Vascular endothelial cadherin (VE-cadherin) is an endothelial cell–specific cadherin that plays an important role in the control of vascular organization. Blocking VE-cadherin antibodies strongly inhibit angiogenesis, and inactivation of VE-cadherin gene causes embryonic lethality due to a lack of correct organization and remodeling of the vasculature. Hence, inhibitors of VE-cadherin adhesive properties may constitute a tool to prevent tumor neovascularization. In this paper, we tested different monoclonal antibodies (mAbs) directed to human VE-cadherin ectodomain for their functional activity. Three mAbs (Cad 5, BV6, BV9) were able to increase paracellular permeability, inhibit VE-cadherin reorganization, and block angiogenesis in vitro. These mAbs could also induce endothelial cell apoptosis in vitro. Two additional mAbs, TEA 1.31 and Hec 1.2, had an intermediate or undetectable activity, respectively, in these assays. Epitope mapping studies show that BV6, BV9, TEA 1.31, and Hec 1.2 bound to a recombinant fragment spanning the extracellular juxtamembrane domains EC3 through EC4. In contrast, Cad 5 bound to the aminoterminal domain EC1. By peptide scanning analysis and competition experiments, we defined the sequences TIDLRY located on EC3 and KVFRVDAETGDVFAI on EC1 as the binding domain of BV6 and Cad 5, respectively. Overall, these results support the concept that VE-cadherin plays a relevant role on human endothelial cell properties. Antibodies directed to the extracellular domains EC1 but also EC3-EC4 affect VE-cadherin adhesion and clustering and alter endothelial cell permeability, apoptosis, and vascular structure formation.
Introduction
Vascular endothelial cadherin (VE-cadherin) is an endothelial cell–specific cadherin that plays a major role in the organization of intercellular adherens junctions.1 A null mutation of VE-cadherin gene induces embryonic lethality at day 9.5 to 10 of development due to a lack of assembly and remodeling of the vasculature.2,3 In VE-cadherin−/− mice, endothelial cells undergo apoptosis and lose the capacity to respond to protective signals induced by vascular endothelial cell growth factor (VEGF).2 Consistent with these observations, VE-cadherin blocking antibodies are able to inhibit angiogenesis in vitro4 5 and tumor vascularization in vivo in mice (F. Liao, P. Bohlen, and D. J. Hicklin, unpublished observations, 2000).
The molecular basis of cadherin homophilic binding has not yet been fully elucidated. Cadherins are single-pass transmembrane glycoproteins that associate as cis-dimers on the cell surface and then combine to form a linear zipperlike structure that promotes homophilic intercellular adhesion.6-12
The extracellular region of classical cadherins is composed of 5 homologous domains (numbered EC1 to EC5).13 Several studies are consistent with the concept that the aminoterminal region corresponding to domain EC1 of cadherins is responsible for homophilic recognition. Blocking monoclonal antibodies (mAbs) directed to E-, P-, and N-cadherin have been found to bind within this region.14 Studies using multiple-point mutations and chimeric proteins showed that the aminoterminal 113 amino acid (AA) region is responsible for homophilic adhesive specificity between cadherins.14
The crystal structure of N-cadherin EC1 supported the idea that homophilic adhesion of antiparallel cis-dimers occurs through N-terminal domains.10 In addition, recent studies brought evidence that cis-dimers may form through the interaction of EC1 and EC2.15
At variance with these reports, others suggest that the ectodomain of cadherins may present multiple adhesive contacts and that other regions may influence cadherin-adhesive properties.16
In this paper, we analyze 5 mAbs directed to the human VE-cadherin extracellular region for their activity on a set of in vitro biologic assays using human endothelial cells. We found that 3 mAbs have a significant effect on endothelial cell permeability, in vitro angiogenesis, and endothelial cell apoptosis. One of these mAbs, Cad 5, bound to a peptidic region contained in the extracellular domain EC1; and BV6 and BV9, bound in EC3 or EC3-EC4, respectively. These data support the idea that VE-cadherin sequence contains multiple domains able to modulate its adhesive and clustering properties. VE-cadherin blocking mAbs may be useful tools to study the role of endothelial cell junctions in permeability control and angiogenesis.
Materials and methods
Cell culture
Endothelial cells were harvested from human umbilical veins and cultured as previously described.17
Antibodies and reagents
Mouse mAbs against the extracellular domain of human VE-cadherin were as follows: clone TEA 1.31,18,19 clone BV6,18,19 clone BV9,18,19 Cad 5 (Transduction Laboratories, Lexington, KY), and Hec 1.2.20 The mAbs were used as purified immunoglobulin G (IgG). A concentration of 50 μg/mL of each mAb was selected as saturating concentration. This was tested at flow cytometry using endothelial cells in suspension incubated with increasing concentrations of the mAbs. A total of 50 μg/mL of each mAb gave maximal fluorescence intensity, and higher concentrations did not further increase this parameter.21 A polyclonal antiserum (VECR1-5) was raised against a bacterially expressed entire extracellular region of human VE-cadherin and affinity-purified using the same immunogen coupled to CNBr-Sepharose (Pharmacia, Piscataway, NJ).
Rhodamine-conjugated secondary antibodies (reactive with either mouse or rabbit IgG) were purchased from Dakopatts (Glostrup, Denmark). Antihistidine mAb was purchased from Amersham-Pharmacia (Uppsala, Sweden).
Peroxidase-conjugated goat antimouse IgG antibody was used for immunoblotting (Jackson Immunoresearch Laboratories, West Grove, PA).
After sodium dodecyl sulfate–polyacrylamide gel electrophoresis and blotting, the biotynilated proteins were detected by streptavidin–horseradish peroxidase (Biospa Division, Milan, Italy).
Development of peroxidase activity was performed using an enhanced chemiluminescence kit (Amersham-Pharmacia).
Immunofluorescence staining
The procedure for immunofluorescence microscopy analysis of endothelial cell monolayer has been described previously.17 Briefly, endothelial cell monolayers were cultured on fibronectin-coated glass coverslips, fixed, permeabilized, and then labeled with a VE-cadherin antibody followed by rhodamine-conjugated secondary antibody. Fluorescence was detected with a Zeiss Axiophot microscope and photographed using T-Max 3200 film.
Fluorescence flow cytometric analysis was performed by a FACStar Plus apparatus (Becton Dickinson, Mountain View, CA) using a fluorescein isothiocynate (FITC)–conjugated goat antimouse antiserum (Jackson ImmunoResearch Laboratories) as described in detail elsewhere.21
Calcium switch assay
Cells grown to confluency on glass coverslips as described previously22 were incubated with 5 mM ethyleneglycotetraacetic acid (EGTA) at 37°C for 30 minutes. EGTA was then removed and Ca++ restored adding fresh medium either in the presence or in absence of mAbs (50 μg/mL). One hour later, cells were fixed and processed for immunofluorescence as described above.
Permeability assay
Permeability across endothelial cell monolayers was measured in Transwell units (with polycarbonate filter, 0.4 μm pore; Corning Costar, Cambridge, MA) as described previously.18 23 Briefly, endothelial cells were cultured for 3 to 4 days to confluency, and then mAbs (50 μg/mL) were added in the upper compartment followed by the addition of FITC-dextran (1 mg/mL, average molecular mass 40 000; Sigma Chemical). At the indicated time points, 50 μL samples were taken from the lower compartment and the fluorescence was measured (492/520 nm, absorption/emission wavelengths).
In some experiments, EGTA (5 mM, final concentration) was added both to the lower and upper compartments for 30 minutes at 37°C. Then, as described above, EGTA was removed and Ca++ restored in the presence or absence of mAbs (50 μg/mL). FITC-dextran was added 1 hour after addition of the mAbs, and aliquots were collected from the lower compartment at the indicated time points and assayed by fluorimetry.
Expression and purification of VE-cadherin recombinant fragments
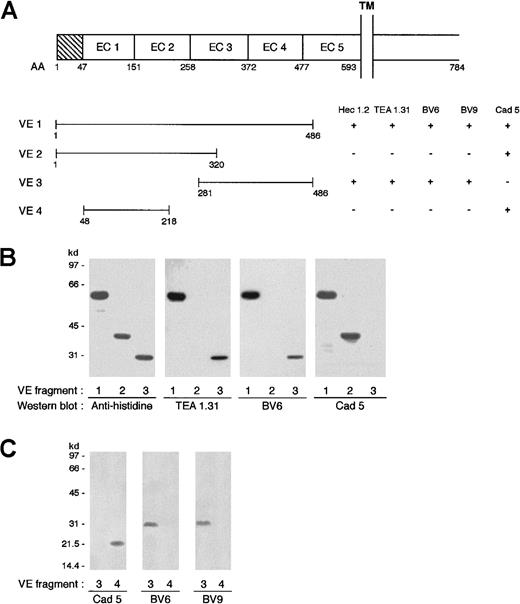
VE1, VE2, VE3, and VE4 fragments, corresponding to the 1 to 486, 1 to 320, 281 to 486, and 48 to 218 AA residues, respectively, of human VE-cadherin (Figure 5) were cloned by polymerase chain reaction amplification. Complementary DNAs were subcloned into the bacterial pQE-32 expression vector (Qiagen, Germany) to produce HIS-tagged VE-cadherin fragments. The fragments were expressed and purified according to the published Qiagen protocol.
The same fragments were also expressed using the TNT Quick Coupled Transcription/Translation System (Promega, Madison, WI). Using this system, biotinylated lysine residues are incorporated into nascent protein during translation.
In few experiments (Figure 6), VE1 fragment was produced as a chimeric protein fused to the Fc portion of human IgG1. VE1-Ig chimera was constructed by cloning the complementary DNA encoding the extracellular portion corresponding to the 1-to-486 AA into the pIg1 eukaryotic expression vector24 using a previously described method24 25 for the expression and purification steps.
Epitope mapping
Peptide scanning was performed following the previously described procedure.26 Peptides were chemically synthesized as arrays of N-terminally acetylated and C-terminally covalently immobilized products on cellulose sheets derivatized with amino–polyethylene glycol anchors by the SPOT synthesis technique using a model ASP222 spotting robot (Abimed Analysen-Technik, Langenfeld, Germany). Peptide arrays included overlapping pentadecapeptides, with an offset of 3 AA residues, spanning the entire extracellular domain of VE-cadherin. Binding of mAbs to peptide spots was detected using a goat antimouse IgG peroxidase-conjugated antibody and followed by enhanced chemiluminescence detection system.
Peptide competition experiments were described elsewhere.24 Briefly, enzyme-linked immunosorbent assay (ELISA) plates were coated with recombinant VE1-Ig chimera (1 μg/mL) and then blocked with 2% bovine serum albumin. BV6 and BV9 mAbs (0.5 μg/mL) were preincubated for 1 hour with different peptides (range 1-500 μM) and were then added to coated plates, followed by peroxidase-conjugated goat antimouse IgG (Jackson ImmunoResearch Laboratories).
Apoptosis
To induce apoptosis, endothelial cells were seeded at 2 × 105 cells in 24-well dishes in 1 mL serum-free MCDB-131 medium (Life Technologies, Paisley, United Kingdom) supplemented with 1% bovine serum albumin and insulin-transferrin-selenium (Life Technologies). Cells were cultured for 72 hours either in the absence or in the presence of VEGF-A (R&D Systems, Minneapolis, MN), 30 ng/mL. Antibodies to VE-cadherin (50 μg/mL) were added 24 hours after seeding and daily thereafter. Apoptosis was quantitated by measuring DNA fragmentation (TUNEL detection method, Boehringer Mannheim, Germany).2
Capillary tube assay
Briefly, human fibrinogen (5 mg/mL solution in distilled water, Sigma code F4883) was dialyzed against triethanolamine-buffered saline (10 mM Tris-HCl, pH 7.4; 150 mM NaCl) to eliminate sodium citrate and stored in aliquots at −20°C. To make the underlying fibrin gel, 500 μL fibrinogen solution (3 mg/mL in triethanolamine-buffered saline with one-tenth volume 10 × minimal essential medium, pH 8.5) was placed into each 24-well culture plate, and human thrombin (Sigma, code T6884) was added to a final concentration of 3 U/mL. Endothelial cells (2.5 × 105/mL per well) were seeded on the top of the polymerized gel and cultured for 24 hours in medium 199 (Life Technologies) with 20% newborn calf serum (Life Technologies) containing endothelial cell growth supplement (50 μg/mL) and VEGF (30 ng/mL). Monclonal antibodies (50 μg/mL) were added for 30 minutes when indicated. The medium was then aspirated and another fibrin gel overlying the monolayer was prepared including mAbs (50 μg/mL) in the fibrinogen solution before clotting, as described.4 Fresh medium containing the same concentration of mAbs was added over the gel, and the formation of tubes was followed by phase contrast microscopy.
Results
Effects of VE-cadherin mAbs on adherens junction organization and permeability
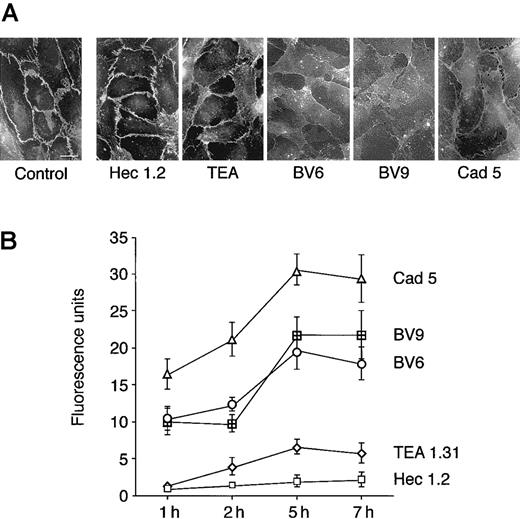
As shown in Figure 1A, mAbs BV6, BV9, and Cad-5 induced redistribution of VE-cadherin from intercellular junctions, while Hec 1.2 and TEA were inactive. Previous work28 showed that VE-cadherin disappearance from junctions was due to diffusion of the molecule on the cell membrane and not to its internalization. Paracellular permeability of endothelial cell monolayers to FITC-dextran was significantly increased by mAbs Cad 5, BV9, and BV6 (Figure 1B). The time course of the effect showed a detectable increase in permeability within 1 hour after the addition of the mAbs, with maximal permeability reached at 5 hours. In contrast, mAb TEA 1.31 showed only a slight effect on permeability, and Hec 1.2 had no effect.
Antibodies directed to VE-cadherin increase endothelial permeability.
(A) Endothelial cells grown to confluency on glass coverslips were exposed to mAbs (50 μg/mL). Seven hours later, cells were fixed and processed for immunofluorescence analysis of VE-cadherin distribution (bar = 20 μm). (B) FITC-dextran passage through endothelial cell monolayers on Transwell filters was measured at different times after incubation with VE-cadherin mAbs. The mAb code is on the right. The background permeability values measured in absence of the mAbs was subtracted from each point of the curves. Values are mean ± SEM of at least 4 replicates of a typical experiments out of 5 performed. The permeability values obtained with Cad 5, BV6, and BV9 were always significantly different (P < .01) from the values obtained with Hec 1.2 by analysis of variance and the Duncan test. The permeability values obtained with TEA 1.31 were significantly different (P < .05) from Hec 1.2 only at 5 hours.
Antibodies directed to VE-cadherin increase endothelial permeability.
(A) Endothelial cells grown to confluency on glass coverslips were exposed to mAbs (50 μg/mL). Seven hours later, cells were fixed and processed for immunofluorescence analysis of VE-cadherin distribution (bar = 20 μm). (B) FITC-dextran passage through endothelial cell monolayers on Transwell filters was measured at different times after incubation with VE-cadherin mAbs. The mAb code is on the right. The background permeability values measured in absence of the mAbs was subtracted from each point of the curves. Values are mean ± SEM of at least 4 replicates of a typical experiments out of 5 performed. The permeability values obtained with Cad 5, BV6, and BV9 were always significantly different (P < .01) from the values obtained with Hec 1.2 by analysis of variance and the Duncan test. The permeability values obtained with TEA 1.31 were significantly different (P < .05) from Hec 1.2 only at 5 hours.
A saturating concentration of the mAbs (50 μg/mL) was used in all the assays. Higher concentrations did not produce a more marked effect (not shown).
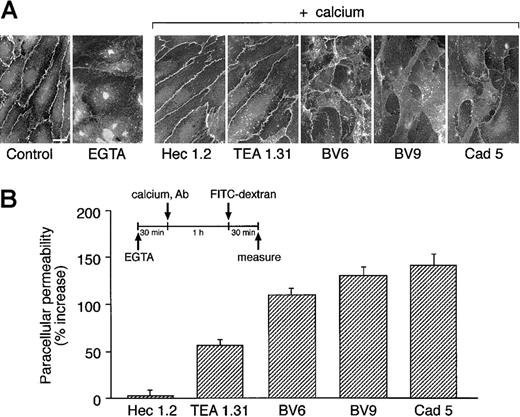
The mAbs were further studied in a Ca++ switch assay. Endothelial cells were exposed to EGTA for 30 minutes to fully disrupt VE-cadherin clustering at cell-to-cell contacts28 (Figure2A). Calcium concentration was then restored, and the capacity of the cells to reorganize VE-cadherin at junctions was examined in the absence or presence of mAbs. As shown in Figure 2A, Hec 1.2 and TEA 1.31 did not prevent VE-cadherin reclustering, while the presence of BV9, Cad 5 and, to a lower extent, BV6 inhibited a full reorganization of intercellular adherens junctions.
Antibodies to VE-cadherin inhibit VE-cadherin clustering after Ca++ switch.
(A) Endothelial cell monolayers were exposed to 5 mM EGTA for 30 minutes. EGTA was then removed and Ca++ restored adding fresh culture medium either in the presence or in absence of mAbs. One hour later, cells were fixed and processed for immunofluorescence microscopy. The presence of VE-cadherin at junctions was detected by adding a polyclonal antibody VECR1-5. EGTA induced disappearance of VE-cadherin from cell-to-cell contacts. Addition of Ca++restored junctional localizaton of VE-cadherin in cells cultured in absence of mAbs (not shown) or in the presence of Hec 1.2 or TEA 1.31. VE-cadherin remained in large part outside the intercellular junctions when cells were cultured in the presence of BV6, BV9, and Cad 5 (bar = 20 μm). (B) Endothelial cells grown on Transwell filters were incubated with EGTA for 30 minutes. To allow junction reorganization, Ca++ was then restored in the presence or absence of mAbs (see above). FITC-dextran was added 1 hour after Ca++ restoration, and permeability was measured 30 minutes later. Data are expressed as percentage increase in permeability in comparison to cells maintained in the absence of mAbs and are means ± SEM of 4 replicates from a typical experiment out of 3 performed.
Antibodies to VE-cadherin inhibit VE-cadherin clustering after Ca++ switch.
(A) Endothelial cell monolayers were exposed to 5 mM EGTA for 30 minutes. EGTA was then removed and Ca++ restored adding fresh culture medium either in the presence or in absence of mAbs. One hour later, cells were fixed and processed for immunofluorescence microscopy. The presence of VE-cadherin at junctions was detected by adding a polyclonal antibody VECR1-5. EGTA induced disappearance of VE-cadherin from cell-to-cell contacts. Addition of Ca++restored junctional localizaton of VE-cadherin in cells cultured in absence of mAbs (not shown) or in the presence of Hec 1.2 or TEA 1.31. VE-cadherin remained in large part outside the intercellular junctions when cells were cultured in the presence of BV6, BV9, and Cad 5 (bar = 20 μm). (B) Endothelial cells grown on Transwell filters were incubated with EGTA for 30 minutes. To allow junction reorganization, Ca++ was then restored in the presence or absence of mAbs (see above). FITC-dextran was added 1 hour after Ca++ restoration, and permeability was measured 30 minutes later. Data are expressed as percentage increase in permeability in comparison to cells maintained in the absence of mAbs and are means ± SEM of 4 replicates from a typical experiment out of 3 performed.
This effect was quantified by measuring FITC-dextran passage through endothelial monolayers after Ca++ restoration (Figure 2B). The presence of BV6, BV9, or Cad 5 prevented a full reestablishment of paracellular barrier function. TEA 1.31 had a moderate but detectable effect, while Hec 1.2 had no activity.
Effect of VE-cadherin mAbs on endothelial cell apoptosis
We recently reported that blocking VE-cadherin mAbs prevented the protective effect of VEGF on endothelial cell apoptosis induced by the absence of serum.2
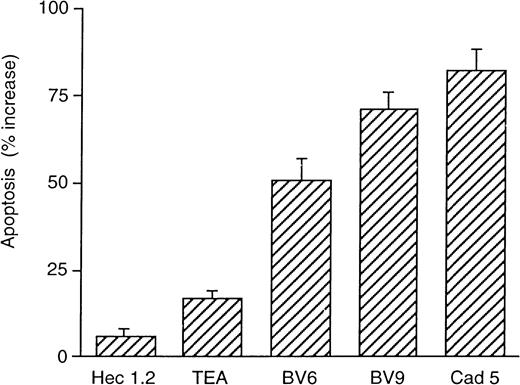
As reported in Figure 3, in the presence of VEGF, BV6, BV9, and Cad 5 strongly increased endothelial cell apoptosis. TEA 1.31 showed a poor but still measurable activity, while Hec 1.2 was inactive.
Monoclonal antibodies directed to VE-cadherin prevent the protective effect of VEGF on endothelial cell apoptosis.
Endothelial cells were maintained without serum in the absence or presence of mAbs for 48 hours; 63% ± 7% control cells underwent apoptosis, and this percentage was not significantly modified by addition of the mAbs (not shown). When VEGF (30 ng/mL) was included in the absence of mAbs, apoptotic endothelial cells decreased to 31% ± 2%. Addition of the mAbs together with VEGF increased the apoptotic index. The values in the figure are expressed as percentage increase in apoptosis in comparison to cells cultured in absence of the mAbs and in the presence of VEGF. Data are means ± SEM of 4 replicates from a typical experiment out of 4 performed.
Monoclonal antibodies directed to VE-cadherin prevent the protective effect of VEGF on endothelial cell apoptosis.
Endothelial cells were maintained without serum in the absence or presence of mAbs for 48 hours; 63% ± 7% control cells underwent apoptosis, and this percentage was not significantly modified by addition of the mAbs (not shown). When VEGF (30 ng/mL) was included in the absence of mAbs, apoptotic endothelial cells decreased to 31% ± 2%. Addition of the mAbs together with VEGF increased the apoptotic index. The values in the figure are expressed as percentage increase in apoptosis in comparison to cells cultured in absence of the mAbs and in the presence of VEGF. Data are means ± SEM of 4 replicates from a typical experiment out of 4 performed.
Effect of VE-cadherin mAbs on the formation of vascularlike structures in vitro
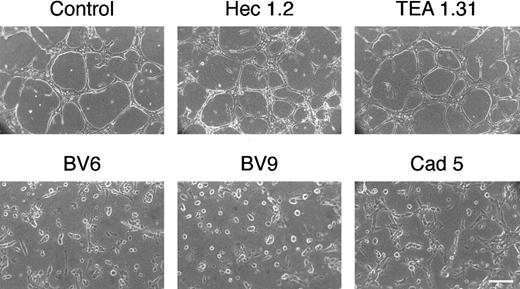
In fibrin gels, Cad 5, BV6, and BV9 blocked cord formation, and TEA 1.31 and Hec 1.2 did not induce any detectable change in comparison to control cultures (Figure4). In this assay, the cells were kept in the presence of serum (see “Materials and methods”) to reduce apoptosis induced by the mAbs (Figure 3). Within 48 hours, apoptosis induced by addition of BV6 or BV9 was still low and not significantly increased in this system (not shown).
Monoclonal antibodies to VE-cadherin inhibit the organization of vascularlike structures in endothelial cells.
Endothelial cell monolayers were cultured in a 3D fibrin gel (see “Materials and methods”). After 48 hours, cells reorganize in cords and tubes. In the presence of mAbs to VE-cadherin BV6, BV9, and Cad 5 (50 μg/mL), the formation of the cord network was blocked. Monoclonal antibodies TEA 1.31 or Hec 1.2 (50 μg/mL) did not change the reorganization pattern as compared with control (bar = 30 μm).
Monoclonal antibodies to VE-cadherin inhibit the organization of vascularlike structures in endothelial cells.
Endothelial cell monolayers were cultured in a 3D fibrin gel (see “Materials and methods”). After 48 hours, cells reorganize in cords and tubes. In the presence of mAbs to VE-cadherin BV6, BV9, and Cad 5 (50 μg/mL), the formation of the cord network was blocked. Monoclonal antibodies TEA 1.31 or Hec 1.2 (50 μg/mL) did not change the reorganization pattern as compared with control (bar = 30 μm).
Identification of binding epitope of VE-cadherin mAbs
To identify the epitope of VE-cadherin mAbs, recombinant fragments of the protein were produced (Figure5A). By Western blot and immunoprecipitation analysis (Figure 5B,C), TEA 1.31, BV6, and BV9 recognized a VE-cadherin fragment containing EC3 and EC4 (fragment VE3) but not EC1 and EC2 (fragments VE2 and VE4). In contrast, Cad 5 mAb bound only to EC1 and EC2 (VE2, VE4) and not to EC3 and EC4 (VE3) (Figure 5A). The boundaries among domains 1 to 5 reported in Figure 5A derive from alignment of the VE-cadherin AA sequence with that of other classic cadherins (E-, N-, and P-cadherins) as reported previously29 and in a review by Yagi and Takeichi.13
Different mAbs bind to different regions of VE-cadherin.
(A) Schematic representation of VE-cadherin fragments spanning different regions of the protein ectodomain and the mAb binding domain. TM indicates transmembrane domain; EC, extracellular domain. (B) Western blot analysis of mAbs binding to VE-cadherin fragments produced in Escherichia coli. Antihistidine antibody recognized the 3 fragments VE1, VE2, and VE3, which migrated at molecular weights of 60, 40, and 30 kd, respectively. Monoclonal antibodies TEA 1.31 and BV6 bound to VE1 and VE3 but not VE2. mAb Cad 5 bound to VE1 and VE2. (C) Immunoprecipitation analysis of VE3 and VE4 obtained by protein synthesis in vitro. Biotinylated lysine residues are incorporated into nascent proteins during translation. Fragments VE3 and VE4 were immunoprecipitated with Cad 5, BV6, and BV9, and after sodium dodecyl sulfate–polyacrylamide gel electrophoresis and electroblotting, the biotinylated proteins were visualized by streptavidin–horseradish peroxidase. Cad 5 bound to VE4 while BV6 and BV9 bound to VE3 only.
Different mAbs bind to different regions of VE-cadherin.
(A) Schematic representation of VE-cadherin fragments spanning different regions of the protein ectodomain and the mAb binding domain. TM indicates transmembrane domain; EC, extracellular domain. (B) Western blot analysis of mAbs binding to VE-cadherin fragments produced in Escherichia coli. Antihistidine antibody recognized the 3 fragments VE1, VE2, and VE3, which migrated at molecular weights of 60, 40, and 30 kd, respectively. Monoclonal antibodies TEA 1.31 and BV6 bound to VE1 and VE3 but not VE2. mAb Cad 5 bound to VE1 and VE2. (C) Immunoprecipitation analysis of VE3 and VE4 obtained by protein synthesis in vitro. Biotinylated lysine residues are incorporated into nascent proteins during translation. Fragments VE3 and VE4 were immunoprecipitated with Cad 5, BV6, and BV9, and after sodium dodecyl sulfate–polyacrylamide gel electrophoresis and electroblotting, the biotinylated proteins were visualized by streptavidin–horseradish peroxidase. Cad 5 bound to VE4 while BV6 and BV9 bound to VE3 only.
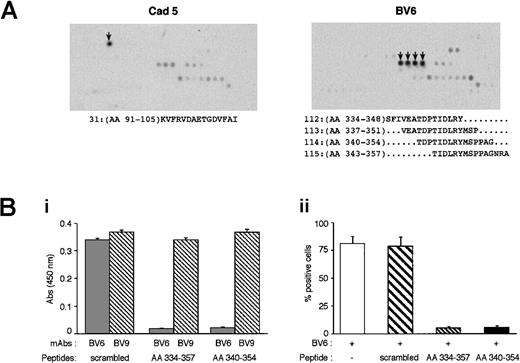
To identify more precisely the epitopes of the mAbs, we studied their capacity to bind different peptidic sequences spanning the extracellular domain of VE-cadherin. As reported in Figure6A, BV6 bound to 4 peptides sharing the same 6-AA sequence Thr-Ile-Asp-Leu-Arg-Tyr (TIDLRY). This sequence spans 343 to 348 AA of domain EC3 of VE-cadherin ectodomain.
Identification of the peptidic sequence recognized by BV6.
(A) Peptide scan of VE-cadherin extracellular region. Peptides of 15 AA spanning the entire extracellular domain of the protein were synthesized and tested for mAb recognition (arrows indicate the peptide spots recognized).41 mAb Cad 5 bound to peptide 31: KVFRVDAETGDVFAI; mAb BV6 bound to peptides 112 to 115 containing the sequence TIDLRY. (B) The peptides containing TIDLRY corresponding to VE-cadherin sequences AA 334 to 357 and AA 340 to 354 and the scrambled peptide (APRTDIAEINDGYPVTSMRFPALS) were tested in competition ELISA experiments. (Bi) The binding of soluble BV6 and BV9 (0.5 μg/mL) to purified VE-cadherin recombinant fragment (VE1-Ig, 1 μg/mL) coated on plastic was tested in the presence or absence of the peptides (200 μM). TIDLRY peptides blocked BV6 but not BV9 binding. (Bii) Flow cytometric analysis of BV6 binding to endothelial cells expressing VE-cadherin. Peptides containing TIDLRY sequence but not scrambled peptides blocked the binding of BV6.
Identification of the peptidic sequence recognized by BV6.
(A) Peptide scan of VE-cadherin extracellular region. Peptides of 15 AA spanning the entire extracellular domain of the protein were synthesized and tested for mAb recognition (arrows indicate the peptide spots recognized).41 mAb Cad 5 bound to peptide 31: KVFRVDAETGDVFAI; mAb BV6 bound to peptides 112 to 115 containing the sequence TIDLRY. (B) The peptides containing TIDLRY corresponding to VE-cadherin sequences AA 334 to 357 and AA 340 to 354 and the scrambled peptide (APRTDIAEINDGYPVTSMRFPALS) were tested in competition ELISA experiments. (Bi) The binding of soluble BV6 and BV9 (0.5 μg/mL) to purified VE-cadherin recombinant fragment (VE1-Ig, 1 μg/mL) coated on plastic was tested in the presence or absence of the peptides (200 μM). TIDLRY peptides blocked BV6 but not BV9 binding. (Bii) Flow cytometric analysis of BV6 binding to endothelial cells expressing VE-cadherin. Peptides containing TIDLRY sequence but not scrambled peptides blocked the binding of BV6.
Cad 5 bound to KVFRVDAETGDVFAI. This mAb was raised against a fragment spanning 26 to 194 AA of VE-cadherin EC1, and the identified peptidic epitope is contained in this sequence.
To confirm BV6 binding specificity for TIDLRY, 2 peptides containing this sequence and a scrambled peptide were tested in competition experiments. As reported in Figure 6Bi, TIDLRY-containing peptides but not the scrambled peptide were able to block the binding of BV6 to a recombinant VE1-Ig chimera. Different concentrations of the peptides were used ranging from 1 to 500 μM, and the calculated inhibitory concentration of 50% for TIDLRY peptides was 12.6 to 13.4 μM.
The scrambled peptide did not show dose-dependent competition for BV6 binding. The 3 peptides were inactive on BV9 binding up to 500 μM.
Besides purified recombinant fragments, TIDLRY-containing peptides blocked BV6 binding to native VE-cadherin, ie, expressed on cultured endothelial cells measured by flow cytometry analysis (Figure 6Bii).
The TIDLRY peptides or the scrambled peptide (up to a concentration of 500 μM) did not change paracellular permeability of endothelial monolayers (measured as in Figure 1) (data not shown), and they did not prevent VE-cadherin reassembly at intercellular contacts after Ca++ switch (measured as in Figure 2) (data not shown).
A general summary of the results is reported in Table 1.
The effects of different VE-cadherin antibodies on endothelial cell activities
| mAbs . | Permeability . | Ca++switch . | Apoptosis . | Tube formation . | Domain . |
|---|---|---|---|---|---|
| Cad 5 | +++ | +++ | +++ | +++ | EC1 |
| BV9 | ++ | +++ | +++ | +++ | EC3-EC4 |
| BV6 | ++ | ++ | ++ | +++ | EC3 |
| TEA 1.31 | + | + | + | − | EC3-EC4 |
| Hec 1.2 | − | − | − | − | EC3-EC4 |
| mAbs . | Permeability . | Ca++switch . | Apoptosis . | Tube formation . | Domain . |
|---|---|---|---|---|---|
| Cad 5 | +++ | +++ | +++ | +++ | EC1 |
| BV9 | ++ | +++ | +++ | +++ | EC3-EC4 |
| BV6 | ++ | ++ | ++ | +++ | EC3 |
| TEA 1.31 | + | + | + | − | EC3-EC4 |
| Hec 1.2 | − | − | − | − | EC3-EC4 |
− indicates no effect; +, barely detectable; ++, intermediate; +++, strong activity. For the actual data see the corresponding figures.
Discussion
VE-cadherin has multiple functions in endothelial cells.1 2 Different blocking antibodies may interfere with these activities to a different extent, and this may be related to the specific region of the molecule to which they bind.
In this paper, we first compared 5 available mAbs directed to the extracellular region of VE-cadherin for their effect on several endothelial cell biologic tests.
We found that 3 mAbs (Cad 5, BV9, and BV6) were significantly active in all the assays, while the 2 remaining mAbs, TEA 1.31 and Hec 1.2, showed moderate or no activity, respectively.
Comparing the effect of the mAbs on VE-cadherin clustering (Figures 1A,2A), the most active (Cad 5, BV9, and BV6) were also those more effective in increasing permeability (Figures 1B and 2B). This was true measuring disruption of preorganized junctions (Figure 1) or measuring the ability of the mAbs to prevent the reorganization of EGTA-disrupted VE-cadherin clusters (Figure 2).
TEA 1.31 is an exception because it did not visibly inhibit VE-cadherin localization at junctions in both conditions (Figures 1A and 2A) yet slightly increased paracellular permeability. Possibly, immunofluorescence microscopy does not detect subtle changes in VE-cadherin clustering and organization, which may, however, lead to a significant increase in permeability to high molecular weight solutes. Also, in other conditions, as upon histamine activation of endothelial cells, a marked increase in paracellular permeability could be observed without major changes in VE-cadherin distribution at junctions.23
TEA 1.31 showed a slightly more marked effect on permeability when the cells were pretreated with EGTA. This means that this mAb is more effective in preventing correct homophilic interactions of VE-cadherin molecules during the organization of the cluster than in disrupting preconstituted junctional structures.
The mechanism of action of VE-cadherin in promoting angiogenesis and vasculogenesis in the embryo was explained in part by the capacity of this molecule to protect endothelial cells from apoptosis.2 It was therefore important to test whether the mAbs could inhibit VE-cadherin antiapoptotic effect in human cells. We found that mAbs BV6, BV9, and Cad 5 were indeed able to prevent the protective effect of VEGF on apoptosis.
The mAbs also inhibited endothelial tube formation in fibrin gels. The effect of the mAbs may be due to inhibition of VE-cadherin adhesive properties but also to induction of apoptosis. The presence of serum in this assay significantly reduced the susceptibility of the cells to apoptosis, and BV6 and BV9 did not increase significantly this parameter. This suggests that the effect observed in this particular assay is mostly due to the block of VE-cadherin–mediated adhesion.
Others found that a VE-cadherin blocking mAb (Cad 5) was able to inhibit tube formation in a fibrin gel, and they brought direct evidence for VE-cadherin binding to fibrin.4 30 It is possible that the block of this interaction leads to a lack of a correct anchorage of the cells to the matrix, causing disruption of vascularlike structures.
In the attempt to define the molecular basis of mAb activity, we analyzed their binding to different recombinant fragments of the protein. The mAbs TEA 1.31, Hec 1.2, BV6, and BV9 bound to a membrane domain of VE-cadherin spanning EC3 and EC4. Peptide scanning analysis showed that BV6 recognizes the peptidic sequence TIDLRY on EC3. In contrast, Cad 5 mAb bound to the fragment that includes EC1 and, by peptide scan, it recognizes a peptide contained in this region.
We have been unable to precisely define the epitope of the other blocking mAb BV9 by peptide scanning. A possibility is that BV9 binds to a conformational epitope that may be lost in short peptides of VE-cadherin. Competition experiments with peptides containing TIDLRY showed that BV9 binding to VE-cadherin was unaffected, indicating that it recognizes a different domain.
In general, the mechanisms underlying homophilic binding functions of cadherins are poorly understood.
The precise mechanism through which BV9 and BV6 binding to EC3-EC4 influences VE-cadherin homophilic adhesion is still unknown, but different possibilities may be considered.
Monoclonal antibody binding may change VE-cadherin conformation and, as a consequence, the availability of the aminoterminal region for homophilic binding. Conformational changes of other adhesive molecules by antibody/ligand binding were reported to induce activation or inhibition of their adhesive properties.31-37
BV6 or BV9 may also affect VE-cadherin association in cis by steric hindrance and alter the formation of the zipper structure promoting cell-to-cell recognition and binding. Indeed, data obtained in the Ca++ switch assay indicate that BV6 and BV9 may inhibit the reassembly of VE-cadherin at intercellular junctions.
Another interesting possibility comes from the recent paper of Sivasankar et al.16 This work brings evidence that C-cadherin presents multiple adhesive contacts along the extracellular domain for the interdigitated antiparallel proteins. The block of one of these sites may affect the strength of recognition of cadherin molecules and facilitate the junctional rupture.
Other data in the literature may be accommodated in this last picture. The mAb DECMA directed to E-cadherin38,39 or a mAb to chicken N-cadherin, which are strong inhibitors of intercellular adhesion,40 both bind to regions outside EC1 and EC2 and closer to the cell membrane.
It is possible that EC1 interacts with EC3-EC4 and depending on their orientation form dual or multimeric aggregates. Thus, antibodies directed against either one of these epitopes may inhibit VE-cadherin–adhesive properties. Binding may involve a 2-stage process of initial docking or alignment of the 2 molecules along their length followed by specific engagement of the binding sites in domain EC1 and EC3-4.
In conclusion, inhibition of VE-cadherin adhesion properties by blocking antibodies affects several endothelial cell–specific functions, which may include the maintenance of barrier properties or formation of vascular structures in vitro. Interestingly, antibodies directed to different epitopes of the molecules present a comparable inhibitory effect, suggesting that the protein has multiple biologically important domains.
We thank A. Tiepold for help in the SPOT peptide synthesis.
Supported in part by Associazione Italiana per la Ricerca sul Cancro, Consiglio Nazionale delle Ricerche (CNR grant 97.01299 PF49); the European Community (BMH4 CT983380, BIO4CT 980337, QLG1-CT-1999-01136, and QLK3-CT-1999-00020); and Ministero dello SANITA' RF99.72 and RF99.52 and MURST 9906317157-003.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Elisabetta Dejana, Istituto FIRC di Oncologia Molecolare, Via Serio 21, 20139 Milano, Italy; e-mail:dejana@ifom-firc.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal