Abstract
Eotaxin is a potent inducer of eosinophil chemotaxis and was considered as a selective ligand of the CC chemokine receptor 3 (CCR3), which is expressed on eosinophils, basophils, and Th2 lymphocytes. This study shows that eotaxin also interacts with CCR2 and CCR5 and can, thus, affect the responses of monocytes, which express both receptors. In human monocytes pretreatment with eotaxin decreased responsiveness to MCP-1, a selective ligand for CCR2, as well as to RANTES and MIP-1β, which bind to CCR5. Similar effects were obtained with transfected cells expressing CCR2 or CCR5, but here a difference became apparent: Eotaxin triggered CCR5 at a concentration of 100 nM but not CCR2 even at 1 μM, suggesting an antagonistic effect on this receptor. In agreement with this observation, eotaxin induced internalization of CCR5 but not of CCR2 in human monocytes and transfected cells. Binding studies showed that eotaxin displaces125 I-MCP-1 from monocytes in a concentration-dependent manner, and functional experiments showed that eotaxin inhibits MCP-1-induced chemotaxis and enzyme release. The results demonstrate that eotaxin is a CCR5 agonist and a CCR2 antagonist. The present findings suggest a role of eotaxin in the fine-tuning of cellular responses occurring at sites of allergic inflammation, in which both MCP-1 and eotaxin are produced.
Introduction
Eotaxin was originally described as a potent and selective stimulus for eosinophil leukocytes inducing eosinophil migration in vitro and accumulation in vivo.1,2 These effects were shown to be mediated via chemokine receptor 3 (CCR3) that is highly expressed on eosinophils, basophils, and Th2 lymphocytes3-7 and, thus, mainly involved in allergic inflammation.8,9 Pathologic conditions, especially reactions that are dominated by Th2-type cytokines, were found to induce the expression of eotaxin10-12 and MCP-1 in several tissues.13-15 It was also reported that eosinophils secrete MCP-1 after stimulation with C5a, suggesting a mechanism for the production of this chemokine in infiltrates elicited by eotaxin.16 In addition to eosinophils, basophils, and Th2 lymphocytes, which are typically found in allergic inflammations and express CCR3, these infiltrates also contain monocytes that lack CCR3.17 Starting from the observation that eotaxin elicits changes in intracellular free calcium ([Ca++]i) and chemotaxis in human blood monocytes, we studied its effects on chemokine receptors known to occur in these cells, namely CCR1, CCR2, CCR5, and CXCR4. In this study, we show that eotaxin binds to CCR2 and CCR5, inducing opposite effects: antagonism on CCR2 and agonistic activities on CCR5. Our data indicate that eotaxin has a wider pathophysiologic role than previously assumed and may regulate monocyte responses during allergic inflammation.
Materials and methods
Reagents
All chemokines were synthesized as described.18 Monoclonal phycoerythrin (PE)-conjugated anti-CCR2 antibody, secondary antibodies, and isotype-matched immunoglobulins were purchased from R&D Systems (London, United Kingdom). Anti-CCR3 (clone 7B11)17 and anti-CCR5 (clone 5C7)19 antibodies were kindly provided by LeukoSite Inc (Cambridge, MA).
Cells
Monocytes (90% to 95% purity with 5% to 10% contaminating lymphocytes) were isolated from buffy coats of donor blood (Central Laboratory of the Swiss Red Cross, Bern, Switzerland) by centrifugation on Ficoll-Paque and Percoll gradients.20 For some experiments, monocytes were cultured overnight under standard conditions in RPMI 1640 supplemented with 10% fetal calf serum (FCS), 10% nonessential amino acids, 1 mM sodium pyruvate, 0.05 mM β-mercaptoethanol, 50 U/mL penicillin, 50 mg/mL streptomycin, and 2 mM glutamine (all from GIBCO BRL, Rockville, MD). The cells were detached from the dishes by incubation for 10 minutes on ice with 10 mM EGTA in phosphate-buffered saline (PBS), followed by 10 minutes at 37°C in 10 mM EDTA, and finally washed twice in PBS.
Murine pre-B 300-19 cells stably expressing human CCR1, CCR2b, or CCR5 were obtained by transfection. The corresponding receptor complementary DNAs (cDNAs) were cloned into theSmaI/XbaI site of Srαpuro (kindly provided by Dr F. Arenzana-Seisdedos, Institute Pasteur, Paris, France). The cells (5 × 106) were transfected by electroporation with 20 μg cDNA. Positive clones were selected in the presence of 1.5 μg/mL puromycin (Sigma, St. Louis, MO) and tested for chemokine responsiveness by monitoring [Ca++]i changes. Expression of CCR2b and CCR5 was also assessed by flow cytometry.
Functional assays
Chemotaxis was assayed in 48-well chambers (Neuro Probe, Cabin John, MD) by using polyvinylpyrrolidone-free polycarbonate Nucleopore membranes with 5 μm pores. N-acetyl-β-D-glucosaminidase release was assayed in monocytes as described previously.21 [Ca++]i changes were assessed in Fura-2-loaded cells.22 Receptor desensitization was determined by monitoring [Ca++]i changes in response to sequential stimulation with chemokines at 90-second intervals.
Chemokine receptor expression and internalization
Chemokine receptor expression in freshly isolated or cultured monocytes was analyzed by flow cytometry (FACScan, Becton Dickinson, San Jose, CA). The cells were incubated for 20 minutes with 0.1% human immunoglobulin G (IgG; Sandoglobulin; Novartis, Basel, Switzerland) in FACS buffer (2% FCS, 0.1% sodium azide in PBS), washed with FACS buffer, and then incubated for 30 minutes in FACS buffer with PE-conjugated anti-CCR2b, or anti-CCR3 or anti-CCR5, followed by a PE-conjugated goat antimouse antibody. Isotype-matched immunoglobulins were used as control. For determination of chemokine-induced receptor internalization, the cells were incubated for 45 minutes at 37°C with 1 μM eotaxin, MCP-1, or RANTES in FACS buffer without sodium azide before staining. Controls were performed under the same conditions at 4°C. The chemokines used did not affect antibody binding to the receptors.
Receptor binding
MCP-1 was iodinated with the Bolton Hunter reagent (Amersham Pharmacia Biotech, Uppsala, Sweden) according to the manufacturer's instructions. For competition binding studies, freshly isolated monocytes or CCR2b-transfected cells (4 × 106) in 120 μL RPMI-1640 containing 20 mM HEPES, 1% bovine serum albumin (BSA), pH 7.1 were incubated on ice for 90 minutes with 0.8 nM125I-MCP-1 in the presence of increasing concentrations of unlabeled MCP-1 or eotaxin. The cells were then pelleted by centrifugation through 6% BSA in PBS, and the cell-bound radioactivity was determined by gamma-counting. The data were analyzed with PRISM software (GraphPad Software, San Diego, CA), using nonlinear regression in a one-binding-site model.
Results
Eotaxin acts on human monocytes
As shown in Figure 1, eotaxin induced [Ca++]i changes in human monocytes. Responses were significant at concentrations of 300 nM or higher. After pretreatment with eotaxin, monocytes showed a decreased responsiveness to MCP-1, RANTES, and MIP-1β (Figure 1A), suggesting that eotaxin interacts with CCR2, the receptor for MCP-1, and with CCR5 that binds RANTES and MIP-1β. Flow cytometric analysis of our preparations confirmed former evidence by Heath et al17 that monocytes do not express CCR3, the eotaxin receptor (data not shown). Figure 1B shows the [Ca++]i changes obtained when eotaxin was applied after pretreatment of the cells with one of the other chemokines. Ca++ mobilization by eotaxin was virtually abrogated by pretreatment with RANTES or MIP-1β but was not affected by pretreatment with MCP-1, suggesting that eotaxin acts via CCR5 but not via CCR2. Further experiments were performed with pre-B cells transfected with single human chemokine receptors known to be expressed on monocytes. Eotaxin was inactive on cells expressing CCR2b but decreased the responsiveness to MCP-1 (Figure2A). In contrast, eotaxin induced a [Ca++]i rise in cells expressing CCR5 (Figure2B). The rate of the [Ca++]i rise (percentage of FURA-2 saturation per second) was calculated. Table1 summarizes the quantitative effects of pretreatment of the cells with one chemokine on the rate of the [Ca++]i rise induced by a second chemokine. The data are presented as relative values (percentage of control without pretreatment). To rule out the involvement of unidentified eotaxin-triggered receptors on the transfected cells as well as further chemokine receptors expressed on monocytes, namely CCR1 or CXCR4, cells expressing these receptors and untransfected 300-19 pre-B cells were analyzed and failed to respond to eotaxin up to the concentration of 1 μM (data not shown). These results indicate that eotaxin acts on monocytes via CCR5 and is an antagonist for CCR2.
Eotaxin-induced [Ca++]ichanges in monocytes.
FURA-2-loaded monocytes were stimulated sequentially at 90-second intervals with 1 μM eotaxin followed by 10 nM MCP-1, 30 nM RANTES, or 10 nM MIP-1β (A) or vice versa (B), and [Ca++]i-dependent fluorescence changes were recorded. The tracings are representative of 2 to 4 independent experiments performed under identical conditions with cells from different donors.
Eotaxin-induced [Ca++]ichanges in monocytes.
FURA-2-loaded monocytes were stimulated sequentially at 90-second intervals with 1 μM eotaxin followed by 10 nM MCP-1, 30 nM RANTES, or 10 nM MIP-1β (A) or vice versa (B), and [Ca++]i-dependent fluorescence changes were recorded. The tracings are representative of 2 to 4 independent experiments performed under identical conditions with cells from different donors.
[Ca++]i changes in CCR2- and CCR5-transfected cells.
Murine pre-B cells stably expressing human CCR2 or CCR5 were loaded with FURA-2, and [Ca++]i-dependent fluorescence changes were recorded after sequential stimulation with 1 μM eotaxin and 10 nM MCP-1 (for CCR2-expressing cells, A) or with 1 nM MIP-1β (for CCR5-expressing cells, B). Controls were run under the same conditions, using buffer instead of eotaxin. The tracings are representative of 2 independent experiments with each cell line.
[Ca++]i changes in CCR2- and CCR5-transfected cells.
Murine pre-B cells stably expressing human CCR2 or CCR5 were loaded with FURA-2, and [Ca++]i-dependent fluorescence changes were recorded after sequential stimulation with 1 μM eotaxin and 10 nM MCP-1 (for CCR2-expressing cells, A) or with 1 nM MIP-1β (for CCR5-expressing cells, B). Controls were run under the same conditions, using buffer instead of eotaxin. The tracings are representative of 2 independent experiments with each cell line.
Effect of chemokine pretreatment of monocytes and transfected cells expressing CCR2 or CCR5
| Cells . | Pretreatment . | Rate of [Ca++]i rise after stimulation . | |||
|---|---|---|---|---|---|
| Eotaxin . | MCP-1 . | RANTES . | MIP-1β . | ||
| Monocytes | |||||
| None | 100 | 100 | 100 | 100 | |
| Eotaxin | 57 | 64 | 33 | ||
| MCP-1 | 100 | 50 | |||
| RANTES | 0 | 50 | |||
| MIP-1β | 35 | 50 | |||
| CCR2-transfected cells | |||||
| None | 100 | ||||
| Eotaxin | 21 | ||||
| CCR5-transfected cells | |||||
| None | 100 | ||||
| Eotaxin | 22 | ||||
| Cells . | Pretreatment . | Rate of [Ca++]i rise after stimulation . | |||
|---|---|---|---|---|---|
| Eotaxin . | MCP-1 . | RANTES . | MIP-1β . | ||
| Monocytes | |||||
| None | 100 | 100 | 100 | 100 | |
| Eotaxin | 57 | 64 | 33 | ||
| MCP-1 | 100 | 50 | |||
| RANTES | 0 | 50 | |||
| MIP-1β | 35 | 50 | |||
| CCR2-transfected cells | |||||
| None | 100 | ||||
| Eotaxin | 21 | ||||
| CCR5-transfected cells | |||||
| None | 100 | ||||
| Eotaxin | 22 | ||||
The cells were stimulated sequentially with different chemokines, and changes of intracellular free calcium ([Ca++]i) were monitored as shown in Figures1 and 2. The rate of the [Ca++]i rises in the absence of stimulation was set to 100%, and the percentage rate obtained after prestimulation was calculated.
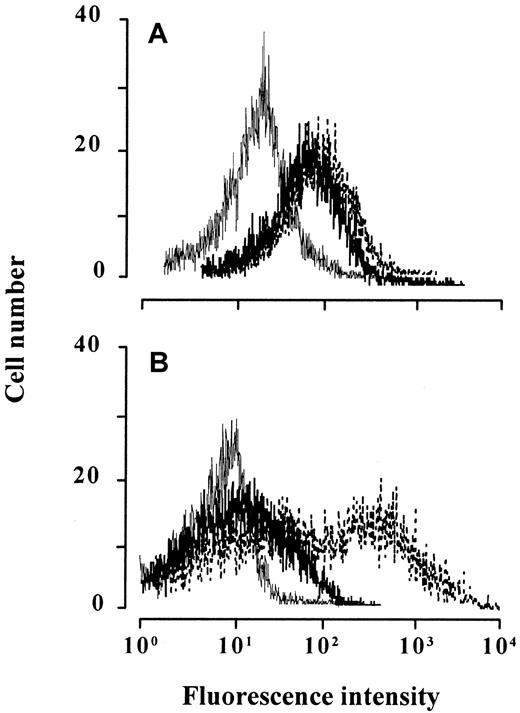
Eotaxin-induced receptor internalization
Chemokines with agonistic activity, in contrast to antagonists, induce the internalization of their receptors. The surface expression of CCR2 and CCR5 was determined on monocytes by flow cytometry before and after treatment with 1 μM eotaxin. As shown in Figure3, eotaxin markedly decreased the level of CCR5 but did not influence the level of CCR2. Eotaxin-dependent CCR5 internalization was obtained in monocytes that were cultured overnight to enhance CCR5 expression prior to the experiment. The uptake of CCR5 but not of CCR2 was also observed when pre-B-cell transfectants expressing one or the other receptor were treated with eotaxin, whereas the total amount of each receptor detected by a combined intracellular and surface staining remained unchanged (data not shown). Together, these findings support the suggestion that eotaxin is an antagonist for CCR2 and an agonist for CCR5.
Chemokine-induced CCR2 and CCR5 internalization on monocytes.
Monocytes immediately after isolation (A) or cultured overnight (B) were treated with 1 μM eotaxin (thick line), the agonists (thin line) MCP-1 (A) or RANTES (B), or medium alone (broken line) for 60 minutes, stained with anti-CCR2 (A) or anti-CCR5 (B), and analyzed by flow cytometry. The histograms show the fluorescence intensity of relative cell number. Tracings are representative of 3 experiments performed with monocytes from different donors.
Chemokine-induced CCR2 and CCR5 internalization on monocytes.
Monocytes immediately after isolation (A) or cultured overnight (B) were treated with 1 μM eotaxin (thick line), the agonists (thin line) MCP-1 (A) or RANTES (B), or medium alone (broken line) for 60 minutes, stained with anti-CCR2 (A) or anti-CCR5 (B), and analyzed by flow cytometry. The histograms show the fluorescence intensity of relative cell number. Tracings are representative of 3 experiments performed with monocytes from different donors.
Eotaxin is a CCR2 antagonist
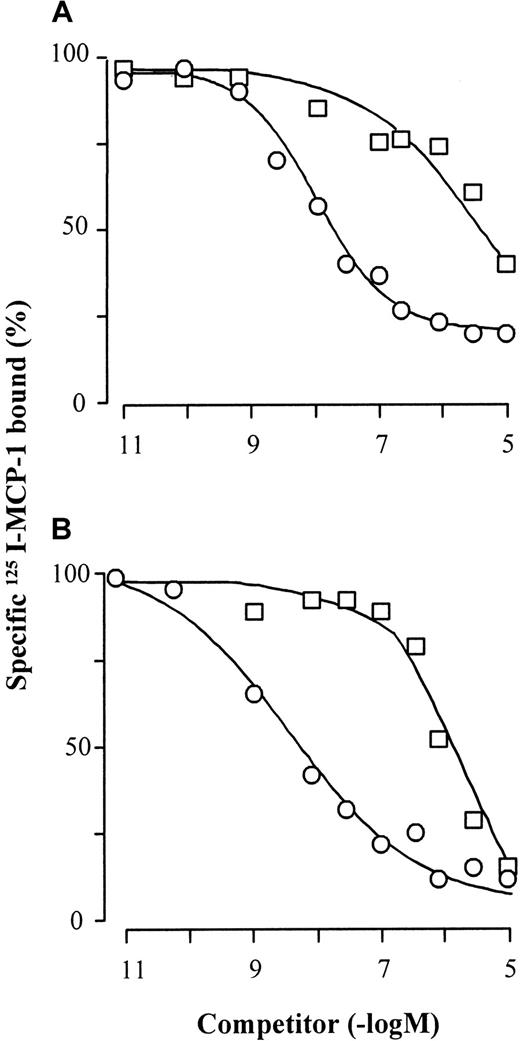
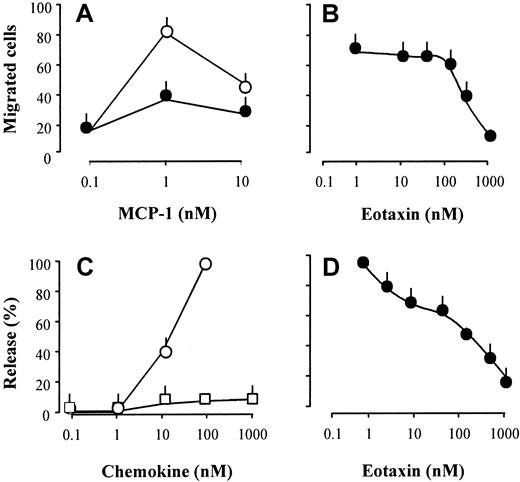
The effects of eotaxin were further explored by receptor binding and functional studies. Eotaxin competed for the binding of125I-MCP-1 on monocytes and CCR2b-transfected cells (Figure4). The ligand displacement curves were parallel to those obtained with unlabeled MCP-1, but the affinity of eotaxin was about 2 orders of magnitude lower than that of the CCR2 selective agonist. This difference is in agreement with the moderate potency of eotaxin as inhibitor of [Ca++]imobilization induced by MCP-1. Migration and enzyme release assays were performed to assess further the effects of eotaxin on monocyte functions. Pretreatment of monocytes with eotaxin inhibited MCP-1-elicited chemotaxis and N-acetyl-β-D-glucosaminidase release in a concentration-dependent manner with half-maximal effects between 100 and 300 nM (Figure 5). In agreement with the former evidence, eotaxin was devoid of agonistic activity. The competition for MCP-1 binding and the inhibition of MCP-1-induced responses, which were obtained in a similar concentration range, indicate again that eotaxin is a CCR2 antagonist.
125I-MCP-1 binding competition on monocytes and CCR2-transfected cells.
Monocytes (A) or CCR2-transfected cells (B) were incubated on ice for 90 minutes with 0.8 nM 125I-MCP-1 in the presence of increasing concentrations of unlabeled MCP-1 (○) or eotaxin (■). The cells were then pelleted by centrifugation through 6% BSA in PBS, and cell-bound radioactivity was counted. The curves (means of duplicate values) are representative of 1 of 4 experiments performed with monocytes from different donors and 3 experiments performed with CCR2 transfectants.
125I-MCP-1 binding competition on monocytes and CCR2-transfected cells.
Monocytes (A) or CCR2-transfected cells (B) were incubated on ice for 90 minutes with 0.8 nM 125I-MCP-1 in the presence of increasing concentrations of unlabeled MCP-1 (○) or eotaxin (■). The cells were then pelleted by centrifugation through 6% BSA in PBS, and cell-bound radioactivity was counted. The curves (means of duplicate values) are representative of 1 of 4 experiments performed with monocytes from different donors and 3 experiments performed with CCR2 transfectants.
Inhibition of CCR2-dependent chemotaxis (A,B) and enzyme release (C,D).
(A) MCP-1–induced chemotaxis of monocytes in the presence (●) or absence (○) of 300 nM eotaxin. (B) Concentration-dependent inhibition of monocyte chemotaxis in response to 1 nM MCP-1 by eotaxin. (C) MCP-1 (○) or eotaxin (■) inducedN-acetyl-β-d-glucosaminidase release in cytochalasin B-treated monocytes. (D) Concentration-dependent inhibition of N-acetyl-β-d-glucosaminidase release in cytochalasin B-treated monocytes in response to 10 nM MCP-1 by eotaxin. Each panel shows mean values of 3 experiments performed with monocytes from different donors (bars indicate SEM).
Inhibition of CCR2-dependent chemotaxis (A,B) and enzyme release (C,D).
(A) MCP-1–induced chemotaxis of monocytes in the presence (●) or absence (○) of 300 nM eotaxin. (B) Concentration-dependent inhibition of monocyte chemotaxis in response to 1 nM MCP-1 by eotaxin. (C) MCP-1 (○) or eotaxin (■) inducedN-acetyl-β-d-glucosaminidase release in cytochalasin B-treated monocytes. (D) Concentration-dependent inhibition of N-acetyl-β-d-glucosaminidase release in cytochalasin B-treated monocytes in response to 10 nM MCP-1 by eotaxin. Each panel shows mean values of 3 experiments performed with monocytes from different donors (bars indicate SEM).
Discussion
In this study, we show that eotaxin, a presumed selective agonist for CCR3, acts as a competitive antagonist on CCR2 and as an agonist on CCR5. The apparent affinity of eotaxin to both receptors is moderate (ie, up to 2 orders of magnitude less than its affinity to CCR3). Both effects, however, are concentration dependent, and the antagonism toward CCR2 is sufficient to reduce the main functional responses of monocytes, chemotaxis and enzyme release to minimal levels. Our observation that eotaxin stimulates CCR5-bearing cells is in agreement with recently reported data by Blanpain et al23 on transfected cells, showing that eotaxin binds to CCR5. Binding or activities of eotaxin on CCR2 have not been reported before.
The present results indicate that eotaxin has potential activities beyond the recruitment of cells expressing CCR3, namely eosinophils,3,4 Th2 lymphocytes,6,7 and possibly basophils5 to sites of allergic inflammation and certain parasitic infestations. Eotaxin was originally considered a major mediator of pathology in allergic conditions and a prime target for therapeutic intervention.8 However, it was found that the so-called eotaxin receptor, CCR3, recognizes a large number of CC chemokines including MCP-2, MCP-3, MCP-4, RANTES, eotaxin-2, and eotaxin-3,24 and we now show that eotaxin also binds, albeit with lower affinity, to CCR2 and CCR5. Inhibition of the interaction of eotaxin with CCR3 was considered as a promising therapeutic strategy for allergic reactions. Despite the present evidence for interaction of eotaxin with other chemokine receptors, blockade of CCR3 still appears a worthy goal of therapy.
CCR2 and CCR5, like CCR3, are receptors for inflammatory chemokines. They are both expressed on monocytes, macrophages, and interleukin 2-activated T lymphocytes, and CCR2 is additionally found on basophils. Ligands that activate CCR2 (ie, MCP-1, MCP-2, MCP-3, and MCP-4) have been characterized as major stimuli for release of enzymes by monocytes and natural killer cells21,25 and of histamine and leukotrienes by basophils.5 Owing to its antagonistic function on CCR2, eotaxin inhibits the release of inflammatory enzymes and possibly leukotrienes and histamine and thus displays anti-inflammatory effects. In this perspective eotaxin can be regarded as a chemokine with a dual activity in chemokine-driven inflammation.
We thank Andrea Blaser for expert technical assistance and Dr Pius Loetscher for useful discussion. This work was also supported by the Helmut Horten Foundation.
Supported by grant 31-055996.98 from the Swiss National Science Foundations to M.B. and by grant OG8/1-1 from the Deutsche Forschungsgemeinschaft to P.O.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mariagrazia Uguccioni, Institute for Research in Biomedicine, 6500 Bellinzona, Switzerland; e-mail:mariagrazia.uguccioni@irb.unisi.ch.

![Fig. 1. Eotaxin-induced [Ca++]ichanges in monocytes. / FURA-2-loaded monocytes were stimulated sequentially at 90-second intervals with 1 μM eotaxin followed by 10 nM MCP-1, 30 nM RANTES, or 10 nM MIP-1β (A) or vice versa (B), and [Ca++]i-dependent fluorescence changes were recorded. The tracings are representative of 2 to 4 independent experiments performed under identical conditions with cells from different donors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/7/10.1182_blood.v97.7.1920/6/m_h80710851001.jpeg?Expires=1769153986&Signature=w2sqCKX2nPiQPcwTJe8IO5FLacZNZFy7oBygwbhy~cKqjVJLZRJ04Tvh9VRash-08b9I~cvAc3iPL1KnPY-Y5~jkIGTf6h6i75MRPsV747pqCLiK9uPFV-0gvKxxA1JXdbv-wrBy9etm6sRfEeBL9zBQM-iG7jOUuO3-MDDUjutG1LlPSgpeUJIcB8OAyjx5BlTyw9GTqebmyvVkHRxWv1pg~fKzCZcS5c3JyiAUXny4hZ6uWhpM2l7OKaUB0bRJRtbt7jlIOTJWzJHmf~ltQQ5oInAmbDqQpJ2A2QSrniJVmRWtwiYuqkz86EaLKM~jjR4v70Gr4sePmCLiGJ7cBw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. [Ca++]i changes in CCR2- and CCR5-transfected cells. / Murine pre-B cells stably expressing human CCR2 or CCR5 were loaded with FURA-2, and [Ca++]i-dependent fluorescence changes were recorded after sequential stimulation with 1 μM eotaxin and 10 nM MCP-1 (for CCR2-expressing cells, A) or with 1 nM MIP-1β (for CCR5-expressing cells, B). Controls were run under the same conditions, using buffer instead of eotaxin. The tracings are representative of 2 independent experiments with each cell line.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/7/10.1182_blood.v97.7.1920/6/m_h80710851002.jpeg?Expires=1769153986&Signature=vV3dajBH2VSOQ37yiMeUxXwegQ~jZ4sShfejfObO06SBRFCVrumHVucHUjxNFXxu~N7Vct6s3p1qH~VVu-9INk4Ip09cnns4PPAggvGm-id27UEtGf6Sj3ZlLSXg1x9OmbQ0aeIG3Ef10xy~HBygo8b74y2sXKoT5HjPYzDYnBXYjYpC-CuajgY2nJerpSNwNVUPUTDjpF2NBm2HnfBDgpRBYFNAwt2ByxW2yVFNj8zTSADAWZDDjx~keHrguOQiMbTfKLjjvXB~BPBD3hdDpDhLM-yb6hFA5ypF0qd6QgdAMYvGJFBoLqsM5tZxemzrTq635-tfpB4-oTI8X2lSLQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal