 hour. The proteolytic reaction was stopped by centrifugation and its efficacy assessed by SDS-PAGE.
Electroelutionof HDL proteins
The HDL proteins were isolated by electroelution from gel slices essentially as previously described.27 Briefly, 1 mg protein of delipidated HDL was subjected to SDS-PAGE on unreduced conditions. The gel was stained with copper and the detected bands (Mr = 56 000-66 000, 50 000, 28 000, and 18 000) were cut, destained, and eletroeluted for 5 hours in 50 mM Tris-HCl and 384 mM glycine (pH 8.3) containing 5 mM EDTA and 0.1% SDS. SDS was discarded by precipitating proteins in acetone. Proteins were lyophilized and resuspended in 0.1 M Tris-HCl (pH 7.4) containing 0.1 M NaCl, 1 mM NaN3, 1 mM EDTA, and 2 M guanidinium chloride and then dialyzed in PBS. Alternatively, proteins from delipidated HDL were solubilized in 0.1 M Tris-HCl pH 7.4 containing 0.1 M NaCl, 1 mM NaN3, 1 mM EDTA, and 2 M guanidinium chloride and subjected to gel filtration on Superdex S75 (75 × 1.6 cm, Pharmacia) equilibrated in the same buffer. Fractions corresponding to Mr = 28 000 were pooled, concentrated and dialyzed in PBS for testing their inhibitory activity. Fractions were analyzed by Western blot for their content in apo A-I using mouse mAb from Calbiochem-Novabiochem.
MessengerRNA analysis
Total RNA was isolated from THP-1 cells and monocytes with TRIzol reagent (Life Technologies, Basel, Switzerland) according to the manufacturer's procedure. Two and 10 μg total RNA were used to quantify messenger RNAs (mRNAs) in monocytes and THP-1 cells, respectively, using a commercially available “RNase protection assay system” kit with hck2 template set (Pharmingen, San Diego, CA) to which an antisense riboprobe for TNF-α was added. TNF-α antisense riboprobe was obtained from TNF-α complementary DNA (cDNA) template prepared with SP6 RNA polymerase after linearizing pSP64/hTNF plasmid provided by Dr C. V. Jongeneel (Ludwig Institute for Cancer Research, Lausanne, Switzerland).
Results
Human serum inhibited T-cell signaling of both monocytes and THP-1 cells
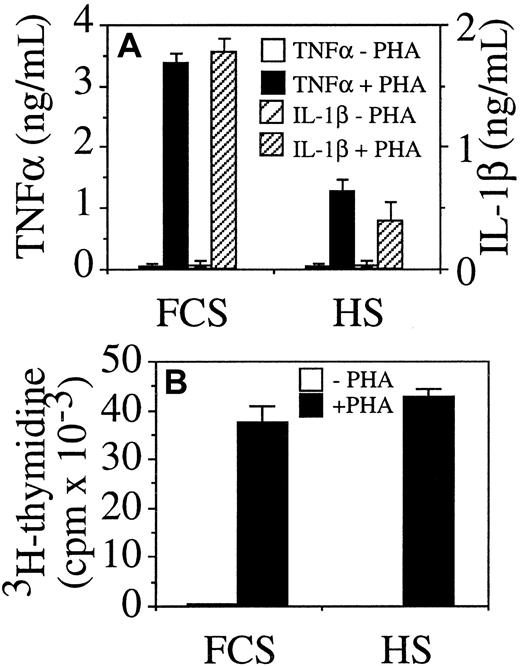
To assess whether HS displayed an anti-inflammatory activity by inhibiting cytokine production, unseparated PBMC were stimulated by PHA in medium supplemented with either 10% FCS or HS. In the presence of HS, the production of both TNF-α and IL-1β was inhibited in PBMC cultured with HS as compared with FCS (Figure1A), but cell proliferation was similar in HS and FCS (Figure 1B). Because it was likely that cytokine production by PBMC was induced in monocytes by direct contact with stimulated T lymphocytes, the inhibitory effect of HS was ascertained in several culture systems in which either fixed, stimulated T lymphocytes or HUT-78 T cells, or plasma membranes from the latter cells were added to peripheral blood monocytes or THP-1 monocytic cells. The induced production of TNF-α and IL-1β was measured (Figure 2).
Human serum inhibits TNF-α and IL-1β production in PHA-stimulated PBMC.
PBMC (4 × 105 cells/200 μL/well) were stimulated with 1 μg/mL PHA in medium completed with either FCS or HS. (A) TNF-α and IL-1β were measured in supernatant after 48 hours of incubation; and (B) proliferation (3H-thymidine incorporation) was measured after 72 hours.
Human serum inhibits TNF-α and IL-1β production in PHA-stimulated PBMC.
PBMC (4 × 105 cells/200 μL/well) were stimulated with 1 μg/mL PHA in medium completed with either FCS or HS. (A) TNF-α and IL-1β were measured in supernatant after 48 hours of incubation; and (B) proliferation (3H-thymidine incorporation) was measured after 72 hours.
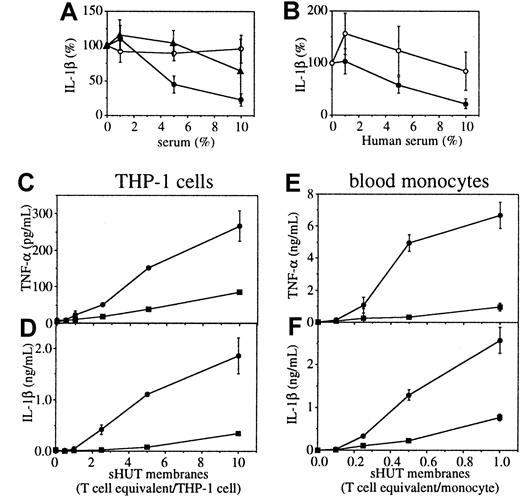
Inhibition of T-cell signaling of monocytes and THP-1 cells by HS.
(A) THP-1 cells were activated for 48 hours by fixed, stimulated T lymphocytes at a cellular ratio of 8 T lymphocytes/THP-1 cell in the presence of increasing doses of HS (closed circles), FCS (open circles), or cord blood serum (closed trianlges). (B) THP-1 cells were activated for 48 hours by either fixed, stimulated HUT-78 cells (fsHUT-78) at a cellular ratio of 8 HUT-78 cells/THP-1 cell (closed symbols) or 10 μg/mL LPS and 5 ng/mL PMA (open symbols) in the presence of increasing doses of HS. THP-1 cells (C,D) and monocytes (E,F) were activated for 48 hours by increasing doses of membranes isolated from stimulated HUT-78 cells in the presence (closed squares) or absence of 10% HS (closed circles). TNF-α (C,E) and IL-1β (D,F) were measured in culture supernatants. Results represent mean ± SD, n = 3 except in panel B where n = 7. In panels A and B, 100% represents the production of IL-1β after 48 hours of culture in the absence of inhibitor.
Inhibition of T-cell signaling of monocytes and THP-1 cells by HS.
(A) THP-1 cells were activated for 48 hours by fixed, stimulated T lymphocytes at a cellular ratio of 8 T lymphocytes/THP-1 cell in the presence of increasing doses of HS (closed circles), FCS (open circles), or cord blood serum (closed trianlges). (B) THP-1 cells were activated for 48 hours by either fixed, stimulated HUT-78 cells (fsHUT-78) at a cellular ratio of 8 HUT-78 cells/THP-1 cell (closed symbols) or 10 μg/mL LPS and 5 ng/mL PMA (open symbols) in the presence of increasing doses of HS. THP-1 cells (C,D) and monocytes (E,F) were activated for 48 hours by increasing doses of membranes isolated from stimulated HUT-78 cells in the presence (closed squares) or absence of 10% HS (closed circles). TNF-α (C,E) and IL-1β (D,F) were measured in culture supernatants. Results represent mean ± SD, n = 3 except in panel B where n = 7. In panels A and B, 100% represents the production of IL-1β after 48 hours of culture in the absence of inhibitor.
Stimulated T lymphocytes isolated from peripheral blood were fixed with paraformaldehyde and added to THP-1 cells in complete RPMI medium supplemented with either HS or FCS, that is, a final serum concentration of 20%. In these conditions, T lymphocytes triggered the production of IL-1β in THP-1 cells that was inhibited in a dose-dependent manner by adult HS but not by FCS (Figure 2A). This demonstrates the presence of an inhibitory activity in HS. Human cord blood serum (CBS) was only slightly inhibitory at 10% concentration (Figure 1A), suggesting that the inhibitory factor was only present in adult serum. To ascertain the specificity of this inhibitory activity for contact-mediated activation of monocytes, THP-1 cells were activated by fixed, stimulated HUT-78 cells or by lipopolysaccharide (LPS) and PMA in the presence of increasing concentrations of HS. In these conditions, HS inhibited in a dose-dependent manner IL-1β production in THP-1 cells when triggered by stimulated T cells, but not when triggered by LPS and PMA (Figure 2B). This demonstrates that the inhibitory activity of HS was directed to T-cell signaling of THP-1 cells and that freshly isolated T lymphocytes and the HUT-78 cell line expressed activating factor(s) on stimulation (Figure 2A,B). Plasma membranes of stimulated T cells induced both TNF-α and IL-1β production in THP-1 cells and freshly isolated monocytes (Figure 2C-F). Monocytes were more sensitive to contact activation by membranes of stimulated HUT-78 cells than were THP-1 cells, the latter requiring a 10-fold higher amount of membranes for activation. Indeed, IL-1β and TNF-α production was triggered by isolated membranes at an amount equivalent to a cellular ratio as low as 0.1 stimulated T cell/monocyte (Figure 2E,F), whereas in THP-1 cells the cytokine production was significantly elevated at a ratio of 2 stimulated T cell/THP-1 cell (Figure 2C,D). Similar levels of IL-1β were induced in both types of cell (Figure 2D,F), whereas levels of TNF-α were 20-fold lower in THP-1 cells than in monocytes. HS inhibited the production of TNF-α and IL-1β production in both THP-1 cells and monocytes. These data substantiate (1) the high potency of direct contact with stimulated T cells in triggering TNF-α and IL-1β production by monocytes and (2) the hypothesis that inhibitory factors were present in HS. To further identify HS inhibitory component(s) and mechanism(s) of inhibition, THP-1 cells and membranes from stimulated HUT-78 cells were used for the sake of convenience.
Isolation of HS factor inhibiting T-cell signaling of monocytes
To identify the inhibitory factor(s), HS was fractionated by serial chromatography on Blue Sepharose fast flow, Q Sepharose fast flow, phenyl Sepharose 6 fast flow, and Superdex 200 (Pharmacia). The latter revealed one peak of inhibitory activity that was recovered in fractions 23 to 26 (Figure 3A), representing a Mr = 179 × 103 ± 46 × 103 (mean ± SD, n = 4 inhibitory fractions). On analysis by SDS-PAGE the inhibitory activity correlated with the enrichment of a 28-kd protein band (Figure 3A,B). According to N-terminal microsequencing this band was consistent with apo A-I, the main protein component of HDL. This accounted for the discrepancy in Mr analysis between gel filtration by which intact HDL were separated and SDS-PAGE in which HDL proteins were separated (Figure 3A,B). These data demonstrate that the inhibitor was associated with HDL particles.
Superdex 200 elution profile and SDS-PAGE analysis of serial chromatography fractionation of HS.
(A) Inhibitory fractions eluted from Phenyl Sepharose HP were pooled, concentrated, and subjected to gel filtration on Superdex 200 equilibrated in PBS. The column was calibrated with the molecular weight marker kit for gel filtration chromatography (Sigma). Fractions were tested for their protein contents (optical density [OD] 280 nm, dashed line) and inhibitory activity (closed circles). (B) Inhibitory fractions were pooled after each step and 10 μg protein aliquots were loaded per lane on 10% acrylamide gel: (a) HS; (b) breakthrough of Blue-Sepharose; (c) pool of inhibitory fractions from Q Sepharose; (d) pool of inhibitory fractions from Phenyl Sepharose HP; and (e) pool of inhibitory fractions from Superdex 200; the gel was stained with Coomassie blue.
Superdex 200 elution profile and SDS-PAGE analysis of serial chromatography fractionation of HS.
(A) Inhibitory fractions eluted from Phenyl Sepharose HP were pooled, concentrated, and subjected to gel filtration on Superdex 200 equilibrated in PBS. The column was calibrated with the molecular weight marker kit for gel filtration chromatography (Sigma). Fractions were tested for their protein contents (optical density [OD] 280 nm, dashed line) and inhibitory activity (closed circles). (B) Inhibitory fractions were pooled after each step and 10 μg protein aliquots were loaded per lane on 10% acrylamide gel: (a) HS; (b) breakthrough of Blue-Sepharose; (c) pool of inhibitory fractions from Q Sepharose; (d) pool of inhibitory fractions from Phenyl Sepharose HP; and (e) pool of inhibitory fractions from Superdex 200; the gel was stained with Coomassie blue.
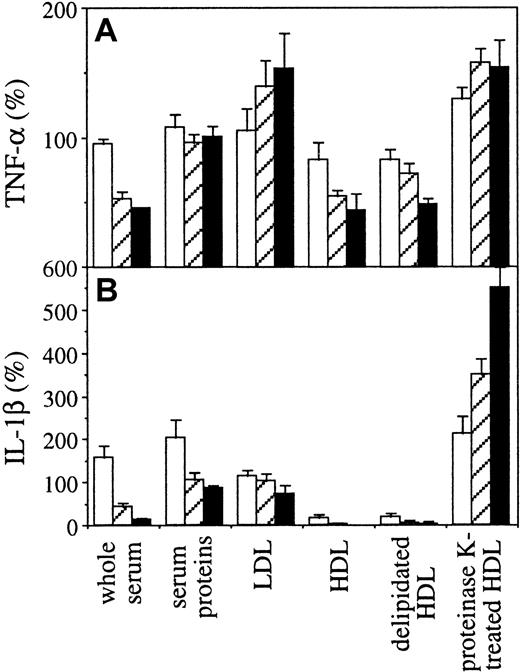
To confirm that HDL displayed inhibitory activity, isolated lipoproteins and serum proteins were tested for their inhibitory activity in THP-1 cells activated by membranes of stimulated HUT-78 cells. The inhibitory activity was recovered in HDL, whereas LDL and serum proteins did not display inhibition (Figure4). To determine whether the inhibition was due to a proteic or a lipidic component, HDL were subjected to either delipidation or proteolytic treatment with proteinase K. HDL proteins obtained by diethylether/methanol treatment displayed a high inhibitory activity, whereas HDL lipids obtained after proteolytic digestion with proteinase K were no longer inhibitory (Figure 4). This demonstrates that the production of both TNF-α and IL-1β was inhibited by HDL proteins (Figure 4A,B).
Presence of the inhibitory activity in protein fraction of HDL.
HS was fractionated by high-density centrifugation and the inhibitory activity of HDL, LDL, and serum protein fractions was analyzed. Isolated HDL were further subjected to either delipidation (delipidated HDL) or proteolytic digestion with proteinase K (proteinase K–treated HDL). The inhibitory activity of fractions was compared to HS (whole serum). The final protein concentration for whole serum and serum proteins was 7 mg/mL (black columns), 3.5 mg/mL (hatched columns), and 0.7 mg/mL (white columns). The final protein concentration for LDL was 0.04 mg/mL (black columns), 0.02 mg/mL (hatched columns), and 0.004 mg/mL (white columns). HDL and delipidated HDL was 0.2 mg/mL (black columns), 0.1 mg/mL (hatched columns), and 0.02 mg/mL (white columns). The amount of proteinase K–treated HDL was estimated according to the protein concentration before proteolysis and was similar to untreated HDL. Results are expressed as percentage, 100% being the production of IL-1β or TNF-α measured in the absence of inhibitor (mean ± SD, n = 3).
Presence of the inhibitory activity in protein fraction of HDL.
HS was fractionated by high-density centrifugation and the inhibitory activity of HDL, LDL, and serum protein fractions was analyzed. Isolated HDL were further subjected to either delipidation (delipidated HDL) or proteolytic digestion with proteinase K (proteinase K–treated HDL). The inhibitory activity of fractions was compared to HS (whole serum). The final protein concentration for whole serum and serum proteins was 7 mg/mL (black columns), 3.5 mg/mL (hatched columns), and 0.7 mg/mL (white columns). The final protein concentration for LDL was 0.04 mg/mL (black columns), 0.02 mg/mL (hatched columns), and 0.004 mg/mL (white columns). HDL and delipidated HDL was 0.2 mg/mL (black columns), 0.1 mg/mL (hatched columns), and 0.02 mg/mL (white columns). The amount of proteinase K–treated HDL was estimated according to the protein concentration before proteolysis and was similar to untreated HDL. Results are expressed as percentage, 100% being the production of IL-1β or TNF-α measured in the absence of inhibitor (mean ± SD, n = 3).
Apo A-I is the HS inhibitor of T-cell signaling of monocytes
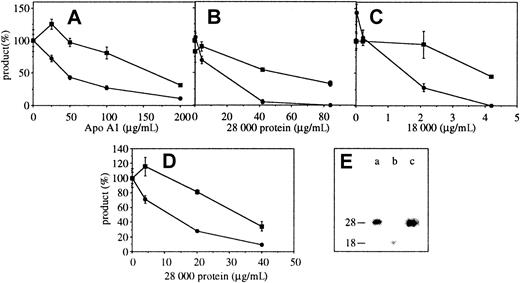
To determine whether apo A-I would display the inhibitory activity, various preparations were tested. Commercially available apo A-I inhibited the production of IL-1β and to a lesser extent TNF-α HP-1 cells activated by membranes of HUT-78 cells in a dose-dependent manner (Figure 5A), suggesting that apo A-I displayed the inhibitory activity. However, because apo A-I preparation contained 3% unidentified contaminants (according to the supplier) other preparations of apo A-I were required to confirm this result. Proteins from delipidated HDL were subjected to preparative SDS-PAGE. After copper-staining, bands (Mr: 56 000-66 000, 50 000, 28 000, and 18 000) were excised and electroeluted. The inhibitory activity was recovered in 28 000 and 18 000 bands, which inhibited the production of both IL-1β and TNF-α (Figure 5B,C). All inhibitory fractions contained apo A-I as demonstrated by Western blot analysis (Figure 5E), establishing that apo A-I was the inhibitor of T-cell signaling of monocytes. Indeed, it is very unlikely that another HDL apolipoprotein should display the same characteristics as apo A-I in terms of size of protein and proteolytic fragment (Figure 5E, lanes a and b). Alternatively, proteins from delipidated HDL were subjected to gel filtration on Superdex S75. Pooled fractions corresponding to Mr = 28 000 ± 10 000 displayed the inhibitory activity (Figure 5D). These fractions contained apo A-I as determined by Western blot analysis (Figure 5E, lane c), providing additional proof that apo A-I was the inhibitor.
Apo A-I inhibits the production of TNF-α and IL-1β in THP-1 cells activated by membranes of stimulated HUT-78 cells.
(A) THP-1 cells were activated by membranes of stimulated HUT-78 cells in the presence of increasing concentrations of Apo A-I purchased from Sigma. After 48 hours, TNF-α and IL-1β were measured in culture supernatants. Results represent mean ± SD, n = 3. (B,C) THP-1 cells were activated by membranes of stimulated HUT-78 cells in the presence of increasing concentrations of proteins electroeluted from preparative SDS-PAGE of delipidated HDL: panel B, Mr = 28 000, and panel C, Mr = 18 000. (D) THP-1 cells were activated by membranes of stimulated HUT-78 cells in the presence of increasing concentrations of apo A-I (Mr = 28 000) isolated by gel filtration on Superdex S75. After 48 hours, TNF-α (closed squares) and IL-1β (closed circles) were measured in culture supernatants. Results represent mean ± SD, n = 3, 100% being the amount of cytokine produced in the absence of inhibitor. (E) Isolated fractions that were tested for inhibitory activity were analyzed for their contents in apo A-I by Western blot: (a) 28 000 electroeluted band tested in panel B, (b) 18 000 electroeluted band tested in panel C, and (c) 28 000 protein recovered from Superdex S75 gel filtration and tested in panel D.
Apo A-I inhibits the production of TNF-α and IL-1β in THP-1 cells activated by membranes of stimulated HUT-78 cells.
(A) THP-1 cells were activated by membranes of stimulated HUT-78 cells in the presence of increasing concentrations of Apo A-I purchased from Sigma. After 48 hours, TNF-α and IL-1β were measured in culture supernatants. Results represent mean ± SD, n = 3. (B,C) THP-1 cells were activated by membranes of stimulated HUT-78 cells in the presence of increasing concentrations of proteins electroeluted from preparative SDS-PAGE of delipidated HDL: panel B, Mr = 28 000, and panel C, Mr = 18 000. (D) THP-1 cells were activated by membranes of stimulated HUT-78 cells in the presence of increasing concentrations of apo A-I (Mr = 28 000) isolated by gel filtration on Superdex S75. After 48 hours, TNF-α (closed squares) and IL-1β (closed circles) were measured in culture supernatants. Results represent mean ± SD, n = 3, 100% being the amount of cytokine produced in the absence of inhibitor. (E) Isolated fractions that were tested for inhibitory activity were analyzed for their contents in apo A-I by Western blot: (a) 28 000 electroeluted band tested in panel B, (b) 18 000 electroeluted band tested in panel C, and (c) 28 000 protein recovered from Superdex S75 gel filtration and tested in panel D.
HDL interact with stimulated T cells through apo A-I S
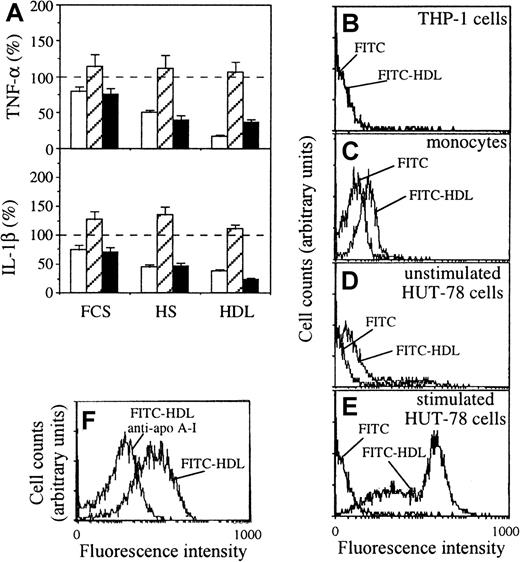
To establish whether the inhibitory activity of HDL-associated apo A-I could be due to binding to stimulated T cells or THP-1 cells, either membranes isolated from stimulated HUT-78 cells or THP-1 cells were preincubated in the presence or absence of FCS, HS, or isolated HDL. After washing, the residual activating capacity of membranes from stimulated HUT-78 cells was assessed on THP-1 cells. The inhibition of TNF-α and IL-1β production was observed only when membranes of stimulated HUT-78 cells were incubated with HS or HDL but not when THP-1 cells were incubated with either FCS, HS, or HDL (Figure6A). Consequently the inhibitory activity of HS and HDL was mainly directed to the activating factor(s) expressed at the surface of stimulated T cells.
Analysis of HDL binding to cells.
(A) Inhibition of T-cell signaling by binding of HDL to membranes of stimulated HUT-78 cells; either membranes of stimulated HUT-78 cells (white columns), THP-1 cells (hatched columns), or both (black columns) were preincubated in the absence (−) or presence of FCS (10%), HS (10%), or HDL (0.32 mg/mL protein) for 45 minutes on ice; after washing treated (hatched and black columns) and untreated (white columns) THP-1 cells were cultured in the presence of treated (white and black columns) or untreated (hatched columns) membranes of stimulated HUT-78 cells; TNF-α (top) and IL-1β (bottom) production was measured in 48-hour culture supernatants. Results are expressed as percentage, 100% (dashed line) being the production of IL-1β or TNF-α measured in the absence of inhibitor, mean ± SD, n = 6. (B-F) Binding of unconjugated FITC and FITC-HDL (0.1 mg/mL) was assessed by flow cytometry on THP-1 cells (B), isolated human monocytes (C), unstimulated HUT-78 cells (D), and stimulated HUT-78 cells (E). FITC was used as a negative control. (F) Binding of FITC-HDL (10 μg/mL) to stimulated HUT-78 cells in the presence or absence of purified anti–apo A-I antibodies (100 μg/mL).
Analysis of HDL binding to cells.
(A) Inhibition of T-cell signaling by binding of HDL to membranes of stimulated HUT-78 cells; either membranes of stimulated HUT-78 cells (white columns), THP-1 cells (hatched columns), or both (black columns) were preincubated in the absence (−) or presence of FCS (10%), HS (10%), or HDL (0.32 mg/mL protein) for 45 minutes on ice; after washing treated (hatched and black columns) and untreated (white columns) THP-1 cells were cultured in the presence of treated (white and black columns) or untreated (hatched columns) membranes of stimulated HUT-78 cells; TNF-α (top) and IL-1β (bottom) production was measured in 48-hour culture supernatants. Results are expressed as percentage, 100% (dashed line) being the production of IL-1β or TNF-α measured in the absence of inhibitor, mean ± SD, n = 6. (B-F) Binding of unconjugated FITC and FITC-HDL (0.1 mg/mL) was assessed by flow cytometry on THP-1 cells (B), isolated human monocytes (C), unstimulated HUT-78 cells (D), and stimulated HUT-78 cells (E). FITC was used as a negative control. (F) Binding of FITC-HDL (10 μg/mL) to stimulated HUT-78 cells in the presence or absence of purified anti–apo A-I antibodies (100 μg/mL).
To confirm that the inhibitory factor(s) interacted with surface factors on stimulated T cells, isolated HDL was labeled with FITC, and its binding to different cell types was assessed by flow cytometry. No binding of FITC-HDL was observed on THP-1 cells (Figure 6B), whereas fluorescence of monocytes was slightly enhanced when incubated with FITC-HDL as compared to unconjugated FITC control (Figure 6C). A low level of binding of FITC-HDL to unstimulated HUT-78 cells was observed, whereas stimulated HUT-78 cells bound FITC-HDL and displayed 2 fluorescent peaks suggesting the presence of at least 2 different HDL binding sites (Figure 6D,E). At a lower FITC-HDL concentration, a single fluorescent peak was observed. In the presence of anti–apo A-I antibodies, a shift toward lower fluorescence intensity was observed, demonstrating that HDL interacted with stimulated T cells via apo A-I–specific binding (Figure 6F). Together these results show that HDL interacted preferentially with stimulated T cells, implying that the inhibitory activity of apo A-I was directed to surface factors on T cells.
Optimal inhibition of mRNA steady-state required the addition of apo A-I simultaneously or shortly after the stimulus
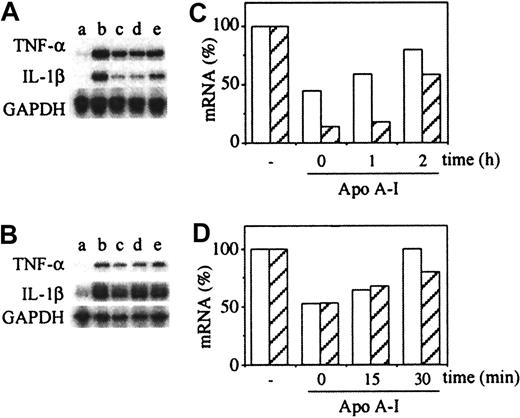
To further elucidate the mechanism of action of apo A-I the inhibitory effect of isolated apo A-I on steady-state levels of TNF-α and IL-1β mRNA was assessed. THP-1 cells were stimulated with membranes of stimulated HUT-78 cells in the presence of apo A-I added at the indicated time. Apo A-I diminished the steady-state levels of TNF-α and IL-1β mRNA in THP-1 cells activated with membranes of stimulated HUT-78 cells (Figure 7A,C). The inhibition of TNF-α mRNA was less pronounced than that of IL-1β mRNA, correlating with the data obtained at the level of protein production. To achieve optimal inhibition of cytokine mRNA steady-state levels apo A-I had to be added simultaneously or shortly after activation by membranes. Similar results were observed with peripheral blood monocytes activated by membranes of stimulated T lymphocytes (Figure 7B,D) in which steady-state levels of both TNF-α and IL-1β mRNAs were diminished by apo A-I, although mRNA induction set in more rapidly in monocytes than in THP-1 cells. On monocytes inhibition of mRNA steady-state levels was also optimal when apo A-I was added together with membranes or shortly after (Figure 7D). No inhibition was observed when apo A-I was added after 30 minutes of activation. The latter results demonstrate that apo A-I inhibited contact-mediated activation of monocytes regardless of the cell type, confirming the data in Figure 2.
Apo A-I decreases the steady-state levels of TNF-α and IL-1β mRNA.
(A,B) Autoradiogram of RNAse protection assay. (A) THP-1 cells (5 × 106 cell/mL) untreated (a) or activated by membranes of stimulated HUT cells (200 μg protein/mL) during 3 hours (b-e) in the presence or absence of apo A-I (200 μg/mL) (c-e), which was added at different time points of THP-1 activation: c (0 hours); d (1 hour); and e (2 hours). (B) Monocytes (10 × 106cell/ml) untreated (a) or activated by membranes of stimulated T lymphocytes (40 μg protein/mL) during 1 hour (b-e) in the presence or absence of apo A-I (200 μg/mL) (c-e), which was added at different time points of THP-1 activation: c (0 minute); d (15 minutes); and e (30 minutes). (C,D) Densitometric analysis of autoradiography A and B, respectively, normalized with the densitometry of GAPDH mRNA = 1, and expressed as percentage, 100% being the mRNA level of activated THP-1 cells (C) or monocytes (D) in the absence of inhibitor. TNF-α (white columns); IL-1β (hatched columns). Results are representative of 3 different experiments.
Apo A-I decreases the steady-state levels of TNF-α and IL-1β mRNA.
(A,B) Autoradiogram of RNAse protection assay. (A) THP-1 cells (5 × 106 cell/mL) untreated (a) or activated by membranes of stimulated HUT cells (200 μg protein/mL) during 3 hours (b-e) in the presence or absence of apo A-I (200 μg/mL) (c-e), which was added at different time points of THP-1 activation: c (0 hours); d (1 hour); and e (2 hours). (B) Monocytes (10 × 106cell/ml) untreated (a) or activated by membranes of stimulated T lymphocytes (40 μg protein/mL) during 1 hour (b-e) in the presence or absence of apo A-I (200 μg/mL) (c-e), which was added at different time points of THP-1 activation: c (0 minute); d (15 minutes); and e (30 minutes). (C,D) Densitometric analysis of autoradiography A and B, respectively, normalized with the densitometry of GAPDH mRNA = 1, and expressed as percentage, 100% being the mRNA level of activated THP-1 cells (C) or monocytes (D) in the absence of inhibitor. TNF-α (white columns); IL-1β (hatched columns). Results are representative of 3 different experiments.
Apo A-I inhibits TNF-α and IL-1β production
in antigen-activated PBMC
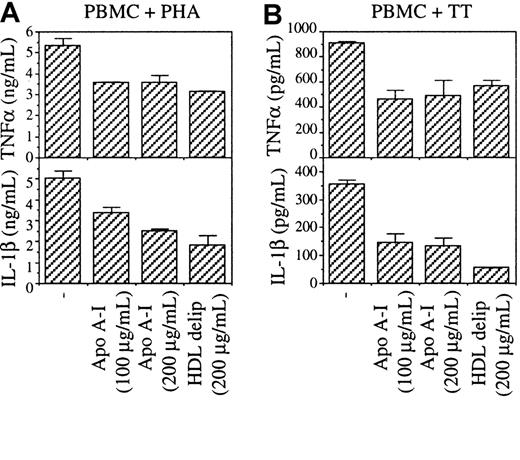
To confirm that apo A-I was the factor responsible for the inhibition of cytokine production in PBMC stimulated by PHA, PBMC were stimulated by PHA or tetanus toxoid (TT) in the presence or absence of apo A-I and delipidated HDL. Although cytokine production was lower in TT-stimulated than in PHA-stimulated PBMC, in both conditions TNF-α and IL-1β production was inhibited by either apo A-I or delipidated HDL (Figure 8). TNF-α production was inhibited to a lesser extent than that of IL-1β, confirming results of Figure 1. This result demonstrates that (1) the inhibition of cytokine production by unfractionated HS depicted in Figure 1 was due to apo A-I, and (2) that apo A-I inhibits monocyte activation regardless of the T-cell stimulus suggesting that antigen-specific and mitogen stimulation induced similar activating factors on T lymphocytes, although resulting in different levels of expression. Furthermore, these results confirm that contact between monocytes and stimulated T cells was required for the induction of TNF-α and IL-1β in PBMC.
Apo A-I inhibits TNF-α and IL-1β in PBMC stimulated by either PHA or TT.
PBMC (4 × 105 cells/200 μL/well) were stimulated by (A) 1 μg/mL PHA or (B) 10 μg/mL TT in the presence of the indicated doses of apo A-I and HDL. Delip indicates delipidated.
Apo A-I inhibits TNF-α and IL-1β in PBMC stimulated by either PHA or TT.
PBMC (4 × 105 cells/200 μL/well) were stimulated by (A) 1 μg/mL PHA or (B) 10 μg/mL TT in the presence of the indicated doses of apo A-I and HDL. Delip indicates delipidated.
Discussion
The present study reveals a new anti-inflammatory activity elicited by apo A-I, a known “negative” acute-phase protein. The inhibitory activity was absent in FCS and CBS that contain low amounts of apo A-I.28 Although the mechanism of action of apo A-I is not fully elucidated, the present results suggest that apo A-I exerts most of its inhibitory activity by specifically blocking the interaction between T cells and monocytes. The blockade or inhibition of TNF-α and IL-1 in vivo has proved successful in treating human diseases such as rheumatoid arthritis, Crohn disease, and other immunoinflammatory disorders.29-35 Therefore, blocking the production of these cytokines at a more distal level by apo A-I (ie, at the level of monocyte activation) may constitute a new therapeutic approach.
The inhibitory activity of apo A-I seems to be specifically directed to T-cell signaling of monocytes because activation of THP-1 cells by other stimuli (LPS and PMA) was not affected by HS. However, in peripheral blood monocytes stimulated by LPS the inhibition of IL-1β and TNF-α production was slight (20%-30%) in the presence of 200 μg/mL apo A-I (ie, in the presence of LPS, the production of IL-1β and TNF-α was 1.8 ± 0.1 ng/mL and 2.6 ± 0.2 ng/mL, respectively; in the presence of apo A-I the LPS-induced production of the latter was 1.5 ± 0.1 ng/mL and 2.3 ± 0.2 ng/mL, respectively). In the same experiment, cytokine production induced by membranes of stimulated HUT-78 cells was inhibited by about 75% (ie, in the presence of membranes of stimulated HUT-78 cells, the production of IL-1β and TNF-α was 3.1 ± 0.5 ng/mL and 6.0 ± 0.4 ng/mL, respectively; in the presence of apo A-I the contact-induced production of the latter was 0.7 ± 0.1 ng/mL and 1.6 ± 0.2 ng/mL, respectively). Numerous studies have shown that HDL exhibit anti-inflammatory functions in endotoxemia in vivo and in vitro.36,37 Indeed, LPS-binding protein transfers LPS to HDL through binding to apo A-I.38-40 Once bound to HDL, LPS activity is partially inhibited. Thus, HDL exert anti-inflammatory functions in 2 different systems of monocyte activation, that is, by LPS and by cellular contact. However, the inhibition of contact activation of monocytes seems to be more efficient than that induced by LPS. Indeed, in whole blood assays carried out under LPS-free, sterile conditions, IL-1β production was detected only when LPS was added to the blood; PHA or bovine tuberculin purified protein derivative only induced small amounts of TNF-α and interferon-γ in T lymphocytes.17
The present data demonstrate that HDL interact with stimulated T cells via binding of apo A-I. A specific HDL binding site on human lymphocytes has been described but not identified.41Together with the premise that apo A-I is a major player in the reverse transport of cholesterol, this suggests that apo A-I may disturb the lipid organization of a raft membrane microdomain containing the activating factor(s),42,43 thus abolishing the activating capacity of stimulated T cells. The latter hypothesis is, however, very unlikely because contact-mediated activation of monocytes by paraformaldehyde-fixed, stimulated T cells was efficiently inhibited by HS. According to flow cytometry analyses (Figure 6), HDL also bind monocytes in accordance with data showing the binding of HDL (HDL3) to monocytes in PBMC.24 It is therefore possible that HDL also affect monocyte activation by directly modulating the level of activation of the latter cells. Indeed, when added shortly after the stimulus apo A-I still decreased cytokine mRNA steady-state levels, although to a lower extent than when added at t = 0 (Figure 7). Although this might be due to the premise that membranes of stimulated T cells deliver sustained activation signals, it does not discard the possibility of a direct effect of the inhibitor on monocytes/THP-1 cells. Despite the fact that HDL binding or functional activity was not observed on resting THP-1 cells, the latter may express HDL receptors once differentiated (activated).44 Therefore, the present study demonstrates that HDL-associated apo A-I can inhibit the production of proinflammatory cytokines on activation of monocytes by contact with stimulated T cells through 2 different pathways: (1) the main pathway consisting of the blockade of the binding of the T cell activating factor to its receptor on monocytes, thus inhibiting the expression of both TNF-α and IL-1β; and (2) another pathway involving direct effects of HDL-associated apo A-I on monocytes through the binding to a receptor. Although the latter pathway remains to be demonstrated, these dual effects may account in part for the discrepancy in the extents of inhibition of TNF-α and IL-1β production in THP-1 cells.
No interaction has been described between apo A-I or HDL and the different surface molecules that have been claimed to be involved in T-cell signaling of monocytes. It is unlikely that CD40L should be the apo A-I ligand because this molecule is not detected on either HUT-78 cells. Furthermore, blocking antibodies to CD40L (5C8, a kind gift from Dr P. E. Lipsky, University of Texas, Dallas, TX) failed to inhibit contact-mediated activation of monocytes, whereas in the same experiment apo A-I inhibited 85% to 90% of cytokine production (unpublished data, January 2000). An assumption is that apo A-I ligand belongs to a family of HDL plasma membrane receptors.45However, because HDL receptor proteins such as SR-BI or CD36 are not specific for a particular apolipoprotein (ie, they interact with apo A-I, apo A-II, apo C, HDL, and LDL45,46) and because LDL do not display inhibitory activity toward T-cell signaling of monocytes, it is unlikely that apo A-I ligand(s) on stimulated T cells belong(s) to this HDL receptor family. On the other hand, specific HDL-binding proteins (HB1 and HB2) have been described that are expressed in rat liver plasma membrane and human blood monocytes.24 HB2 is homologous with ALCAM, a cell-adhesion molecule belonging to the immunoglobulin superfamily,45 whose function has not been clearly elucidated. Because apo A-I is involved in the binding of HDL to HB2 it is possible that the HDL receptor on stimulated T cells displays some homology with the HB2 protein family. Recently, an epithelial protein (cubilin) was shown to display high affinity for apo A-I.47 Whether the latter 460-kd protein and HB2 are expressed in stimulated T lymphocytes remains to be determined. The identification of HDL-associated apo A-I receptor(s) on stimulated T cells may lead to the elucidation of the mechanism of action of the inhibitor of T-cell signaling of monocytes.
Apo A-I inhibits contact-mediated monocyte activation by stimulated T cells or T lymphocytes stimulated by various stimuli, eg,antigen-specific (TT), PHA, or a mixture of both PHA and PMA. This suggests that similar factors interacting with apo A-I are induced at the surface of stimulated T cells by either stimuli. Furthermore, it is likely that similar activating factors are expressed by different types of T cell, freshly isolated T lymphocytes, or HUT-78 cells, because apo A-I displays inhibitory activity in both systems. However, because the levels of activation of monocytes differ as a function of the stimulus,4,7 8 the expression levels of the activating factors may differ as a function of the type of T cells and their stimuli.
The inhibition of T-cell signaling of monocytes might be important in maintaining a low level of monocyte activation within the bloodstream, although static conditions used in this study might not reflect shear stress induced by blood flow. However, variations of apo A-I concentration were observed in systemic lupus erythematosus, an inflammatory disease of autoimmune etiology,48 in which apo A-I plasma concentrations were diminished. This decrease was associated with the presence of anti–apo A-I antibodies in 32% of patients.49 Recently, it was shown that the inflammatory condition in juvenile rheumatoid arthritis was associated with hypo-high density lipoproteinemia50 and a significant decrease in apo A-I concentration in patient plasma. In rheumatoid arthritis, the levels of circulating apo A-I and HDL cholesterol in untreated patients are lower than in normal controls.51-53In contrast, apo A-I is enhanced in synovial fluid of patients with rheumatoid arthritis,54 although its concentrations remained 10-fold lower in synovial fluid than in plasma. The elevation of apo A-I levels in synovial fluid of patients with rheumatoid arthritis was accompanied by an enhancement in cholesterol, suggesting an infiltration of HDL particles in the inflamed joint. This putative regulatory mechanism might, however, be overpowered by serum amyloid A (SAA), a positive acute-phase protein that is produced in the rheumatoid arthritis synovium.55,56 Indeed, SAA can displace apo A-I from HDL, and HDL-associated SAA displays proinflammatory activity.57 58
Increasing evidence strongly supports the contention that inflammatory responses are an integral part of atherosclerosis.59Indeed, monocyte-macrophages and T lymphocytes are present at all stages of lesion development, and the earliest lesion (fatty streak) is composed predominantly of macrophages and T lymphocytes.60Therefore, T lymphocyte-signaling of monocytes may occur in atherosclerosis. Gene transfer of apo A-I reduces atherosclerosis in several mouse models.61,62 This was usually attributed to the function of HDL-associated apo A-I in lipid metabolism and transport. However, our study suggests that HDL fulfill protective functions at several levels in atherosclerosis including the decrease in contact-mediated monocyte activation by T lymphocytes. Furthermore, the premise that the incidence of atherosclerotic heart disease is higher in patients with systemic lupus erythematosus and rheumatoid arthritis63 is in agreement with the inverse correlation of the concentration of HDL with the incidence of atherosclerosis.
In conclusion, we have identified a novel anti-inflammatory function for HDL-associated apo A-I. This may be a general mechanism of protection against the activation of monocytes in inflammatory conditions when stimulated T lymphocytes are found in the bloodstream, as well as an important counterregulatory mechanism to macrophage activation by leakage of plasma proteins into the inflamed tissue. A new concept emerging from the present results is the importance of “negative” acute-phase proteins such as apo A-I as anti-inflammatory molecules able to block T-cell signaling of macrophages and thus to inhibit TNF-α and IL-1β production. Because HDL are also able to inhibit cytokine production triggered by LPS in monocytes, this study demonstrates that HDL display anti-inflammatory functions in both acute and chronic inflammation aiming at diminishing the extent of monocyte activation.
We thank Mrs M.-T. Kaufmann and Dr Hsien-Sen Lu for skillful technical assistance, Drs R. Rezzonico, C. Chizzolini, and R. W. James for advice and discussions.
Division of Immunology and Allergy (Hans Wilsdorf Laboratory), Department of Internal Medicine, University Hospital, Genève, Switzerland; and Amgen, Thousand Oaks, CA.
Submitted August 25, 2000; accepted December 21, 2000.
Supported in part by a grant from the Swiss National Science Foundation (no. 31-50930-97), the Hans Wilsdorf Foundation, and a grant from the Swiss Society for Multiple Sclerosis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Danielle Burger, Clinical Immunology Unit, University Hospital, 24 rue Micheli-du-Crest, CH-1211 Genève 14, Switzerland; e-mail: danielle.burger@hcuge.ch.

![Fig. 3. Superdex 200 elution profile and SDS-PAGE analysis of serial chromatography fractionation of HS. / (A) Inhibitory fractions eluted from Phenyl Sepharose HP were pooled, concentrated, and subjected to gel filtration on Superdex 200 equilibrated in PBS. The column was calibrated with the molecular weight marker kit for gel filtration chromatography (Sigma). Fractions were tested for their protein contents (optical density [OD] 280 nm, dashed line) and inhibitory activity (closed circles). (B) Inhibitory fractions were pooled after each step and 10 μg protein aliquots were loaded per lane on 10% acrylamide gel: (a) HS; (b) breakthrough of Blue-Sepharose; (c) pool of inhibitory fractions from Q Sepharose; (d) pool of inhibitory fractions from Phenyl Sepharose HP; and (e) pool of inhibitory fractions from Superdex 200; the gel was stained with Coomassie blue.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/8/10.1182_blood.v97.8.2381/5/m_h80810884003.jpeg?Expires=1769153811&Signature=hnz7XjvEInWHibmyC1c6Af23JC6AJHRcMGdhsZWIzZLmJinKpi6SgZbNb5TqshslHJDAqDYJYCyFkOi-a3RH9EzSi8yXWxe-H7M0iQgMZBgsM7EbwTxJM1K2iB-ILPZcUJsgesde9wRUJRk501v4ImUS3gFwAZCbB~cSXbBxXa8ycY4FTFCulSD-G5OYQXfHkqLCW--c4FrWXnJs0bqdcNmw-6EIABj1ZTwh2jYUHlHcu1fXLQuIrWlOt5TuutUvHPMcSd3-ogpvgHe6ttRjdpGQmCeD6psA3pvk6GL1TI4PdsBhPa1mrHcx91Jf0Azma9vuncog3PPTCUlAF4WqGg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal