Abstract
Cytokines play an essential role during early T-cell development. However, the mechanisms controlling cytokine signaling in developing thymocytes have not been elucidated. Cytokine receptor signaling can be modulated by suppressor of cytokine signaling-1 (SOCS-1), which acts as a negative regulator of Janus kinases. SOCS-1 is normally expressed throughout thymocyte development; however, retroviral-mediated overexpression of SOCS-1 in fetal liver–derived hematopoietic progenitors prevented their progression beyond the earliest stage of T-cell development. Further analysis revealed that SOCS-1 expression is transiently suppressed following pre-T-cell receptor (TCR) signaling. Moreover, constitutive expression of SOCS-1 abrogated pre-TCR– mediated expansion of immature thymocytes but did not interfere with differentiation. These findings reveal that SOCS-1 serves to regulate cytokine signaling at critical checkpoints during early T-cell development.
Introduction
Cytokines play an important role in the regulation of cell differentiation, proliferation, survival, and migration. These effects are mediated by the binding of cytokines to specific cell-surface receptors, which activate Janus kinases (JAKs), leading to phosphorylation and nuclear translocation of signal transducers and activators of transcription (STATs).1 Severe lymphocyte developmental defects are observed in mice deficient for various JAKs and STATs, illustrating the requirement for stringent regulation of cytokine signaling pathways. For example,JAK3−/− mice display a severe reduction of αβ T cells and a complete elimination of γδ T cells,2-6 demonstrating that, in addition to signals transduced by antigen receptors, T-cell development is critically dependent on cytokine signaling.2-6
Hematopoietic progenitors migrate from the fetal liver or the adult bone marrow to the thymus, where commitment to the T-cell lineage occurs.7 The earliest thymic precursors lack surface expression of the CD4 and CD8 coreceptors and are thus termed double-negative (DN) thymocytes. This subset can be subdivided based on the ordered surface expression of CD25 (interleukin-2 receptor α chain [IL-2Rα]) and CD117 (c-Kit): CD25−CD117+ (DNI), CD25+CD117+ (DNII), CD25+CD117− (DNIII), and CD25−CD117− (DNIV). Cells within the DNI subset can give rise to cells of the T, natural killer, and B-lymphocyte lineages and may also differentiate into thymic dendritic cells.7 Progression from the DNI stage to the DNIII stage is thought to be regulated by signals provided through cytokine receptors, among which c-Kit and the IL-7R appear to be critical.8 Transition from the DNIII to the DNIV stage occurs following the productive rearrangement of a T-cell receptorβ (TCRβ) gene, leading to rescue from apoptosis, intense proliferation, allelic exclusion at the TCRβ gene locus, and transcriptional activation of the TCRα gene loci. In addition, thymocytes down-regulate CD25 surface expression and begin to express CD4 and CD8 and thus differentiate into CD4+CD8+ double-positive (DP) thymocytes.9 This process, known as β selection, is controlled by the pre-TCR, a multisubunit receptor complex composed of a TCRβ chain, the invariant pTα chain, and noncovalently associated CD3 and TCRζ chains.9,10 Whereas the importance of pre-TCR signaling in regulating the transition from the DNIII to DP stage has been clearly demonstrated,9,11 much less is known regarding the requirement for cytokine signaling at this crucial step of T-cell development. Indeed, cytokines are generally considered to be dispensable for β selection, although it is recognized that IL-7 enhances the survival of DNIII thymocytes by increasing expression of the antiapoptotic protein Bcl-2.12 13 In addition, despite the considerable progress made in identifying the cytokines and signaling pathways responsible for promoting the development and maturation of early thymic progenitors, little is known of how these signals are themselves regulated or integrated with pre-TCR–derived signals.
Recently, a new family of negative regulators of JAK-STAT signaling was identified,14 providing a potential mechanism for the regulation of cytokine responses. Proteins belonging to this family are termed suppressors of cytokine signaling (SOCS) and are characterized by the presence of a Src homology 2 (SH2) domain and a carboxyl-terminal conserved domain called the SOCS box. At present, 8 members of the SOCS family have been identified, suppressor of cytokine signaling-1 (SOCS-1) to SOCS-7 and CIS (cytokine-inducible SH2-containing protein). Expression of SOCS-1 is induced by several cytokines, including IL-2, IL-3, IL-4, IL-6, IL-13, granulocyte-monocyte colony-stimulating factor, erythropoietin, interferon-γ (IFN-γ), leukemia inhibitory factor (LIF), and stem cell factor (SCF; c-Kit ligand).15,16 SOCS-1 can bind to all 4 members of the JAK family through interaction with the JH1 kinase domain, thereby inhibiting the activation of the JAKs and their subsequent signaling cascades. SOCS-1 is expressed mainly in the thymus and spleen,17 suggesting a potential role in regulating T-cell development. The generation ofSOCS-1−/− mice by several groups17-21 shed light on the function of this molecule in vivo. SOCS-1−/− mice display growth retardation and die 3 weeks after birth with fatty degeneration of the liver and monocytic infiltration of visceral organs.14Lymphocytes in the thymus and spleen ofSOCS-1−/− mice exhibit increased apoptosis, which correlates with increased expression of the proapoptotic protein Bax within these cells and results in an age-dependent loss in thymic cellularity principally affecting the DP population. The perinatal lethality phenotype observed in SOCS-1−/− mice is rescued by disruption of the IFN-γ locus, suggesting that SOCS-1 is a potent suppressor of IFN-γ. However, the precise role played by SOCS-1 during T-cell development could not be determined from these experiments, because the primary effects produced by SOCS-1 deficiency could not be distinguished from secondary effects of IFN-γ dysregulation.17 21
To gain insight into the possible function of SOCS-1 during T-cell development, we overexpressed SOCS-1 in fetal liver–derived hematopoietic progenitors by retrovirus-mediated gene transfer and examined their ability to give rise to T cells in fetal thymic organ culture (FTOC). Although SOCS-1 is normally expressed throughout thymocyte development, constitutive expression of SOCS-1 in hematopoietic progenitors prevented their progression beyond the earliest stage of T-cell development. Further analysis revealed that SOCS-1 expression is transiently suppressed following pre-TCR signaling. Moreover, overexpression of SOCS-1 in DNIII thymocytes abrogated pre-TCR–mediated expansion of DNIV thymocytes but did not interfere with differentiation to the DP stage of T-cell development. Thus, SOCS-1 down-regulation may allow for the proliferative burst associated with pre-TCR signaling, suggesting that this outcome can be functionally dissociated from allelic exclusion, survival, and differentiation. Our findings suggest that SOCS-1 may regulate β selection by maintaining DNIII thymocytes in a state of cytokine unresponsiveness, halting their expansion until they receive a signal from the pre-TCR to release the inhibitory effects of SOCS-1.
Materials and methods
Mice
Timed-pregnant CD1 mice were purchased from Charles River Canada (Saint-Constant, QC), and fetuses were obtained at day 14 of gestation.RAG2−/− Ly5.1 congenic mice22 andRAG2−/− × γc−/−mice23 were kindly provided by Dr P. Poussier (University of Toronto, ON). Mice were bred and maintained in our animal facility.
Cell lines
The RetroPack PT67 cell line was purchased from Clontech (Palo Alto, CA); the GP+E 86 retroviral packaging cell line24 was obtained from Dr P. Ohashi (University of Toronto). GP+E 86 retroviral packaging cells stably transfected with the empty MIEV vector25,26 were produced as follows. PT67 retroviral packaging cells were transfected with 1 μg MIEV retroviral vector DNA using Effectene transfection reagent (Qiagen, Mississauga, ON). Retroviral supernatant was harvested 48 hours postinfection, diluted 2-fold with fresh culture medium (see below), and supplemented with 4 μg/mL hexadimethrine bromide (Sigma-Aldrich Canada, Oakville, ON) to infect GP+E 86 cells. Stable retrovirus-producing GP+E 86 cells expressing enhanced green fluorescent protein (GFP) were isolated by flow cytometry 48 hours later. GP+E 86 cells infected with the MIEV–SOCS-1 or with the MIEV–SOCS-1:SH2* vectors have been described.16 PT67 and GP+E 86 cell lines were maintained in complete high-glucose Dulbecco modified Eagle medium (Sigma-Aldrich) supplemented with 10% fetal calf serum (FCS) (Life Technologies, Burlington, ON). The IL-7–dependent cell line B23, obtained from Dr C. Paige (University of Toronto), was grown in complete RPMI medium (Life Technologies) supplemented with 10% FCS and culture supernatant from F15A cells containing IL-7 (gift from Dr C. Paige). B23 cells stably transfected with the empty MIEV vector or the MIEV–SOCS-1 vector have been described.16
Flow cytometry
For flow cytometry, fluorescein isothiocynate (FITC)-, phycoerythrin (PE)-, Cy-Chrome–, allophycocyanin (APC)-, or biotin-conjugated antibodies were purchased from Pharmingen (Mississauga, ON). Flow cytometry was performed as previously described.27 To obtain purified subsets of DN thymocytes, single-cell suspensions from day 14 CD1 fetal thymic lobes were stained with anti-CD25–FITC, anti-CD117–PE, and biotinylated lineage markers (CD3ε, CD4, CD8, B220, Gr-1, Mac-1, DX-5), followed by APC-conjugated streptavidin. Purified DP and single-positive (SP) thymocytes were obtained by staining single-cell suspensions from adult CD1 thymus with anti-CD4–FITC, anti-TCRβ–PE, and anti-CD8–APC; DP cells were defined by a CD4+CD8+TCRβIntphenotype, and SP cells were defined by a CD4+CD8−TCRβHigh or CD4−CD8+TCRβHigh phenotype. Cells were sorted using a Coulter Elite flow cytometer (Coulter Electronics, Montréal, QC); in all cases, sort purity was more than 99%.
RNA preparation and reverse transcriptase–polymerase chain reaction
Total RNA was prepared from purified thymocyte populations using the RNeasy Mini kit (Qiagen). Complementary DNA (cDNA) was produced with the Omniscript RT kit (Qiagen) using random hexamers. Polymerase chain reaction (PCR) was performed in a final volume of 25 μL containing 1 × PCR buffer (Qiagen), 0.5 mM deoxyribonucleoside triphosphate, 2.5 U HotStar Taq polymerase (Qiagen), and appropriate dilutions of cDNA. For SOCS-1 reverse transcriptase (RT)-PCR, 1 × Q solution (Qiagen) was added. Cycle conditions were 95°C for 15 minutes, followed by 35 cycles of 94°C for 30 seconds, annealing for 30 seconds at the indicated temperature, and 72°C for 30 to 40 seconds, depending on the size of the expected PCR product. After a final incubation at 72°C for 10 minutes, reactions were run on a 1.6% agarose gel, and PCR products were visualized by ethidium bromide staining. The β-actin cDNA was amplified using the β-actin forward (GTGGGCCGCTCTAGGCACCAA) and reverse (CTCTTTGATGTCACGCACGATTTC) oligonucleotides, with annealing at 55°C, generating a 539–base-pair (bp) product. CD45 cDNA was amplified using the CD45 forward (CTACGCAAAGCACGGCCTG) and reverse (TCGAGTCTGCGTTGTCCCAC) oligonucleotides, with annealing at 52°C, generating a 340-bp product. SOCS-1 cDNA was amplified using the SOCS-1 forward (TCCTCGTCCTCGTCTTCGTC) and reverse (AAGCCATCTTCACGCTGAGC) oligonucleotides, with annealing at 58°C, generating a 279-bp product. TCR-Cα cDNA was amplified using the TCR-Cα forward (AGAACCTGCTGTGTACCAGTTAA) and reverse (CATGAGCAGGTTAAATCCGGCT) oligonucleotides, with annealing at 55°C, generating a 350-bp product.
Retrovirus infection
Single-cell suspensions from day 14 fetal liver were depleted of cells expressing high levels of CD24 (heat-stable antigen) by complement-mediated lysis. Briefly, cell suspensions were incubated with J11d hybridoma28 culture supernatant for 5 minutes at 4°C and lysed by addition of freshly reconstituted Low-Tox-H rabbit complement (Cedarlane Laboratories, Hornby, ON) and incubation at 37°C for 30 minutes. Dead cells and contaminating erythrocytes were removed using Lympholyte-M (Cedarlane Laboratories). Subsequently, 1 × 106 to 3 × 106 purified hematopoietic precursors were seeded onto GP+E 86 monolayers in 60-mm tissue culture dishes in culture medium supplemented with a further 5% FCS, 4 μg/mL hexadimethrine bromide, IL-3, IL-6, IL-7, and SCF (R&D Systems, Minneapolis, MN). Cells were cocultured for 24 hours at 37°C, and then CD117+GFP+ cells were isolated by flow cytometry. Equal numbers of sorted cells, 3 × 103 to 5 × 103, were transferred into deoxyguanosine (dGuo)-treated CD1 fetal thymus lobes by hanging drop in an inverted Terasaki plate for 24 hours at 37°C. Thymic organs were subsequently cultured in standard FTOC conditions, as previously described.29 Single-cell suspensions from the FTOCs were prepared at the indicated times, and thymocytes were analyzed by flow cytometry, as described above.
Retrovirus infection of developing thymocytes in intact organ cultures
Day 14 fetal thymus lobes from CD1 orRAG2−/− mice were cocultured with GP+E 86 cells in a high oxygen–supported submersion culture. Briefly, GP+E 86 cells were seeded onto flat-bottom 96-well microtiter plates (2 × 104 cells/well) and allowed to adhere by incubation at 37°C for 16 hours, after which time the cells were irradiated (3000 rad) to prevent further proliferation. The culture medium was replaced with fresh culture medium supplemented with a further 5% FCS and 2 μg/mL hexadimethrine bromide. Fetal thymus lobes were placed onto the GP+E 86 monolayers, and cultures were conducted for 2 to 3 days in an atmosphere containing 70% O2, 25% N2, and 5% CO2.30 31 Thymus lobes were then washed and further cultured in standard FTOC conditions. At the indicated time, single-cell suspensions were prepared and thymocytes counted and analyzed by flow cytometry as described above.
Antibody treatment of mice and fetal thymus lobes
Neonatal RAG2−/− mice (< 8 days) were injected intraperitoneally with 10 μg/g body weight purified anti-CD3ε monoclonal antibody (mAb) (Pharmingen). Injections were given 36 hours or 72 hours prior to harvest to obtain CD25Low or ISP (immature SP) and DP thymocytes, respectively.32 Fetal thymus lobes were prepared fromRAG2−/− fetuses at day 14 of gestation and infected by submersion culture as indicated. The lobes were then transferred in regular FTOC conditions with or without 10 μg/mL purified anti-CD3ε mAb, similar to the method of Levelt et al.33 After 8 days, single-cell suspensions were prepared. Cell counts were performed, and cells were analyzed by flow cytometry as described above.
Cell proliferation assay
Microcultures were prepared by seeding B23 cells in 96-well microtiter plates (104 cells/well) in a final volume of 100 μL. After 48 hours or 72 hours, 3.7 × 104Bq/well of [3H]thymidine (Dupont NEN, Boston, MA) was added to one set of triplicate cultures, and cells were incubated for a further 6 hours. The amount of [3H]thymidine incorporated was determined by transferring cell lysates onto glass fiber filter mats, followed by scintillation counting.
Results
Expression of SOCS-1 during T-cell development
Thymocytes isolated from CD1 fetal thymic lobes and adult thymus were separated according to the surface expression of coreceptors CD4 and CD8 into DN, DP, and SP populations. The DN population was further fractionated according to surface expression of CD25 and CD117 by flow cytometry. Total RNA was prepared and reverse transcribed to cDNA. The amount of SOCS-1 cDNA was assayed by semiquantitative PCR and normalized according to β-actin cDNA levels (Figure1). We were able to detect SOCS-1 cDNA in all subpopulations, indicating that SOCS-1 is expressed at all stages of T-cell development (Figure 1).
SOCS-1 is expressed at all stages of T-cell development.
Thymocytes from CD1 fetal thymic lobes or adult thymus were fractionated according to surface expression of CD25 and CD117 (DNI-DNIV) or CD4 and CD8 (DP and SP). The cDNAs were prepared from total RNA, and serial 3-fold dilutions of cDNAs were analyzed for expression of β-actin and SOCS-1 mRNA by RT-PCR. As control (H2O), RT-PCR reactions lacking template were amplified simultaneously.
SOCS-1 is expressed at all stages of T-cell development.
Thymocytes from CD1 fetal thymic lobes or adult thymus were fractionated according to surface expression of CD25 and CD117 (DNI-DNIV) or CD4 and CD8 (DP and SP). The cDNAs were prepared from total RNA, and serial 3-fold dilutions of cDNAs were analyzed for expression of β-actin and SOCS-1 mRNA by RT-PCR. As control (H2O), RT-PCR reactions lacking template were amplified simultaneously.
Overexpression of SOCS-1 abrogates T-cell differentiation from hematopoietic precursors
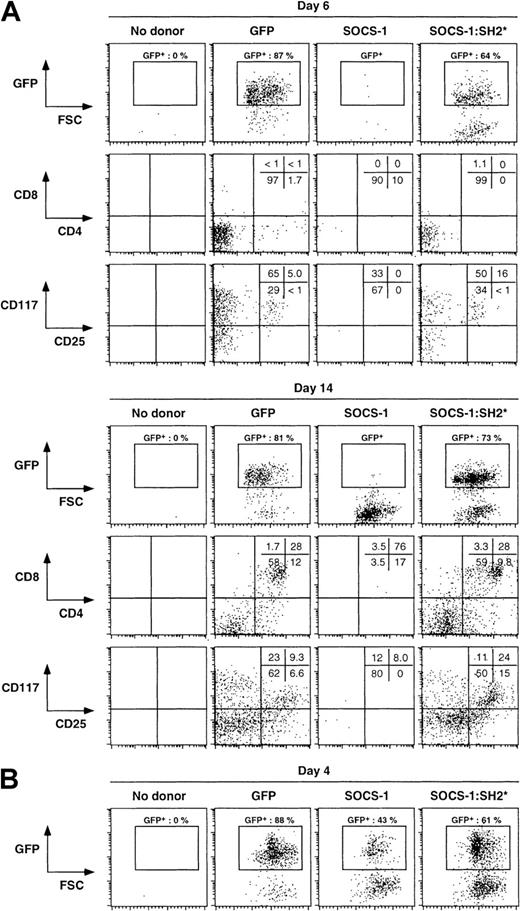
To examine the role of SOCS-1 during the earliest steps of T-cell commitment, we introduced wild-type SOCS-1 by retroviral gene transfer into day 15 fetal liver–derived hematopoietic progenitors (Figure2A). Infected cells were sorted for GFP and CD117 expression, and GFP+ CD117+ cells were transferred into fetal thymic lobes, which were subsequently cultured in standard FTOC conditions (Figure 2B). The GFP+cells contained within the organ cultures were analyzed on days 6 and 14 by flow cytometry for surface expression of CD4 and CD8 or of CD25 and CD117 (Figure 3A). We were unable to recover significant numbers of GFP+ cells from SOCS-1–infected progenitors in FTOCs at either day 6 or 14 (Figure3A). In contrast, progenitors infected with GFP alone gave rise to progeny that developed normally, reaching the DNI/II stage at day 6 and then progressing through the DNIII/IV stages to the DP and SP stage by day 14 (Figure 3A). To determine whether the effects of SOCS-1 required a functional SH2 domain, we introduced a mutant form of SOCS-1 harboring a loss-of-function mutation within the phosphotyrosine binding site in the SOCS-1 SH2 domain (SOCS-1:SH2*) (Figure 3A). We observed normal T-cell development in the GFP+SOCS-1:SH2*–infected cells, demonstrating that the effects of SOCS-1 on early thymic development require a functional SH2 domain. To ensure that the failure to detect SOCS-1–infected progenitors in FTOC was not due simply to an inability to colonize fetal thymic lobes, we analyzed FTOC at day 4 and were able to detect GFP+ SOCS-1–infected cells (Figure 3B). This indicates that these progenitors seeded the thymi but failed to progress beyond the DNI stage.
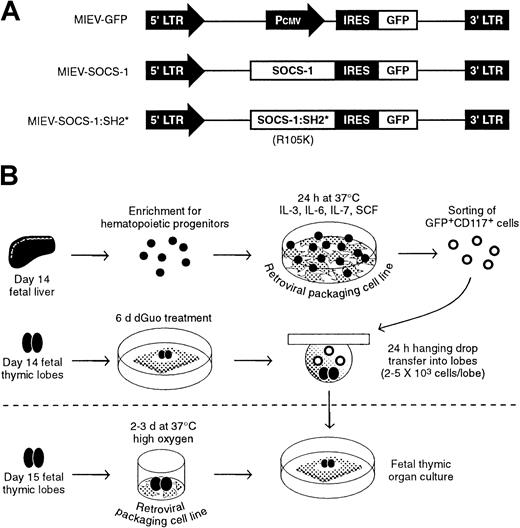
Experimental strategy.
(A) Retrovirus constructs were generated by subcloning cDNA for wild-type SOCS-1 or mutated SOCS-1 lacking a functional SH2 domain (SOCS-1:SH2*) into the MIEV retroviral vector. The presence of an internal ribosomal entry site (IRES) upstream of a GFPreporter gene leads to the production of bicistronic mRNA under the control of the 5′ long terminal repeat (LTR). As control, a retroviral vector was generated containing only the Pcmvpromoter derived from the pcDNA3.1 plasmid. (B) Experimental procedure for retroviral gene transfer into fetal liver–derived hematopoietic precursors (top) or fetal thymic lobes (bottom). Hematopoietic precursors were enriched from fetal liver by complement-mediated depletion of CD24+ cells. Precursors were then cocultured on a monolayer of GP+E 86 retroviral packaging cells. The following day, infected cells were sorted based on the coexpression of GFP and CD117 and subsequently introduced into dGuo-treated fetal thymic lobes to allow T-cell differentiation to occur. Alternatively, fetal thymic lobes were cocultured with GP+E 86 cells and subsequently cultured under normal FTOC conditions.
Experimental strategy.
(A) Retrovirus constructs were generated by subcloning cDNA for wild-type SOCS-1 or mutated SOCS-1 lacking a functional SH2 domain (SOCS-1:SH2*) into the MIEV retroviral vector. The presence of an internal ribosomal entry site (IRES) upstream of a GFPreporter gene leads to the production of bicistronic mRNA under the control of the 5′ long terminal repeat (LTR). As control, a retroviral vector was generated containing only the Pcmvpromoter derived from the pcDNA3.1 plasmid. (B) Experimental procedure for retroviral gene transfer into fetal liver–derived hematopoietic precursors (top) or fetal thymic lobes (bottom). Hematopoietic precursors were enriched from fetal liver by complement-mediated depletion of CD24+ cells. Precursors were then cocultured on a monolayer of GP+E 86 retroviral packaging cells. The following day, infected cells were sorted based on the coexpression of GFP and CD117 and subsequently introduced into dGuo-treated fetal thymic lobes to allow T-cell differentiation to occur. Alternatively, fetal thymic lobes were cocultured with GP+E 86 cells and subsequently cultured under normal FTOC conditions.
Overexpression of SOCS-1 abrogates T-cell differentiation from hematopoietic progenitors.
Fetal liver–derived hematopoietic precursors were infected with the indicated retrovirus constructs and introduced into dGuo-treated FTOC. (A) Single-cell suspensions were obtained from fetal thymic lobes at day 6 or 14 of culture and analyzed for surface expression of CD4 and CD8 or of CD25 and CD117; percentages of cells in each quadrant are shown. The analysis was confined to infected donor cells by gating on GFP expression, as indicated by the rectangular gate in the GFP versus forward light scatter (FSC) dot plots. (B) Single-cell suspensions were obtained from fetal thymic lobes at day 4 of culture and analyzed for the presence of GFP-expressing cells. As control in each set of experiments, fetal thymic lobes receiving no donor cells were also analyzed.
Overexpression of SOCS-1 abrogates T-cell differentiation from hematopoietic progenitors.
Fetal liver–derived hematopoietic precursors were infected with the indicated retrovirus constructs and introduced into dGuo-treated FTOC. (A) Single-cell suspensions were obtained from fetal thymic lobes at day 6 or 14 of culture and analyzed for surface expression of CD4 and CD8 or of CD25 and CD117; percentages of cells in each quadrant are shown. The analysis was confined to infected donor cells by gating on GFP expression, as indicated by the rectangular gate in the GFP versus forward light scatter (FSC) dot plots. (B) Single-cell suspensions were obtained from fetal thymic lobes at day 4 of culture and analyzed for the presence of GFP-expressing cells. As control in each set of experiments, fetal thymic lobes receiving no donor cells were also analyzed.
Overexpression of SOCS-1 impairs T-cell differentiation from committed thymic precursors
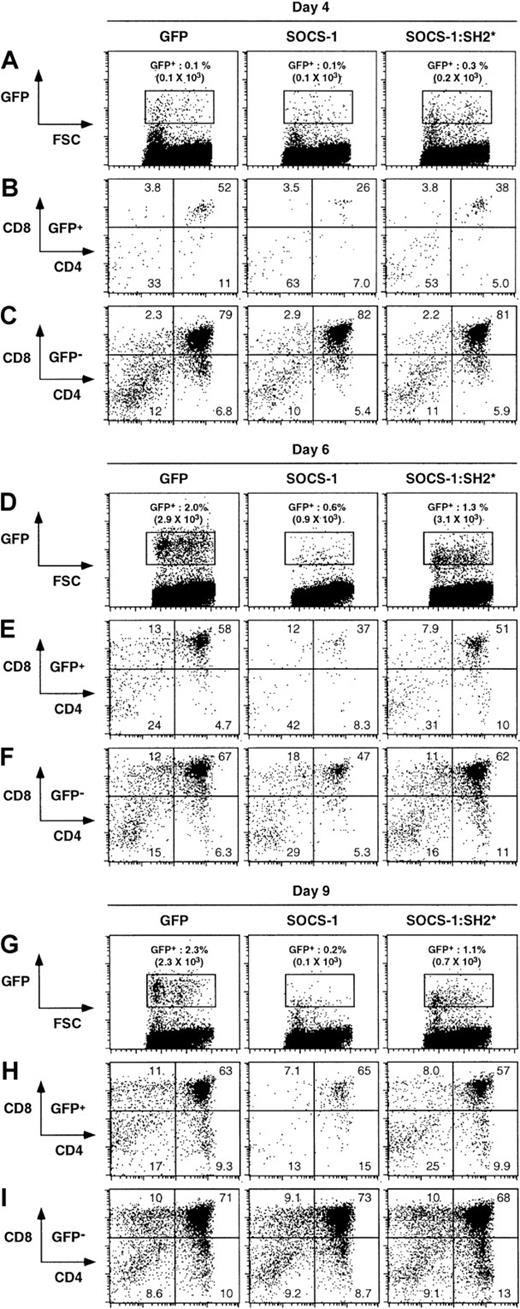
To determine the capacity of SOCS-1 to influence later stages of T-cell development, we cocultured day 14 fetal thymi with SOCS-1, SOCS-1:SH2*, or GFP (control) retroviral packaging cell lines for 3 days. This allowed us to study the effects of SOCS-1 overexpression after the DNI-to-DNII transition had occurred but prior to the DNIII-to-DP transition, because fetal thymi from day 14 embryos contain only DN thymocytes.7 Thymic lobes were then transferred to standard FTOC conditions for 1, 3, or 6 days. In all cases, GFP− populations (ie, noninfected cells) progressed through the DN, DP, and SP stages of thymocyte development (Figure4, rows C, F, and I). The development of control GFP- and SOCS-1:SH2*–infected cells paralleled the normal development observed with noninfected cells (Figure 4, rows B, E and H). We also observed GFP+ SOCS-1–infected thymocytes at each developmental stage. However, in contrast to the accumulation of GFP+ cells observed in GFP- and SOCS-1:SH2*–infected thymic lobes, there was no significant increase in the number of GFP+ SOCS-1–infected cells by day 9 (Figure 4, rows A and G; absolute number of GFP+ cells per lobe are shown in parentheses). These results suggest that either most DN thymocytes were infected after 3 days of coculture and subsequently differentiated into DP thymocytes but failed to undergo the normal burst of proliferation or suggest that only a few DN thymocytes were successfully infected and subsequently underwent normal expansion. The latter hypothesis is unlikely, because the frequency of GFP+ cells at day 4 of culture was similar in SOCS-1–infected thymic lobes compared with GFP-infected lobes (Figure 4, row A). Moreover, when FTOCs were analyzed at an earlier time point (day 0-2), the frequency of GFP+ cells in SOCS-1–infected lobes was higher than in GFP-infected lobes (data not shown).
Overexpression of SOCS-1 impairs T-cell differentiation from committed thymic precursors.
Fetal thymic lobes were infected with the indicated retrovirus constructs for 3 days and subsequently cultured for a further 1, 3, or 6 days. Single-cell suspensions were obtained from fetal thymic lobes at the indicated time and analyzed for surface expression of CD4 and CD8; percentages of cells in each quadrant are shown. The analysis was confined to infected donor cells by gating on GFP expression, as indicated by the rectangular gate in the GFP versus forward light scatter (FSC) dot plots; cell counts for the absolute number of GFP+ cells per lobe are shown in parentheses. Data obtained with uninfected (GFP−) cells are provided as a control for the ability of the thymic lobes to sustain normal T-cell development.
Overexpression of SOCS-1 impairs T-cell differentiation from committed thymic precursors.
Fetal thymic lobes were infected with the indicated retrovirus constructs for 3 days and subsequently cultured for a further 1, 3, or 6 days. Single-cell suspensions were obtained from fetal thymic lobes at the indicated time and analyzed for surface expression of CD4 and CD8; percentages of cells in each quadrant are shown. The analysis was confined to infected donor cells by gating on GFP expression, as indicated by the rectangular gate in the GFP versus forward light scatter (FSC) dot plots; cell counts for the absolute number of GFP+ cells per lobe are shown in parentheses. Data obtained with uninfected (GFP−) cells are provided as a control for the ability of the thymic lobes to sustain normal T-cell development.
Expression of SOCS-1 is reduced upon pre-TCR signaling
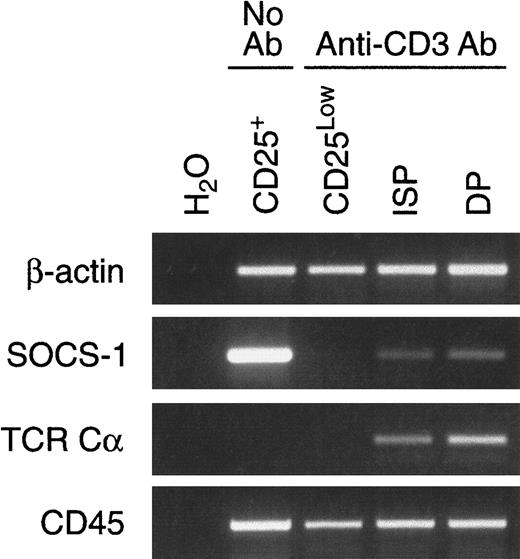
The apparent decrease in SOCS-1 expression observed in DNIV thymocytes (Figure 1) together with the observation that forced expression of SOCS-1 impaired the pre-TCR–dependent proliferation occurring during the transition from the DNIII to the DP stage (Figure4) suggested that the expression of SOCS-1 might need to be down-regulated during this transition. Because a preparation of DNIV cells from normal mice consists of a heterogeneous population of cells, we took advantage of an experimental model of pre-TCR activation first developed by Levelt et al33 to study the expression of SOCS-1 messenger RNA (mRNA) following pre-TCR signaling. This model is based on the use of RAG-deficient mice, in which thymocytes are arrested at the DNIII stage due to an inability to rearrange theTCR genes. Cross-linking of CD3 at the surface of these cells with anti-CD3ε mAb mimics pre-TCR signaling and allows thymocytes to progress to the DP stage. DNIII cells (CD25+) were purified from untreated RAG2−/− mice by cell sorting, whereas CD25Low, ISP (CD4+CD8- or CD4-CD8+), and DP thymocytes were obtained from anti-CD3ε–treatedRAG2−/− mice. The expression of SOCS-1 mRNA from each population was analyzed by semiquantitative reverse transcriptase–polymerase chain reaction (RT-PCR) and normalized against β-actin mRNA levels (Figure 5). SOCS-1 was abundantly expressed in the CD25+ (DNIII) population but was undetectable in CD25Low cells following pre-TCR signaling. SOCS-1 expression was again detectable in the ISP and DP subsets. The presence of germline TCRα transcripts (TCR Cα), which normally follows successful β selection, was detected in ISP and DP thymocytes, thus confirming that anti-CD3ε treatment initiated a normal developmental program. Moreover, to ensure that the loss of SOCS-1 mRNA was selective and not the result of nonspecific down-regulation of multiple mRNA species, CD45 expression was detected in all subsets (Figure 5).
Expression of SOCS-1 is suppressed following pre-TCR signaling.
Neonatal RAG2−/− mice were injected intraperitoneally with anti-CD3ε mAb. Injections were given 36 or 72 hours prior to harvest to obtain CD25Low or ISP and DP thymocytes, respectively. CD25+ thymocytes were obtained from untreated RAG2−/− mice. The cDNAs were prepared from total RNA and analyzed by RT-PCR for expression of β-actin, SOCS-1, TCRα (germline transcripts), and CD45 mRNA. As control (H2O), RT-PCR reactions lacking template were amplified simultaneously.
Expression of SOCS-1 is suppressed following pre-TCR signaling.
Neonatal RAG2−/− mice were injected intraperitoneally with anti-CD3ε mAb. Injections were given 36 or 72 hours prior to harvest to obtain CD25Low or ISP and DP thymocytes, respectively. CD25+ thymocytes were obtained from untreated RAG2−/− mice. The cDNAs were prepared from total RNA and analyzed by RT-PCR for expression of β-actin, SOCS-1, TCRα (germline transcripts), and CD45 mRNA. As control (H2O), RT-PCR reactions lacking template were amplified simultaneously.
Overexpression of SOCS-1 hinders pre-TCR–driven proliferation of immature thymocytes
To determine whether failure to down-regulate SOCS-1 expression might affect the maturation of thymocytes from the DNIII to the DP stage, we constitutively expressed SOCS-1 in RAG-deficient thymocytes. Fetal thymic lobes from RAG2−/− day 14 embryos were cocultured with GFP or SOCS-1 retroviral packaging cell lines for 2 days and then placed in standard FTOC conditions in the presence of anti-CD3ε mAb. After 6 days, thymic cellularity was assessed and cells were analyzed for CD4 and CD8 expression. Both GFP- and SOCS-1–infected thymocytes were able to give rise to DP cells (Figure6). However, whereas the percentage of GFP- and SOCS-1–infected DP cells were similar (56% and 55%, respectively), SOCS-1–expressing DP thymocytes represented a 6-fold smaller fraction of the total thymic cellularity compared with GFP-infected DP cells (Figure 6, right panel). Analysis of infectedRAG2−/− thymic lobes after 2 days of coculture with the retroviral packaging lines demonstrated that GFP+DNIII thymocytes were present at a higher frequency in SOCS-1–infected thymic lobes than in GFP-infected thymic lobes (data not shown). Therefore, the reduced number of SOCS-1–infected DP thymocytes could not be explained by a decrease in the number of SOCS-1–infected DNIII thymocytes prior to stimulation with anti-CD3ε mAb. These observations suggest that the forced expression of SOCS-1 in DNIII thymocytes does not prevent pre-TCR–mediated differentiation but, rather, results in the failure of these cells to expand in response to this stimulus.
Overexpression of SOCS-1 hinders pre-TCR–driven expansion of immature thymocytes.
Fetal thymic lobes from RAG2−/− mice were infected with the indicated retrovirus constructs for 2 days and subsequently cultured for a further 6 days in the presence of anti-CD3ε mAb. Single-cell suspensions were obtained from fetal thymic lobes and analyzed for surface expression of CD4 and CD8; percentages of cells in each quadrant are shown. Results obtained for uninfected (GFP−) and infected (GFP+) donor cells are presented; cell counts for the absolute number of GFP+ cells per lobe are shown in parentheses. The ratio—calculated from the total thymic cellularity—of infected cells (GFP+) to uninfected cells (GFP−) expressing CD4/CD8 coreceptors is provided.
Overexpression of SOCS-1 hinders pre-TCR–driven expansion of immature thymocytes.
Fetal thymic lobes from RAG2−/− mice were infected with the indicated retrovirus constructs for 2 days and subsequently cultured for a further 6 days in the presence of anti-CD3ε mAb. Single-cell suspensions were obtained from fetal thymic lobes and analyzed for surface expression of CD4 and CD8; percentages of cells in each quadrant are shown. Results obtained for uninfected (GFP−) and infected (GFP+) donor cells are presented; cell counts for the absolute number of GFP+ cells per lobe are shown in parentheses. The ratio—calculated from the total thymic cellularity—of infected cells (GFP+) to uninfected cells (GFP−) expressing CD4/CD8 coreceptors is provided.
The γc family of cytokines is not required for transition to the CD4+CD8+ stage
SOCS-1 and other members of the SOCS family have been shown to suppress cytokine signaling. We therefore hypothesized that SOCS-1 might interfere with the proliferative burst normally triggered by the pre-TCR by blocking cytokine signaling in DNIII thymocytes. Although the survival and proliferation of DNI and DNII thymocytes is known to be critically dependent on SCF and IL-7, little is known about the role of cytokines during subsequent stages of intrathymic T-cell development. However, there is evidence that γc-dependent cytokines (IL-2, IL-7, IL-9, and IL-15) might play a role at the DNIII stage: IL-7Rα/γc receptors are expressed at this stage34,35; DNIII and DNIV thymocytes are severely depleted in IL-7/IL-7Rα/γc–deficient mice36; and γc−/− × pTα−/− mice demonstrate a complete arrest in thymocyte development, whereas the absence of pTα alone allows T-cell development to proceed, albeit with reduced efficiency.36,37 At present, IL-7 is the only γc-dependent cytokine for which a role in T-cell development has clearly been demonstrated.8 SOCS-1 has been shown to be a potent inhibitor of a variety of cytokines, including IL-6, IFN-γ, LIF, and SCF,14 16 but to date its activity in modulating IL-7/IL-7Rα/γc signaling has not been examined. To determine whether SOCS-1 could interfere with this signaling pathway, we analyzed the proliferation of the IL-7–dependent B-cell line B23 in the presence or absence of ectopically expressed SOCS-1 (Figure7A). A profound block in proliferation was observed in B23 cells overexpressing SOCS-1, demonstrating that SOCS-1 potently interfered with the induction of DNA synthesis following IL-7 stimulation.
The γc family of cytokines is not required for transition to the CD4+CD8+ stage.
(A) The IL-7–dependent cell line B23 was infected with the MIEV–SOCS-1 retroviral vector or with the empty MIEV vector as control. Stably infected cells were obtained by sorting for GFP expression. Cells were then cultured for the indicated time and pulsed with [3H]thymidine for 6 hours to measure cell proliferation. Mean values ± SD for triplicate samples are given. (B) Newborn RAG2−/− andRAG2−/− × γc−/− mice were injected with anti-CD3ε mAb or with control Ab (Armenian hamster [Ahm] IgG). After 3 days, single-cell suspensions were prepared from the thymus and analyzed for surface expression of CD4 and CD8 by flow cytometry; percentages of cells in each quadrant are shown. Absolute cell numbers were calculated (mean ± SD) from 3 to 6 mice of each genotype. (C) Role of SOCS-1 during early T-cell development. A simple scheme of early T-cell development is shown that is adapted from Di Santo et al36; solid and dashed lines indicate where overexpression of SOCS-1 affects a complete or partial block in T-cell development, respectively. Bold circles represent cycling cells.
The γc family of cytokines is not required for transition to the CD4+CD8+ stage.
(A) The IL-7–dependent cell line B23 was infected with the MIEV–SOCS-1 retroviral vector or with the empty MIEV vector as control. Stably infected cells were obtained by sorting for GFP expression. Cells were then cultured for the indicated time and pulsed with [3H]thymidine for 6 hours to measure cell proliferation. Mean values ± SD for triplicate samples are given. (B) Newborn RAG2−/− andRAG2−/− × γc−/− mice were injected with anti-CD3ε mAb or with control Ab (Armenian hamster [Ahm] IgG). After 3 days, single-cell suspensions were prepared from the thymus and analyzed for surface expression of CD4 and CD8 by flow cytometry; percentages of cells in each quadrant are shown. Absolute cell numbers were calculated (mean ± SD) from 3 to 6 mice of each genotype. (C) Role of SOCS-1 during early T-cell development. A simple scheme of early T-cell development is shown that is adapted from Di Santo et al36; solid and dashed lines indicate where overexpression of SOCS-1 affects a complete or partial block in T-cell development, respectively. Bold circles represent cycling cells.
To ascertain whether IL-7 or other γc-dependent cytokines regulate the differentiation and expansion of thymocytes triggered by the pre-TCR, we examined the ability of RAG2−/−and RAG2−/− × γc−/− mice to give rise to DP thymocytes upon stimulation with anti-CD3ε mAb. Neonate mice were injected intraperitoneally with anti-CD3ε mAb or with control mAb. After 3 days, thymic cellularity was measured, and thymocytes were analyzed for surface expression of CD4 and CD8. In bothRAG2−/− andRAG2−/− × γc−/− mice, stimulation with anti-CD3ε mAb resulted in the production of DP thymocytes, with a comparable distribution of thymocytes among the DN, ISP, and DP subsets (Figure 7B). In addition, the relative increase in cellularity was identical in RAG2−/− andRAG2−/− × γc−/− mice (Figure 7B). The 100-fold decrease in total cell numbers inRAG2−/− × γc−/− mice compared with RAG2−/− mice is due to the absence of IL-7R signaling during the DNI and DNII stages.38 These results suggest that γc-mediated signals are not necessary for the proliferative burst observed following pre-TCR signaling.
Discussion
The data presented in this study demonstrate that SOCS-1 intervenes at 2 critical checkpoints during early T-cell development. The first checkpoint occurs at the transition from the DNI to the DNII stage, where SOCS-1 may modulate c-Kit– and γc-derived signals. The second checkpoint occurs at the transition from the DNIII to the DP stage and involves signaling through the pre-TCR complex, leading to SOCS-1 down-regulation and thymocyte proliferation. Overexpression of SOCS-1 at the first checkpoint abrogates further T-cell development, whereas constitutive expression of SOCS-1 at the second checkpoint impedes cell proliferation but does not prevent differentiation. These observations are summarized in Figure 7C.
The differentiation and proliferation of early thymic precursors is guided in part by signals delivered through cytokine receptors. Among the large number of cytokines produced by the thymic stroma, IL-7 and SCF have emerged as the dominant cytokines governing the early stages of thymopoiesis.38-41 Although transition from the DNI to the DNII stage can proceed in the absence of IL-7 or SCF signaling, it is abrogated when both of these signaling pathways are inoperative. We observed that fetal liver progenitors constitutively expressing SOCS-1 could not reconstitute FTOCs, indicating that this process depends on signaling pathways inhibited by SOCS-1. The developmental arrest of thymocytes at the DNI stage observed with SOCS-1 overexpression is reminiscent of the phenotype of c-kit−/− × γc−/−mice41 and is consistent with our findings that SOCS-1 blocks signaling through both c-Kit16 and the IL-7 receptor (Figure 7A). Our results suggest that either differentiation or self-renewal were affected—and not survival directly—because SOCS-1–infected (GFP+) cells could be detected after 4 days in FTOC. Nevertheless, it is important that SOCS-1 overexpression may not only affect the pathways known to be regulated by SOCS-1 but may also inhibit other signaling cascades normally under the control of other SOCS family members or perhaps certain pathways in which SOCS-1 overexpresion may have an undue effect.
The ability of SOCS-1 to inhibit the differentiation or renewal of hematopoietic stem cells within the thymus suggested that it might also inhibit stem cell function within the bone marrow. We tested this possibility by injecting fetal liver hematopoietic progenitors infected either with SOCS-1, SOCS-1:SH2*, or GFP retrovirus into sublethally irradiated RAG-deficient hosts. Whereas both SOCS-1:SH2*– and GFP-infected hematopoietic progenitors repopulated the marrow and periphery of host mice, SOCS-1–infected progenitors failed to reconstitute all hematopoietic lineages, including the erythrocyte, granulocyte, megakaryocyte, and lymphocyte lineages (data not shown). Thus, survival, self-renewal, differentiation, and migration of hematopoietic stem cells may be adversely affected by constitutive overexpression of SOCS-1.
There is ample evidence to support a model of T-cell development in which thymocyte survival, differentiation, and proliferation are regulated by a series of overlapping signals provided by cytokine and T-cell receptors.8,36,37,42 At each stage of development, efficient thymopoiesis appears to require at least 2 such signals. For instance, the transition from the DNI stage to the DNII stage requires the synergistic action of IL-7 and SCF. This “2-signal” requirement persists throughout the existence of the T cell. Indeed, activation of naive T cells in the periphery necessitates signaling not only through the TCR but also through costimulatory receptors such as CD28.43 At the transition from the DNIII stage to the DP stage, there again appears to be a requirement for 2 separate signals, because overexpression of SOCS-1 curbs the proliferative burst normally induced by pre-TCR signaling. In support of this notion, it was recently shown that transgenic mice overexpressing SOCS-1 display a reduction in thymic cellularity, owing to an apparent block in thymocyte development at the transition from the DNIII to the DP stage.44 However, the role of SOCS-1 at the earliest stages of T-cell development could not be assessed in this study, and a direct link between pre-TCR signaling and SOCS-1 regulation was not investigated.
Whereas pre-TCR signals are clearly essential for the generation of DP thymocytes, the role of cytokine receptor signaling for this process remains uncertain. It has been proposed that γc-dependent cytokines might function only to maintain CD117Low/-CD25+ thymocyte viability for a short period to allow for the rearrangement of the TCRgenes to occur.37 This view is supported by in vivo experiments showing that the proliferation of DNIII thymocytes is not affected in the absence of the IL-7R38 and by in vitro experiments demonstrating that IL-7 improves survival but not proliferation of DNI-DNIII thymocytes through the induction of Bcl-2 expression.13 In agreement with these previous studies, we show that differentiation and expansion of anti-CD3ε–treatedRAG−/− thymocytes can proceed normally in the absence of γc-dependent cytokine signaling, suggesting that other cytokines, acting through non-γc–dependent receptors, assist in the transition to the DP stage. Notwithstanding, the data presented here indicate that SOCS-1 can interfere with these alternate cytokine signaling cascades that appear to be necessary for the proliferation of thymocytes following β selection (Figure 7C).
Our results further support a key role for SOCS-1 in providing additional regulation at the β selection checkpoint. First, we show that SOCS-1 mRNA is expressed throughout T-cell development, except for a transient period following pre-TCR signaling when SOCS-1 expression becomes undetectable. This finding is unique, because no other surface receptor has been shown to negatively regulate SOCS-1 expression. Indeed, signaling by a wide variety of cytokine and growth factor receptors increases the level of SOCS-1 transcripts.14Second, we show that overexpression of SOCS-1 hinders the mitogenic stimuli transduced by the pre-TCR. Together, these observations indicate that signaling pathways sensitive to inhibition by SOCS-1 participate in promoting the maturation of DNIII thymocytes to the DP stage and that SOCS-1 may regulate these signaling cascades in vivo. This model is consistent with the thymic hypocellularity phenotype observed in SOCS-1−/− mice.17Indeed, the lack of SOCS-1 might be predicted to lead to disordered DNA recombination and replication at the DNIII stage, leading in turn to massive intrathymic cell death.45,46 Recently, Li et al47 demonstrated that mitogenesis of peripheral T cells is similarly regulated through a TCR/SOCS axis. However, in contrast to pre-T cells that down-regulate SOCS-1 to enhance cell proliferation, peripheral T cells increase expression of CIS (another SOCS family member) upon stimulation through the TCR, resulting in improved proliferation and survival of the activated T cell.47Thus, both the pre-TCR and TCR share the ability to regulate the expression of SOCS family proteins.
The recent discovery that SOCS-1 inhibits apoptosis and promotes activation of the p38 mitogen–activated protein kinase (MAPK) induced by TNF-α48 reveals that SOCS-1 also regulates signaling by MAPKs. Activation of p38 MAPK appears to be required for the earliest stages of T-cell development, but inactivation must occur for DNIII thymocytes to differentiate into DP thymocytes.50Hence, the ability of SOCS-1 to promote the activation of p38 MAPK might contribute to the developmental arrest of thymocytes unable to assemble a pre-TCR and therefore unable to down-regulate SOCS-1 expression. Thus, the pre-TCR might trigger the expansion of pre-T cells not only through a direct activation of the ERK MAPK50 but also by an indirect inactivation of p38 MAPK through inhibition of SOCS-1 expression.
These results reveal a novel role for cytokine signaling acting in concert with pre-TCR signals to allow efficient proliferation of TCRβ-expressing thymocytes. We propose a model whereby SOCS-1 functions to maintain DNIII thymocytes in a state of cytokine unresponsiveness, thereby halting thymocytes at the DNIII stage until they receive a signal to release the inhibitory effects of SOCS-1. In this manner, β selection would be enforced because thymocytes lacking a functional TCRβ chain would be prevented from proceeding to the next developmental stage. The function of the pre-TCR might then be not only to provide direct mitogenic stimuli but also to enable thymocytes to respond to signals provided by the thymic stroma. This notion may shed light on the finding that RAG−/−thymocytes can proliferate and differentiate upon treatment with anti-CD3ε mAb in vivo or in FTOC but not when treated in cell suspension.51 Taken together, our findings reveal a novel role for SOCS-1 in regulating checkpoints during early T-cell development. In particular, SOCS-1 appears to interfere with mitogenic pathways used by the pre-TCR but not with the differentiative signals associated with β selection.
We thank Drs Alison Michie and Philippe Poussier for critical review of this manuscript. S.T. is the recipient of a Doctoral Research Award from the Medical Research Council of Canada. R.R. and J.C.Z.-P. are the recipients of a Scientist Award from the Medical Research Council of Canada.
Supported by grants from the Canadian Institutes of Health Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Juan Carlos Zúñiga-Pflücker, Dept of Immunology, University of Toronto, 1 King's College Circle, Toronto, ON M5S 1A8, Canada; e-mail: jc.zuniga.pflucker@utoronto.ca.



![Fig. 7. The γc family of cytokines is not required for transition to the CD4+CD8+ stage. / (A) The IL-7–dependent cell line B23 was infected with the MIEV–SOCS-1 retroviral vector or with the empty MIEV vector as control. Stably infected cells were obtained by sorting for GFP expression. Cells were then cultured for the indicated time and pulsed with [3H]thymidine for 6 hours to measure cell proliferation. Mean values ± SD for triplicate samples are given. (B) Newborn RAG2−/− andRAG2−/− × γc−/− mice were injected with anti-CD3ε mAb or with control Ab (Armenian hamster [Ahm] IgG). After 3 days, single-cell suspensions were prepared from the thymus and analyzed for surface expression of CD4 and CD8 by flow cytometry; percentages of cells in each quadrant are shown. Absolute cell numbers were calculated (mean ± SD) from 3 to 6 mice of each genotype. (C) Role of SOCS-1 during early T-cell development. A simple scheme of early T-cell development is shown that is adapted from Di Santo et al36; solid and dashed lines indicate where overexpression of SOCS-1 affects a complete or partial block in T-cell development, respectively. Bold circles represent cycling cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/8/10.1182_blood.v97.8.2269/5/m_h80810941007.jpeg?Expires=1769084912&Signature=DPPOMITF~hBPf~QyPioFvMWz8kwLT-XomQ7f2LVOYLW4av6fehdrK7x-6iJvLsDUO2hXiTwm~LoLJ8hjsURRovjAOgHCeccaEyHxLZcK6MaoWUWQnzCKgCfEzQKroP~24ZYKf5daLNoGIA7fLIzeVLE0uXnlgZWdNxX01zefJMrp5pev1cEDASQJ40OmbgPAWTdGZ8PmXRIkmGxDZU3DV-hLr5jgqo05frG1oRsdRvfiZsbA30xfKdfr~GoDi7F3CZLQRTwQVBsxrZFDJpkeddpP7hX~GHVbpogsbT5QbRta6bj29YTdiv6K5izYOSBPIZ8KfAGEGUrnEUweFCwY8A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal