The α subunit of the human granulocyte-macrophage colony-stimulating factor (GM-CSF) receptor has several isoforms that result from alternative splicing events. Two forms, α-1 and α-2, have intracytoplasmic sequences that are identical within a membrane-proximal domain but differ completely distally. Variant and mutated GM-CSF receptor α subunits, along with the β subunit (βc protein) were expressed in M1 murine leukemia cells. and the ability of the receptors to signal for differentiation events and to activate Jak/Stat signaling pathways was examined. All cell lines expressing both α and βc proteins exhibited high-affinity binding of radiolabeled human GM-CSF. Receptor α subunits with intact membrane-proximal intracellular domains could induce expression of the macrophage antigen F4/80 and down-regulate the expression of CD11b. Addition of recombinant human GM-CSF to cells expressing α-1 subunits induced the expression of CD86 and tyrosine phosphorylation of Jak-2 and its putative substrates SHPTP-2, Stat-5, and the GM-CSF receptor βc subunit. Cells containing α subunits that lacked a distal domain (term-3) or had the alternatively spliced α-2 distal domain showed markedly decreased ability to support tyrosine phosphorylation of Jak-2 and its substrates or to up-regulate CD86. Ligand binding induced stable association of the α-1 subunit and βc protein. In contrast, the α-2 subunit did not stably associate with the βc subunit. These data identify potential molecular mechanisms for differential signaling of the α-1 and α-2 proteins. The association of unique signaling events with the 2 active GM-CSF α subunit isoforms offers a model for variable response phenotypes to the same ligand.
Introduction
The mechanisms by which hematopoietic cytokines effect multiple biologic responses have been the subjects of many studies.1 Granulocyte-macrophage colony-stimulating factor (GM-CSF) stimulates both the growth and the differentiation of hematopoietic cells. The GM-CSF receptor consists of an α and a β subunit. The α subunit binds GM-CSF, whereas the β subunit complexes with the α protein and reconstitutes the high-affinity receptor. Eight alternatively spliced variants of the GM-CSF α subunit have been detected. The α-1 subunit (400 amino acids) is largely external and has a short, 54–amino acid internal tail.2 In contrast, the α-2 subunit is 410 amino acids long.3 In the α-2 subunit, the C-terminal 25 amino acids of the α-1 sequence are replaced by a 35–amino acid stretch that is rich in serine and proline residues. Six additional splicing variants of the α subunit lack the transmembrane and intracellular domains, and some of these are likely secreted.4-7
Receptors for IL-3, IL-5, and GM-CSF share a common β subunit (βc) but each receptor has a unique α subunit. These cytokines generally induce similar signal transduction events and response phenotypes when tested in cell cultures. Nevertheless, certain differences have been described. In factor-dependent human leukemia cells, IL-3 and GM-CSF induce proliferation by protein kinase C–dependent mechanisms, whereas IL-5–induced proliferation is not inhibited by protein kinase C antagonists.8 In a subline of HL60 cells that expresses receptors for both GM-CSF and IL-5, the former cytokine was able to stimulate leukotriene synthesis, but the latter was ineffective.9 In some cell lines GM-CSF is able to prevent cell death induced by the phosphatidylinositol-3–kinase inhibitor wortmannin, but IL-3 is ineffective.10 Other cell lines can be induced to differentiate by GM-CSF, whereas IL-3 supports blast-like proliferation.11 Because all 3 cytokine receptors share a common βc receptor component, differences in their response phenotypes likely result from signaling events regulated by the α subunits. Furthermore, because the membrane-proximal regions of the α subunits are highly homologous, differences in response phenotypes could be particularly dependent on signaling events regulated by the variable carboxy-terminal domains of the various α subunits.
Specific regions of the GM-CSF receptor βc subunit have been shown to be important in the binding of signal transduction intermediates12,13 and the regulation of response phenotypes.14,15 In contrast, the α subunit is less well studied. The α subunit protein does not appear to bind Jak-2 directly.13 However, it does regulate GM-CSF–induced responses in hematopoietic cells. Deletion of the entire α intracytoplasmic domain blocked GM-CSF–induced cell growth and differentiation.16 These effects appear to require a proline-rich membrane-proximal domain that can also be found in the α subunits of the IL-3 and IL-5 receptors.
Few data exist examining the differential signaling events triggered by the GM-CSF receptor α-1 and α-2 subunits. Both receptor isoforms appear to be expressed in the primary and immortalized hematopoietic cell lines examined to date.17 Both α subunits can support the propagation of a proliferation signal in factor-dependent murine cells.17 Thus, it is unclear whether there are unique biologic or biochemical events mediated by the alternative isoforms of the GM-CSF receptor.
To further evaluate the function of the α-1 and α-2 subunits and the importance of specific segments of the α subunits in signaling, we have produced murine leukemia cells containing the human GM-CSF receptor common βc subunit in combination with variant or mutant α subunits. Our results suggest the existence of at least 2 signaling domains of the α subunit that variably regulate Jak/Stat signaling and the expression of the differentiation antigens F4/80, CD11b, and CD86.
Materials and methods
Cell lines and culture methods
The murine leukemia cell line M1 was used for all experiments. Cells were maintained in Dulbecco modified Eagle medium (DMEM) with 10% fetal calf serum. For studies of clonogenic growth, the cells were plated at 200 cells/35-mm dish, in DMEM 10% fetal calf serum, and 0.3% agar. Seven days later colonies were counted with an inverted-stage microscope and scored for number and morphology.
Plasmids and gene transduction
cDNAs encoding the α-1 subunit and the common βcsubunit of the human GM-CSF receptor have previously been described,2,14 as have the truncated mutants term-1 and term-3.18 The cDNA for the human α-2 subunit was kindly provided by Dr Colin Sieff.3 A chimeric α-1 subunit protein (hereafter GM5), in which the C-terminal amino acids have been replaced by the corresponding amino acids of the human IL-5 receptor α subunit protein, was constructed by a polymerase chain reaction–based technique, using an IL-5 receptor α chain cDNA provided by Dr J. Tavernier. All α subunit cDNAs were ligated into the retroviral transduction plasmid pLXSN19 and sequenced fully to ensure fidelity of the sequence. The βc subunit cDNA was in the mammalian expression plasmid pEF/BOS.20
The human GM-CSF receptor βc cDNA was introduced into M1 cells by electroporation, and a single clone (M1/βc #7) was used for all subsequent experiments requiring coexpression of α and βc subunits.
α subunits were then expressed in M1/βc cells by retroviral transduction. Plasmids encoding α subunit variants were packaged in the amphotropic packaging cell line PA317, as described previously.21 Briefly, recombinant retroviruses were produced by transiently transfecting HEK293 cells with plasmids encoding pol and ecotrophic env, along with pLXSN plasmid containing retroviral backbone, gag sequences, and transgene of choice. Supernatant from the transfected cells was used to infect PA317 cells, and pools of retrovirus-producing packaging cells were selected by growth in G418. M1/βc cells were cocultivated with packaging cells for 48 hours, and nonadherent cells were removed and selected in G418. At least 3 independently derived pools of retrovirally infected cells were produced for each βc–α subunit combination. Parental M1 cells were also cocultivated with packaging cells to produce pools of cells expressing only α-1 or α-2 subunits.
Reagents
Recombinant human GM-CSF (rhGM-CSF) was from Immunex (Seattle, WA), and MG132 and tyrosine kinase inhibitors were from Alexis (San Diego, CA). Sodium iodide I 125–labeled rhGM-CSF was obtained from DuPont (Boston, MA).
Receptor characterization
Transduced M1 cells were characterized initially by flow cytometry, using an anti-βc monoclonal antibody (Chemicon, Temecula, CA) and an anti-α subunit monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Functional characterization was performed by radioligand-binding studies using the program Ligand for Scatchard analysis.
Immunochemical studies
Signal transduction events associated with the various receptors were characterized by combined immunoprecipitation–immunoblotting experiments. Cells were cultured with rhGM-CSF (100 ng/mL) for various periods and then were washed once in ice-cold phosphate-buffered saline with 1 mM sodium orthovanadate. Pelleted cells were lysed in detergent buffer (20 mM Tris, pH 7.5, 100 mM NaCl, 5 mM EDTA, 1% Triton X-100, 1 mM sodium orthovanadate, 50 mM NaF, plus protease inhibitors), clarified by centrifugation, and used for immunochemical studies. Lysate derived from approximately 20 million cells was used for each immunoprecipitation. The following antibodies were used: anti–Jak-2, anti–SHPTP-2, anti-Stat-5 (Santa Cruz); anti-βc(Chemicon [monoclonal], Santa Cruz [polyclonal]), and antiphosphotyrosine (UBI, Lake Placid, NY). Detection was with either protein G-peroxidase or anti-(species) IgG-peroxidase conjugates, followed by enhanced chemiluminescence reagents (Amersham, Piscataway, NJ).
For coimmunoprecipitation studies, cell pellets (typically derived from 30 × 106 cells) were lysed in coimmunoprecipitation buffer (50 mM Tris, pH 7.5, 150 mM NaCl, NP-40 1%, 1 mM EDTA, 1% glycerol, 1 mM dithiothreitol, and protease inhibitors). The precipitates were washed twice in high-salt buffer (coimmunoprecipitation buffer with 500 mM NaCl, 0.1% NP-40) and twice in low-salt buffer (coimmunoprecipitation buffer with 0.1% NP-40, but no NaCl). Precipitates were then solubilized and analyzed by immunoblotting with the appropriate antibodies.
Differentiation studies
Pools of M1 cells were treated with rhGM-CSF (100 ng/mL) in culture for 3 days. Differentiation of M1 cells in response to cytokine treatment was assessed through flow cytometry and morphology. In each flow cytometry experiment, fluorescence with specific antibodies was compared with that from an isotype-matched control antibody. Specific fluorescence was quantitated by comparing the 2 curves through Kolmogorov–Smirnoff analysis. The parameter D/s(n) was used to represent the overall difference between the curves and, hence, specific fluorescence. The antibodies: anti-F4/80 and anti-CD11b (Biosource, Camarillo, CA) and anti-CD86 (Caltag, Burlingame, CA) were used for this analysis. Morphology was assessed on Giemsa-stained cytocentrifuge preparations.
Results
Expression of human GM-CSF receptors in M1 leukemia cells
The human GM-CSF receptor βc cDNA was introduced into M1 cells by electroporation, along with a puromycin resistance plasmid. A single clone of puromycin-resistant cells, in which expression of the βc protein was detected by flow cytometry, was used for all subsequent studies in which βc and α subunits were coexpressed. These M1/βc cells were cocultured with retroviral packaging lines that produced amphotropic viruses encoding 5 variant human GM-CSF receptor α subunits (Figure 1). The intracytoplasmic domain of the α-1 subunit of the receptor is 54 amino acids long. In comparison, in the α-2 splicing variant, the C-terminal 25 amino acids of the α-1 cytoplasmic domain are replaced by 35 amino acids rich in serine and proline. Three additional α subunit mutants were created by polymerase chain reaction–based mutagenesis. In the term-3 α subunit, a stop codon was inserted after 34 intracytoplasmic amino acids, and in the term-1 α subunit, a stop codon was placed after only 5 amino acids of the intracytoplasmic portion of the receptor. Finally, a GM5 α subunit was constructed containing the first 18 amino acids of the GM-CSF receptor α subunit intracytoplasmic domain (including the critical proline residues) and 36 amino acids derived from the carboxy terminal amino acids of the IL-5 α subunit.22 Additional pools of M1 cells, expressing only the α-1 or α-2 subunits, were also prepared.
Sequence of variant and mutant GM-CSF receptor α subunits
. The α-1 and α-2 isoforms are natural splicing variants of the α subunit. They differ completely from their C-terminal amino acids (italics). The GM5 chimeric α subunit consists of sequences from the proximal proline-rich domain of the GM-CSF receptor α subunit (bold) fused to distal sequences from the human IL-5 α subunit (underlined). The term-3 mutant α subunit lacks the C-terminal amino acids because of placement of a stop codon after the codons for 34 intracellular amino acids. The term-1 mutant α subunit has only 5 intracellular amino acids.
Sequence of variant and mutant GM-CSF receptor α subunits
. The α-1 and α-2 isoforms are natural splicing variants of the α subunit. They differ completely from their C-terminal amino acids (italics). The GM5 chimeric α subunit consists of sequences from the proximal proline-rich domain of the GM-CSF receptor α subunit (bold) fused to distal sequences from the human IL-5 α subunit (underlined). The term-3 mutant α subunit lacks the C-terminal amino acids because of placement of a stop codon after the codons for 34 intracellular amino acids. The term-1 mutant α subunit has only 5 intracellular amino acids.
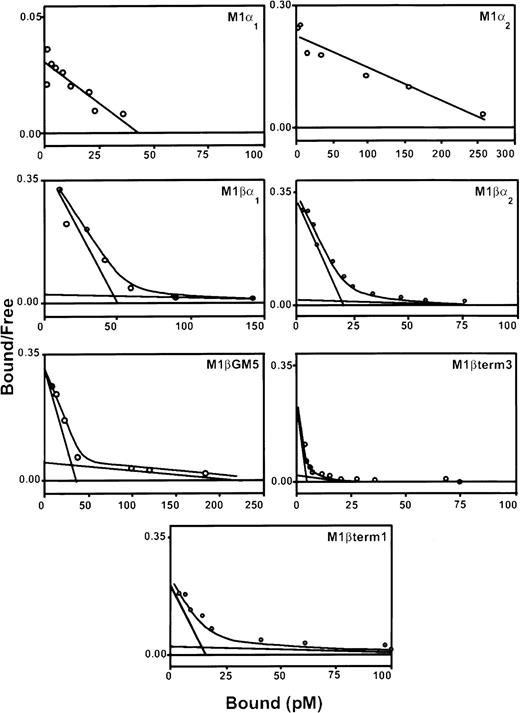
Three pools of G418-resistant cells were isolated and characterized for each α subunit variant. Expression of the α subunit proteins, as detected by flow cytometry, was variable (data not shown). All pools of transduced cells contained specific binding sites for 125I–rhGM-CSF (Table1; Figure2). Scatchard analysis demonstrated both high-affinity and low-affinity binding sites for cells coexpressing α and βc subunits. The high-affinity binding of each of these receptor complexes was similar to that reported for natural GM-CSF receptors (kd = 10-66 pM). In contrast, M1/α-1 and M1/α-2 cells did not express high-affinity binding sites but showed a single class of low-affinity binding sites (kd = 1.2-2.2 nM).
Binding affinity of human GM-CSF receptors in M1 cells expressing human βc and various α subunit constructs
| Cell line . | High affinity . | Low affinity . | ||
|---|---|---|---|---|
| kd(pM)* . | Sites† . | kd (nM) . | Sites . | |
| M1/α-1 | 0 | 0 | 2.2 | 22 415 |
| M1/α-2 | 0 | 0 | 1.2 | 17 200 |
| M1/βc/α-1 | 10 | 3130 | 1.1 | 12 320 |
| M1/βc/α-2 | 50 | 3160 | 3.0 | 14 300 |
| M1/βc/GM5 | 10 | 4190 | 0.9 | 20 105 |
| M1/βc/term-3 | 66 | 2560 | 8.8 | 24 600 |
| M1/βc/term-1 | 56 | 3520 | 7.2 | 32 800 |
| Cell line . | High affinity . | Low affinity . | ||
|---|---|---|---|---|
| kd(pM)* . | Sites† . | kd (nM) . | Sites . | |
| M1/α-1 | 0 | 0 | 2.2 | 22 415 |
| M1/α-2 | 0 | 0 | 1.2 | 17 200 |
| M1/βc/α-1 | 10 | 3130 | 1.1 | 12 320 |
| M1/βc/α-2 | 50 | 3160 | 3.0 | 14 300 |
| M1/βc/GM5 | 10 | 4190 | 0.9 | 20 105 |
| M1/βc/term-3 | 66 | 2560 | 8.8 | 24 600 |
| M1/βc/term-1 | 56 | 3520 | 7.2 | 32 800 |
Values are the means of duplicate determinations from a pool of 3 independently derived pools of retrovirally transduced cells.
Sites per cell.
Scatchard analysis of 125I-labeled rhGM-CSF binding to M1 cells expressing α and βc subunits of GM-CSF receptor.
Cells were incubated with decreasing concentrations of labeled rhGM-CSF in the presence or absence of 100-fold excess of unlabeled rhGM-CSF, on ice for 3 hours. Bound and free rhGM-CSF fractions were separated by centrifugation through fetal calf serum. The bound and free fractions were quantitated in a gamma-counter. Scatchard transformation of the data was performed assuming 100% binding ability of labeled ligand. Graphs show representative curves from a single pool of transduced cells. At least 3 pools were produced for each receptor combination.
Scatchard analysis of 125I-labeled rhGM-CSF binding to M1 cells expressing α and βc subunits of GM-CSF receptor.
Cells were incubated with decreasing concentrations of labeled rhGM-CSF in the presence or absence of 100-fold excess of unlabeled rhGM-CSF, on ice for 3 hours. Bound and free rhGM-CSF fractions were separated by centrifugation through fetal calf serum. The bound and free fractions were quantitated in a gamma-counter. Scatchard transformation of the data was performed assuming 100% binding ability of labeled ligand. Graphs show representative curves from a single pool of transduced cells. At least 3 pools were produced for each receptor combination.
GM-CSF regulates expression of differentiation antigens in M1 leukemia cells
The murine leukemia cell line M1 has commonly been used to identify signaling events associated with macrophage-like differentiation. A wide variety of cytokines can induce cell adhesion, macrophage-like morphology, and terminal differentiation with clonal extinction in this line. Treatment of M1/βc/α-1 cells and M1/βc/GM5 cells with rhGM-CSF produced an increase in size, a decrease in nuclear/cytoplasmic ratio, and a moderate degree of vacuolization. However there was no decrease or increase in growth rate in suspension culture. Furthermore, no decrease in plating efficiency or clonogenic growth in semisolid medium, dispersed colony morphology in semisolid medium, or other evidence of terminal differentiation or clonal extinction was seen. Treatment of M1/βc/α-2, M1/βc/term-1, M1/βc/term-3, M1/α-1, or M1/α-2 cells with rhGM-CSF had no obvious effect on cell morphology (data not shown).
We further examined the expression of several monocyte–dendritic cell differentiation markers in cytokine-treated M1 cells. Neither untreated nor rhGM-CSF–treated cells expressed major histocompatibility complex class II, CD80, or CD40 proteins on their membranes. All cell pools expressed the antigen recognized by the antidendritic cell antibody NLDC145.23 Cytokine treatment neither increased nor decreased expression of this marker.
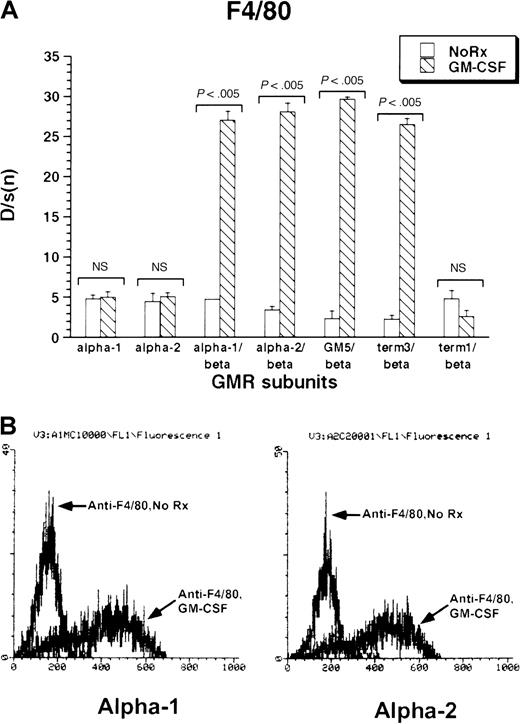
The monocytic–dendritic protein F4/80 was minimally expressed under basal conditions by M1 cells. GM-CSF treatment induced strong expression of F4/80 in all M1/βc/α cell lines containing at least the membrane-proximal 18 amino acids of the GM-CSF receptor α subunits (M1/βc/α-1, M1/βc/α-2, M1/βc/GM5, and M1/βc/term-3; Figure 3A-B). The M1/βc/term-1 cells, which lack almost the entire intracytoplasmic portion of the α subunit, showed no up-regulation of F4/80 in response to cytokine treatment. Similarly, cells expressing only α-1 or α-2 subunit proteins did not show cytokine-dependent expression of F4/80 antigen.
Expression of F4/80 antigen in rhGM-CSF–treated M1 cells expressing α or βc subunits, or both, of GM-CSF receptor.
Cells were incubated with rhGM-CSF 100 ng/mL for 3 days, then analyzed by flow cytometry using anti-F4/80 or an irrelevant isotype-matched control antibody. (A) Bars show mean ± SD of D/s(n) (derived from Kolmogorov-Smirnoff analysis) of 3 independently derived pools. (B) Histograms of F4/80 fluorescence with or without rhGM-CSF treatment in M1/βc/α-1 (left) and M1/βc/α-2 (right) cells.
Expression of F4/80 antigen in rhGM-CSF–treated M1 cells expressing α or βc subunits, or both, of GM-CSF receptor.
Cells were incubated with rhGM-CSF 100 ng/mL for 3 days, then analyzed by flow cytometry using anti-F4/80 or an irrelevant isotype-matched control antibody. (A) Bars show mean ± SD of D/s(n) (derived from Kolmogorov-Smirnoff analysis) of 3 independently derived pools. (B) Histograms of F4/80 fluorescence with or without rhGM-CSF treatment in M1/βc/α-1 (left) and M1/βc/α-2 (right) cells.
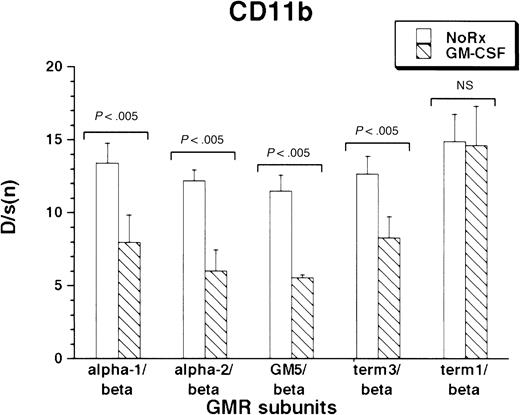
CD11b was expressed under basal conditions by the M1 cells. As with F4/80 antigen, all M1/βc/α cells containing at least the proximal intracytoplasmic portion of the α subunit were able to regulate CD11b expression in response to cytokine treatment. (Figure4). Interestingly, rhGM-CSF suppressed expression of the protein. As before, the M1/βc/term-1 cells did not respond to cytokine treatment.
Expression of CD11b antigen in rhGM-CSF–treated M1/βc cells with various α subunits.
Cells were incubated with rhGM-CSF 100 ng/mL for 3 days, then analyzed by flow cytometry using anti-CD11b or an irrelevant isotype-matched control antibody. Bars show mean ± SD of D/s(n) (derived from Kolmogorov-Smirnoff analysis) of 3 independently derived pools.
Expression of CD11b antigen in rhGM-CSF–treated M1/βc cells with various α subunits.
Cells were incubated with rhGM-CSF 100 ng/mL for 3 days, then analyzed by flow cytometry using anti-CD11b or an irrelevant isotype-matched control antibody. Bars show mean ± SD of D/s(n) (derived from Kolmogorov-Smirnoff analysis) of 3 independently derived pools.
All cell lines expressed low levels of CD86 antigen, an immune costimulatory molecule highly expressed by activated dendritic cells. Treatment of M1/βc/α-1 and M1/βc/GM5 cells with rhGM-CSF for 3 days led to a noticeable increase in CD86 expression (Figure5A-B). In contrast, M1/βc/term-1 and M1/βc/term-3 cells showed no significant change in CD86 expression after cytokine treatment. M1/βc/α-2 cells actually showed a slight, but statistically significant, decline in expression of the costimulatory molecule after treatment with rhGM-CSF. Again, M1 cells expressing only α-1 or α-2 did not show cytokine-dependent expression of CD86 in response to rhGM-CSF treatment. Thus CD86 expression paralleled morphologic differentiation in response to cytokine treatment.
Expression of CD86 antigen in rhGM-CSF–treated M1 cells expressing α or βc subunits, or both, of GM-CSF receptor.
Cells were incubated with rhGM-CSF 100 ng/mL for 3 days, then analyzed by flow cytometry using anti-CD86 or an irrelevant isotype-matched control antibody. (A) Bars show mean ± SD of D/s(n) (derived from Kolmogorov-Smirnoff analysis) of 3 independent derived pools. (B) Histograms of CD86 fluorescence with and without rhGM-CSF treatment in M1/βc/α-1 (left) and M1/βc/α-2 (right) cells.
Expression of CD86 antigen in rhGM-CSF–treated M1 cells expressing α or βc subunits, or both, of GM-CSF receptor.
Cells were incubated with rhGM-CSF 100 ng/mL for 3 days, then analyzed by flow cytometry using anti-CD86 or an irrelevant isotype-matched control antibody. (A) Bars show mean ± SD of D/s(n) (derived from Kolmogorov-Smirnoff analysis) of 3 independent derived pools. (B) Histograms of CD86 fluorescence with and without rhGM-CSF treatment in M1/βc/α-1 (left) and M1/βc/α-2 (right) cells.
Regulation of Jak phosphorylation by the GM-CSF α subunit
Our initial results suggested that the variable C-terminal domain of the GM-CSF receptor α subunit could regulate morphologic differentiation and expression of CD86. We next sought to determine whether different signal transduction events could be associated with signaling through the various α/βc receptor complexes and their response phenotypes.
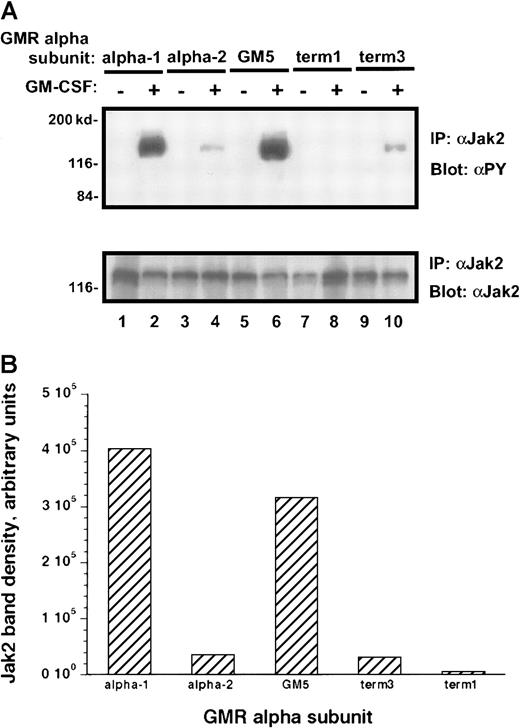
Treatment of the transduced M1 leukemia cells with rhGM-CSF resulted in prompt tyrosine phosphorylation of the Jak-2 protein (Figure6A). Interestingly, the M1/βc/α-1 and M1/βc/GM5 cells demonstrated more than 10 times as much tyrosine phosphorylation of the Jak-2 protein as did the M1/βc/α-2 and M1/βc/term-3 cells, even though the amount of total Jak-2 protein in these cells was similar (Figure 6A-B). M1/βc/term-1 cells again failed to demonstrate any response to cytokine treatment, suggesting that the intracytoplasmic portion of the receptor is necessary for Jak/Stat activation. A time-course analysis after cytokine treatment showed that the difference in phosphotyrosine content of Jak-2 isolated from M1/βc/α-1 and M1/βc/α-2 cells persisted at least 1 hour (Figure 7). In this experiment, the level of Jak-2 protein declined by 40% to 50% in the M1/βc/α-2 cells after cytokine treatment. This finding was inconsistently seen (compare with Figure 6) and could not account for the near-complete absence of Jak-2 tyrosine phosphorylation in these cells. The time course of the faint Jak-2 phosphorylation in M1/βc/α-2 cells (peak at 10 minutes, decline thereafter) was similar to that seen with M1/βc/α-1 cells. Thus it is unlikely that the differences in phosphorylation result from untimely sampling.
Tyrosine phosphorylation of Jak-2 in M1/βccells with various α subunits.
(A) Immunoprecipitation–immunoblotting analysis. Cells were treated with rhGM-CSF 100 ng/mL for 10 minutes. Lysates were then subjected to immunoprecipitation with anti–Jak-2, followed by blotting with antiphosphotyrosine (top panel). The blot was then stripped and reprobed with anti–Jak-2 (bottom panel). (B) Quantitation by densitometry of tyrosine-phosphorylated Jak-2 band after rhGM-CSF treatment.
Tyrosine phosphorylation of Jak-2 in M1/βccells with various α subunits.
(A) Immunoprecipitation–immunoblotting analysis. Cells were treated with rhGM-CSF 100 ng/mL for 10 minutes. Lysates were then subjected to immunoprecipitation with anti–Jak-2, followed by blotting with antiphosphotyrosine (top panel). The blot was then stripped and reprobed with anti–Jak-2 (bottom panel). (B) Quantitation by densitometry of tyrosine-phosphorylated Jak-2 band after rhGM-CSF treatment.
Time course of Jak-2 tyrosine phosphorylation in M1/βc/α-1 and M1/βc/α-2 cells, after rhGM-CSF treatment.
Cells were treated with rhGM-CSF 100 ng/mL for the indicated periods, then immunoprecipitated with anti–Jak-2, followed by blotting with antiphosphotyrosine (top panel). The blot was then stripped and reprobed with anti–Jak-2 (bottom panel).
Time course of Jak-2 tyrosine phosphorylation in M1/βc/α-1 and M1/βc/α-2 cells, after rhGM-CSF treatment.
Cells were treated with rhGM-CSF 100 ng/mL for the indicated periods, then immunoprecipitated with anti–Jak-2, followed by blotting with antiphosphotyrosine (top panel). The blot was then stripped and reprobed with anti–Jak-2 (bottom panel).
Effect of variant and mutant α subunits on GM-CSF–induced tyrosine phosphorylation of putative Jak-2 substrates
To examine whether the observed differences in activation of Jak-2 were reflected in differential phosphorylation of potential Jak-2 substrates, we studied phosphorylation of the human βcsubunit after the addition of rhGM-CSF to the transduced cells. Tyrosine phosphorylation of the βc subunit in response to cytokine was markedly different between cells containing the α-1 protein and those with the α-2 protein. Among all the α subunit variant pools, the level of phosphorylation of the βcsubunit paralleled the level of activation of Jak-2 (Figure8A-B). Thus, even though Jak-2 is constitutively associated with the βc subunit in these M1 clones (see below), the carboxy terminal portion of the α receptor is important in regulating rhGM-CSF–mediated tyrosine phosphorylation of the βc subunit.
Tyrosine phosphorylation of βc subunit protein in M1/βc cells with various α subunits.
(A) Immunoprecipitation–immunoblotting analysis. Cells were treated with rhGM-CSF 100 ng/mL for 10 minutes. Lysates were then immunoprecipitated with anti-βc (monoclonal), followed by blotting with anti-phosphotyrosine (top panel). The blot was then stripped and reprobed with anti-βc (polyclonal; bottom panel). (B) Quantitation by densitometry of tyrosine-phosphorylated βc subunit band after rhGM-CSF treatment.
Tyrosine phosphorylation of βc subunit protein in M1/βc cells with various α subunits.
(A) Immunoprecipitation–immunoblotting analysis. Cells were treated with rhGM-CSF 100 ng/mL for 10 minutes. Lysates were then immunoprecipitated with anti-βc (monoclonal), followed by blotting with anti-phosphotyrosine (top panel). The blot was then stripped and reprobed with anti-βc (polyclonal; bottom panel). (B) Quantitation by densitometry of tyrosine-phosphorylated βc subunit band after rhGM-CSF treatment.
Because Stat-5 proteins play an essential role in mediating signal transduction by the Jak kinases, the ability of each of these α subunits to regulate Stat phosphorylation after rhGM-CSF treatment was examined (Figure 9A-B). When compared to the α-1 and GM5 clones, incubation of the α-2 or term-3 subunit containing M1 clones with rhGM-CSF stimulated 50% lower levels of tyrosine phosphorylation of Stat-5. This decrease in phosphorylation was less than the decrease in Jak-2 phosphorylation. In the latter case α-2 and term-3 receptor complexes induced no more than 10% of the level of Jak-2 phosphorylation induced in the α-1 and GM5 subunits containing clones. These results could suggest that only small amounts of Jak-2 phosphorylation are sufficient to regulate the phosphorylation of larger amounts of Stat-5 or that the level of Stat-5 phosphorylation reflects the activities of other tyrosine kinases.24
Tyrosine phosphorylation of Stat-5 protein in M1/βc cells with various α subunits.
(A) Immunoprecipitation–immunoblotting analysis. Cells were treated with rhGM-CSF 100 ng/mL for 10 minutes. Lysates were then immunoprecipitated with anti–Stat-5. Precipitated proteins were then solubilized, divided, and subjected to immunoblotting with either antiphosphotyrosine (top panel) or anti–Stat-5 (bottom panel). (B) Quantitation by densitometry of tyrosine-phosphorylated Stat-5 band after rhGM-CSF treatment.
Tyrosine phosphorylation of Stat-5 protein in M1/βc cells with various α subunits.
(A) Immunoprecipitation–immunoblotting analysis. Cells were treated with rhGM-CSF 100 ng/mL for 10 minutes. Lysates were then immunoprecipitated with anti–Stat-5. Precipitated proteins were then solubilized, divided, and subjected to immunoblotting with either antiphosphotyrosine (top panel) or anti–Stat-5 (bottom panel). (B) Quantitation by densitometry of tyrosine-phosphorylated Stat-5 band after rhGM-CSF treatment.
Results similar to those obtained with other Jak-2 substrates were seen when the dual SH2-containing phosphatase SHPTP-2 was immunoprecipitated from the rhGM-CSF–treated cells (Figure10A-B). Those M1 cell lines containing the α-1 and GM-5 receptors had markedly increased phosphorylation of SHPTP-2, whereas the term-3 and α-2 cell lines had approximately half as much tyrosine phosphorylation. Again, the term-1–containing M1 cells showed no cytokine-directed phosphorylation of SHPTP-2.
Tyrosine phosphorylation of SHPTP-2 protein in M1/βc cells with various α subunits.
(A) Immunoprecipitation–immunoblotting analysis. Cells were treated with rhGM-CSF 100 ng/mL for 10 minutes. Lysates were then immunoprecipitated with anti–SHPTP-2. Precipitated proteins were then solubilized, divided, and subjected to immunoblotting with either antiphosphotyrosine (top panel) or anti–SHPTP-2 (bottom panel). (B) Quantitation by densitometry of tyrosine-phosphorylated SHPTP-2 band after rhGM-CSF treatment.
Tyrosine phosphorylation of SHPTP-2 protein in M1/βc cells with various α subunits.
(A) Immunoprecipitation–immunoblotting analysis. Cells were treated with rhGM-CSF 100 ng/mL for 10 minutes. Lysates were then immunoprecipitated with anti–SHPTP-2. Precipitated proteins were then solubilized, divided, and subjected to immunoblotting with either antiphosphotyrosine (top panel) or anti–SHPTP-2 (bottom panel). (B) Quantitation by densitometry of tyrosine-phosphorylated SHPTP-2 band after rhGM-CSF treatment.
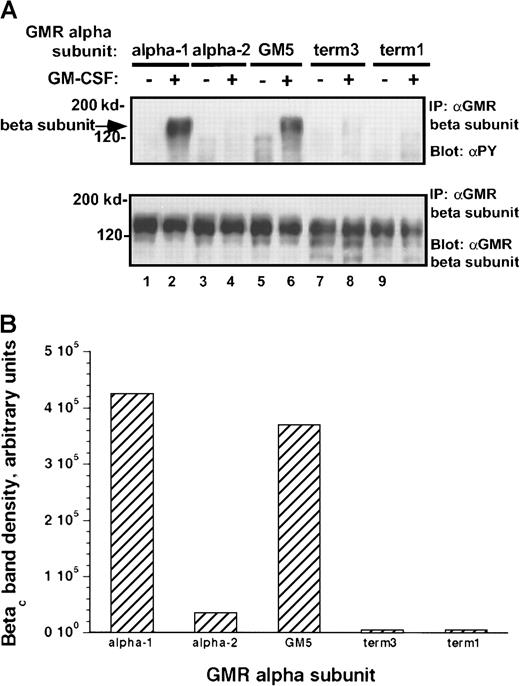
α subunits differ in their ability to form stable complexes with βc protein
The human GM-CSF receptor exists as a preformed complex, consisting of variable combinations of the α subunit, the βc receptor protein, and the tyrosine kinase Jak-2.25 26 We examined receptor complexes in the presence and absence of ligand binding. Complex formation between the α subunits and the βc subunit differed markedly among the various samples (Figure 11A). Neither α subunit form appeared to be constitutively associated with the βc receptor protein (under our assay conditions). After ligand binding, the α-1 subunit formed a complex with the larger receptor component, which was stable in the face of stringent washing conditions. In contrast, no stable complex between the α-2 protein and the βc subunit was detected after ligand binding. Differences in α/βc complex formation did not appear to result from variation in the amount of α chain expressed by the cells (Figure11C). Jak-2 was constitutively associated with the receptor βc subunit in both cell lines and was not modified by ligand binding (Figure 11B).
Complex formation among components of human GM-CSF receptor
. (A) Association of GM-CSF receptor α subunits with βcprotein. Cells were treated with rhGM-CSF 100 ng/mL for 10 minutes. Lysates were then immunoprecipitated with anti-βcpolyclonal antibody. Precipitated proteins were solubilized and subjected to immunoblotting with anti-α subunit antibody (top panel). The blot was stripped and reprobed with anti-βc(polyclonal; bottom panel). (B) Association of Jak-2 with βc protein. Cells were treated with rhGM-CSF as above. Lysates were immunoprecipitated with anti-βc polyclonal antibody. Precipitated proteins were then solubilized, divided, and subjected to immunoblotting with either anti–Jak-2 (top panel) or anti-βc (polyclonal; bottom panel). (C) Expression of α subunit proteins in transduced M1 cells. Lysates from either M1/βc/α-1 or M1/βc/α-2 cells were subjected to immunoprecipitation with an anti-α subunit antibody (α GM-CSF receptor) or an irrelevant isotype-matched antibody (NC). Precipitated proteins were then subjected to immunoblotting with anti-α subunit antibody. The heavy band at approximately 55 kd represents IgG heavy chain.
Complex formation among components of human GM-CSF receptor
. (A) Association of GM-CSF receptor α subunits with βcprotein. Cells were treated with rhGM-CSF 100 ng/mL for 10 minutes. Lysates were then immunoprecipitated with anti-βcpolyclonal antibody. Precipitated proteins were solubilized and subjected to immunoblotting with anti-α subunit antibody (top panel). The blot was stripped and reprobed with anti-βc(polyclonal; bottom panel). (B) Association of Jak-2 with βc protein. Cells were treated with rhGM-CSF as above. Lysates were immunoprecipitated with anti-βc polyclonal antibody. Precipitated proteins were then solubilized, divided, and subjected to immunoblotting with either anti–Jak-2 (top panel) or anti-βc (polyclonal; bottom panel). (C) Expression of α subunit proteins in transduced M1 cells. Lysates from either M1/βc/α-1 or M1/βc/α-2 cells were subjected to immunoprecipitation with an anti-α subunit antibody (α GM-CSF receptor) or an irrelevant isotype-matched antibody (NC). Precipitated proteins were then subjected to immunoblotting with anti-α subunit antibody. The heavy band at approximately 55 kd represents IgG heavy chain.
Discussion
Hematopoietic cytokine receptors often exhibit multiple isoforms. The human GM-CSF receptor α subunit exists in at least 8 isoforms, which result from alternative splicing. Six of these appear to have no intracytoplasmic sequences,4-7 and 5 lack the transmembrane domain as well. Two forms, α-12 and α-2,3 have intracytoplasmic sequences that are able to transduce signals. Similarly, several membrane-associated and soluble isoforms of the IL-5 receptor α subunit have been described.22 Recently, a novel isoform of the common βc receptor protein has been characterized.27 As a result of alternative splicing, this receptor protein has a deletion in the intracytoplasmic domain, resulting in impaired proliferative signaling. However, no previous reports have identified differences in signaling events or response phenotypes for α subunit variants.
Previous studies comparing the α-1 and the α-2 GM-CSF receptor proteins demonstrated that both subunits were able to support the proliferation of factor-dependent murine hematopoietic cells treated with human GM-CSF.17 To determine whether the α subunit variants could differentially regulate maturation, we introduced the receptor cDNAs into M1 murine leukemia cells, which demonstrate cytokine-dependent differentiation. Human GM-CSF treatment of cells with receptor complexes incorporating α-1 proteins induced morphologic changes and increases in F4/80 antigen and CD86 protein expression but a decrease in CD11b expression. There was no evidence of terminal macrophage-like differentiation (clonal extinction, dispersed colony morphology) when cells were cultured with rhGM-CSF in semisolid medium. The cells did, however, retain the ability to differentiate because treatment of every pool with leukemia inhibitory factor dramatically up-regulated CD11b expression and induced clonal extinction with dispersed colony morphology in semisolid medium (data not shown).
Although the α-1 and α-2 receptor complexes did support some similar response phenotypes, important differences were seen. M1/βc/α-1 cells increased their expression of CD86 in response to rhGM-CSF, whereas M1/βc/α-2 cells slightly (but significantly) decreased display of this costimulatory molecule. These data demonstrate that the distal C-terminal domain of the GM-CSF receptor α subunit can regulate phenotypic responses after ligand binding. They also suggest that the C-terminus of the α-2 subunit is actively involved in propagating transmembrane signals because the term-3 mutant α subunit, which lacks a distal domain, had no statistically significant effect on CD86 expression. We have also noted in M1 cells that both α-1 and α-2 receptor proteins can support cytokine-induced expression of the pim-1 kinase, whereas term-3 α subunits cannot (data not shown). At present we cannot determine whether the α-2 receptor complex transmits signals for unique differentiation or proliferation phenotypes. The lack of a clear morphologic response in M1/βc/α-2 cells may be a reflection of the limited spectrum of potential differentiation response phenotypes in M1 cells. Expression of the variant GM-CSF receptor proteins in other cell lines could result in wider differences of response phenotypes than we have seen in the M1 cells.
The basis for the different response phenotypes supported by α-1– and α-2–GM-CSF receptors appears to involve Jak/Stat signaling. Both the α-2– and the term-3–containing complexes were markedly less efficient at inducing tyrosine phosphorylation of Jak-2 than were receptors with α-1 and GM5 α subunits. This effect was amplified by additional differences in tyrosine phosphorylation of putative Jak-2 substrates. It is unknown what minimal level of Jak-2 activation–tyrosine phosphorylation is required to propagate an effective signal. Therefore, the α-2 and term-3 receptors could be supporting some degree of Jak-2–dependent response. However, the correlation between phenotypic responses (CD86 expression, morphologic differentiation) and biochemical responses (tyrosine phosphorylation of Jak-2 and substrates) was exact for the 5 cell lines examined, suggesting that phenotype and biochemical events were linked.
The biochemical events underlying the differences in Jak/Stat signaling by the various receptor complexes are unclear. Certainly receptor number alone is not an adequate explanation. Although the term-3 α subunit was expressed in lower numbers than the other functional receptors, this is unlikely to account for its relatively poor Jak-2 activation. The numbers of high-affinity binding sites on M1/βc/term-3 cells were still higher than typically found on normal hematopoietic cells, and the receptors were equally capable of inducing F4/80 antigen expression as the other variants. Furthermore, M1/βc/α-2 cells also showed impaired Jak-2 activation, even though they had higher receptor density than did the M1/βc/α-1 or M1/βc/GM5 cells. More rapid degradation of phosphorylated Jak-2 in M1/βc/α-2 cells appears an unlikely explanation. Treatment with the proteasome inhibitor MG132, reported to increase levels of tyrosine phosphorylated proteins,28 had no effect on Jak-2 phosphorylation in M1/βc/α-2 cells (data not shown), and Jak-2 appeared equally able to bind to the βc protein in the presence of either α-1 or α-2 subunits.
Our data suggest that the molecular mechanisms underlying differential signaling may involve the stability of α/βc complexes. After ligand binding, the α-1 subunit clearly was more stably associated with βc protein than was the α-2 subunit. These differences may result from the secondary structure of the C-terminal domain of the α subunits. Both the α-1 and the GM5 α chains were able to support intense tyrosine phosphorylation of Jak-2 and its substrates and up-regulation of CD86. There are no areas of high homology in the membrane-distal region, though both proteins show alternating charged and hydrophobic amino acids in this area. Secondary structure analysis does show, though, that both receptor molecules are likely to have a β strand configuration near the C-terminus. In contrast, the α-2 subunit is predicted to lack this secondary structure. Possibly, the carboxy terminal domain of the α-2 protein destabilizes receptor complexes, leading to impaired mutual phosphorylation of the associated Jak-2 molecules after ligand binding.
The current studies extend our previous work in mapping significant domains of the GM-CSF receptor α subunit.16 18 Our data suggest the existence of 2 domains (Figure12). A membrane-proximal domain (amino acids 346-370) contains important, conserved proline residues. The distal domain extends from amino acid 370 to the C-terminus and differs entirely between the α-1 and α-2 subunits. The current report demonstrates that the primary (and likely, secondary) structure of the distal domain, as much as its presence or absence, can modulate signal transduction events and ligand-dependent responses.
Schematic diagram representing GM-CSF receptor α subunit domains.
Receptor regions are identified at left (TM, transmembrane domain). Biochemical events (within minutes) or response phenotypes (within hours to days) dependent on the various domains are identified. Data are from Matsuguchi et al,16 Polotskaya et al,18 and the present report (*).
Schematic diagram representing GM-CSF receptor α subunit domains.
Receptor regions are identified at left (TM, transmembrane domain). Biochemical events (within minutes) or response phenotypes (within hours to days) dependent on the various domains are identified. Data are from Matsuguchi et al,16 Polotskaya et al,18 and the present report (*).
Although distinct biochemical events can be associated with these domains, it is more difficult to generalize about response phenotypes. The participation of the distal domain in supporting proliferation is clearly cell-type dependent. Thus the term-3 mutant, containing only the proximal domain, is able to fully signal for cell growth in BaF3 cells18 but not in WT19 cells.16Differentiation is a complex process involving many molecular events. We here find that a particular form of the distal domain is required for morphologic changes and expression of CD86. As we have seen before,16 however, the proximal domain is adequate to support expression of the differentiation antigen F4/80. Likely, the degree of differentiation reflects the sum of many signaling events, some of which may show marked quantitative differences based on the stability of the involved receptor complexes.
Although distal regions of the GM-CSF receptor α subunit appear to modulate Jak-2 activation, tyrosine kinases activated by the proximal domain of the α subunit are unknown. We have used kinase inhibitors to explore the signaling events behind expression of F4/80, which requires the membrane-proximal domain of the α subunit. The tyrosine kinase inhibitor tyrphostin AG490 was able to completely inhibit F4/80 expression at doses between 10 and 40 μM, whereas tyrphostin A10 had no effect (data not shown). Tyrphostin AG490 has been claimed to be a specific inhibitor of the Jak-229 and Jak330 kinases, though it can also inhibit the epidermal growth factor receptor kinase.31 Under our test conditions, tyrphostin AG490 had no effect on rhGM-CSF–induced Jak-2 tyrosine phosphorylation in M1/βc/α-1 cells, even at doses that completely blocked F4/80 up-regulation. These results imply that the proximal domain of the α subunit activates signaling events that include a tyrosine kinase distinct from Jak-2. We did not find any evidence of activation of the Jak-family kinases Jak-1 or Tyk-2 after rhGM-CSF treatment of our cells (data not shown). Possibilities might include a member of the src family of tyrosine kinases.
Our data demonstrate the existence of 2 distinct domains on the human GM-CSF receptor α subunit and provide evidence for distinct biologic and biochemical activities of the 2 receptor α subunit isoforms. It is possible that differential signaling through the variant receptors will provide a mechanism for a wider range of response phenotypes after exposure of the cells to GM-CSF.
We thank Drs G. Begley, D. Metcalf, and N. Nicola for their encouragement in these studies and for graciously supplying important reagents. We also thank Drs C. Sieff and J. Tavernier for generously providing cDNA clones, and John J. Cooper for superb technical assistance.
Supported in part by National Institutes of Health grants CA45672 (M.B.L.) and DK44741 (A.S.K.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michael Lilly, Department of Medicine, Loma Linda University, Loma Linda, CA 92354; e-mail: mlilly@som.llu.edu.








This feature is available to Subscribers Only
Sign In or Create an Account Close Modal