Abstract
Kaposi sarcoma–associated herpesvirus (KSHV) is associated with KS, primary effusion lymphoma (PEL), and multicentric Castleman disease. Reactivation of KSHV in latently infected cells and subsequent plasma viremia occur before the development of KS. Intracellular signaling pathways involved in KSHV reactivation were studied. In latently infected PEL cells (BCBL-1), KSHV reactivation in single cells was determined by quantitative flow cytometry. Viral particle production was determined by electron microscope analyses and detection of minor capsid protein in culture supernatants. Agents that mobilized intracellular calcium (ionomycin, thapsigargin) induced expression of KSHV lytic cycle-associated proteins and led to increased virus production. Calcium-mediated virus reactivation was blocked by specific inhibitors of calcineurin-dependent signal transduction (cyclosporine, FK506). Similarly, calcium-mediated virus reactivation in KSHV-infected dermal microvascular endothelial cells was blocked by cyclosporine. Furthermore, retroviral transduction with plasmid DNA encoding VIVIT, a peptide specifically blocking calcineurin-NFAT interactions, inhibited calcium-dependent KSHV reactivation. By contrast, chemical induction of lytic-phase infection by the phorbol ester 12-O-tetradecanoyl-phorbol-13-acetate was blocked by protein kinase C inhibitors, but not by calcineurin inhibitors. In summary, calcineurin-dependent signal transduction, an important signaling cascade in vivo, induces calcium-dependent KSHV replication, providing a possible target for the design of antiherpesvirus strategies in KSHV-infected patients.
Introduction
Infection with the γ-herpesvirus Kaposi sarcoma–associated herpesvirus (KSHV), also known as human herpesvirus 8 (HHV-8), is causally involved in KS, primary effusion lymphoma (PEL), and some forms of multicentric Castleman disease.1-5 The detection of KSHV in peripheral blood leukocytes is strongly predictive of the future development of KS.6-9 Circulating B cells form the major virus reservoir in blood,10-13 though KSHV has been found in monocytes, CD8+ T cells, and CD34+ cells.11,14-16 Active replication in blood and subsequent plasma viremia likely disseminate the virus throughout the body, leading to infection of dermal lymphatic endothelial cells, which are believed to be precursor cells for KS.5 Furthermore, in KS lesions, tumor spindle cells are latently infected by KSHV; a small subpopulation of lytically infected cells produces virus and lytic viral gene products, which may contribute to the maintenance or progression of KS lesions. In general, understanding the pathways that activate virus replication (latent-to-lytic switch), in blood as well as in KS lesions, is an important issue in fully understanding KS pathogenesis.
In experimental systems to date, KSHV infection of primary B cells in vitro has been inefficient and unstable.13,17 However, latently infected B cell lines, derived from patients with PEL, and infected immortalized endothelial cells are useful tools to study KSHV reactivation.18-23 Expression of the KSHV lytic cycle-associated immediate-early ORF 50 protein (Rta) is sufficient to induce the entire lytic cycle of active viral replication.24 Ionomycin, a Ca++-ionophore, is known to induce expression of the ORF 50 protein.25 26This suggests that calcium-dependent signaling pathways may play a role in virus reactivation.
In the immune system, calcium signaling is essential for the expression of many inducible genes encoding cytokines and cell surface receptors.27,28 One of the enzymes activated by a sustained rise in [Ca++]i is calcineurin, a ubiquitously expressed serine-threonine phosphatase. Activation of the Ca++-calcineurin signaling cascade is triggered by the engagement of T and B cell antigen receptors, Fc receptors, and receptors coupled to certain heterotrimeric G proteins.28Drugs that inhibit calcineurin—eg, cyclosporine (CsA) and tacrolimus (FK506)—are in clinical use to prevent the rejection of transplanted organs or for control of autoimmune disorders.29 Earlier studies showed involvement of calcineurin in Ca++-dependent reactivation of Epstein-Barr virus (EBV),30 another member of the γ-herpesvirus family. Here, we investigated whether calcineurin-dependent signaling is involved in KSHV reactivation.
Materials and methods
Cell lines and reagents
KSHV-positive/EBV-negative PEL cell line BCBL-120(NIH AIDS Research and Reagent Program, Rockville, MD) was grown in RPMI 1640 medium (Gibco BRL, Gaithersburg, MD) containing 10% (vol/vol) fetal bovine serum (Gibco), 2 mM l-glutamine (Gibco), 100 U/mL penicillin (Gibco), 100 μg/mL streptomycin (Gibco), 10 mM HEPES (Gibco), and 5 × 10−5 M 2-mercaptoethanol (Sigma Chemical, St Louis, MO). Immortalized human dermal microvascular endothelial cells (DMVEC), generated as described,23 were cultured in endothelial-SFM medium (Gibco) supplemented with 10% human AB serum (Sigma), 100 U/mL penicillin (Gibco), 100 μg/mL streptomycin (Gibco), 2 mM l-glutamine (Gibco), 25 μg/mL endothelial cell growth supplement (Becton Dickinson, Bedford, MA), 40 μg/mL heparin (Sigma), and 200 μg/mL G418 (Gibco). Ionomycin, thapsigargin, cyclosporine, polybrene and 12-O-tetradecanoyl-phorbol-13-acetate (TPA) were obtained from Sigma. FK506 was provided by Fujisawa Healthcare (Deerfield, IL). Bisindolylmaleimide I (BIM) and staurosporine (SS) were purchased from Calbiochem (La Jolla, CA). Anti-KSHV monoclonal antibodies (mAbs) anti–PF-8 and anti-gpK8.1 were provided by Dr B. Chandran (University of Kansas Medical Center, Kansas City, KS) and Dr B. Forghani (California Department of Health Services, Berkeley, CA). Enhanced green fluorescence protein (EGFP) and EGFP-VIVIT expression plasmids were kindly given by Dr A. Rao (Harvard Medical School, Cambridge, MA).
Analysis of KSHV lytic protein expression by flow cytometry
BCBL-1 cells were seeded into culture flasks (2-3 × 105 cells/mL) and grown for 24 hours. Then inducers were added, and cells were cultured for another 1 to 4 days. Flow cytometric analysis of early- and late-lytic KSHV expression in viable single cells was performed as described previously.22 Briefly, cells were suspended in phosphate-buffered saline (PBS) containing 0.1% (wt/vol) bovine serum albumin (BSA) and 0.05% (wt/vol) NaN3. Dead cells were stained with Dead Red (Live/Dead kit; Molecular Probes, Eugene, OR) for 20 minutes at 4°C, and cells were washed 3 times. Cells were fixed and permeabilized using the Cytofix/Cytoperm kit (Pharmingen, San Diego, CA) and washed with Permwash buffer (Pharmingen) containing 2% (vol/vol) human AB+ serum (Sigma). Cells were labeled with mouse monoclonal antibodies (1 μg/mL) directed against KSHV22 for 30 minutes at 4°C, followed by incubation with fluorescein isothiocyanate (FITC)- or phycoerythrin-labeled goat antimouse IgG (Caltag Laboratories, Burlingame, CA). Cells were washed 3 times, resuspended in PBS containing 0.1% (wt/vol) bovine serum albumin and 0.05% (wt/vol) NaN3, and analyzed for fluorescence on a FACScan flow cytometer (Becton Dickinson, Mountain View, CA). Dead cells were identified by Dead Red (Molecular Probes) fluorescence and excluded from all analyses.
Retroviral transduction
Expression plasmids encoding EGFP or the EGFP-VIVIT fusion protein are described.31 DNA encoding EGFP or EGFP-VIVIT was ligated into the retroviral vector pCLNCX, kindly provided by Dr P. Robbins (National Cancer Institute, Bethesda, MD). 293-GP cells were transfected in RPMI containing the various retroviral vectors together with an expression plasmid encoding the VSV envelope (pMDG), using lipofectamine for 3 to 4 hours at 37°C. Medium was then replaced by RPMI containing 10% fetal bovine serum, and, after an additional 48 hours, supernatants (R-sup) were collected, filtered (0.45 μM), and diluted 1:2 with culture medium. For transduction experiments, BCBL-1 cells (750 000 cells/mL) were incubated overnight with R-sup in the presence of 8 μg/mL polybrene. Cells were washed and further cultured in standard RPMI medium (see “Cell lines and reagents”). After an additional 24 hours, 10% to 15% of the cells were green fluorescent, as determined by flow cytometry. Similar transduction efficiencies and levels of green fluorescence were obtained using either the EGFP or the EGFP-VIVIT retroviral construct. Positively transduced cells were further selected by adding 500 μg/mL G418 to the culture medium.
Transmission electron microscopy
Cells were fixed overnight in neutral buffered 2.5% (vol/vol) glutaraldehyde, mixed, and pelleted in warm agar, which was then cooled overnight to harden. The cells were post-fixed in 1% OsO4, dehydrated in graded ethanol and propylene oxide, and embedded in Spurr epoxy. Semithin 1-μm plastic sections were stained with methylene blue, azure II, and basic fuchsin for light microscopic selection of blocks for thinning. Thin sections were stained with uranyl acetate and lead citrate and examined on an electron microscope at 60 kV. Samples were blinded, and the number of cells showing ultrastructural characteristics of virion formation was scored.
Analysis of virus production by Western blot
As described,32 cell culture supernatants were collected, and the virus was pelleted by ultracentrifugation (100 000g, 1 hour). Virus pellets were solubilized in 40 μL sodium dodecyl sulfate sample buffer containing dithiothreitol (50 mM) and heated at 70°C for 10 minutes. Equal volumes were applied to 10% to 14% polyacrylamide gels and subjected to electrophoresis (Novex, San Diego, CA). Proteins were transferred to nitrocellulose, and the resultant blots were probed with affinity-purified rabbit polyclonal antibody to the KSHV minor capsid protein (mCP) followed by alkaline phosphatase-conjugated goat antirabbit IgG (Promega, Madison, WI). Protein bands were visualized with Western blue substrate for alkaline phosphatase (Promega). A purified virus preparation (Advanced Biotechnologies, Columbia, MD) was used as a positive control.
Immunofluorescence assay for KSHV protein expression in DMVEC
Immunofluorescence assays were performed as described previously.23 Briefly, DMVEC monolayers were fixed in 95% ethanol–5% glacial acetic acid, permeabilized with 0.5% Triton X-100, and blocked with 20% normal goat serum in PBS for 20 minutes. Monolayers were stained with anti–PF-8 mAb followed by FITC-conjugated goat antimouse secondary mAbs. For staining with anti-gpK8.1 mAbs, monolayers were fixed with 2% paraformaldehyde (pH 7.4) in PBS followed by blocking and staining essentially as described above. Finally, cells were mounted in SlowFade antifade reagent in 50% glycerol (Molecular Probes) and examined on a Nikon fluorescence microscope.
Statistical analysis
Data were analyzed statistically using the 2-tailed Student t test with the levels of significance indicated.
Results
Mobilization of intracellular calcium reactivates KSHV
in BCBL-1 cells
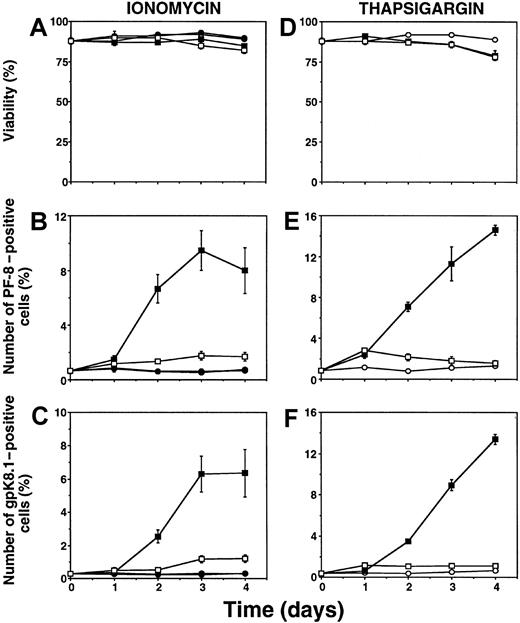
To quantitate KSHV lytic infection, the number of cells expressing KSHV lytic proteins was determined by flow cytometry. KSHV-positive BCBL-1 cells were incubated with calcium-mobilizing agents or TPA. At different time points, cells were fixed, permeabilized, and labeled with specific mAbs directed against the KSHV early lytic viral accessory protein PF-8 or the late-lytic viral envelope glycoprotein gpK8.1.22 Incubation with the Ca++-ionophore ionomycin (1 μg/mL) induced the expression of PF-8 (Figure1B) and gpK8.1 (Figure 1C). Similar results were obtained with 5 nM thapsigargin (Figure 1E-F), a compound that increases [Ca++]i by impeding its sequestration into intracellular calcium stores and by allowing additional entry of extracellular calcium through store-operated calcium channels in the plasma membrane.33-35 Addition of the Ca++-chelator ethylene glycol-bis(b-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA) dissipates the inward Ca++-gradient over the plasma membrane and prevents sustained rises in [Ca by Ca++-mobilizing agents. EGTA blocked the ability of ionomycin or thapsigargin to induce KSHV lytic protein expression (Figure 1). Conversely, EGTA did not prevent KSHV reactivation induced by the phorbol ester TPA (not shown). All agents used did not cause significant cellular toxicity (Figure 1A, D).
Ca++-mobilizing agents induce expression of KSHV lytic proteins in BCBL-1 cells.
Cells were incubated with 1 μg/mL ionomycin (A-C) or 5 nM thapsigargin (D-F) for several days. At various time points, cells were harvested, labeled with Dead Red, washed, fixed, permeabilized, and labeled with anti–PF-8 or anti-gpK8.1 mAbs followed by FITC-conjugated secondary Abs. Cells were examined for fluorescence by flow cytometry. The viable cell population was examined for PF-8 (B, E) or gpK8.1 (C, F) expression, as described previously.22 Unstimulated cells (○), 1 mM EGTA (●), ionomycin or thapsigargin (▪), ionomycin or thapsigargin + EGTA (■). Data represent mean ± SEM of at least 3 separate experiments.
Ca++-mobilizing agents induce expression of KSHV lytic proteins in BCBL-1 cells.
Cells were incubated with 1 μg/mL ionomycin (A-C) or 5 nM thapsigargin (D-F) for several days. At various time points, cells were harvested, labeled with Dead Red, washed, fixed, permeabilized, and labeled with anti–PF-8 or anti-gpK8.1 mAbs followed by FITC-conjugated secondary Abs. Cells were examined for fluorescence by flow cytometry. The viable cell population was examined for PF-8 (B, E) or gpK8.1 (C, F) expression, as described previously.22 Unstimulated cells (○), 1 mM EGTA (●), ionomycin or thapsigargin (▪), ionomycin or thapsigargin + EGTA (■). Data represent mean ± SEM of at least 3 separate experiments.
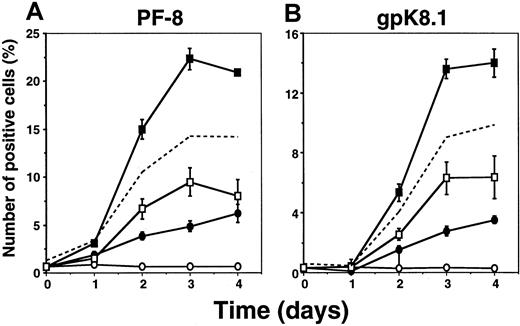
We also examined the relation between Ca++-dependent induction and induction by TPA. Incubation with ionomycin, in combination with a suboptimal dose of TPA (3 nM),22synergistically induced high numbers of cells expressing PF-8 (Figure2A) or gpK8.1 (Figure 2B). Synergistic effects on KSHV reactivation were also observed combining thapsigargin and TPA induction (not shown).
Synergy between Ca++-and TPA-dependent induction of KSHV lytic protein expression.
Viable BCBL-1 cells stimulated with 1 μg/mL ionomycin, 3 nM TPA, or both were examined for PF-8 (A) or gpK8.1 (B) expression. Unstimulated cells (○), TPA (●), ionomycin (■), TPA + ionomycin (▪). The dotted line with no symbols represents the sum of the number of positive cells induced by TPA (●) and the number of positive cells induced by ionomycin (■). Data represent mean ± SEM of at least 3 separate experiments.
Synergy between Ca++-and TPA-dependent induction of KSHV lytic protein expression.
Viable BCBL-1 cells stimulated with 1 μg/mL ionomycin, 3 nM TPA, or both were examined for PF-8 (A) or gpK8.1 (B) expression. Unstimulated cells (○), TPA (●), ionomycin (■), TPA + ionomycin (▪). The dotted line with no symbols represents the sum of the number of positive cells induced by TPA (●) and the number of positive cells induced by ionomycin (■). Data represent mean ± SEM of at least 3 separate experiments.
Specific inhibition of calcineurin blocks Ca++-mediated KSHV reactivation in BCBL-1 cells
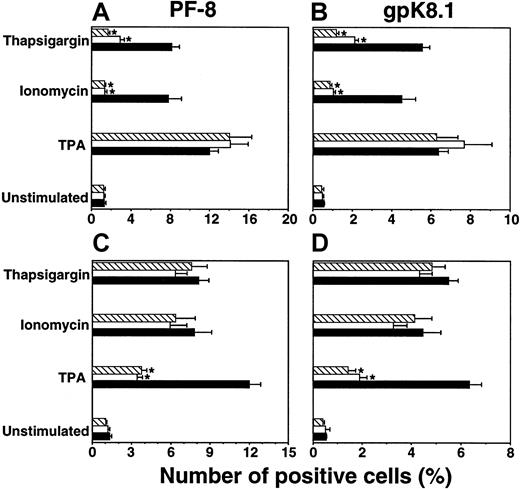
To determine the intracellular pathways involved in Ca++-dependent KSHV reactivation, the effects of specific pharmacologic inhibitors were investigated. Inhibiting agents were added 2 hours before inducers of KSHV reactivation. Three days after induction, cells were harvested and examined for lytic KSHV protein expression by flow cytometry. The calcineurin inhibitors CsA (0.5 μM) or FK506 (0.1 μM) prevented Ca++-dependent expression of PF-8 and gpK8.1 (Figure 3A,B). By contrast, TPA-induction was not affected by calcineurin inhibitors (Figure 3A-B). Instead, specific inhibition of TPA-mediated reactivation occurred with protein kinase C (PKC) inhibitors BIM (1 μM) or low-dose SS (10 nM), whereas no effects of these PKC inhibitors were observed on calcium-dependent induction (Figure 3C-D). Effects of all inhibitors used were not due to an increase in cellular toxicity (not shown).
Inhibition of calcineurin blocks Ca++-dependent induction of KSHV lytic protein expression.
BCBL-1 cells were stimulated with ionomycin (1μg/mL), thapsigargin (5 nM), or TPA (15 nM) in the presence of calcineurin or PKC inhibitors. After 3 days, viable cells (ie, Dead Red–negative cells) were examined for PF-8 (A, C) or gpK8.1 (B, D) expression. For experiments with calcineurin inhibitors (A, B): no inhibitors (▪), +0.5 μM CsA (■), +0.1 μM FK506 (▧). For experiments with PKC inhibitors (C, D): no inhibitors (▪), +10 nM SS (■), +1 μM BIM (▧). Data represent mean ± SEM of at least 3 separate experiments. *P < .01 compared to concurrent induction without inhibitor.
Inhibition of calcineurin blocks Ca++-dependent induction of KSHV lytic protein expression.
BCBL-1 cells were stimulated with ionomycin (1μg/mL), thapsigargin (5 nM), or TPA (15 nM) in the presence of calcineurin or PKC inhibitors. After 3 days, viable cells (ie, Dead Red–negative cells) were examined for PF-8 (A, C) or gpK8.1 (B, D) expression. For experiments with calcineurin inhibitors (A, B): no inhibitors (▪), +0.5 μM CsA (■), +0.1 μM FK506 (▧). For experiments with PKC inhibitors (C, D): no inhibitors (▪), +10 nM SS (■), +1 μM BIM (▧). Data represent mean ± SEM of at least 3 separate experiments. *P < .01 compared to concurrent induction without inhibitor.
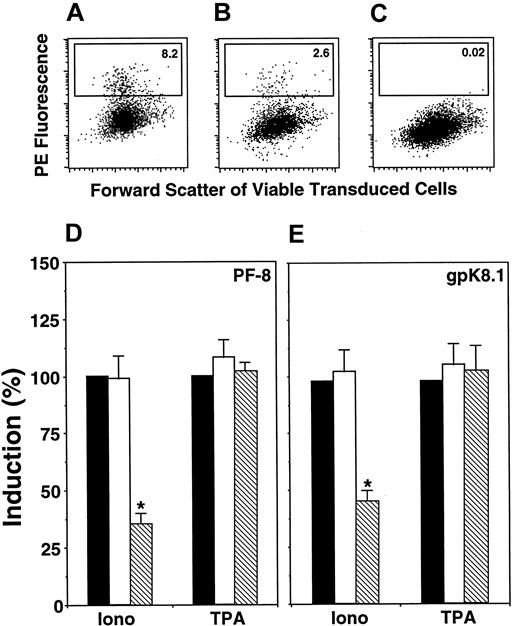
Calcineurin inhibitors CsA and FK506 block different functions of calcineurin. To determine the specific function involved in calcium-dependent KSHV reactivation, BCBL-1 cells were transduced with retroviruses encoding the fusion protein EGFP-VIVIT (fusion between EGFP and the peptide MAGPHPVIVITGPHEE, as described previously31) or EGFP alone. VIVIT competitively inhibits interaction between calcineurin and the nuclear factor of activated T cells (NFAT) family of transcription factors without affecting other calcineurin functions. Viable cells expressing EGFP were gated, and within this population the percentage of PF-8– or gpK8.1-positive cells was determined (Figure4A-C). VIVIT significantly inhibited calcium-dependent induction of KSHV lytic-protein expression by 50% to 70% (Figure 4D-E) compared to nontransduced cells or cells expressing EGFP. By contrast, TPA induction was not affected by VIVIT (Figure4D-E). Transduced cells exhibited identical low levels of reactivation in the absence of inducers (less than 1%), as observed in nontransduced cells.
Calcineurin-binding peptide VIVIT inhibits Ca++-dependent induction of KSHV lytic protein expression.
BCBL-1 cells were retrovirally transduced with EGFP or EGFP-VIVIT fusion protein (see “Materials and methods”). Then cells were incubated with ionomycin (1 μg/mL) or TPA (15 nM). After 3 days, cells were labeled with Dead Red, fixed, permeabilized, and labeled with anti–PF-8 or anti-gpK8.1 mAbs followed by PE-conjugated secondary antibodies. Green fluorescent viable cells were gated and examined for the expression of KSHV lytic proteins. Expression of PF-8 after induction by ionomycin in EGFP- (A) or EGFP-VIVIT–transduced (B) cells. (C) Isotype control for ionomycin-induced EGFP-VIVIT cells. The number of cells within the defined region reflects the percentage of PF-8–positive cells. Ionomycin or TPA induction of PF-8– (D) or gpK8.1-positive (E) cells in EGFP-VIVIT– (▧) and EGFP-transduced (■) cells compared to concurrent induction in nontransduced cells (▪). Data represent mean ± SEM of 4 separate experiments. *P < .01 compared to induction by ionomycin in EGFP-transduced or nontransduced cells.
Calcineurin-binding peptide VIVIT inhibits Ca++-dependent induction of KSHV lytic protein expression.
BCBL-1 cells were retrovirally transduced with EGFP or EGFP-VIVIT fusion protein (see “Materials and methods”). Then cells were incubated with ionomycin (1 μg/mL) or TPA (15 nM). After 3 days, cells were labeled with Dead Red, fixed, permeabilized, and labeled with anti–PF-8 or anti-gpK8.1 mAbs followed by PE-conjugated secondary antibodies. Green fluorescent viable cells were gated and examined for the expression of KSHV lytic proteins. Expression of PF-8 after induction by ionomycin in EGFP- (A) or EGFP-VIVIT–transduced (B) cells. (C) Isotype control for ionomycin-induced EGFP-VIVIT cells. The number of cells within the defined region reflects the percentage of PF-8–positive cells. Ionomycin or TPA induction of PF-8– (D) or gpK8.1-positive (E) cells in EGFP-VIVIT– (▧) and EGFP-transduced (■) cells compared to concurrent induction in nontransduced cells (▪). Data represent mean ± SEM of 4 separate experiments. *P < .01 compared to induction by ionomycin in EGFP-transduced or nontransduced cells.
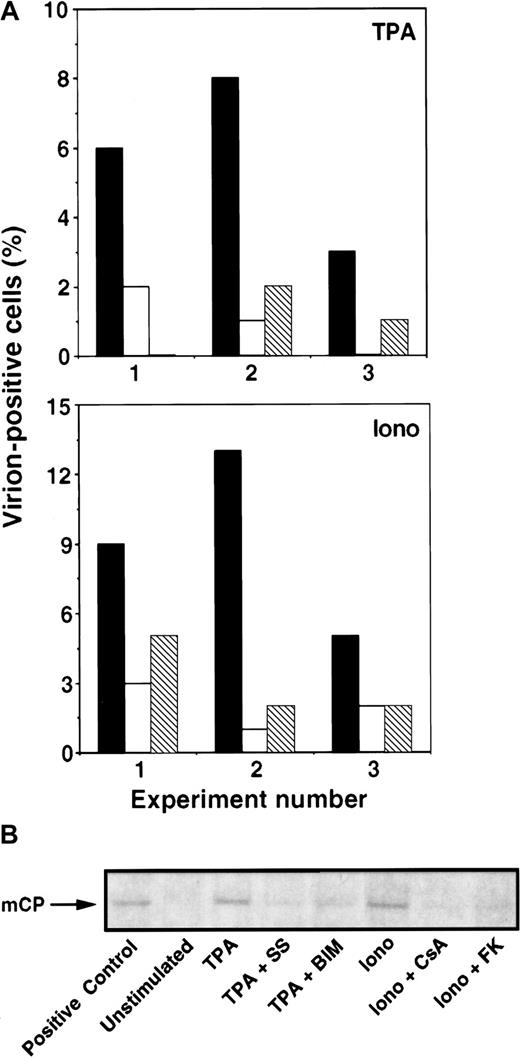
Next, we assessed whether the observed changes in KSHV lytic protein expression correlated with virus particle production. First, cells were examined by transmission electron microscopy (TEM) for evidence of virus formation. Three days after induction in the presence or absence of inhibitors, the percentage of cells with viral particles was determined. Of importance, the identities of the samples were unknown to the person performing the TEM analyses. Consistently, calcium- or TPA-dependent induction of virion-positive cells was inhibited by the calcineurin inhibitors (CsA, FK506) or PKC inhibitors (BIM, SS), respectively (Figure 5A). Second, 3 or 4 days after treatment with inducers and inhibitors, culture supernatants were collected and virus particles were spun down by ultracentrifugation. Virus pellets were lysed and examined by Western blot analysis using a polyclonal antibody against the KSHV minor capsid protein (mCP). Again, calcium- or TPA-dependent induction of virus particle production was inhibited by calcineurin inhibitors (CsA, FK506) or PKC inhibitors (BIM, SS), respectively (Figure 5B).
Calcineurin blockers inhibit Ca++-dependent induction of KSHV production.
BCBL-1 cells were incubated with ionomycin (1 μg/mL) or TPA (15 nM) in the presence of calcineurin or PKC inhibitors. (A) On day 3, KSHV production was determined by enumerating virion-positive cells by TEM analyses. Results of 3 individual experiments are shown (x-axis: 1-3). Top panel: TPA (▪), TPA + SS (■), TPA + BIM (▧). Note that the result of TPA + BIM in experiment 1 and TPA + SS in experiment 3 is 0%. Bottom panel: ionomycin (▪), ionomycin + CsA (■), ionomycin + FK506 (▧). Without inducers, virion-positive cells were rarely observed (less than 0.5%). (B) On day 3 or 4, KSHV production was determined by measuring virus particle accumulation in cell culture supernatants by Western blot analyses. Cell culture supernatants were collected, and viral particles were pelleted by ultracentrifugation. Virus pellets were solubilized in equal volumes of sample buffer, subjected to gel electrophoresis, and blotted onto nitrocellulose membranes. The KSHV mCP was labeled with anti-mCP rabbit polyclonal antibody, followed by an antirabbit IgG antibody coupled to alkaline phosphatase and visualized with Western blue. A commercially available purified virus preparation was used as positive control. Data shown are representative of 3 separate experiments.
Calcineurin blockers inhibit Ca++-dependent induction of KSHV production.
BCBL-1 cells were incubated with ionomycin (1 μg/mL) or TPA (15 nM) in the presence of calcineurin or PKC inhibitors. (A) On day 3, KSHV production was determined by enumerating virion-positive cells by TEM analyses. Results of 3 individual experiments are shown (x-axis: 1-3). Top panel: TPA (▪), TPA + SS (■), TPA + BIM (▧). Note that the result of TPA + BIM in experiment 1 and TPA + SS in experiment 3 is 0%. Bottom panel: ionomycin (▪), ionomycin + CsA (■), ionomycin + FK506 (▧). Without inducers, virion-positive cells were rarely observed (less than 0.5%). (B) On day 3 or 4, KSHV production was determined by measuring virus particle accumulation in cell culture supernatants by Western blot analyses. Cell culture supernatants were collected, and viral particles were pelleted by ultracentrifugation. Virus pellets were solubilized in equal volumes of sample buffer, subjected to gel electrophoresis, and blotted onto nitrocellulose membranes. The KSHV mCP was labeled with anti-mCP rabbit polyclonal antibody, followed by an antirabbit IgG antibody coupled to alkaline phosphatase and visualized with Western blue. A commercially available purified virus preparation was used as positive control. Data shown are representative of 3 separate experiments.
Calcineurin mediates Ca++-dependent reactivation of KSHV
in microvascular endothelial cells
Apart from B cells, KSHV has tropism for endothelial cells.5 Therefore, calcium-dependent virus reactivation was investigated in DMVEC infected with KSHV.23 Almost all cells were latently infected with KSHV, as determined by immunofluorescence assay with antibodies against the latency-associated nuclear antigen LANA (not shown). Three-day incubation with ionomycin (1 μg/mL) in the presence of a suboptimal dose of 3 nM TPA induced a large increase in the number of cells expressing PF-8 and gpK8.1 (Figure 6, Table1). This increase in KSHV reactivation was completely blocked by CsA (Figure 6, Table 1). In these cells ionomycin alone or low-dose TPA (3 nM) alone had no effect or a minimal effect on KSHV reactivation, respectively (Table 1). Adherent cell monolayers remained intact under the various treatments, as observed by phase-contrast light microscopy (not shown).
Calcineurin mediates Ca++-dependent KSHV reactivation in DMVEC.
Immortalized DMVEC monolayers were infected with KSHV, as described previously.23 Three days after incubation with ionomycin, low-dose TPA, or both, monolayers were fixed, permeabilized, and blocked with 20% normal goat serum. Then monolayers were stained with anti–PF-8 mAb followed by FITC-conjugated goat antimouse secondary mAbs. Representative fields were photographed on a Nikon fluorescence microscope (magnification, 10×). Unstimulated cells (A), 1 μg/mL ionomycin (B), 3 nM TPA (C), TPA + ionomycin (D), TPA + 0.5 μM CsA (E), TPA + ionomycin + CsA (F).
Calcineurin mediates Ca++-dependent KSHV reactivation in DMVEC.
Immortalized DMVEC monolayers were infected with KSHV, as described previously.23 Three days after incubation with ionomycin, low-dose TPA, or both, monolayers were fixed, permeabilized, and blocked with 20% normal goat serum. Then monolayers were stained with anti–PF-8 mAb followed by FITC-conjugated goat antimouse secondary mAbs. Representative fields were photographed on a Nikon fluorescence microscope (magnification, 10×). Unstimulated cells (A), 1 μg/mL ionomycin (B), 3 nM TPA (C), TPA + ionomycin (D), TPA + 0.5 μM CsA (E), TPA + ionomycin + CsA (F).
Calcineurin mediates reactivation of Kaposi sarcoma–associated herpesvirus in dermal microvascular endothelial cells
| . | PF-8 . | gpK8.1 . | ||
|---|---|---|---|---|
| . | + CsA . | . | + CsA . | |
| Unstimulated | 3.2 ± 2.7 | 3.4 ± 2.4 | < 1 | < 1 |
| Iono | 2.9 ± 2.4 | 3.0 ± 1.8 | < 1 | < 1 |
| TPA | 8.3 ± 2.3 | 8.5 ± 2.0 | 1.5 ± 0.2 | 1.5 ± 0.1 |
| TPA + Iono | 32 ± 4.6* | 7.9 ± 3.4† | 8.3 ± 3.3* | 1.3 ± 0.1† |
| . | PF-8 . | gpK8.1 . | ||
|---|---|---|---|---|
| . | + CsA . | . | + CsA . | |
| Unstimulated | 3.2 ± 2.7 | 3.4 ± 2.4 | < 1 | < 1 |
| Iono | 2.9 ± 2.4 | 3.0 ± 1.8 | < 1 | < 1 |
| TPA | 8.3 ± 2.3 | 8.5 ± 2.0 | 1.5 ± 0.2 | 1.5 ± 0.1 |
| TPA + Iono | 32 ± 4.6* | 7.9 ± 3.4† | 8.3 ± 3.3* | 1.3 ± 0.1† |
Immortalized DMVEC monolayers were infected with KSHV and stimulated with ionomycin, low-dose TPA, or both in the presence or absence of CsA. After 3 days, the percentage of DMVEC expressing either PF-8 or gpK8.1 was determined. Data shown are mean ± SD of 5 (PF-8 expression) or 10 (gpK8.1 expression) fields.
CsA indicates cyclosporine; iono, ionomycin; TPA, 2-O-tetradecanoyl-phorbol-13-acetate; DMVEC, dermal microvascular endothelial cells; KSHV, Kaposi sarcoma–associated herpesvirus.
P < .001 compared to induction by TPA only.
P < .001 compared to induction by TPA + ionomycin in the absence of CsA.
Discussion
Human herpesviruses often persist in hosts within latently infected cells without causing disease or symptoms. However, virus reactivation can occur, leading to viremia and an increased risk for disease development. Understanding the intracellular signaling pathways underlying human herpesvirus reactivation may provide new ways to suppress virus reactivation and control viral pathogenesis. In this report, we show that calcineurin-dependent signal transduction is involved in KSHV reactivation in latently infected cells.
Calcineurin-dependent signaling after an increase in intracellular calcium is a universal biologic mechanism translating extracellular signals to specific cellular gene transcription.27,28 This process can occur through physiologic stimulation of specific cell surface receptors and in pathophysiologic conditions such as ischemia or other forms of tissue damage.36,37 In B cells, engagement of the antigen receptor is coupled to calcineurin activation.38-40 In the KSHV-infected B cell line BCBL-1, however, expression of surface immunoglobulin is lost. Therefore, sustained increases in intracellular calcium were mimicked using various common calcium-mobilizing agents.28 We showed that the mobilization of intracellular calcium in BCBL-1 cells induced KSHV lytic gene expression and production of virus particles. Accordingly, removal of the calcium stimulus by EGTA abolished virus reactivation.
Highly specific calcineurin blockers (eg, CsA and FK506) are used in vivo to prevent unwanted immune reactivity in patients who undergo transplantation or to reduce immune hyperreactivity in patients with autoimmune diseases.29 We demonstrated that calcineurin blockers prevented KSHV lytic protein expression and inhibited virus production on calcium mobilization, whereas virus reactivation induced by other means (ie, by the phorbol ester TPA) was not inhibited. Moreover, TPA induction was sensitive to PKC inhibitors, which did not affect calcium-mediated virus reactivation. These results imply that, on calcium mobilization, the activation of calcineurin is sufficient to reactivate the complete lytic life cycle of KSHV.
Activated calcineurin dephosphorylates NFAT transcription factors, which then translocate to the nucleus to initiate gene transcription.28 Calcineurin-NFAT signaling can be enhanced by activation of PKC-dependent signal transduction pathways. Synergy between both signaling pathways is explained by the fact that NFAT-dependent gene transcription can be more effective when complexed to other PKC-inducible transcription factors, such as AP-1.28 Accordingly, activation of both pathways in latently infected BCBL-1 cells resulted in a synergistic effect on KSHV reactivation. To further define the role of calcineurin in viral reactivation, we examined the effect of the recently developed high-affinity, calcineurin-binding peptide VIVIT.31 VIVIT interferes with binding of calcineurin to members of the NFAT transcription factor family without affecting other calcineurin activities. Expression of VIVIT in BCBL-1 cells rendered them significantly less susceptible to calcium-mediated KSHV reactivation while exerting no effect on virus reactivation by phorbol esters. Suboptimal expression levels of VIVIT or involvement of additional signaling pathways may explain why VIVIT did not fully block calcium-dependent virus reactivation. Nevertheless, the data imply that the site for calcineurin-NFAT interactions is predominantly involved in calcium-dependent virus reactivation.
Apart from B cell tropism, latent KSHV is also found in endothelial-like cells within KS lesions.5 41Interestingly, we show here that calcium mobilization also induced KSHV reactivation in latently infected endothelial cells, though this was dependent on the presence of low-dose TPA. This suggests that virus reactivation in DMVEC requires a PKC-inducible factor, which in BCBL-1 cells may already be present and active or simply not needed. Again, calcium-mediated KSHV reactivation was completely blocked by CsA, implicating calcineurin involvement. Taken together, we found that calcineurin-dependent virus reactivation is involved in various cell types relevant to KS pathogenesis.
Recent studies in vitro suggested KSHV reactivation by proinflammatory cytokines, in particular interferon (IFN)-γ, as possibly relevant for KS pathogenesis.42 43 Using our FACS detection system, however, physiologic concentrations of all proinflammatory cytokines tested (IFN-γ, tumor necrosis factor-α, IL-1β, IL-6) had a minimal effect or no effect on KSHV reactivation in BCBL-1 cells (ie, less than a 2-fold increase in the number of cells undergoing KSHV reactivation). These findings, though, do not necessarily rule out an effect of these cytokines within KS lesions in vivo, thereby possibly engaging calcineurin- or PKC-dependent signaling pathways.
Calcineurin-NFAT signaling is essential for lymphocyte activation in vivo. In human immunodeficiency virus disease, patients often have evidence of highly activated immune systems, including abnormal activation of B cells with consequent hyperglobulinemia.44-46 In lymph nodes, this activated B cell response is reflected by follicular B cell hyperplasia with an increase in the number of secondary germinal centers.47 In this situation, calcineurin-NFAT signaling may promote the reactivation of latent KSHV residing in B cells and contribute to KS development.48 49 Future studies will be directed toward the relation between the extent of lymphocyte activation and KSHV viremia.
In summary, calcineurin-dependent signal transduction reactivates KSHV on the mobilization of intracellular calcium in latently infected cells. The use of available drugs to block calcineurin is likely to be limited because of the broad spectrum of immune suppression and toxic side effects. However, the future development of specific inhibitors for individual NFAT family members may provide the means to selectively suppress virus replication without adversely affecting essential calcineurin-dependent cellular functions.
We thank Anjana Rao, Mark Udey, Stephen Katz, and Jeffrey Cohen for expert scientific advice and Harry Schaefer for preparation of the figures.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Andrew Blauvelt, Dermatology Branch, National Cancer Institute, Bldg 10/Rm 12N238, 10 Center Dr MSC 1908, Bethesda, MD 20892-1908; e-mail: blauvelt@box-b.nih.gov.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal