Abstract
Coronary atherosclerosis is a major cause of death in industrialized countries. Monocytes, which play a key role in atherosclerosis, migrate into the vessel wall, presumably guided by specific chemoattractant and adhesion molecules. A compelling candidate for this role is the chemokine receptor CX3CR1, which is expressed on monocytes and acts as either a chemotactic receptor or an adhesion molecule, depending on whether its ligand, fractalkine, is presented free or membrane bound. A common variant of CX3CR1 was recently identified, encoded by the alleles I249 and M280, which form a common I249M280 haplotype. When CX3CR1 genotypes were analyzed in 151 patients with acute coronary syndromes and in 249 healthy controls, CX3CR1 I249 heterozygosity was associated with a markedly reduced risk of acute coronary events, independent of established acquired coronary risk factors (eg, smoking, diabetes). The adjusted odds ratio for this allele was 0.43 (95% confidence interval, 0.26-0.72; P = .001). Consistent with this, functional analysis of peripheral blood mononuclear cells showed that CX3CR1 I249 heterozygosity was associated with a significant decrease in the number of fractalkine binding sites per cell. The results show that CX3CR1 I249 is an independent genetic risk factor for coronary artery disease and that CX3CR1 may be involved in the pathogenesis of atherosclerotic disease.
Introduction
It is now widely accepted that inflammatory pathways play a major role in the pathogenesis of atherosclerosis. In particular, monocyte/macrophage accumulation and activation in the vessel wall appear to be critical events, not only during the initial phase of plaque formation,1 but also in chronic lesion progression and during acute complications such as plaque rupture and thrombosis.2 The molecular mechanisms responsible for monocyte accumulation in plaque are likely to include chemokines and their receptors because these molecules are major regulators of specific leukocyte trafficking. The CX3C chemokine fractalkine (FKN) and its 7–transmembrane domain G-protein–coupled receptor CX3CR1 are particularly compelling candidates. FKN is a unique chemokine because it exists in both a soluble form and in a membrane-anchored form, for example on the surface of interleukin-1–activated and tumor necrosis factor–activated endothelium.3 Membrane-bound FKN directly mediates the capture and firm adhesion of CX3CR1-expressing leukocytes, thus providing a novel pathway for leukocyte activation.4 Soluble FKN has leukocyte chemotactic activity.
Recently, we identified 2 common single-nucleotide polymorphisms in the open reading frame of CX3CR1. The polymorphisms are nonsynonymous substitutions causing relatively conservative amino acid changes (V249I and T280M; single-letter amino acid code) in the CX3CR1 protein. The 2 polymorphisms are in strong linkage disequilibrium, forming a common I249M280haplotype.5 It is interesting that functional CX3CR1 analysis showed that FKN binding was reduced in peripheral blood mononuclear cells (PBMCs) from patients with human immunodeficiency virus (HIV) homozygous for the I249M280haplotype. Here we report that allele I249 is associated with a reduced risk of acute coronary artery disease as well as altered CX3CR1 expression and ligand-binding affinity.
Patients, materials, and methods
Subjects
The patients and control subjects gave their informed written consent for the study, which was approved by the Pitié-Salpêtrière ethics committee. The subjects have been described in detail in a recent paper.6 Briefly, we enrolled 151 white patients with acute coronary syndromes (134 with myocardial infarction [MI] and 17 with unstable angina [UA]), all less than 65 years of age, who had been admitted to Bichat Hospital coronary care unit (ACABI study: Accidents Coronaires Aigus BIchat). MI and UA were diagnosed on the basis of usual criteria, as described by Moatti et al.7 Among the patients with UA, those with angiography-proved ≥50% diameter stenosis were selected. Two hundred forty-nine age- and sex-matched controls were recruited among hospital employees and blood donors. As expected, risk factors for coronary heart disease (including current or recent smoking, hypercholesterolemia, diabetes mellitus, hypertension, and obesity) were more frequent among cases than controls.6
Screening for polymorphisms
The CX3CR1 gene T280M and V249I mutations were identified after amplification of a 588–base pair (bp) sequence with primers A (5′-CCGAGGTCCTTCAGGAAATCT-3′) and B (5′-TCAGCATCAGGTTCAGGAACTC-3′). The T280M and V249I polymorphisms, which were checked on the same amplified fragment, each disrupted a restriction site for the enzymesBsmBI and Psp1406I, respectively. The polymerase chain reaction (PCR) mixture contained 25 pmol of each primer, 200 μM dNTPs (Life Technologies, Cergy-Pontoise, France), 300 ng genomic DNA, 1 × PCR buffer (ATGC, Marne la Vallée, France), and 0.25 U Taq polymerase (Super Taq; ATGC) in a final volume of 50 μL. The reaction was run in a PTC100AGVHB thermal cycler (MJ Research, Watertown, MA) with 1 minute of denaturation at 94°C, followed by 34 cycles of 30 seconds' denaturation at 94°C, 40 seconds' annealing at 50°C, and 55 seconds' extension at 72°C. The PCR products were digested for 2 hours at 55°C withBsmBI and at 37°C with Psp1406I and checked on 2.5% agarose gels. Two restriction sites for BsmBI are present at positions 216 and 291 of the normal strand (T280), which was completely digested into 3 fragments of 75, 216, and 297 bp; the second site is disrupted in the mutated strand (M280), thus displaying only 2 fragments of 216 and 372 bp. In heterozygous subjects, 4 bands (75, 216, 297, and 372 bp) were present. The V249I polymorphism was detected using Psp1406I. One restriction site for Psp1406I is present at position 205 of the normal strand, which was split into 2 fragments of 205 and 383 bp. This site was disrupted in the mutated strand, which remained undigested (588 bp).
Receptor binding assay
Binding experiments were carried out using 125I-FKN (specific activity = 2200 Ci/mmol protein; Amersham, Saclay, France). PBMCs were isolated from heparinized venous blood from healthy volunteers by one-step centrifugation on a Ficoll separating solution (Biochrom KG, Berlin, Germany). One million PBMCs were incubated in duplicate with increasing amounts of 125I-labeled FKN (0.02-2 nM) in the presence or absence of a 500-fold excess of unlabeled recombinant human FKN (TEBU, Le Perray en Yvelines, France) in Hanks' buffered salt solution (HBSS; Life Technologies, Cergy Pontoise, France) containing 1 mg/mL bovine serum albumin and 0.01% azide, pH 7.4, in a total volume of 200 μL. After incubation for 2 hours at 37°C, unbound chemokine was separated from cells by washing with 1 mL HBSS containing 10% sucrose; γ emissions were then counted in the cell pellet. Nonspecific binding represented less than 10% of total binding and was subtracted from total binding to define specific FKN binding.
Statistical analysis
The Hardy-Weinberg equilibrium was tested using a χ2 test with 1 degree of freedom. A logistic regression analysis was performed with Systat statistical software (SPSS, Chicago, IL) to determine the association of the genotypes with acute coronary events after accounting for sex, age, smoking, and other risk factors (hypercholesterolemia, diabetes mellitus, hypertension, and obesity). The genotype was included in the equation as a 2-class variable (ie, carrying or not carrying the I allele, coded 0 or 1) or as a 3-class variable corresponding to the 3 genotypes (coded 1, 2, and 3). Interactions between risk factors and genotypes were also tested by logistic regression. Binding parameters (Bmax and Kd) were compared between genotypes using analysis of variance (performed between the groups carrying the VV, VI, and II genotypes), followed by the protected least significant difference Fisher test. Results are expressed as mean ± SEM.
Results
The 151 patients (134 with MI and 17 with UA) and 249 controls were well matched in terms of age and sex (Table1). All of the subjects were white. As expected, risk factors for coronary heart disease (including current smoking, hypercholesterolemia, diabetes mellitus, hypertension, and obesity) were more frequent among cases than among controls (P < .005).
General characteristics and selected risk factors for coronary heart disease in patients and controls
| Characteristic . | Cases (n = 151) . | Controls (n = 249) . |
|---|---|---|
| Age (y) | 47.3 ± 7.1 | 48.1 ± 7.1 |
| Range | 27–64 | 28–65 |
| Male sex | 135 (89.4) | 223 (89.5) |
| Current or recent smoker | 118 (78.1)* | 86 (34.5) |
| Hypertension | 33 (21.8)* | 24 (9.6) |
| Hypercholesterolemia | 35 (23.2)* | 20 (8.0) |
| Diabetes | 16 (10.6)* | 5 (2.0) |
| Body mass index ≥ 27.3 kg/m2 | 66 (46.1)* | 57 (22.9) |
| Characteristic . | Cases (n = 151) . | Controls (n = 249) . |
|---|---|---|
| Age (y) | 47.3 ± 7.1 | 48.1 ± 7.1 |
| Range | 27–64 | 28–65 |
| Male sex | 135 (89.4) | 223 (89.5) |
| Current or recent smoker | 118 (78.1)* | 86 (34.5) |
| Hypertension | 33 (21.8)* | 24 (9.6) |
| Hypercholesterolemia | 35 (23.2)* | 20 (8.0) |
| Diabetes | 16 (10.6)* | 5 (2.0) |
| Body mass index ≥ 27.3 kg/m2 | 66 (46.1)* | 57 (22.9) |
Values are mean ± SEM, range, or number of patients (%).
P < .005.
The frequencies of the V249I and T280M polymorphisms showed no deviation from Hardy-Weinberg equilibrium. However, a statistically significant difference in genotype frequencies was observed in cases compared with controls. The adjusted odds ratios (ORs) associated with the presence of the M280 (TM + MM versus TT genotype) and I249 alleles (VI + II versus VV genotype) were 0.49 (95% confidence interval [CI], 0.27-0.89; P = .002) and 0.43 (95% CI, 0.26-0.72; P = .001), respectively (Table2). Thus, these alleles were associated with a reduced risk of acute coronary events. To calculate the OR for each genotype carrying the I249 or M280 allele, we also tested the genotype as a 3-class variable. The adjusted ORs were 0.47 (95% CI, 0.26-0.88; P < .02) and 0.68 (95% CI, 0.09-5.45;P = .7) for the TM and MM genotypes, respectively; they were 0.44 (95% CI, 0.26-0.75; P = .003) and 0.39 (95% CI, 0.13-1.19; P = .099) for the VI and II genotypes, respectively. As shown recently,5 the T280M and V249I polymorphisms are in complete linkage disequilibrium and generate 6 combined genotypes of the 9 theoretically possible (Table3) and only 3 haplotypes (V249T280, I249T280, and I249M280). Indeed, all subjects carrying allele M280 also carry allele I249, whereas in some subjects, allele I249 is associated with allele T280. However, when the T280M polymorphism was used in the same logistic equation as the V249I polymorphism, only the effect of the I249 allele remained significant: Adjusted ORs were 0.84 (95% CI, 1.84-0.39; P = .669) and 0.48 (95% CI, 0.92-0.25; P = .028) for the M280 and I249 alleles, respectively. Therefore, we considered that the protective effect was due to the I249 allele and performed subsequent calculations using the V249I genotypes. No significant interaction was found between genotype and any of the adjustment variables (sex, age, smoking, and other risk factors) when adding interaction terms in the logistic regression.
Genotype frequencies of the V249I and T280M polymorphisms of the CX3CR1 gene in cases and controls
| Genotype . | Cases (n = 151) . | Controls (n = 249) . | OR . | 95% CI . | P . |
|---|---|---|---|---|---|
| T280M | |||||
| MM | 3 (2) | 5 (2) | 0.49* | 0.27–0.89 | .002 |
| TM | 25 (17) | 65 (26) | |||
| TT | 123 (81) | 179 (72) | |||
| V249I | |||||
| II | 7 (5) | 19 (7) | 0.43† | 0.26–0.72 | .001 |
| VI | 47 (31) | 104 (42) | |||
| VV | 97 (64) | 126 (51) |
| Genotype . | Cases (n = 151) . | Controls (n = 249) . | OR . | 95% CI . | P . |
|---|---|---|---|---|---|
| T280M | |||||
| MM | 3 (2) | 5 (2) | 0.49* | 0.27–0.89 | .002 |
| TM | 25 (17) | 65 (26) | |||
| TT | 123 (81) | 179 (72) | |||
| V249I | |||||
| II | 7 (5) | 19 (7) | 0.43† | 0.26–0.72 | .001 |
| VI | 47 (31) | 104 (42) | |||
| VV | 97 (64) | 126 (51) |
Values are the number of subjects (%).
Adjusted OR associated with the MM + TM versus the TT genotype.
Adjusted OR associated with the II + VI versus the VV genotype.
Combined genotype frequencies of the V249I and T280M polymorphisms of the CX3CR1 gene in cases and controls
| Combined genotype . | V249I . | T280M . | Cases (n = 151) . | Controls (n = 249) . |
|---|---|---|---|---|
| 1 | VV | TT | 97 (64) | 126 (51) |
| 2 | II | TT | 2 (1) | 2 (1) |
| 3 | II | MM | 3 (2) | 5 (2) |
| 4 | II | TM | 2 (1) | 12 (5) |
| 5 | VI | TT | 24 (16) | 51 (20) |
| 6 | VI | TM | 23 (15) | 53 (21) |
| Combined genotype . | V249I . | T280M . | Cases (n = 151) . | Controls (n = 249) . |
|---|---|---|---|---|
| 1 | VV | TT | 97 (64) | 126 (51) |
| 2 | II | TT | 2 (1) | 2 (1) |
| 3 | II | MM | 3 (2) | 5 (2) |
| 4 | II | TM | 2 (1) | 12 (5) |
| 5 | VI | TT | 24 (16) | 51 (20) |
| 6 | VI | TM | 23 (15) | 53 (21) |
Values are the number of subjects (%).
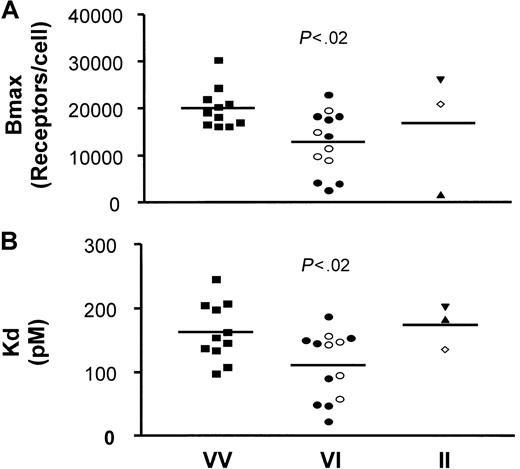
It is important to consider potential mechanisms whereby CX3CR1 could alter predisposition to cardiovascular disease. PBMCs from 27 healthy donors of defined genotypes were tested for FKN binding capacities. From the saturation binding curves (data not shown), we used a nonlinear regression to extrapolate the total number of FKN binding sites (Bmax) and the apparent binding affinity of FKN to PBMCs (Kd) (Figure 1). The binding parameters for individuals with the reference VV genotype appeared more homogeneous than those of the other genotypes. The analysis also revealed a significantly reduced Bmax (Figure1A) when comparing the reference genotype (20 090 ± 1290 sites per PBMC, n = 11) with the VI genotype (12 850 ± 1826 sites per PBMC, n = 13, P < .02). The Bmax values of the VI-TT genotype (12 880 ± 2827 sites per PBMC, n = 8) and VI-TM genotype (12 810 ± 1911 sites per PBMC, n = 5) were similar. Furthermore, Figure 1B shows that the Kd was significantly decreased when comparing PBMCs from individuals with the VV genotype (163 ± 14 pM, n = 11) and with the VI genotype (111 ± 15 pM, n = 13, P < .02). The Kd values of the VI-TT subgroup (104 ± 21.2 pM, n = 8) and the VI-TM subgroup (121 ± 19.3 pM, n = 5) did not differ significantly. These results show that, compared with PBMCs from homozygous VV individuals, PBMCs from heterozygous VI individuals expressed 35% fewer receptors at the cell surface, with an affinity increased by about 30%. Only 3 individuals homozygous for the I249 allele were analyzed, and the binding parameters were highly heterogeneous (16 830 ± 7813 sites per PBMC), with an apparent affinity of 174 ± 21 pM. Thus, a larger study will be needed to accurately compare FKN binding parameters of these individuals with those of other CX3CR1 genotypes.
Binding parameters (Bmax and Kd) of 125I-labeled FKN to PBMCs from VI individuals are reduced compared with PBMCs from VV individuals.
125I-labeled FKN bound specifically to freshly isolated PBMCs. With the use of a nonlinear regression equation based on a single class of FKN binding sites, the total number of FKN binding sites (Bmax) and the apparent FKN affinity (Kd) were extrapolated from the saturation binding curves. Bmax, expressed as the number of FKN binding sites per cell (A), and Kd, expressed as pM (B), were plotted as a function of the CX3CR1 V249I genotypes: VV-TT (▪), VI-TT (●), VI-TM (○), II-TT (▴), II-TM (⋄), and II-MM (▾). Significance by analysis of variance was P < .05 for the Bmax and the Kd values. P values on the figure indicate the significance of the comparison between VV and VI genotypes (protected least significant difference Fisher test). Note that the Bmax and Kd values reported here for the reference genotype (more than10 000 sites per cell with an apparent affinity of 100 pM) are different from those in our previous report (2800 sites per cell with 12 pM affinity).5 The difference may be explained by differences in the study populations: thawed PBMCs from HIV patients in the initial report versus freshly isolated PBMCs from healthy subjects in the present study, and binding conditions of room temperature versus 37°C.
Binding parameters (Bmax and Kd) of 125I-labeled FKN to PBMCs from VI individuals are reduced compared with PBMCs from VV individuals.
125I-labeled FKN bound specifically to freshly isolated PBMCs. With the use of a nonlinear regression equation based on a single class of FKN binding sites, the total number of FKN binding sites (Bmax) and the apparent FKN affinity (Kd) were extrapolated from the saturation binding curves. Bmax, expressed as the number of FKN binding sites per cell (A), and Kd, expressed as pM (B), were plotted as a function of the CX3CR1 V249I genotypes: VV-TT (▪), VI-TT (●), VI-TM (○), II-TT (▴), II-TM (⋄), and II-MM (▾). Significance by analysis of variance was P < .05 for the Bmax and the Kd values. P values on the figure indicate the significance of the comparison between VV and VI genotypes (protected least significant difference Fisher test). Note that the Bmax and Kd values reported here for the reference genotype (more than10 000 sites per cell with an apparent affinity of 100 pM) are different from those in our previous report (2800 sites per cell with 12 pM affinity).5 The difference may be explained by differences in the study populations: thawed PBMCs from HIV patients in the initial report versus freshly isolated PBMCs from healthy subjects in the present study, and binding conditions of room temperature versus 37°C.
Discussion
Our results show that the CX3CR1 I249 allele is associated with a markedly reduced risk of acute coronary events independent of established coronary risk factors. The epidemiologic data correlated with altered FKN binding by PBMCs from I249 heterozygous individuals.
The V249I and T280M polymorphisms, located in the sixth and seventh transmembrane domains of the CX3CR1 protein, respectively, are the first genetic tools for studying the specific role of CX3CR1 in human disease. We previously reported that the CX3CR1 M280 allele was associated with accelerated onset of acquired immunodeficiency syndrome among HIV seroconverters from the SEROCO cohort in France (relative risk = 2.44, P = .016), whereas the I249 allele was not associated with altered HIV disease progression.5 Although the mechanism is not known, it is noteworthy that CX3CR1 has been reported to function in vitro with CD4 as an HIV coreceptor and that the M280 allele is associated with reduced receptor expression on PBMCs from HIV-positive subjects.8 In contrast to HIV, in which accelerated disease progression was restricted to M280 homozygotes, the association of CX3CR1 with acute coronary disorders was noted only for I249 and for heterozygotes. This suggests different effects of the 2 polymorphisms on CX3CR1 function. Our study may have lacked sufficient power to detect an association of M280 with acute coronary disease. A larger study will be necessary to determine disease risk in T280 homozygous subjects who carry the I249 allele alone (II-TT individuals) and to determine whether any supplementary risk is associated with allele M280 in subjects who also carry allele I249 (II-MM individuals).
To test how CX3CR1 polymorphism may affect the risk of acute coronary disease, we examined FKN binding and found that FKN binding-site density on PBMCs from individuals carrying VI genotypes (either VI-TT or VI-TM) was approximately 40% lower (decreased Bmax) than on PBMCs from individuals bearing the reference genotype VV-TT. This would be expected to reduce monocyte adhesion to injured endothelium, and therefore this is a potential mechanism for the reduced risk of acute coronary events associated with this genotype. This hypothesis implies that the small increase (approximately 2-fold) in FKN binding affinity (decreased Kd) for PBMCs observed for I249 heterozygotes has a negligible effect on adhesive and chemotactic function of CX3CR1, which is reasonable because large effects of binding affinity on receptor occupancy will be restricted to a very narrow concentration range of ligand. The binding phenotype of PBMCs from VI heterozygotes is consistent with our previous study of 4 HIV-positive II homozygotes (II-MM compound genotype), in whom FKN binding-site density on PBMCs was only 20% of that of HIV-positive VV-TT controls. In that study, PBMCs from 2 individuals with II-TT compound genotypes also had a reduced FKN binding-site density; however, the difference did not reach statistical significance. In the present study of healthy donors, FKN binding levels on PBMCs from 3 individuals with II-containing compound genotypes were highly variable and not significantly different from those of VV-TT controls. In addition, the binding affinities fell within a narrow range from a 2-fold increase to 3-fold decrease for PBMCs from II-containing compound genotypes in the 2 studies. Further work will be needed to more accurately define FKN binding parameters and FKN function using both primary cells from individuals with these relatively uncommon compound CX3CR1 genotypes, as well as cell lines expressing cloned receptor variants. It will also be important to test whether these alleles are linked to promoter polymorphisms that may alter receptor expression independent of receptor structure.
Ultimately, in vivo models may define the importance of CX3CR1 in atherosclerosis. Recently, CX3CR1-deleted mice have been generated. These animals were viable and fertile and had no spontaneous specific phenotype.9 Placing the CX3CR1-deleted mice in conditions leading to cardiovascular disease may reveal the importance of CX3CR1 in atherosclerosis. Such experiments have already demonstrated a significant role of the monocyte-targeted chemokine monocyte chemoattractant protein-1 (MCP-1) and its specific receptor CCR2 in mouse models of atherogenesis.10 Consistent with this, expression of MCP-1 has been correlated with human coronary artery disease.11 Although many other monocyte-derived chemokine signaling pathways have also been defined and should be considered for potential roles in atherosclerosis, one major candidate protein is CX3CR1.
In conclusion, this study demonstrates that the I249 allele of the CX3CR1 gene is associated with a reduced risk of acute coronary events. The underlying mechanism might involve less efficient interaction between monocytes and injured endothelium in subjects exposed to coronary risk factors, as a result of decreased expression of CX3CR1. These results suggest that agents blocking the FKN–CX3CR1 interaction might help to prevent the onset and progression of atherosclerotic lesions.
Supported by grant 2000005884 from the Fondation de France. S.F. was a recipient of a fellowship from the French Agence Nationale de Recherche sur le SiDA.
D.M. and S.F. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Dominique de Prost, Service d'Hématologie Biologique et Immunologie, Hôpital Louis Mourier AP-HP, 178 rue des Renouillers, 92701 Colombes, France; e-mail:dominique.de-prost@lmr.ap-hop-paris.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal