Neuropilin 1 (NP-1) is a receptor for vascular endothelial growth factor (VEGF) 165 (VEGF165) and acts as a coreceptor that enhances VEGF165 function through tyrosine kinase VEGF receptor 2 (VEGFR-2). Transgenic overexpression of np-1results in an excess of capillaries and blood vessels and a malformed heart. Thus, NP-1 may have a key role in vascular development. However, how NP-1 regulates vascular development is not well understood. This study demonstrates how NP-1 can regulate vasculogenesis and angiogenesis in vitro and in vivo. In homozygous np-1mutant (np-1−/−) murine embryos, vascular sprouting was impaired in the central nervous system and pericardium. Para-aortic splanchnopleural mesoderm (P-Sp) explants fromnp-1−/− mice also had vascular defects in vitro. A monomer of soluble NP-1 (NP-1 tagged with Flag epitope) inhibited vascular development in cultured wild-type P-Sp explants by sequestering VEGF165. In contrast, a dimer of soluble NP-1 (NP-1 fused with the Fc part of human IgG) enhanced vascular development in cultured wild-type P-Sp explants. Moreover, the NP-1–Fc rescued the defective vascular development in culturednp-1−/− P-Sp explants. A low dose of VEGF alone did not promote phosphorylation of VEGFR-2 on endothelial cells from np-1−/− embryos, but simultaneous addition of a low dose of VEGF and NP-1–Fc phosphorylated VEGFR-2 significantly. Moreover, NP-1–Fc rescued the defective vascularity of np-1−/− embryos in vivo. These results suggest that a dimer form of soluble NP-1 delivers VEGF165 to VEGFR-2–positive endothelial cells and promotes angiogenesis.
Introduction
The formation of blood vessels begins during early mammalian embryogenesis with differentiation of mesodermally derived primitive angioblasts into mature endothelial cells (ECs). At first, those ECs form a primitive vascular structure, a process termed vasculogenesis.1 Subsequently, ECs from these primitive vessels proliferate and sprout to form a branching network of capillaries during the process of angiogenesis.2 Finally, mural cells (pericytes and vascular smooth muscle cells) and fibroblasts are recruited around vessels and stabilized vessels are built.3
During the past several years, much progress has been made in defining vasculogenesis and angiogenesis by isolating factors such as a family of vascular EC growth factors (VEGFs), angiopoietins, and ephrins.4,5 Gene-targeting strategies revealed that these factors and their receptors were important in the development of the vascular system. VEGFs and their receptor tyrosine kinases, tyrosine kinase VEGF receptor 1 (VEGFR-1) and tyrosine kinase VEGF receptor 2 (VEGFR-2), are expressed specifically on the surface of ECs and were shown to be required for both vasculogenesis and angiogenesis.6-9 More recently, neuropilin 1 (NP-1) was found to be expressed on ECs and tumor cells10 and shown to be the third VEGF receptor.
In contrast to VEGFR-1 and VEGFR-2, NP-1 binds the 165–amino acid form of VEGF (VEGF165) but not the 121–amino acid form (VEGF121). It was reported that the binding of VEGF165 to NP-1 occurs by means of VEGF exon 7, in contrast to VEGFR-1 and VEGFR-2, which bind VEGF165 by means of VEGF exons 3 and 4, respectively.11-13 When NP-1 was coexpressed on VEGFR-2–positive (VEGFR-2+) ECs, the binding to and chemotactic activity of VEGF165 for these cells were enhanced compared with those for ECs expressing VEGFR-2 alone.10 In vivo, overexpression of the np-1gene in mice led to an excess of capillaries and blood vessels and a malformed heart.14 These results suggest that NP-1 works as a coreceptor with VEGFR-2 on ECs and enhances the function of VEGF165.
Originally, NP-1 was identified as a receptor for the axon chemorepellent semaphorin III.15,16 Semaphorin III can induce neuronal growth-cone collapse and repulsion of neurites in vitro. Studies using nonneuronal cells suggested that semaphorin III and VEGF165 bind NP-1 competitively.17Recombinant semaphorin III inhibited the motility of porcine aortic ECs (PAECs) expressing NP-1 alone or coexpressing VEGFR-2 and NP-1 but not parental PAECs or PAECs expressing VEGFR-2 alone. Although it is not clear whether VEGF165 acts on neuronal cells expressing NP-1, a complex system that diversifies EC development using ligands, such as VEGF; semaphorin III; and receptors, such as VEGFR-2 and NP-1, may exist.
Np-1−/− mutant mice died at about embryonal day (E) 12.5 and showed severe abnormalities in the trajectory of the cranial and spinal efferent fibers that express NP-1.18 It was suggested that embryonic death was caused by anomalies in the cardiovascular system and vascular defects in the central nervous system (CNS).19 Precisely how such vascular defects occur is not clear. In this study, we show that homozygous, NP-1–deficient murine embryos are defective in angiogenesis, especially in the CNS and pericardium. To better analyze the function of NP-1 in ECs, we examined the vasculogenesis and angiogenesis of NP-1–deficient embryos in vitro. Previous studies found that when a para-aortic splanchnopleural mesodermal (P-Sp) region from E8.5 to 9.5 was cultured on OP9 stromal cells, vasculogenesis (the formation of the vascular bed by assembled immature ECs) and angiogenesis (the formation of the vascular network by ECs sprouted from the vascular bed) occurred.20,21Using this culture system, we show that NP-1 is involved in vasculogenesis as well as angiogenesis. Previously, Gagnon et al22 found that a monomer of soluble NP-1 exists in vivo and might function as a VEGF165 antagonist. In this study, we show that a monomer of soluble NP-1 inhibits vascular development in wild-type P-Sp cultures but that a dimer of soluble NP-1 can enhance the proliferation of immature ECs. We also demonstrate that a dimer of soluble NP-1 and VEGF effectively phosphorylate VEGFR-2 on ECs. Finally, we provide evidence that a dimer of soluble NP-1 can rescue defective vascular development in np-1−/− embryos.
Materials and methods
Mice
C57BL/6 mice were purchased from Japan SCL (Shizuoka, Japan).Np-1 heterozygous mutant mice18 were housed in environmentally controlled rooms at Kumamoto University School of Medicine in accordance with university guidelines for animal and recombinant DNA experiments. Genotype analysis of the NP-1 mutants was done by using polymerase chain reaction analysis as described previously.18
Immunohistochemical analysis
Immunohistochemical analysis of embryos was done as described previously.20 Briefly, embryos were fixed in 4% paraformaldehyde at 4°C overnight. The fixed embryos were then rinsed in phosphate-buffered saline (PBS), dehydrated in a methanol series, and stored in 100% methanol at −80°C. The dehydrated embryos were bleached in methanol plus 5% vol/vol hydrogen peroxide for 4 to 5 hours at 4°C. The bleached embryos were rehydrated and blocked in PBS, milk, and Triton X-100 (PBSMT; PBS, 2% instant skim milk, and 0.1% Triton X-100) for 1 hour twice. The embryos were then incubated with a 1:500 dilution of anti–platelet EC adhesion molecule 1 (PECAM-1) antibody (MEC13.3 rat antimouse monoclonal antibody [mAb]; Pharmingen, San Diego, CA) in PBSMT overnight at 4°C. The next day, the embryos were washed with PBSMT at 4°C for 1 hour 5 times and incubated at 4°C with 1 μg/mL horseradish peroxidase–conjugated goat antirat IgG (Biosource, Camarillo, CA) in PBSMT overnight at 4°C. Embryos were then washed with PBSMT for 1 hour 5 times at 4°C and in PBS and Triton (PBST; 0.1% Triton X-100 and PBS) for 20 minutes 3 times at room temperature. Peroxidase staining was done by incubating the embryos in 0.3 mg/mL diaminobenzidine (Dojin Chemical, Kumamoto, Japan) and 0.8 mg/mL nickel chloride (Wako Pure Chemical, Osaka, Japan) in PBST for 20 minutes and then adding hydrogen peroxide at a final concentration of 0.05%. Rinsing in PBST stopped the staining reaction.
Production of recombinant fusion proteins
Recombinant fusion proteins of the full-length extracellular domain of murine surface molecules and the Fc part of human IgG and Flag epitope were designed (Figure 2, Shimizu et al23). NP-1–Fc, CD4-Fc, NP-1–Flag, and control Flag were produced by COS7 cells in serum-free conditioned medium as described previously.23 24 Fc fusion protein was purified over a protein A column (Bio-Rad, Richmond, CA) and Flag fusion protein was purified over an anti-Flag M2 column (Scientific Imaging System; Eastman Kodak, Rochester, NY). Their purity and disulfide-linked dimerization were assessed by Coomassie brilliant Blue staining of 6% sodium dodecyl sulfate (SDS) gels.
In vitro culture of P-Sp
The stromal cell line OP925 was maintained in α modified minimum essential media (α-MEM; Gibco BRL, Gaithersburg, MD) supplemented with 20% fetal-calf serum (FCS; JRH Bioscience, Lenexa, KS). P-Sp explants from E9.5 containing a part of the omphalomesenteric artery were cultured on OP9 stromal cells in 10% FCS and 10−5 M 2-mercaptoetanol (2-ME; Gibco BRL) containing RPMI 1640 (Gibco BRL) with or without full-length VEGF (Pepro Tech EC, London, United Kingdom) and NP-1–Fc or CD4-Fc, and NP-1–Flag or control Flag. After 14 days in culture, an anti-PECAM-1 antibody (MEC13.3 rat antimouse mAb; Pharmingen) was used to visualize ECs.
Quantitative analysis of vascular areas
Immunohistochemical analysis in the P-Sp culture using PECAM-1 antibodies was done as described above. After PECAM-1 immunohistochemical staining, images were integrated by using a color chilled 3CCD camera (Hamamatsu Photonics, Shizuoka, Japan). Image-processing software (NIH Image version 1.62 on a Power Macintosh G3; National Institutes of Health, Bethesda, MD) was used to determine alterations in the size of vascular areas. Three vascular areas from each P-Sp explant were measured under each culture condition. All values are shown as the mean ± SD. P values were calculated using 2-tailed Student t tests.
Cell preparation and flow cytometry
Embryos were staged by means of somite counting. Whole-mount embryos of wild-type and np-1 mutants were dissociated using Dispase II (Boehringer Mannheim, Mannheim, Germany) and drawn through a 23-gauge needle. The cell-staining procedure for the flow cytometry analysis was as described previously.26 The mAbs used in immunofluorescence staining were anti–VEGFR-2 antibody (Pharmingen) and anti–PECAM-1 antibody (Pharmingen). All mAbs were purified and conjugated with phycoerythrin (PE) or biotin. Biotinylated antibodies were visualized with PE-conjugated streptavidin (Gibco BRL) or allophycocyanin-conjugated streptavidin (Caltag, Burlingame, CA). Cells were incubated for 15 minutes on ice with CD16/32 (Fcγ III/II receptor, 1:100 [Fcblock; Pharmingen]) before staining with primary antibody. Cells were then incubated in 5% FCS and PBS (washing buffer) with primary antibody for 30 minutes on ice and washed twice with washing buffer. Secondary antibody was added and the cells were incubated for 30 minutes on ice. After incubation, cells were washed twice with washing buffer and then suspended in washing buffer for fluorescence-activated cell-sorter (FACS) analysis. The stained cells were analyzed and sorted using FACSvantage (Becton Dickinson, San Jose, CA). Sorted VEGFR-2+ PECAM-1–positive (PECAM-1+) cells were cultured on OP9 cells in 10% FCS and 10−5 M 2-ME containing RPMI 1640 supplemented with VEGF (1 ng/mL) and NP-1–Fc (50 μg/mL) or CD4-Fc (50 μg/mL) at 37°C in a 5% carbon dioxide (CO2) incubator.
Immunoprecipitation and immunoblotting
Dissociated cells from E12.5 wild-type or np-1 mutant embryos were cultured on OP9 cells in 10% FCS and 10−5 M 2-ME containing RPMI 1640 supplemented with VEGF (10 ng/mL) and basic fibroblast growth factor (bFGF [10 ng/mL]; Pepro Tech EC) at 37°C in a 5% CO2 incubator. After 7 days, cells were harvested, labeled with PECAM-1–PE and VEGFR-2–biotin antibodies, and sorted using FACSvantage. VEGFR-2+ PECAM-1+ cells were transferred to fibronectin-coated dishes (Iwaki Glass, Chiba, Japan) and cultured in serum-free conditioned medium for 24 hours. ECs of wild-type or np-1 mutants were stimulated with 1 ng/mL VEGF in the presence or absence of 50 μg/mL NP-1–Fc for 10 minutes at 37°C. Immunoprecipitation and immunoblotting were done as described previously.27
Injection of NP-1–Fc into mice
Np-1+/− male and female mice were mated and checked for plugs the next morning (designated as E0.5). From E9.5 to 11.5, pregnant mice were given daily intraperitoneal injections of 150 μL of a 2-mg/mL solution of NP-1–Fc or CD4-Fc (control protein). Embryos were harvested on E12.5 and immunohistochemical analyses on whole-mount embryos or sections was done by using PECAM-1 monoclonal antibody as described previously.20
Results
P-Sp explant culture system reflects the vascular defect innp-1 mutant embryos
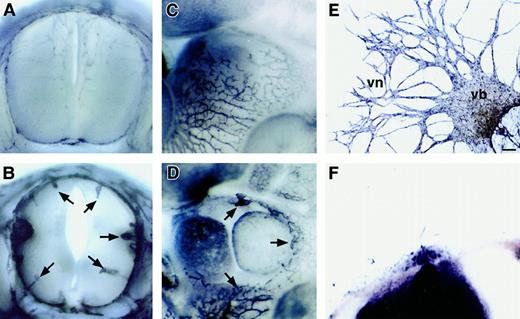
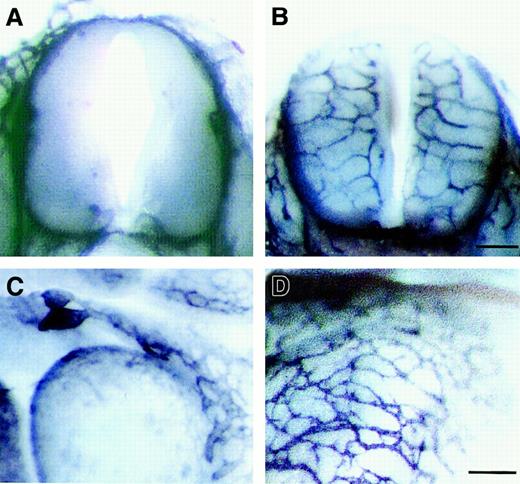
To examine the vascularity of the homozygous np-1mutant (np-1−/−) embryos, E12.0np-1−/− and wild-type (np-1+/+) embryos from the same litter were stained with PECAM-1 mAb to visualize all ECs. No growth retardation was observed in the mutants (data not shown). However, the vascularity of mutant embryos compared with wild-type embryos (Figure1A,C) was impaired in the CNS and pericardial regions (Figure 1B,D). At E12.0, vascular sprouting into the CNS and pericardium was observed in wild-type embryos, whereas few capillaries and branches were observed in the mutants. To examine the vasculogenesis and angiogenesis of the np-1−/−embryos more precisely, P-Sp explants from E9.5 wild-type and homozygous mutant embryos were cultured with OP9 stromal cells supplemented with interleukin (IL) 6, IL-7, stem cell factor, and erythropoietin. We previously reported that this culture system supported the growth of both hematopoietic cells and ECs.20 21 After 10 to 14 days, ECs in the wild-type embryos migrated from the P-Sp explant and formed vascular beds (sheetlike structures) and networks (cordlike structures) on OP9 cells (Figure 1E). In contrast, in P-Sp explants from mutant embryos, neither vascular beds nor networks formed, although a small number of ECs developed (Figure 1F).
P-Sp culture system reflects vascular defects in
np-1 mutant embryos. Spinal cord (A-B) and heart region (C-D) of E12.0 np-1+/+ (A, C) andnp-1−/− (B, D) murine embryos were subjected to immunohistochemical staining with anti–PECAM-1 antibody to visualize all ECs. Capillaries showed little branching and were of large caliber (B, D; arrows). P-Sp explants derived from E9.5 wild-type embryos (E) and mutant embryos (F) were cultured on OP9 stromal cells. Vascular network (vn) formation was defective innp-1−/− P-Sp explants (F), as observed in the CNS and pericardium. The scale bar indicates 100 μm (E-F); vb, vascular bed.
P-Sp culture system reflects vascular defects in
np-1 mutant embryos. Spinal cord (A-B) and heart region (C-D) of E12.0 np-1+/+ (A, C) andnp-1−/− (B, D) murine embryos were subjected to immunohistochemical staining with anti–PECAM-1 antibody to visualize all ECs. Capillaries showed little branching and were of large caliber (B, D; arrows). P-Sp explants derived from E9.5 wild-type embryos (E) and mutant embryos (F) were cultured on OP9 stromal cells. Vascular network (vn) formation was defective innp-1−/− P-Sp explants (F), as observed in the CNS and pericardium. The scale bar indicates 100 μm (E-F); vb, vascular bed.
Structure of NP-1 recombinant proteins
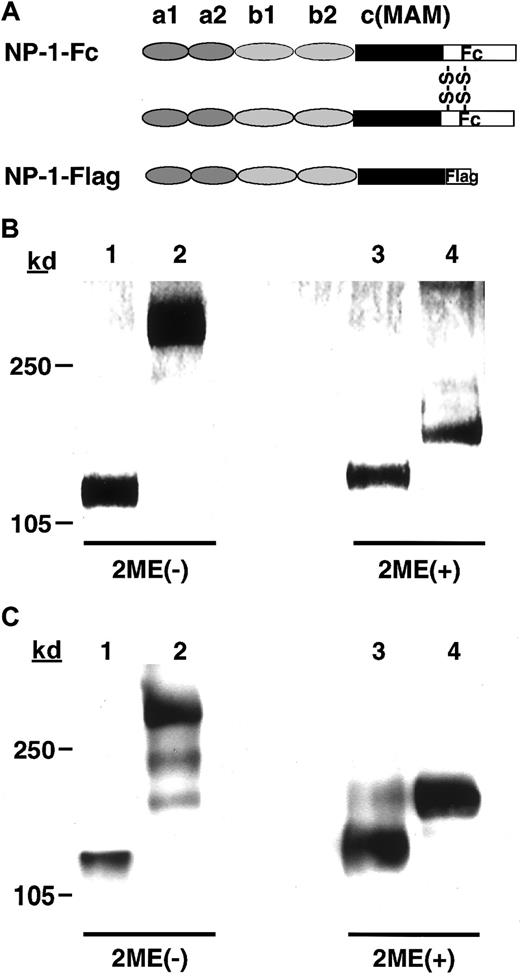
To analyze NP-1 function in vitro in P-Sp cultures, we produced recombinant fusion proteins containing the Fc part of human IgG1 or Flag and the full-length of the NP-1 ectodomain (NP-1–Fc and NP-1–Flag; Figure 2A). We verified the molecular size of each protein by using SDS–polyacrylamide gel electrophoresis. The molecular size of each protein was the expected size under both reducing and nonreducing conditions (Figure 2B). We then confirmed, using Western blotting (reducing and nonreducing conditions), that NP-1–Fc formed a dimer NP-1 and NP-1–Flag formed a monomer NP-1 (Figure 2C).
The structure of recombinant NP-1 proteins.
(A) Schematic representation of the Fc-tagged NP-1 extracellular segment (NP-1–Fc) and Flag-tagged NP-1 extracellular segment (NP-1–Flag). (B) SDS-PAGE analysis of the recombinant proteins stained with Coomassie brilliant blue G-250. (C) Immunoblot of the recombinant proteins with the antirabbit NP-1 antibody.14 Lanes 1-4 correspond to NP-1–Flag (1), NP-1–Fc (2), NP-1–Flag (3), and NP-1–Fc (4) recombinant proteins; 1 and 2 were under nonreducing conditions and 3 and 4 were under reducing conditions.
The structure of recombinant NP-1 proteins.
(A) Schematic representation of the Fc-tagged NP-1 extracellular segment (NP-1–Fc) and Flag-tagged NP-1 extracellular segment (NP-1–Flag). (B) SDS-PAGE analysis of the recombinant proteins stained with Coomassie brilliant blue G-250. (C) Immunoblot of the recombinant proteins with the antirabbit NP-1 antibody.14 Lanes 1-4 correspond to NP-1–Flag (1), NP-1–Fc (2), NP-1–Flag (3), and NP-1–Fc (4) recombinant proteins; 1 and 2 were under nonreducing conditions and 3 and 4 were under reducing conditions.
Effects of soluble NP-1 on vasculogenesis and angiogenesis in P-Sp cultures
We previously reported that when soluble receptors, such as VEGFR-2 or Tie-2, were added to the P-Sp culture system and free ligands were saturated in the culture, EC development was suppressed.20 To test whether soluble NP-1 in P-Sp cultures prevented vascular development by inhibiting the binding of VEGF165 to VEGFR-2, excess amounts of NP-1–Flag or NP-1–Fc were added to the P-Sp cultures of the wild-type embryos. We measured the VEGF concentration in the P-Sp cultures by using enzyme-linked immunosorbent assay and confirmed that 1.2 ± 0.4 ng/mL (n = 5) VEGF was produced in wild-type cultures and 1.5 ± 0.3 ng/mL (n = 5) VEGF was produced in mutant cultures.
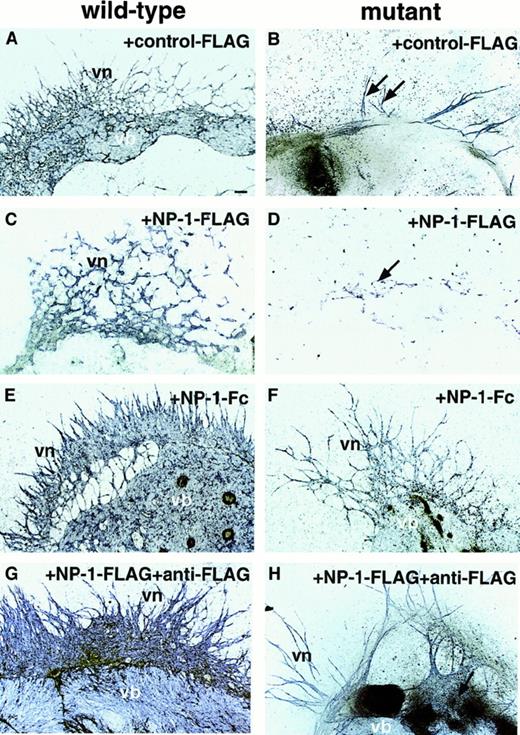
On addition of 50 μg/mL NP-1–Flag to these cultures, vascular-bed formation was suppressed (Figure 3C), but unexpectedly, on addition of 50 μg/mL NP-1–Fc or NP-1–Flag plus anti-Flag IgG (dimer form of NP-1–Flag) to this culture, vascular-bed formation was expanded in the wild-type embryos (Figure 3E,G). Innp-1 mutants, suppressed EC development was completely rescued by addition of NP-1–Fc or NP-1–Flag plus anti-Flag IgG (Figure 3F,H), whereas the same amount of CD4-Fc (data not shown), Flag (Figure 3B), or anti-Flag alone (data not shown) as a control protein had no effect.
Effect of soluble NP-1 in P-Sp cultures.
P-Sp explants derived from E9.5 wild-type embryos (A, C, E, G) and mutant embryos (B, D, F, H) were cultured on OP9 stromal cells. NP-1–Flag (50 μg/mL; C,D,G,H), anti-Flag IgG (50 μg/mL; G-H), NP-1–Fc (50 μg/mL; E-F), or the same amount of control Flag (A-B) was added to this culture. In wild-type embryos, vb formation was suppressed when NP-1–Flag was added (C); however, on addition of NP-1–Fc (E) or NP-1–Flag plus anti-Flag IgG (G), which forms a dimer of NP-1, the vb was expanded compared with that on addition of control Flag (A). In homozygous mutants, vascular formation was slightly suppressed on addition of NP-1–Flag (D; arrow) compared with that on addition of control Flag (B); however, suppressed EC development was rescued by addition of NP-1–Fc (F) or NP-1–Flag plus anti-Flag IgG (H). Similar results were obtained in 4 independent experiments. The scale bar indicates 100 μm.
Effect of soluble NP-1 in P-Sp cultures.
P-Sp explants derived from E9.5 wild-type embryos (A, C, E, G) and mutant embryos (B, D, F, H) were cultured on OP9 stromal cells. NP-1–Flag (50 μg/mL; C,D,G,H), anti-Flag IgG (50 μg/mL; G-H), NP-1–Fc (50 μg/mL; E-F), or the same amount of control Flag (A-B) was added to this culture. In wild-type embryos, vb formation was suppressed when NP-1–Flag was added (C); however, on addition of NP-1–Fc (E) or NP-1–Flag plus anti-Flag IgG (G), which forms a dimer of NP-1, the vb was expanded compared with that on addition of control Flag (A). In homozygous mutants, vascular formation was slightly suppressed on addition of NP-1–Flag (D; arrow) compared with that on addition of control Flag (B); however, suppressed EC development was rescued by addition of NP-1–Fc (F) or NP-1–Flag plus anti-Flag IgG (H). Similar results were obtained in 4 independent experiments. The scale bar indicates 100 μm.
Effect of dimer of soluble NP-1 on the growth of sorted ECs
P-Sp explants contain many types of cells, including hematopoietic cells, endothelial progenitor cells, and other mesenchymal cells. To clarify whether NP-1–Fc affects ECs directly to induce vasculogenesis and angiogenesis, we sorted ECs from E12.5 wild-type and mutant embryos using FACS and cultured them on OP9 cells in the presence or absence of NP-1–Fc. Dual staining with anti–VEGFR-2 and PECAM-1 mAbs revealed that 0.3% of cells in wild-type embryos and 0.5% of cells in mutant embryos were double positive (Figure 4A). VEGFR-2+ PECAM-1+ cells were then cultured on OP9 stromal cells in the presence of 1 ng/mL VEGF (Figure 4B). VEGFR-2+ PECAM-1+ cells from wild-type embryos formed vascular beds and networks (Figure 4Bi), whereas ECs from mutant embryos did not proliferate in the presence of 1 ng/mL VEGF (Figure4Bii). However, 50 μg/mL NP-1–Fc in addition to 1 ng/mL VEGF promoted vascular development (Figure 4Biii) as observed in cultures of ECs from wild-type embryos (Figure 4Bi). The same amount of CD4-Fc had no effect (data not shown). These findings indicate that NP-1–Fc enhances the proliferation of individual ECs through VEGFR-2.
Effect of dimer of soluble NP-1 on sorted ECs.
(A) Murine embryos of E12.5 wild-type and np-1 homozygous mutants were dissociated and stained with PE-conjugated anti–PECAM-1 and biotin-conjugated anti–VEGFR-2 mAbs. Biotin was developed to avidin-allophycocyanin. The stained cells were analyzed and sorted using FACSvantage. Approximately 0.3% of cells derived from wild-type embryos and 0.5% of cells derived from mutant embryos were double positive. (B) Sorted VEGFR-2+ PECAM-1+ cells from E12.5 wild-type (i) and np-1 homozygous mutants (ii-iii) were cultured on OP9 cells. The vascular structure was defective in the ECs from mutant embryos (ii) compared with those from wild-type litter mates (i). NP-1–Fc (50 μg/mL) or CD4-Fc (50 μg/mL) was added to the culture as described above. Vascular formation was rescued in np-1 homozygous mutants by addition of NP-1–Fc (iii), whereas the same amount of CD4-Fc did not have any effect (data not shown). Scale bar indicates 100 μm. (C) Cell lysates of VEGFR-2+ PECAM-1+ ECs fromnp-1+/+ or np-1−/−murine embryos were immunoprecipitated with an anti–VEGFR-2 antibody and subjected to Western blotting using an antiphosphotyrosine mAb (anti-PY). Lane 1 shows results with VEGF (1 ng/mL; wild type); 2, no factor (mutant); 3, VEGF (1 ng/mL; mutant); and 4, VEGF (1 ng/mL plus NP-1–Fc [50 μg/mL]; mutant). The VEGFR-2+PECAM-1+ ECs from np-1+/+ ornp-1−/− embryos were challenged by using VEGF with or without NP-1–Fc. In mutant embryos, phosphorylation of VEGFR-2 was induced by addition of a low dose of VEGF and NP-1–Fc (lane 4; arrowhead), whereas no factor (lane 2) or VEGF alone (lane 3) did not induce phosphorylation of VEGFR-2. Lane 1 was used as a positive control (wt). The lower panel shows the amount of immunoprecipitated VEGFR-2 confirmed by Western blotting using anti–VEGFR-2 mAb.
Effect of dimer of soluble NP-1 on sorted ECs.
(A) Murine embryos of E12.5 wild-type and np-1 homozygous mutants were dissociated and stained with PE-conjugated anti–PECAM-1 and biotin-conjugated anti–VEGFR-2 mAbs. Biotin was developed to avidin-allophycocyanin. The stained cells were analyzed and sorted using FACSvantage. Approximately 0.3% of cells derived from wild-type embryos and 0.5% of cells derived from mutant embryos were double positive. (B) Sorted VEGFR-2+ PECAM-1+ cells from E12.5 wild-type (i) and np-1 homozygous mutants (ii-iii) were cultured on OP9 cells. The vascular structure was defective in the ECs from mutant embryos (ii) compared with those from wild-type litter mates (i). NP-1–Fc (50 μg/mL) or CD4-Fc (50 μg/mL) was added to the culture as described above. Vascular formation was rescued in np-1 homozygous mutants by addition of NP-1–Fc (iii), whereas the same amount of CD4-Fc did not have any effect (data not shown). Scale bar indicates 100 μm. (C) Cell lysates of VEGFR-2+ PECAM-1+ ECs fromnp-1+/+ or np-1−/−murine embryos were immunoprecipitated with an anti–VEGFR-2 antibody and subjected to Western blotting using an antiphosphotyrosine mAb (anti-PY). Lane 1 shows results with VEGF (1 ng/mL; wild type); 2, no factor (mutant); 3, VEGF (1 ng/mL; mutant); and 4, VEGF (1 ng/mL plus NP-1–Fc [50 μg/mL]; mutant). The VEGFR-2+PECAM-1+ ECs from np-1+/+ ornp-1−/− embryos were challenged by using VEGF with or without NP-1–Fc. In mutant embryos, phosphorylation of VEGFR-2 was induced by addition of a low dose of VEGF and NP-1–Fc (lane 4; arrowhead), whereas no factor (lane 2) or VEGF alone (lane 3) did not induce phosphorylation of VEGFR-2. Lane 1 was used as a positive control (wt). The lower panel shows the amount of immunoprecipitated VEGFR-2 confirmed by Western blotting using anti–VEGFR-2 mAb.
To examine the role of NP-1–Fc in VEGFR-2 activation, we evaluated phosphorylation of VEGFR-2 on the ECs. Whole E12.5 wild-type and mutant embryos were dissociated and then cultured on OP9 stromal cells in the presence of 10 ng/mL VEGF and bFGF for 7 days. The VEGFR-2+PECAM-1+ ECs were then sorted with FACS, seeded on fibronectin-coated dishes, cultured for 24 hours under serum-free conditions, and subsequently challenged with VEGF. Although ECs from mutant embryos expressed slightly more VEGFR-2 than those from wild-type embryos (Figure 4A), this difference was lost after culturing in vitro (data not shown). Cell lysates were immunoprecipitated with an anti–VEGFR-2 antibody and then subjected to Western blotting using an antiphosphotyrosine mAb. Phosphorylation of VEGFR-2 was induced by addition of a low dose of VEGF and NP-1–Fc in ECs from np-1mutants (Figure 4C; lane 4). VEGF alone did not induce phosphorylation of VEGFR-2 in ECs from np-1 mutants (Figure 4C; lane 3), whereas VEGF alone did induce phosphorylation in samples from wild-type embryos (Figure 4C; lane1).
Synergistic effect of VEGF and dimer of soluble NP-1 on EC growth
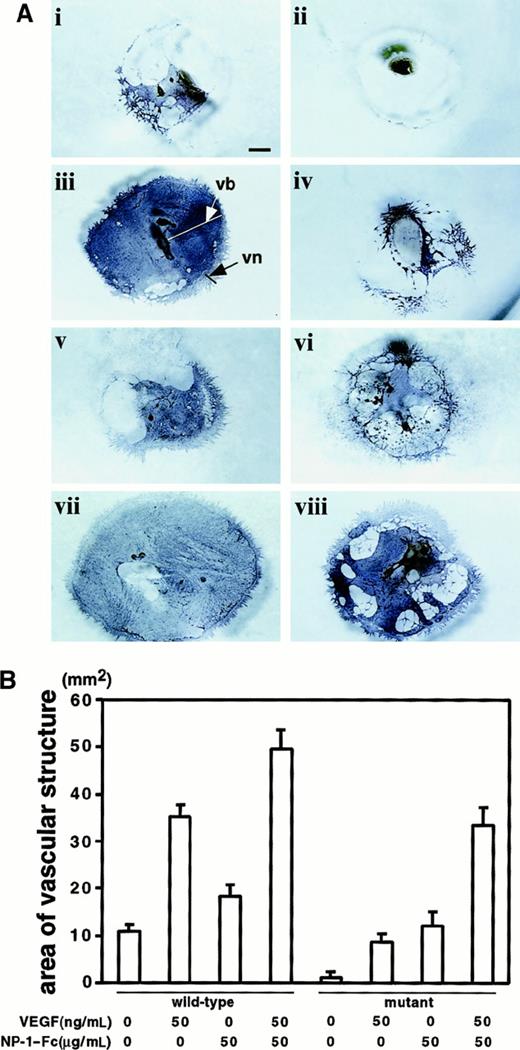
We next examined the synergistic effect of NP-1–Fc and VEGF in P-Sp cultures. P-Sp explants from wild-type (Figure5Ai,iii,v,vii) and mutant (Figure5Aii,iv,vi,viii) embryos on E9.5 were cultured on OP9 stromal cells as described above. The suppressed vasculature in the culture ofnp-1−/− embryos (Figure 5Aii) was rescued by addition of 50 ng/mL VEGF (Figure 5Aiv) or 50 μg/mL NP-1–Fc (Figure5Avi). The pattern of the rescue with VEGF differed from that with NP-1–Fc. When VEGF alone was added to P-Sp cultures ofnp-1−/− embryos (Figure 5Aiv), the vascular bed was poorly formed compared with that of wild-type embryos (Figure5Ai). In contrast, on addition of NP-1–Fc, vascular-bed and vascular-network formation rose to a level comparable to that in wild-type embryos (Figure 5Ai,vi). Of particular note, vascular formation was expanded by simultaneous addition of VEGF and NP-1–Fc in both wild-type (Figure 5Avii) and np-1 mutant explants (Figure 5Aviii). Vascular areas from each P-Sp explant were measured under each culture condition. Quantitative analyses showed that they were increased in the presence of VEGF and NP-1–Fc (Figure 5B). This suggests that VEGF and NP-1–Fc work synergistically in vascular formation.
Synergistic effect of VEGF and dimer of soluble NP-1 in P-Sp cultures.
(A) P-Sp explants derived from E9.5 wild-type and np-1homozygous mutant murine embryo litter mates were cultured on OP9 stromal cells. Formation of the vb and vn was defective in mutant embryo explants (ii) compared with those from wild-type litter mates (i). NP-1–Fc (50 μg/mL), VEGF (50 ng/mL), or both were added to this culture system as described above. The vb was expanded innp-1+/+ embryos on addition of VEGF (iii) or NP-1–Fc (v). Suppressed vasculature in the culture ofnp-1−/− embryos (ii) was partly rescued on addition of 50 ng/mL VEGF (iv) and was completely rescued on addition of 50 μg/mL NP-1–Fc (vi). Simultaneous application of VEGF and NP-1–Fc enhanced the formation of the vb innp-1+/+ (vii) andnp-1−/− (viii) P-Sp cultures. Similar results were obtained in 3 independent experiments. The scale bar indicates 1 mm. (B) The vascular areas shown in Figure 5A were calculated using NIH Image software version 1.62. The mean ± SD vascular areas per explant obtained from 3 independent experiments were as follows: (i) 10.8 ± 1.48 mm2, (ii) 1.1 ± 0.55 mm2, (iii) 35.2 ± 2.39 mm2, (iv) 7.7 ± 1.68 mm2, (v) 18.3 ± 2.48mm2, (vi) 12.1 ± 3.03mm2, (vii) 49.7 ± 3.91 mm2, and (viii) 33.5 ± 3.77 mm2.
Synergistic effect of VEGF and dimer of soluble NP-1 in P-Sp cultures.
(A) P-Sp explants derived from E9.5 wild-type and np-1homozygous mutant murine embryo litter mates were cultured on OP9 stromal cells. Formation of the vb and vn was defective in mutant embryo explants (ii) compared with those from wild-type litter mates (i). NP-1–Fc (50 μg/mL), VEGF (50 ng/mL), or both were added to this culture system as described above. The vb was expanded innp-1+/+ embryos on addition of VEGF (iii) or NP-1–Fc (v). Suppressed vasculature in the culture ofnp-1−/− embryos (ii) was partly rescued on addition of 50 ng/mL VEGF (iv) and was completely rescued on addition of 50 μg/mL NP-1–Fc (vi). Simultaneous application of VEGF and NP-1–Fc enhanced the formation of the vb innp-1+/+ (vii) andnp-1−/− (viii) P-Sp cultures. Similar results were obtained in 3 independent experiments. The scale bar indicates 1 mm. (B) The vascular areas shown in Figure 5A were calculated using NIH Image software version 1.62. The mean ± SD vascular areas per explant obtained from 3 independent experiments were as follows: (i) 10.8 ± 1.48 mm2, (ii) 1.1 ± 0.55 mm2, (iii) 35.2 ± 2.39 mm2, (iv) 7.7 ± 1.68 mm2, (v) 18.3 ± 2.48mm2, (vi) 12.1 ± 3.03mm2, (vii) 49.7 ± 3.91 mm2, and (viii) 33.5 ± 3.77 mm2.
In vivoeffect of dimer of soluble NP-1 on np-1mutant embryos
To examine the in vivo effect of NP-1–Fc, pregnant mice were given daily intraperitoneal injections of 150 μL of a 2 mg/mL solution of either NP-1–Fc or CD4-Fc from E9.5 to 11.5. Embryos were harvested on E12.5 and immunohistochemical analysis was done on whole-mount embryos or sections by using PECAM-1 mAb to observe the vascularity of the embryos. We obtained 11np-1−/− embryos and 56np-1+/+ or np-1+/−embryos from 6 dams given injections of NP-1–Fc. Injection of CD4-Fc into the np-1 mutants as a control had no effect (Figure6A,C). On injection of NP-1–Fc, vascularity in np-1+/+ andnp-1+/− embryos was slightly enhanced; however,np-1−/− embryos had substantial recovery of vascularity, and interestingly, the caliber of the rescued capillaries was larger than that of the wild-type embryos (Figure 1A,C and Figure6B,D). We confirmed that NP-1–Fc bound to ECs in CNS and pericardium by staining with antihuman IgG (data not shown). These findings indicate that in np-1−/− embryos, NP-1–Fc affects EC proliferation and migration directly.
Rescue of defective vascularity of
np-1 mutant embryos in vivo by dimer of soluble NP-1. Injection of CD4-Fc as a control had no effect (A, C). On injection of NP-1–Fc, some np-1−/− embryos showed a substantial recovery of vascularity, and interestingly, the caliber of the rescued capillaries (B, D) was larger than those of capillaries in wild-type embryos (Figure 1A,C). Similar results were obtained in 3 independent experiments. The scale bar indicates 150 μm (A-B) or 300 μm (C-D).
Rescue of defective vascularity of
np-1 mutant embryos in vivo by dimer of soluble NP-1. Injection of CD4-Fc as a control had no effect (A, C). On injection of NP-1–Fc, some np-1−/− embryos showed a substantial recovery of vascularity, and interestingly, the caliber of the rescued capillaries (B, D) was larger than those of capillaries in wild-type embryos (Figure 1A,C). Similar results were obtained in 3 independent experiments. The scale bar indicates 150 μm (A-B) or 300 μm (C-D).
Discussion
In this study, we showed that complexes of NP-1–Fc and VEGF165 directly stimulate VEGFR-2 on ECs. As reported previously, when NP-1 was coexpressed with VEGFR-2, the chemotactic activity and mitogenicity for ECs induced by stimulation of VEGF165 were enhanced.10 Moreover, a murine transgenic model in which native np-1 complementary DNA was overexpressed under the transcriptional control of the β-actin promoter revealed an excess of capillaries and blood vessels, dilation of blood vessels, and a malformed heart.14 NP-1 is a transmembrane protein, but recent studies indicated the existence of a soluble monomeric form.22 This soluble NP-1 monomer binds ECs and tumor cells and appears to be a VEGF165antagonist.22 Interestingly, NP-1–Fc alone can enhance phosphorylation of VEGFR-2 in VEGFR-2+ NP-1–negative (NP-1−) ECs from np-1 mutant embryos in the presence of 1 ng/mL VEGF. Moreover, the P-Sp culture system, which allows for vasculogenesis and angiogenesis,20,21 showed that NP-1–Fc promotes proliferation of ECs. Furthermore, NP-1–Fc could rescue suppression of the development of ECs in the CNS and pericardium of np-1−/−embryos. In neuronal guidance, only the extracellular domain of NP-1 is important for mediation of NP-1 signaling.28 These results indicate that the cytoplasmic domain of NP-1 is not essential for signal transduction through VEGFR-2.
Commonly, soluble receptor proteins bind their ligands with affinities similar to those of cognate transmembrane receptors.29 Most soluble receptors for cytokines and growth factors compete with their membrane-bound counterparts for binding of the ligand and are therefore antagonists. In contrast, as in the case of NP-1–Fc, the soluble receptor works as a signal modulator, as it does in hematopoietic cells. IL-6 is one of several molecules that stimulate proliferation of primitive hematopoietic progenitors.30,31 Binding of IL-6 to IL-6 receptor (IL-6R) stimulated the signal-transducing molecule gp130.32Exogenous soluble IL-6R–IL-6 complex stimulated gp130 on hematopoietic cells directly.33
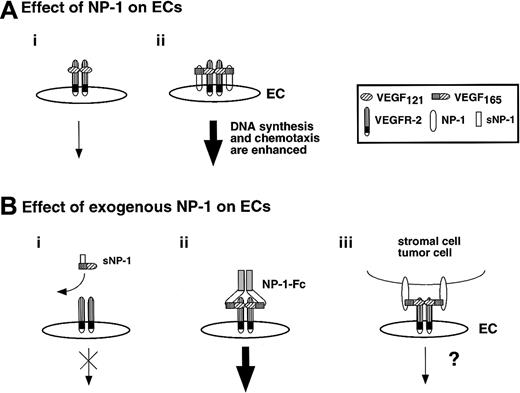
NP-1 is composed of the a/CUB domain, b/FV/VIII domain, c/MAM domain, transmembrane, and a short cytoplasmic domain that does not have an obvious motif. Naturally occurring soluble NP-1 (sNP-1) contains only the a and b domains and does not dimerize. The sNP-1 tagged with Flag epitope (NP-1–Flag) that we constructed in this experiment contains the a, b, and c domains. NP-1–Flag is a monomeric form and works as a suppressor, as was observed with naturally occurring sNP-1 (Figure7Bi). On the other hand, NP-1 tagged with the Fc part of human IgG1 (NP-1–Fc) contains the a, b, and c domains in a dimeric form. Although our soluble NP-1–Fc might not represent what occurs in vivo, this engineered protein indeed enhanced the function of VEGFR-2+ ECs (Figure 7Bii). As far as we know, this is the first model in which a soluble receptor works as a signal modulator for ECs. Although the molecular mechanism allowing a monomer and dimer of NP-1 to work as an antagonist and agonist, respectively, is not known, we think that the findings presented here may have important clinical applications.
Effect of exogenous NP-1 on EC development.
(A) When NP-1 was expressed on ECs, signaling by VEGF165was enhanced through dimerization of NP-1 and VEGFR-2 (ii) compared with situations in which VEGFR-2 alone was expressed on ECs (i). This may have been caused by conformational changes produced by VEGFR-2 on ECs. (B) A monomer of soluble NP-1 inhibited binding of VEGF165 to VEGFR-2 and prevented phosphorylation of VEGFR-2 (i); however, a dimer of soluble NP-1 bound to VEGF and enhanced phosphorylation of VEGFR-2 exogenously (ii). Candidate cells that express NP-1 and affect activation of VEGFR-2 on EC exogenously are neuronal cells, stromal cells in the bone marrow, tumor cells, and so on (iii). However, it is not clear whether the NP-1 on such cell types forms a dimer.
Effect of exogenous NP-1 on EC development.
(A) When NP-1 was expressed on ECs, signaling by VEGF165was enhanced through dimerization of NP-1 and VEGFR-2 (ii) compared with situations in which VEGFR-2 alone was expressed on ECs (i). This may have been caused by conformational changes produced by VEGFR-2 on ECs. (B) A monomer of soluble NP-1 inhibited binding of VEGF165 to VEGFR-2 and prevented phosphorylation of VEGFR-2 (i); however, a dimer of soluble NP-1 bound to VEGF and enhanced phosphorylation of VEGFR-2 exogenously (ii). Candidate cells that express NP-1 and affect activation of VEGFR-2 on EC exogenously are neuronal cells, stromal cells in the bone marrow, tumor cells, and so on (iii). However, it is not clear whether the NP-1 on such cell types forms a dimer.
VEGFR-2 is exclusively expressed on all ECs during embryogenesis; however, NP-1 is not always expressed on ECs (data not shown). As we observed in np-1 mutant embryos, NP-1 is essential for vascular development. These results indicate that exogenous NP-1 may be required for the proliferation of VEGFR-2+NP-1− ECs. In our experiment, 1 ng/mL VEGF alone did not phosphorylate VEGFR-2 in NP-1− ECs from np-1mutant embryos, but on simultaneous addition of NP-1–Fc, 1 ng/mL VEGF could phosphorylate VEGFR-2 in these ECs. This finding indicates that NP-1 contributes to proliferation or migration of ECs where VEGF is not abundantly expressed. Although the existence of a dimer form of soluble NP-1 has not been reported, tumor cells, neuronal cells, and stromal cells in bone marrow have been shown to express NP-1.10 34It is possible that NP-1 on such cell types forms a dimer on the cell surface, binds to VEGF, and then delivers VEGF to NP-1− ECs (Figure 7Biii). The molecular cues that promote dimerization of NP-1 on non–EC-lineage cells should be addressed.
The defective vasculogenesis and angiogenesis that we observed innp-1 mutant embryos was in restricted sites, such as the CNS and pericardium. VEGF expression has been reported to be less abundant in the CNS on E12.5.35 If we hypothesize that exogenous VEGF–NP-1 complexes on non–EC-lineage cells are required for normal vasculogenesis and angiogenesis when tissue distribution of VEGF is limited, it is logical that defective angiogenesis was observed in the CNS of np-1 mutants. In the experiment in which NP-1–Fc was injected into pregnant mice, we did not observe excess capillary formation in wild-type embryos, although defective capillary formation was rescued in np-1 mutant embryos. The effect of NP-1 might depend on the up-regulation of VEGF or VEGFR-2 protein innp-1 mutant embryos. Indeed, Northern blotting analysis showed that VEGF and VEGFR-2 expression in mutant embryos was up-regulated to levels about 1.5 to 2 times that in wild-type embryos (data not shown). Although the existence of organ-specific NP-1 functions, especially in the pericardium and CNS, cannot be ruled out, it appears that VEGF overproduced in np-1 mutants binds to NP-1–Fc and that these VEGF–NP-1 complexes stimulate excess VEGFR-2 on ECs. If such compensational up-regulation of VEGF or VEGFR-2 did not occur in np-1 mutants, more severe defects in vasculogenesis and angiogenesis might be observed.
We thank Dr Fumio Arai, Dr Takeshi Naruse, and Miss Yukari Mukoumatsu for excellent technical assistance and Dr Hiroaki Kodama for providing OP9 stromal cells.
Supported by grants-in-aid for Scientific Research on Priority Areas from the Ministry of Education, Science, and Culture of Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Toshio Suda, Department of Cell Differentiation, Institute of Molecular Embryology and Genetics, Kumamoto University School of Medicine, Honjo 2-2-1, Kumamoto 860-0811, Japan; e-mail:sudato@gpo.kumamoto-u.ac.jp.

![Fig. 4. Effect of dimer of soluble NP-1 on sorted ECs. / (A) Murine embryos of E12.5 wild-type and np-1 homozygous mutants were dissociated and stained with PE-conjugated anti–PECAM-1 and biotin-conjugated anti–VEGFR-2 mAbs. Biotin was developed to avidin-allophycocyanin. The stained cells were analyzed and sorted using FACSvantage. Approximately 0.3% of cells derived from wild-type embryos and 0.5% of cells derived from mutant embryos were double positive. (B) Sorted VEGFR-2+ PECAM-1+ cells from E12.5 wild-type (i) and np-1 homozygous mutants (ii-iii) were cultured on OP9 cells. The vascular structure was defective in the ECs from mutant embryos (ii) compared with those from wild-type litter mates (i). NP-1–Fc (50 μg/mL) or CD4-Fc (50 μg/mL) was added to the culture as described above. Vascular formation was rescued in np-1 homozygous mutants by addition of NP-1–Fc (iii), whereas the same amount of CD4-Fc did not have any effect (data not shown). Scale bar indicates 100 μm. (C) Cell lysates of VEGFR-2+ PECAM-1+ ECs fromnp-1+/+ or np-1−/−murine embryos were immunoprecipitated with an anti–VEGFR-2 antibody and subjected to Western blotting using an antiphosphotyrosine mAb (anti-PY). Lane 1 shows results with VEGF (1 ng/mL; wild type); 2, no factor (mutant); 3, VEGF (1 ng/mL; mutant); and 4, VEGF (1 ng/mL plus NP-1–Fc [50 μg/mL]; mutant). The VEGFR-2+PECAM-1+ ECs from np-1+/+ ornp-1−/− embryos were challenged by using VEGF with or without NP-1–Fc. In mutant embryos, phosphorylation of VEGFR-2 was induced by addition of a low dose of VEGF and NP-1–Fc (lane 4; arrowhead), whereas no factor (lane 2) or VEGF alone (lane 3) did not induce phosphorylation of VEGFR-2. Lane 1 was used as a positive control (wt). The lower panel shows the amount of immunoprecipitated VEGFR-2 confirmed by Western blotting using anti–VEGFR-2 mAb.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/6/10.1182_blood.v97.6.1671/6/m_h80610786004.jpeg?Expires=1769247268&Signature=VTjQxmB8-xDTKT6fC~Gbs1d9H-T8RkTzqIB4WvqEctj3G29656LvZvuNQtYp5YzppF0MtXm3xHGOkuUQHNhd4A4IlAl-ik1lY9J69Kah7vioXVqNe9eLl0EFml5ZwpNFyZWJA4Q0PY~-SN8mZh3mp0d~JK5pttMsZLuU8Pn3-JcoAuFvNGnMB8GN9pRKkXFEpUbv7pgdrbV8quOtItqsWG0BfGi1d4p108lUVM8On0r~-V1fni8vmgk9S7R5m7OBOpyK6T5hq7sUVudTxYw~-2eqof3YDvJzScwm4yCsRFuUeI~IjWSFG2QZxj~43M-gzmf6l8VtV~Q44j5Y4jq5TA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal