To further characterize hematopoietic “replicative stress” induced by bone marrow transplantation (BMT), the cell-cycle status of CD90+/− subsets of marrow CD34+ cells obtained 2 to 6 months after transplantation from 11 fully chimeric recipients was examined. Cycling profiles, derived by flow cytometry after staining with Hoechst 33342 and pyronin Y, were compared with those of 14 healthy marrow donors. Primitive CD34+CD90+cells represented a smaller proportion of CD34+ cells in recipients (10% ± 4% versus 19.6% ± 5.3% in donors;P < .0001) and were more mitotically active, with the proportion of cells in S/G2/M nearly 4-fold higher than in donors (15.6% ± 3% and 4.4% ± 1.6%, respectively;P < .0001). By comparison, there was a modest increase in the proportion of CD34+CD90−progenitors in S/G2/M after BMT (10.9% ± 1% vs 9.6% ± 2% in donors; P = .04). Replicative stress after BMT is borne predominantly by cells in a diminished CD34+CD90+ population.
Introduction
The typical marrow graft contains only a small fraction of the donor's hematopoietic cells, yet is required to sustain hematopoiesis for the lifetime of the recipient. Peripheral blood cell counts in the recipient usually return to the normal range within weeks of bone marrow transplantation (BMT). However, recipients have profound deficits in hematopoietic progenitors (determined by functional assays and absolute CD34+ cell enumeration) persisting for more than 10 years after BMT.1-5 The demonstration of accelerated telomere shortening in leukocytes of BMT recipients,6-8 reflecting an increase in the mitotic rate of bone marrow progenitors and/or stem cells, suggests that hematopoietic reconstitution imposes a “replicative stress” upon the graft. The distribution of this stress within the hematopoietic hierarchy is unknown and is of considerable importance. An increase in the proliferative rate of lineage-committed progenitors (LCPs) may have little consequence for the host, but an increase at the level of the stem cell could lead, through critical telomere shortening or other mechanisms, to cytogenetic instability and the emergence of clonal disorders.9
The majority of human hematopoietic stem/progenitor cells express the CD34 antigen.10 In vitro assays,11-13 a recent clinical trial,14 and experiments in immunodeficient mice11,13 and preimmune sheep15have strongly suggested that most primitive hematopoietic progenitors (PHPs) in humans, and virtually all CD34+ repopulating cells, are contained within the CD90+ subset. Although markedly enriched for PHPs, the CD34+CD90+population is heterogeneous and contains some LCPs. The vast majority of LCPs, however, reside in the larger CD34+CD90− subset.11 12
To establish which progenitors respond to the increased demands on hematopoiesis after BMT, we compared the cell-cycle status of CD34+CD90+ and CD34+CD90− marrow cells from engrafted recipients with values obtained in healthy bone marrow donors.
Study design
Subjects
Eleven recipients of matched sibling donor BMT (median age, 51 years; range, 42-58 years) and 14 healthy marrow donors (median age, 48 years; range, 36-61 years) consented to participate in this ethically approved study between September 1999 and March 2000. Among these 25 subjects were 8 donor/recipient pairs. Indications for BMT were non-Hodgkin lymphoma in 4 patients (B, F, H, J), chronic myeloid leukemia in 4 (E, G, I, K), acute myeloid leukemia in 2 (A, C), and chronic lymphocytic leukemia in 1 (D).
Bone marrow transplants
Our institutional transplantation protocol has been previously published.16 We conditioned 9 patients with busulphan and cyclophosphamide, and 2 with radiation-based regimens. All recipients received unmodified bone marrow grafts containing a median of 3.0 × 108 (range, 1.0-3.8) nucleated cells per kilogram recipient weight. Graft versus host disease prophylaxis consisted of short-course methotrexate and cyclosporine A. Hematopoietic cytokines were not administered to any patient after transplantation or to donors before harvest. In all patients, neutrophil counts recovered to greater than 0.5 × 109/L before day 28.
Chimerism studies
Peripheral blood leukocyte DNA was extracted, and amplification of 8 microsatellite regions performed by the polymerase chain reaction, as described.17 Following gel electrophoresis, hematopoietic chimerism was determined by comparing the size of amplified bands from the recipient at the time of bone marrow aspiration after BMT with the size of bands from donor and recipient samples before BMT. The test allows detection of admixtures of donor and recipient DNA down to the level of 5% to 10%.
Cell-cycle analysis of hematopoietic progenitors
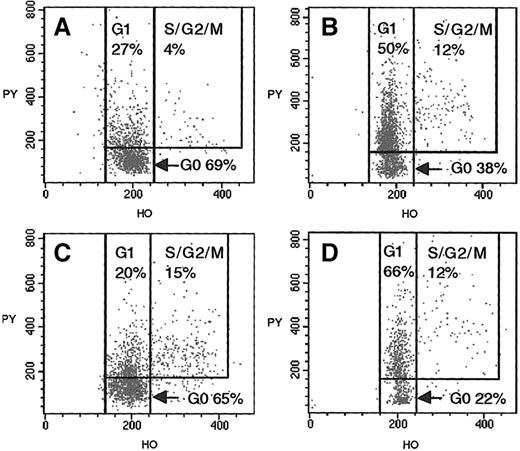
We obtained 5 to 10 mL of bone marrow from 14 donors undergoing bone marrow harvest and from 11 recipients at either 2 months (n = 3) or 6 months (n = 7) after BMT or at both these times (n = 1). CD34+ cells were selected from the low-density mononuclear cell fraction by means of Minimacs CD34 separation columns (Miltenyi Biotec, Auburn, CA).18 CD34+-enriched samples were stained with CD34FITC, CD45PE/CY5, and CD90PE, and the CD90+/− percentages of CD34+ cells were determined by flow cytometry. CD34+CD90+ and CD34+CD90− cells were sorted on a FACSVantage cell sorter (Becton Dickinson Biosciences, San Jose, CA) equipped with Enterprise (Coherent, Santa Clara, CA) and Hene (Spectra-Physics 127, Mountain View, CA) lasers, as previously described.19,20 Sort purity exceeded 94%, and viability 90%, in all cases. Sorted CD34+CD90+ and CD34+CD90− cells were incubated with 1 μg/mL of Hoechst 33342 (stains DNA) and 1 μg/mL pyronin Y (stains RNA), and their cell-cycle status was ascertained by flow cytometry (Figure 1).21-23 A mean of 910 events (range, 300-2126) were gated for CD34+CD90+ cells. The continuous staining pattern observed with pyronin Y prohibits absolute discrimination between CD34+ cells in G0 and G1. To achieve consistent fluorescence-2 (FL2) channel settings, the instrument was calibrated by means of Calibrite beads (BDIS, San Jose, CA). Thereafter, delineation of G0 and G1subsets was accomplished with the discriminator set at a mean FL2 channel setting of 160 (vertical axis, Figure 1). Single-parameter histograms of Hoechst staining were used to confirm discrimination between cells in G0/G1 and those in S/G2/M.
Cell-cycle analysis of CD34+CD90+ and CD34+CD90− progenitors from BMT donor (donor 4) at time of bone marrow harvest, and fully chimeric matched recipient (recipient D) 6 months after BMT.
(A) Flow cytometric analysis of donor CD34+CD90+ cells, after staining with Hoechst 33342 and pyronin Y. Analysis of the cellular uptake of these vital dyes allows the discrimination of G0 (2N DNA + low levels of RNA), G1 (2N DNA + higher levels of RNA), and S/G2/M (greater than 2N DNA + higher levels of RNA) phases of the cell cycle. (B) Donor CD34+CD90− cells. (C) Analysis of recipient's CD34+CD90+ cells 6 months after BMT. (D) Recipient CD34+CD90− cells.
Cell-cycle analysis of CD34+CD90+ and CD34+CD90− progenitors from BMT donor (donor 4) at time of bone marrow harvest, and fully chimeric matched recipient (recipient D) 6 months after BMT.
(A) Flow cytometric analysis of donor CD34+CD90+ cells, after staining with Hoechst 33342 and pyronin Y. Analysis of the cellular uptake of these vital dyes allows the discrimination of G0 (2N DNA + low levels of RNA), G1 (2N DNA + higher levels of RNA), and S/G2/M (greater than 2N DNA + higher levels of RNA) phases of the cell cycle. (B) Donor CD34+CD90− cells. (C) Analysis of recipient's CD34+CD90+ cells 6 months after BMT. (D) Recipient CD34+CD90− cells.
Statistical analysis
Means and standard deviations were calculated. Univariate analyses were performed by means of a 2-tailed independent ttest. A paired 2-tailed t test was applied to data from the 8 matched donor/recipient pairs. Analyses were performed by means of the SAS statistical program (SAS-PC, Version 8.0; SAS Institute, Cary, NC).
Results and discussion
At the time of bone marrow aspiration after BMT, all recipients were complete hematopoietic chimeras. Their median hemoglobin concentration was 104 g/L (range, 93-127 g/L), their white blood cell count 4.3 × 109/L (range, 2.7-9.3 × 109/L), and their platelet count 134 × 109/L (range, 10-264 × 109/L). One recipient was platelet-transfusion–dependent 2 months after BMT.
The proportion of CD34+ marrow cells expressing the CD90 antigen was reduced in these recipients (10% ± 4% compared with 19.6% ± 5.3% in donors; P < .0001). This diminished CD34+ CD90+ population showed marked changes in cell-cycle status after BMT (Tables1 and2). Specifically, 15.6% ± 3% were in S/G2/M compared with 4.4% ± 1.6% of donor cells (P < .0001), and 43.8% ± 13% were in G0 compared with 71.3% ± 12.3% of donor cells (P < .0001). Progenitors tested 2 months after BMT yielded results similar to those assessed at 6 months. When changes in CD90 expression by CD34+ cells and cycling status of CD34+CD90+ progenitors were examined within the 8 matched transplant pairs, the significance of these changes was retained (P < .001 for each analysis).
CD90 expression by bone marrow CD34+ cells, and cycling status of CD34+CD90+ and CD34+CD90− progenitors in bone marrow transplantation donors
| . | Donors . | Mean ± SD . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 11 . | 22 . | 33 . | 44 . | 55 . | 66 . | 77 . | 88 . | 9 . | 10 . | 11 . | 12 . | 13 . | 14 . | ||
| CD34+cells | |||||||||||||||
| % CD90+ | 16 | 22 | 18 | 33 | 19 | 15 | 15 | 28 | 19 | 14 | 20 | 19 | 16 | 21 | 19.6 ± 5.3 |
| % CD90− | 84 | 78 | 82 | 67 | 81 | 85 | 85 | 72 | 81 | 86 | 80 | 81 | 84 | 79 | 80.4 ± 5.3 |
| CD34+CD90+ | |||||||||||||||
| % G0 | 92 | 85 | 58 | 69 | 91 | 65 | 58 | 80 | 77 | 62 | 74 | 58 | 58 | 71 | 71.3 ± 12.3 |
| % S/G2/M | 3 | 6 | 6 | 4 | 2 | 5 | 3 | 5 | 5 | 6 | 3 | 7 | 4 | 2 | 4.4 ± 1.6 |
| CD34+CD90− | |||||||||||||||
| % G0 | 69 | 65 | 33 | 38 | 81 | 29 | 25 | 60 | 56 | 18 | 27 | 38 | 17 | 35 | 42.2 ± 20.3 |
| % S/G2/M | 7 | 10 | 7 | 12 | 7 | 8 | 12 | 13 | 10 | 12 | 9 | 9 | 10 | 8 | 9.6 ± 2.1 |
| . | Donors . | Mean ± SD . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 11 . | 22 . | 33 . | 44 . | 55 . | 66 . | 77 . | 88 . | 9 . | 10 . | 11 . | 12 . | 13 . | 14 . | ||
| CD34+cells | |||||||||||||||
| % CD90+ | 16 | 22 | 18 | 33 | 19 | 15 | 15 | 28 | 19 | 14 | 20 | 19 | 16 | 21 | 19.6 ± 5.3 |
| % CD90− | 84 | 78 | 82 | 67 | 81 | 85 | 85 | 72 | 81 | 86 | 80 | 81 | 84 | 79 | 80.4 ± 5.3 |
| CD34+CD90+ | |||||||||||||||
| % G0 | 92 | 85 | 58 | 69 | 91 | 65 | 58 | 80 | 77 | 62 | 74 | 58 | 58 | 71 | 71.3 ± 12.3 |
| % S/G2/M | 3 | 6 | 6 | 4 | 2 | 5 | 3 | 5 | 5 | 6 | 3 | 7 | 4 | 2 | 4.4 ± 1.6 |
| CD34+CD90− | |||||||||||||||
| % G0 | 69 | 65 | 33 | 38 | 81 | 29 | 25 | 60 | 56 | 18 | 27 | 38 | 17 | 35 | 42.2 ± 20.3 |
| % S/G2/M | 7 | 10 | 7 | 12 | 7 | 8 | 12 | 13 | 10 | 12 | 9 | 9 | 10 | 8 | 9.6 ± 2.1 |
CD90 expression by bone marrow CD34+ cells, and cycling status of CD34+CD90+ and CD34+CD90− progenitors in bone marrow transplantation recipients
| . | Recipients . | Mean ± SD . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 . | B2 . | C3 . | D4 . | D4 . | E5 . | F6 . | G7 . | H8 . | I . | J . | K . | ||
| Months after BMT | 2 | 2 | 2 | 2 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | |
| CD34+cells | |||||||||||||
| % CD90+ | 12 | 19 | 8 | 10 | 7 | 8 | 7 | 15 | 10 | 12 | 5 | 8 | 10 ± 4 |
| % CD90− | 88 | 81 | 92 | 90 | 93 | 92 | 93 | 85 | 90 | 88 | 95 | 92 | 90 ± 4 |
| CD34+CD90+ | |||||||||||||
| % G0 | 49 | 37 | 30 | 55 | 65 | 41 | 21 | 49 | 27 | 48 | 49 | 55 | 43.8 ± 13 |
| % S/G2/M | 15 | 13 | 19 | 13 | 15 | 22 | 19 | 14 | 15 | 11 | 15 | 16 | 15.6 ± 3 |
| CD34+CD90− | |||||||||||||
| % G0 | 71 | 33 | 49 | 34 | 22 | 28 | 53 | 29 | 27 | 40 | 30 | 36 | 37.7 ± 13.8 |
| % S/G2/M | 9 | 12 | 11 | 10 | 12 | 12 | 10 | 12 | 11 | 10 | 11 | 11 | 10.9 ± 1 |
| . | Recipients . | Mean ± SD . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 . | B2 . | C3 . | D4 . | D4 . | E5 . | F6 . | G7 . | H8 . | I . | J . | K . | ||
| Months after BMT | 2 | 2 | 2 | 2 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | |
| CD34+cells | |||||||||||||
| % CD90+ | 12 | 19 | 8 | 10 | 7 | 8 | 7 | 15 | 10 | 12 | 5 | 8 | 10 ± 4 |
| % CD90− | 88 | 81 | 92 | 90 | 93 | 92 | 93 | 85 | 90 | 88 | 95 | 92 | 90 ± 4 |
| CD34+CD90+ | |||||||||||||
| % G0 | 49 | 37 | 30 | 55 | 65 | 41 | 21 | 49 | 27 | 48 | 49 | 55 | 43.8 ± 13 |
| % S/G2/M | 15 | 13 | 19 | 13 | 15 | 22 | 19 | 14 | 15 | 11 | 15 | 16 | 15.6 ± 3 |
| CD34+CD90− | |||||||||||||
| % G0 | 71 | 33 | 49 | 34 | 22 | 28 | 53 | 29 | 27 | 40 | 30 | 36 | 37.7 ± 13.8 |
| % S/G2/M | 9 | 12 | 11 | 10 | 12 | 12 | 10 | 12 | 11 | 10 | 11 | 11 | 10.9 ± 1 |
There was a slight increase in the proportion of CD34+CD90− cells in S/G2/M after BMT (10.9% ± 1% compared with 9.6% ± 2% in donors;P = .04), but the significance of this difference must be regarded as questionable owing to the number of univariate analyses performed. There was no change in the proportion of CD34+CD90− progenitors in G0(Table 1). The increase in proliferation of CD34+CD90+ cells after BMT was such that the proportion of these cells in S/G2/M exceeded the proportion of CD34+CD90− cells in the same phases (P = .003)—a reversal of the pattern seen in steady-state bone marrow.
We offer 3 methodologic caveats. First, phenotypic designations of “primitivity,” such as the one we have used, are limited by inevitable omissions. For example, very rare primitive CD34−Lin− cells are excluded from the CD34+CD90+ population. Second, we have compared phenotypically, but not necessarily functionally, identical cells in steady-state and posttransplant marrow. The functional properties of the CD34+CD90+ population may differ in BMT recipients, in whom functional constituents may be depleted to varying extents. Finally, the method we used for cell-cycle analysis allows precise definition of cells in S/G2/M, but the absolute distinction of cells in G0 and G1on the basis of differential RNA content24 is somewhat arbitrary. The significance of our reported G0 proportions, derived in a consistent manner in these experiments, lies in the difference between donor and recipient values.
We have determined proportions, not absolute numbers, of CD90+/− subsets of CD34+ cells in the marrow of study subjects. The weight of existing evidence suggests that absolute numbers of both subsets are diminished for prolonged periods after transplantation.1-5 Selleri et al5examined the bone marrow of BMT donors and recipients and found 3- to 4-fold fewer CD34+ cells in the marrow of recipients as late as 8 years after BMT. The accentuated decline in the CD90+ subset reported here suggests that this deficit is most pronounced in primitive progenitors, and it anticipates the finding that post-BMT reductions in long-term-culture–initiating cells are more profound, and prolonged, than reductions in LCPs.4 5
Our results imply that early hematopoietic reconstitution after BMT is associated with a compensatory increase in mitotic rate in a population of CD34+CD90+ progenitors. The accelerated proliferation of these cells is the most likely cause of the striking telomere shortening observed in leukocytes of BMT recipients,6-8 though direct correlation must be sought in future studies. Further characterization of these “stressed” CD34+CD90+ cells is required before the consequences of the increase in their mitotic rate may be inferred and tested.
We thank Robert Wynn (Royal Children's Hospital, Manchester, United Kingdom) for many useful discussions and Leslie Steele, Tracy Stockley, and Peter Ray for assessment of hematopoietic chimerism.
Supported by a 1999 Young Investigator Award from the American Society of Clinical Oncology (I.T.), and by a Seed Grant from The Hospital for Sick Children Research Institute.
Submitted June 27, 2000; accepted November 1, 2000.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hans A. Messner, Department of Medical Oncology and Hematology, Rm 5107, 610 University Ave, Toronto, ON M5G 2M9, Canada; e-mail: hans.messner@uhn.on.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal