Interleukin-6 (IL-6), although often regarded as a B-cell differentiation factor, was recently described as the essential survival factor for human plasmablasts in vivo in reactive plasmacytosis. The present study reinvestigated the roles of IL-6 and IL-2 in the generation of plasma cells from human memory B cells in vitro. The cells involved in this differentiation process were identified as preplasmablasts (CD20±CD38±CD138−), plasmablasts (CD20−CD38++CD138−), and early plasma cells (CD20−CD38+++CD138+++). IL-2 or IL-10 induced a strong generation of plasmablasts and early plasma cells (PCs). Compared to IL-2 or IL-10, IL-6 alone was inefficient at PC generation. However, when combined with IL-2 or IL-10, IL-6 enhanced generation of early PCs. Moreover, anti–IL-6 monoclonal antibody markedly reduced IL-2–induced generation of early plasma cells, but not of plasmablasts. These roles of IL-2 and IL-6 were consistent with the difference in the expression of their respective receptors (R). CD25 (IL-2Rα) was increased 72 ± 10-fold on activated B cells, but decreased and then disappeared on plasmablasts. Conversely, CD126 (IL-6Rα) was barely expressed on activated B cells, but increased 18 ± 2-fold on preplasmablasts. Finally, IL-6 enhanced the proliferation (2-fold increase) of IL-2–generated plasmablasts. In conclusion, the data indicate that IL-6 is a growth factor for nonmalignant human plasmablasts.
Introduction
The study of plasma cell (PC) generation remains difficult in humans because these cells are located within bone marrow and represent less than 0.5% of mononuclear cells. PC generation has been investigated in mice using transgenic or knockout models,1,2 whereas studies in humans have essentially involved in vitro models of B-cell activation and differentiation.3-8 Most of these models have supported an essential role for interleukin-2 (IL-2) and IL-10 in the differentiation of B cells previously activated through T-cell contact or upon CD40 or Staphylococcus aureus Cowanstimulation. A recent study by our group showed that reactive plasmacytosis is a very suitable model for in vivo investigation of PC generation from plasmablasts, their progenitors, in humans.9 This model allowed us to isolate and characterize plasmablasts and study their transition into early PCs. The reactive plasmacytoses studied were all associated with high serum levels of C-reactive protein, indicating the presence of systemic IL-6 in excess, and the plasmablasts and early PCs expressed IL-6R (receptor) (CD126) strongly. Finally, IL-6 was found to be the essential survival factor for plasmablasts in this model.
In the transgenic mouse, overexpression or disruption of IL-6 in vivo has profound effects on the synthesis of immunoglobulin (Ig) and PC generation, suggesting a fundamental role of IL-6 in the B-cell immune response.1,2 These observations in mice and humans are in contrast to the apparent lack of IL-6 involvement in the differentiation of B cells in vitro. In fact, IL-6, although unable to support PC differentiation in some models,10,11 was involved in others.5,12-15 When involved, its precise role remained controversial because it was regarded either as a growth16 or differentiation factor.5 17 The present study used the most efficient model of PC generation in vitro—the differentiation of human memory B cells into PCs—to reinvestigate the role of IL-6. Our particular concern was the involvement of IL-2 and IL-6 in the generation of plasmablasts and their transition into early PCs.
Materials and methods
Monoclonal antibodies and cytokines
The following monoclonal antibodies (mAbs) and cytokines were used (manufacturers' clones noted in parentheses): anti-CD138 (B-B4) and anti–IL-6 (BE-8) mAbs (Diaclone Research, Besançon, France); anti-CD25–PE (phycoerythrin) and anti-CD38–PE mAbs (Becton Dickinson, San Jose, CA); unconjugated, PE-conjugated, or fluorescein isothiocyanate (FITC)–conjugated IgG1 control (Immunotech International, Marseilles, France); anti-CD20–FITC, anti-CD126–PE, anti-CD130–PE, FITC-conjugated goat antimouse (GAM), anti-CD2, anti-CD4, and anti-CD8 (Immunotech); anti-BrdU BU5-1 mAb (Cymbus Bioscience, Euromedex, Souffelweyersheim, France); biotinylated anti-IgD (HJ9) and streptavidin-quantum red conjugate (PECY5) (Sigma Chemical, St Louis, MO); anti-CD40L–blocking mAb (gift of Dr P. Garrone, Schering-Plough Co, Dardilly, France); IL-10 (Schering-Plough); anti-CD38 mAb (gift of Dr L. Boumsell, Créteil, France); and IL-6 (Novartis, Basel, Switzerland). B-B4 mAb was conjugated to biotin as previously described.18
Purification of memory B cells
Memory B cells were purified from human tonsils obtained from children and adults undergoing routine tonsillectomy.7Briefly, tonsils were finely minced in phosphate-buffered saline (PBS), and mononuclear cells were isolated by a standard Ficoll-Hypaque gradient method (Eurobio, Les Ulis, France). T cells were depleted by sheep rosetting. The E− fraction was subjected to 2-step depletion of nonmemory B cells using a magnetic activated cell sorter (MACS) (Miltenyi Biotec, Bergisch Gladbach, Germany). For naive B-cell depletion, the E− fraction was incubated with biotinylated antihuman IgD polyclonal antibodies for 15 minutes at 4°C, washed with PBS, and incubated with streptavidin-coated microbeads (Miltenyi) for another 15 minutes at 4°C. After washing, cells were loaded onto a MACS LS+separation column, and the IgD− fraction was collected. Further depletion of germinal center (GC) B cells, monocytes, and remaining T and natural killer (NK) cells was performed as follows: the IgD− fraction was incubated with anti-CD38, anti-CD2, anti-CD4, and anti-CD8 mAbs and then with antimouse IgG-coated magnetic beads. The depletion of labeled cells was performed on a MACS LS+ column. This procedure produced 95% to 99% pure memory B-cell populations.
Two-step culture for plasma cell generation
Memory B cells (1 × 106 cells per well) were first activated for 4 days over a 75-Gy–irradiated CD40L-transfected 3T6 fibroblast monolayer (0.25 × 106 cells per well) in a 24-well plate in 1 mL RPMI 1640 medium and 10% fetal calf serum (FCS) in the presence of 50 U/mL IL-2 and 100 ng/mL IL-10.7 Cells were harvested, washed, and seeded (0.1 × 106 cells per well) in 200 μL RPMI 1640 plus 10% FCS in a 96-well U-bottomed plate with various combinations of cytokines (IL-2, IL-6, or IL-10) or anti–IL-6 mAb (BE-8). In all experiments, 2 μg/mL anti-CD40L–blocking mAb was added to neutralize remaining CD40L+fibroblasts.7 The beginning of the second culture constituted day 0. Supernatants and cells were harvested as indicated.
Cell staining and flow cytometry
For surface immunostaining, cells were incubated at 4°C for 30 minutes with the different mAbs in simple-, double-, or triple-staining in PBS containing 1% bovine serum albumin (BSA) and 0.02% NaN3 (sodium azide) and then washed and fixed in PBS 1% formaldehyde. For unconjugated or biotinylated mAbs, a second incubation was performed prior to fixation with FITC-GAM or PECY5-conjugated streptavidin. Flow cytometry analysis was carried out on a FACScan (fluorescence-activated cell sorter) using CELLQuest software (both from Becton Dickinson). The fluorescence intensity ratio (r) was obtained by dividing the fluorescence of each antigen by the fluorescence of the appropriate control. Apoptotic cells were identified and counted by Apo2.7 staining as previously described.9
The labeling index was performed as previously described (with slight modifications).19 Briefly, 106 cells were incubated with or without (control) 50 μM BrdU for 2 hours at 37°C in a 5% carbon dioxide (CO2) incubator, washed, stained with anti-CD38–PE mAb, and permeabilized overnight in PBS containing 1% paraformaldehyde and 0.01% Tween 20 at 4°C. Permeabilized cells were incubated for 30 minutes at 37°C with 50 Kunitz units of deoxyribonuclease (DNAse) I, washed in 0.5% Tween 20, and labeled for 30 minutes at room temperature with anti–BrdU-FITC mAb in the presence of 10% AB serum. Fluorescence analysis was performed on a FACScalibur flow cytometer (Becton Dickinson).
Proliferation assays
For proliferation assays, 0.037 MBq (1 μCi)3H-TdR (3H-thymidine) was added during the final 48 or 72 hours of culture. Experiments were conducted in triplicate, and results are expressed as cpm ± SD.
Results
Kinetics of plasmablast and plasma cell generation from memory B cells
Purified memory B cells were activated by CD40L+-3T6 fibroblasts in the presence of IL-2 and IL-10 for 4 days and then induced to differentiate (day 0) by removal of CD40L+ fibroblasts.7 During the entire differentiation time course (days 0-10), cells were collected, and their phenotype was determined. As shown in Figure1, 4 critical differentiation stages were defined using triple-staining with CD20, CD38, and CD138. At day 0, all cells had the typical phenotype of activated B cells, ie, CD20+CD38+ (Figure 1A). Beginning in day 1 or 2, a fraction of activated B cells progressively entered into the differentiation process, which was characterized by down-regulation of CD20 expression and up-regulation of CD38 expression (Figure 1B). These cells, designated as preplasmablasts, differentiated first into CD20−CD38++CD138− plasmablasts (Figure 1C,E; day 4) and then into CD20−CD38+++CD138++ early PCs (Figure 1D,F; day 7). The appearance of CD138, a specific PC marker,18,20 characterized the transition from plasmablasts to early PCs, as previously described in reactive plasmacytosis.9 This process was always observed, regardless of the number of PCs obtained (median, 50%; range, 30% to 80%).
Kinetics of plasma cell differentiation.
Resting memory cells were activated for 4 days with CD40L+fibroblasts in the presence of 50 U/mL IL-2 and 100 ng/mL IL-10 as described in “Materials and methods.” At the end of activation, cells were harvested and washed. Activated B cells (day 0) were then recultured for 7 days with IL-2 and IL-10 in the presence of anti-CD40L mAb. The cell phenotype was analyzed in double-staining with anti-CD38–PE mAb and anti-CD20–FITC mAb (A, B) or in triple-staining with anti-CD38–PE, anti-CD20–FITC, and anti-CD138–PECy5 mAbs (C-F). (A) Activated B cells CD20+CD38±; (B) preplasmablasts CD20±CD38+; (C, E) plasmablasts CD20−CD38++CD138−; and (D, F) early plasma cells CD20−CD38+++CD138++. Results are representative of 1 out of more than 10 experiments. Insert: Changes in viable cell number during PC generation. Apoptotic cells were identified and counted by Apo2.7 staining. Results are given as the mean ± SD of 6 experiments.
Kinetics of plasma cell differentiation.
Resting memory cells were activated for 4 days with CD40L+fibroblasts in the presence of 50 U/mL IL-2 and 100 ng/mL IL-10 as described in “Materials and methods.” At the end of activation, cells were harvested and washed. Activated B cells (day 0) were then recultured for 7 days with IL-2 and IL-10 in the presence of anti-CD40L mAb. The cell phenotype was analyzed in double-staining with anti-CD38–PE mAb and anti-CD20–FITC mAb (A, B) or in triple-staining with anti-CD38–PE, anti-CD20–FITC, and anti-CD138–PECy5 mAbs (C-F). (A) Activated B cells CD20+CD38±; (B) preplasmablasts CD20±CD38+; (C, E) plasmablasts CD20−CD38++CD138−; and (D, F) early plasma cells CD20−CD38+++CD138++. Results are representative of 1 out of more than 10 experiments. Insert: Changes in viable cell number during PC generation. Apoptotic cells were identified and counted by Apo2.7 staining. Results are given as the mean ± SD of 6 experiments.
The PCs obtained were designated as early PCs because their CD19+CD20−CD38+++CD45++CD138+++phenotype (data not shown) was reminiscent of that of early PCs during reactive plasmacytosis and in tonsils, but not of the phenotype of CD19+/−CD45weak mature bone marrow PCs.9,18,21 The differentiation process was associated with a decrease of viable cell number, as previously reported (Figure1, insert).7 This decrease resulted from apoptosis of activated B cells and differentiating cells (data not shown). The identification and quantification of each subset of cells (preplasmablasts, plasmablasts, and early PCs) then allowed us to investigate the role of IL-2, IL-6, and IL-10 in the generation of plasmablasts and their transition into early PCs.
IL-6 sustains IL-2– or IL-10–induced generation of plasma cells
PC generation driven by IL-2 + IL-10 was not further enhanced by addition of IL-6, except when IL-10 was reduced to a suboptimal concentration such as 10 ng/mL (data not shown). The role of IL-6 in PC generation was investigated further by culturing activated B cells with IL-2 or IL-10 alone or in combination with IL-6. As shown in Figure2A, IL-6 alone slightly enhanced PC generation (20% vs 12% without cytokine). This effect was detectable between the plasmablast and early PC stages. IL-10 alone induced PC generation that was further enhanced upon addition of IL-6 (30% vs 20% at day 7; Figure 2B), but reduced in the presence of anti–IL-6–blocking BE-8 mAb (8% vs 20% at day 7).
IL-6 sustains the IL-2 generation of PCs.
Activated B cells (day 0) were cultured for up to 10 days with or without 50 U/mL IL-2, 10 ng/mL IL-6, 10 ng/mL IL-10, 50 μg/mL anti–IL-6 BE-8 mAb as indicated in the figure (A-C). (D) Activated B cells (day 0) were cultured for 7 or 10 days with 50 U/ml IL-2. As indicated, 10 ng/mL IL-6 was added either at day 0, 4, or 7; the percentage of PCs was determined at day 7 (for IL-6 added at day 0 or 4) or day 10 (for IL-6 added at day 7). Results show 3 representative experiments out of 5.
IL-6 sustains the IL-2 generation of PCs.
Activated B cells (day 0) were cultured for up to 10 days with or without 50 U/mL IL-2, 10 ng/mL IL-6, 10 ng/mL IL-10, 50 μg/mL anti–IL-6 BE-8 mAb as indicated in the figure (A-C). (D) Activated B cells (day 0) were cultured for 7 or 10 days with 50 U/ml IL-2. As indicated, 10 ng/mL IL-6 was added either at day 0, 4, or 7; the percentage of PCs was determined at day 7 (for IL-6 added at day 0 or 4) or day 10 (for IL-6 added at day 7). Results show 3 representative experiments out of 5.
IL-2 promoted the generation of plasmablasts (41%) that differentiated into early PCs (50% at day 7 and 55% at day 10; Figure 2C). Addition of IL-6 did not modify the number of plasmablasts (41%), but increased the number of early PCs (72% at day 7 and 70% at day 10). In the presence of IL-2 and anti–IL-6 BE-8 mAb, the number of plasmablasts was slightly reduced at day 4 (27% vs 41%), and the number of early PCs was markedly reduced until disappearance (13% at day 7 and 0% at day 10). This effect indicated that autocrine IL-6 was produced and suggested that endogenous IL-6 is critical for the PC generation process. To determine exactly when IL-6 was required, IL-6 was added at day 0, 4, or 7. Figure 2D shows that IL-6 was required between days 0 and 7 and not afterward. IL-6 was most efficient in promoting PC generation when it had been present since day 0 (58% of PCs at day 7 with IL-6 since day 0 vs 44% of PCs with IL-6 since day 4). These results indicate that IL-2 was required for plasmablast generation and IL-6 for PC generation.
IL-6R (CD126) up-regulation is specific for plasmablasts
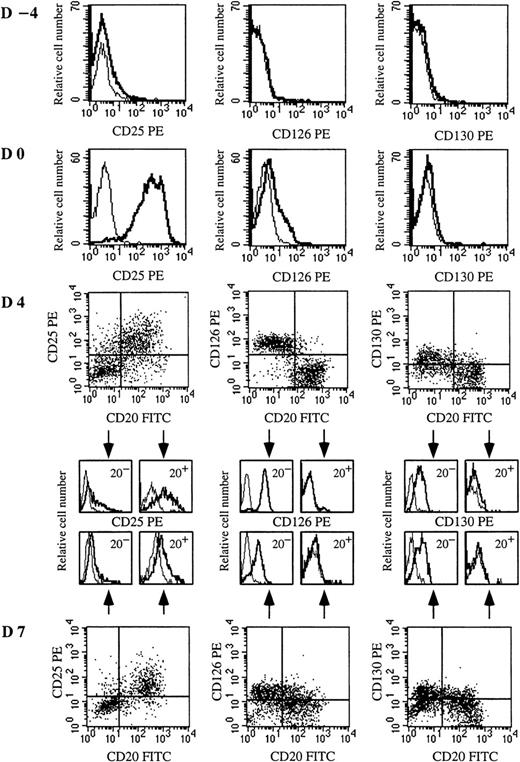
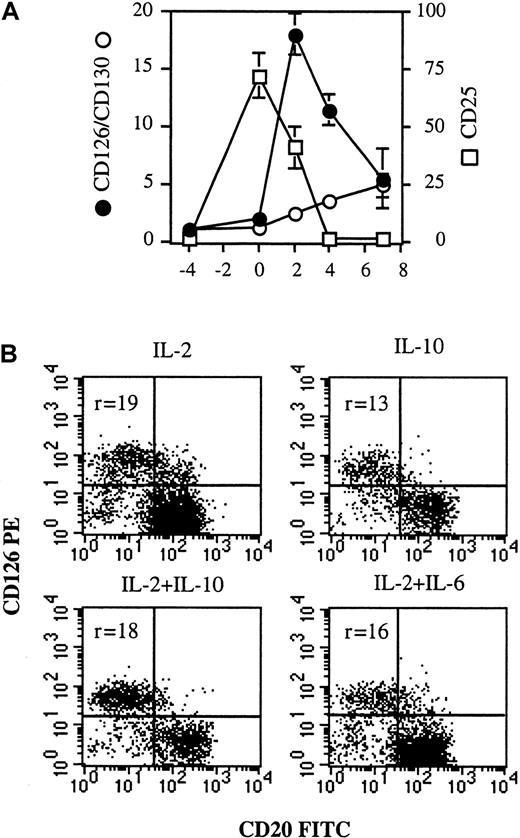
To identify the cellular targets of IL-2 and IL-6, the expression of IL-6R (CD126 and CD130) and IL-2Rα (CD25) was defined from quiescent memory B cells to early PCs (days −4 to 7). As shown in Figure 3, quiescent memory B cells did not express CD126 or CD130 (day −4). CD40 activation induced only slight expression of CD126 and no expression of CD130 (day 0). Conversely, after disruption of CD40 stimulation, CD126 was markedly and specifically upregulated only in cells (CD20− cells) involved in the differentiation process (day 4). CD130 was also up-regulated in CD20− cells. Thus, memory B cells began to express IL-6R once CD40 activation stopped, ie, when they started to differentiate. It is noteworthy that the kinetics of CD126 and CD130 expression was different during the subsequent differentiation stages. The expression of CD126, which was very strong on preplasmablasts (r = 18 ± 1.8), decreased during further maturation into plasmablasts (r = 11.4 ± 1.3) and early PCs (r = 5.5 ± 2.6), but CD130 was continuously enhanced up to the early PC stage (Figures 3 and 4A).
IL-6Rαβ (CD126 and CD130) and IL-2Rα (CD25) are up-regulated on plasmablasts or CD40-activated B cells, respectively.
Resting memory B cells were cultured as described in Figure 1. Expression of CD25, CD126, and CD130 was determined on resting memory B cells (day −4), CD40L-activated B cells (day 0), plasmablasts (day 4), and plasma cells (day 7). IL-2R and IL-6R expression was determined in simple staining at days −4 and 0 because the whole population was CD20+. From day 2, IL-2R and IL-6R expression was determined in double-staining with anti-CD20–FITC and anti-CD25–PE mAbs or anti-CD20–FITC and anti-CD126–PE or anti-CD20–FITC and anti-CD130–PE mAbs, respectively. Overlay histograms represent the immunofluorescence of CD25, CD126, or CD130 (thick line) and the control (thin line). Results show 1 experiment representative out of 4.
IL-6Rαβ (CD126 and CD130) and IL-2Rα (CD25) are up-regulated on plasmablasts or CD40-activated B cells, respectively.
Resting memory B cells were cultured as described in Figure 1. Expression of CD25, CD126, and CD130 was determined on resting memory B cells (day −4), CD40L-activated B cells (day 0), plasmablasts (day 4), and plasma cells (day 7). IL-2R and IL-6R expression was determined in simple staining at days −4 and 0 because the whole population was CD20+. From day 2, IL-2R and IL-6R expression was determined in double-staining with anti-CD20–FITC and anti-CD25–PE mAbs or anti-CD20–FITC and anti-CD126–PE or anti-CD20–FITC and anti-CD130–PE mAbs, respectively. Overlay histograms represent the immunofluorescence of CD25, CD126, or CD130 (thick line) and the control (thin line). Results show 1 experiment representative out of 4.
CD126 up-regulation is specific for plasmablasts and not dependent on cytokine combination.
(A) From days −4 to 0, IL-2R and IL-6R expression was determined in simple-staining. From days 2-7, IL-2R and IL-6R expression was determined in double-staining with anti-CD20–FITC and anti-CD25–PE mAbs or anti-CD20–FITC and anti-CD126–PE or anti-CD20–FITC and anti-CD130–PE mAbs, respectively, and only the phenotype of the CD20− population (the differentiated population) was reported. Results are expressed as the intensity fluorescence ratio mean ± SD of 4 experiments. (B) Activated memory B cells (day 0) were cultured for 2 days in the presence of (1) 50 U/mL IL-2 or (2) 100 ng/mL IL-10 or (3) IL-2 + IL-10 or (4) IL-2 + 10 ng/mL IL-6. CD126 expression was determined as described in Figure 3. Results show 2 representative experiments out of 3.
CD126 up-regulation is specific for plasmablasts and not dependent on cytokine combination.
(A) From days −4 to 0, IL-2R and IL-6R expression was determined in simple-staining. From days 2-7, IL-2R and IL-6R expression was determined in double-staining with anti-CD20–FITC and anti-CD25–PE mAbs or anti-CD20–FITC and anti-CD126–PE or anti-CD20–FITC and anti-CD130–PE mAbs, respectively, and only the phenotype of the CD20− population (the differentiated population) was reported. Results are expressed as the intensity fluorescence ratio mean ± SD of 4 experiments. (B) Activated memory B cells (day 0) were cultured for 2 days in the presence of (1) 50 U/mL IL-2 or (2) 100 ng/mL IL-10 or (3) IL-2 + IL-10 or (4) IL-2 + 10 ng/mL IL-6. CD126 expression was determined as described in Figure 3. Results show 2 representative experiments out of 3.
CD25 was not expressed on quiescent B cells (Figure 3, day −4), but strongly induced upon CD40 activation in the presence of IL-2 and IL-10 (Figure 3, day 0). The expression of CD25 decreased rapidly during subsequent differentiation, disappearing at the CD20−plasmablast stage, whereas residual undifferentiated CD20+B cells retained strong CD25 expression (Figure 3, day 4; and Figure4A). To determine whether CD126 up-regulation was dependent on cytokines or specific to plasmablasts, activated B cells were cultured for 2 days with (1) IL-2 or (2) IL-10 or (3) IL-2 + IL-10 or (4) IL-2 + IL-6. Although a varying proportion of preplasmablasts (CD20− cells) was obtained, depending on the efficiency of cytokines to induce PC generation (Figure 4B), CD126 was always up-regulated.
Our results clearly show that the kinetics of CD25 and CD126 expression differed, confirming that IL-2 and IL-6 had distinct roles in the process. IL-2 was essential to plasmablast generation, and CD25 was highly expressed on activated B cells. IL-6 was involved after the generation of (pre)plasmablasts, and IL-6R was highly expressed on (pre)plasmablasts. Regardless of the number of preplasmablasts obtained (depending on the cytokine combination), up-regulation of CD126 was always observed and would appear to be a hallmark of (pre)plasmablasts.
IL-6 is a growth factor for plasmablasts
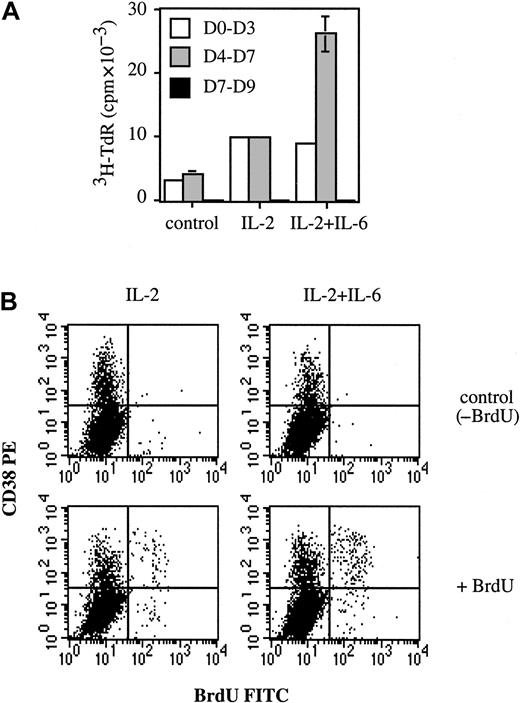
The fact that IL-6 enhanced the number of early PCs induced by IL-2 and anti–IL-6 mAb reduced it, suggested that IL-6 could be a growth and/or survival factor for plasmablasts. Accordingly, IL-6 was added to determine whether PC proliferation would be modified. Proliferation was determined at days 0-3, 4-7, and 7-9. As shown in Figure 5A, thymidine incorporation was detected at days 0-3 and 4-7, but not at days 7-9. This lack of proliferation was consistent with our previous results showing that cells at days 7-9 were nonproliferative early PCs. Figure 5A shows that proliferation at days 0-3 was enhanced 3-fold by IL-2 (9786 ± 76 cpm vs 3165 ± 125 cpm), but no longer stimulated upon addition of IL-6 (8818 ± 80 cpm). In contrast, addition of IL-6 strongly increased (230%) IL-2–induced proliferation at days 4-7 (25 856 ± 2726 cpm vs 9753 ± 117 cpm). These results indicate that IL-2 and IL-6 enhanced proliferation at days 0-3 and 4-7, respectively. The enhancement induced by IL-6 at days 4-7 could only have been due to stimulation of plasmablast proliferation. B cells no longer proliferated and, unlike plasmablasts, did not express IL-6R (Figures 3and 4A). To demonstrate that plasmablasts really constituted the proliferative cellular population, BrdU incorporation was performed at day 4. As shown in Figure 5B, BrdU+ cells were nearly all (more than 80%) found within the CD38++ (plasmablast) population. The labeling index of plasmablasts was 1.9-fold higher in the presence of IL-6, ie, 19.5% (20.5% − 1%) for IL-2 + IL-6 vs 10.2% (10.7% − 0.5%) for IL-2. The labeling index of the nonplasmablast population (CD38− cells) was always below 1%. These results indicate that IL-6 specifically enhanced the labeling index of plasmablasts.
IL-6 is a growth factor for plasmablasts.
(A) Activated B cells (day 0) were cultured alone or with 50 U/mL IL-2 with or without 10 ng/mL IL-6. Proliferation was determined by3H-TdR incorporation from days 0-3, 4-7, or 7-9. Experiments were performed in triplicate wells, and results are expressed as cpm ± SD. Results are representative of one out of 2 experiments. (B) At day 4, cells were incubated for 2 hours at 37°C with or without 1 mM BrdU (control). CD38-PE and BrdU-FITC staining was performed as described in “Materials and methods.” Results are representative of 1 experiment out of 2.
IL-6 is a growth factor for plasmablasts.
(A) Activated B cells (day 0) were cultured alone or with 50 U/mL IL-2 with or without 10 ng/mL IL-6. Proliferation was determined by3H-TdR incorporation from days 0-3, 4-7, or 7-9. Experiments were performed in triplicate wells, and results are expressed as cpm ± SD. Results are representative of one out of 2 experiments. (B) At day 4, cells were incubated for 2 hours at 37°C with or without 1 mM BrdU (control). CD38-PE and BrdU-FITC staining was performed as described in “Materials and methods.” Results are representative of 1 experiment out of 2.
Discussion
IL-6 has generally been regarded as a B-cell differentiation factor in vitro, whereas our group recently showed that it is a survival factor for highly proliferative plasmablasts in reactive plasmacytosis in vitro. The present study reinvestigated the role of IL-6 in PC generation in vitro from activated memory B cells rather than plasmablasts. Phenotypic analysis throughout the differentiation process allowed successive identification of CD20+CD38±CD138− activated B cells, CD20±CD38+CD138−preplasmablasts, CD20−CD38+++CD138− plasmablasts, and CD20−CD38+++CD138+++ early PCs. As in reactive plasmacytosis, syndecan-1 (CD138) was the only molecule that distinguished early PCs from plasmablasts. Most studies of PC generation in vitro have been documented on the basis of Ig secretion, without any real evaluation of the nature of the Ig-secreting cells obtained, ie, plasmablasts versus early PCs. However, our study reinvestigated the role of IL-2 and IL-6 in PC generation relative to the generation of plasmablasts and early PCs.
Compared to IL-2 or IL-10, IL-6 alone was inefficient in inducing PC generation. However, when combined with IL-2 or IL-10, IL-6 enhanced early PCs at day 7, but not plasmablasts at day 4. The fact that this enhancement effect was no longer detectable when IL-6 was added at day 7 suggests that PC survival was dependent on other factors.12,15,22 Moreover, as anti–IL-6 BE-8 mAb decreased the number of early PCs obtained, endogenous IL-6, though undetectable by enzyme-linked immunosorbent assay (ELISA) (data not shown), was involved in IL-2 or IL-10 generation of PCs. The origin of endogenous IL-6 was not investigated, but autocrine IL-6 may have been produced following activation of B cells. Other studies have shown that CD40 stimulation of B cells induced IL-6 production.23 24
Previous reports described IL-2 as a growth and differentiation factor for B cells, although the cellular targets of IL-2 could not be identified because of a lack of phenotypic characterization.25-27 The present study indicates that the role of IL-2 in the B-cell differentiation process is to generate plasmablasts from activated B cells. Finally, IL-10 has also been described as a growth and differentiation factor for B cells.28 In our study, activated B cells differentiated into plasmablasts and early PCs in the presence of 100 ng/mL IL-10, and addition of IL-6 or neutralization of endogenous IL-6 did not subsequently modify the process (data not shown). Nevertheless, when IL-10 was reduced to suboptimal concentrations (eg, 10 ng/mL), the number of early PCs obtained was enhanced by IL-6 and decreased by anti–IL-6 mAb. This lack of IL-6 involvement in PC generation in the presence of high concentrations of IL-10 may have been due to the existence of a partly shared signaling pathway for IL-6 and IL-10 involving signal transducer and activator of transcription (STAT)-3 activation (as described in hepatocytes).29 This shared pathway could have been fully stimulated by high concentrations of IL-10.
The respective effects of IL-2 and IL-6 were confirmed by the kinetics of IL-6R (CD126 and CD130) and IL-2Rα (CD25). CD25 and CD126 expression was regulated differentially and discriminated activated memory B cells (CD25+++CD126−) from plasmablasts (CD25−CD126++) and subsequently from early PCs (CD25−CD126+). Weaker up-regulation of CD25 was previously reported on tonsillar B cells stimulated by CD40.30 In our experiment, CD25 up-regulation disappeared quickly for cells specifically involved in the differentiation process. These results suggest that IL-2 has a preferential effect during the early stages of activation and proliferation of B cells and their differentiation into preplasmablasts. Quiescent memory B cells did not express CD126, which was induced weakly upon CD40 activation. CD126 expression on naive and germinal center B cells was previously reported.31
However, our results show for the first time that differentiation following CD40 signal disruption was associated with strong expression of CD126. This expression was detectable 24 hours after CD40 signal disruption and was observed in the presence of IL-2 or IL-10 alone, which suggests that CD40 disruption could have been the CD126-inducing factor. CD126 expression was very strong on preplasmablasts and then decreased progressively. It is noteworthy that the disappearance of CD126 was associated with a marked release of sCD126, an IL-6 agonist (data not shown). Strong expression of CD126 on plasmablasts appears to be characteristic of these cells because CD126 was also highly expressed in vivo on plasmablasts from reactive plasmacytoses.9 Thus, the effect of IL-6 on PC generation observed here suggests that the cellular target of IL-6 was plasmablasts, which is consistent with the kinetics of its receptor expression. The fact that PC number was increased by IL-6 and decreased by anti–IL-6 mAb (BE-8) suggests that IL-6 could be a growth factor for plasmablasts. In fact, IL-6 enhanced the proliferation of plasmablasts generated by IL-2, but IL-6 could also have been a survival factor for IL-2–generated plasmablasts because the neutralization of autocrine IL-6 decreased the number of early PCs to zero, which indicated that cell viability was not maintained. Moreover, our previous work showed that IL-6 was the essential survival factor for plasmablasts in vivo in reactive plasmacytoses.9
Taken together, our results indicate that IL-6 is a growth and survival factor, but not a differentiation factor, for plasmablasts. IL-6 has been described both as a B-cell growth and differentiation factor, although differentiation was evaluated essentially through Ig secretion, regardless of the cells obtained (plasmablasts or early PCs).5,16,17 Conversely, in vivo nonmalignant or malignant PC expansions have all been shown to be IL-6–dependent. Nonmalignant PC expansions, such as reactive plasmacytoses, were IL-6 dependent both in vivo and in vitro.9,32-34 Malignant PC expansions, ie, multiple myeloma, plasma cell leukemia, and Castleman disease, were also IL-6 dependent, and these PC expansions were reduced with anti–IL-6 treatment.35-39 Furthermore, the overall level of IgG was significantly reduced in IL-6 KO mice, again indicating a central role for IL-6 in PC generation in vivo.2 Thus, our current data in vitro are in good agreement with the central role played by IL-6 in supporting nonmalignant or malignant PC expansions in vivo.
The absolute need for IL-6 in IL-2–induced PC generation from memory B cells was previously suggested by Dubois et al.40 Although the mechanism was not investigated, these authors found that IL-2–induced Ig synthesis was almost totally stopped when anti-CD126 mAb was added. They also determined that PC generation from naive B cells was dependent on IL-12, rather than IL-6, which could explain why some studies have not found IL-6 involvement in B-cell differentiation. Moreover, PC generation was not previously documented in terms of plasmablasts versus PCs because CD138 expression was not reported. As the role of IL-6 is only detectable after plasmablast generation, studies ending at the plasmablast stage would not have found any role for IL-6. Furthermore, the involvement of IL-6 was more apparent when IL-6 was neutralized (though nearly undetectable) than when it was added, especially in the presence of IL-10. Taken together, these observations could explain why IL-6 was not previously identified as an essential growth and survival factor for plasmablasts.
In conclusion, this study shows that IL-2 promotes plasmablast generation and that IL-6 is a growth (and survival) factor for plasmablasts rather than a differentiation factor for B cells or plasmablasts. This role of IL-6 is consistent with its involvement in benign and malignant PC expansions in vivo in humans.
The authors are grateful to Dr K. Thielemans (Brussels, Belgium) and Dr P. Garrone (Schering-Plough, Dardilly, France) for providing CD40L-3T6 transfectant and anti-CD40L mAb, respectively, and to Dr N. Robillard (Nantes, France) for assistance in BrdU experiments. G.J. was supported by the Association pour la Recherche contre le Cancer (ARC).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Catherine Pellat-Deceunynck, INSERM U463, Institut de Biologie, 9 quai Moncousu, 44093 Nantes Cedex 01, France; e-mail: cpellat@nantes.inserm.fr.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal