Mpl is the thrombopoietin (TPO) receptor. The current molecular understanding of how Mpl activation stimulates proliferation of megakaryocyte-lineage cells is based largely on the engineered expression of Mpl in nonmegakaryocyte-lineage cell lines. However, the relevance of these findings to Mpl signaling in primary megakaryocyte-lineage cells remains largely unknown. Therefore, a system was developed to study Mpl function in primarympl−/−megakaryocyte-lineage cells. Expressing avian retroviral receptors on the surfaces of mammalian cells overcomes their natural block to avian retroviral infection; 815 bp of human GPIIb regulatory sequence was used to generate transgenic mice with megakaryocyte-lineage expression of the subgroup A avian leukosis virus receptor, TVA. Avian retroviral infection of unfractionated bone marrow from these mice is restricted to megakaryocyte-lineage cells. The transgenic mice were crossed to anmpl−/−background generatingGPIIb-tva+mpl−/−mice. By using avian retroviruses to express wild-type or mutant Mpl on the surfaces of primary megakaryocyte-lineage cells, it was demonstrated that (1) the 10 membrane-proximal, cytoplasmic amino acids of Mpl are required for TPO-induced proliferation; (2) Y582F mutation confers a proliferative advantage over wild-type Mpl and imparts a constitutive anti-apoptotic signal; (3) truncating the 50 C-terminal Mpl amino acids reduces but does not eliminate TPO-induced mitogen-activated protein kinase activation, yet it does not alter the synergistic effect of stem cell factor on TPO-induced proliferation; and (4) TPO-induced proliferation of early, primary megakaryocyte-lineage cells does not require Stat-5 phosphorylation. The system reported provides an improved approach for Mpl structure–function studies, and the method can be applied to any hematopoietic lineage.
Introduction
Thrombopoietin (TPO), the Mpl ligand, is the primary physiologic regulator of megakaryocytopoiesis.1-3Genetic disruption of TPO or mpl leads to an 85% to 90% reduction in circulating platelets.4-6 Like other members of the hematopoietic receptor superfamily, Mpl lacks intrinsic tyrosine kinase activity and is thought to be activated by TPO-induced homodimerization.7-9 The Mpl extracellular domain contains 2 cytokine receptor homology domains, each characterized by 2 pairs of membrane-distal cysteines and a membrane-proximal WSXWS motif. The 121-amino acid intracellular domain of the 625-amino acid murine Mpl contains membrane-proximal Box1 and Box2 motifs characteristic of members of the hematopoietic receptor superfamily. There are 5 intracellular tyrosines.
The current understanding of how TPO engagement of Mpl activates intracellular signaling pathways and regulates megakaryocytopoiesis comes largely from the heterologous expression of wild-type and mutant Mpl in nonmegakaryocyte-lineage immortalized cell lines.10-15 These studies suggest that the membrane-proximal intracellular domain provides the primary proliferative signal that requires activation of JAK/STAT signaling, whereas the membrane-distal portion of the intracellular domain is thought to be more important for maturation, possibly by prolonged activation of the Raf/MAPK/Erk signaling pathway (reviewed in Drachman et al16). However, this simple model of Mpl activity must be re-evaluated in light of the recent demonstration that mice lacking the membrane-distal half of the Mpl intracellular domain have full megakaryocyte-lineage maturation, including normal platelet counts and agonist-induced fibrinogen binding.17 The problems of translating intracellular signaling data from various immortalized cell lines to physiologically relevant primary cells and the related need to confirm cell line findings in primary cells have been emphasized by others.15,16 18-21 We therefore sought to develop a new system for studying Mpl signaling in primary megakaryocyte-lineage cells that lack endogenous Mpl expression.
Avian retroviruses fail to replicate in mammalian cells because of a block at viral entry and a block in viral assembly. However, expression of the subgroup A avian leukosis virus (ALV-A) receptor, TVA, on the surfaces of mammalian cells confers susceptibility to ALV-A infection.22,23 Furthermore, tissue-specific expression of TVA in transgenic mice allows for selective infection of TVA-expressing cells by avian retroviral vectors.24-27 Herein we describe a new transgenic mouse in which 815 bp human GPIIb 5′-regulatory sequence has been used to direct megakaryocyte-lineage restricted TVA expression. After crossing to an mpl−/−genetic background, unfractionated bone marrow from theGPIIb-tva+mpl−/− mice can be infected with avian retroviral vectors to express wild-type or mutant Mpl on the surface of primary megakaryocyte-lineage cells that lack endogenous Mpl expression. The system described can be applied to a broad range of Mpl structure–function studies, and the results presented underscore the value of performing Mpl signaling studies in primary cells.
Materials and methods
Mice
The tv-a cDNA sequence contained in aBglII–BamHI fragment from pCB6-tva950 (kindly provided by Paul Bates, University of Pennsylvania, Philadelphia) was ligated into pIIbLUC (kindly provided by Paul Bray, Baylor College of Medicine, Houston, TX). This generated pGPIIb-TVA in which thetv-a cDNA is placed directly downstream of an 815-bpGPIIb regulatory sequence (nucleotides −783 to +32 from aSacII site to the GPIIb initiation ATG). The tv-atranslation initiation codon is located at the precise position normally occupied by the initiation codon in GP-IIb (Figure1). A 1.8-kb fragment containing 815 bp of GP-IIb regulatory sequence linked to the tv-acDNA was injected into oocytes using standard procedures.27Mpl−/− mice were described previously.4
Generation of TVA-expressing transgenic mice.
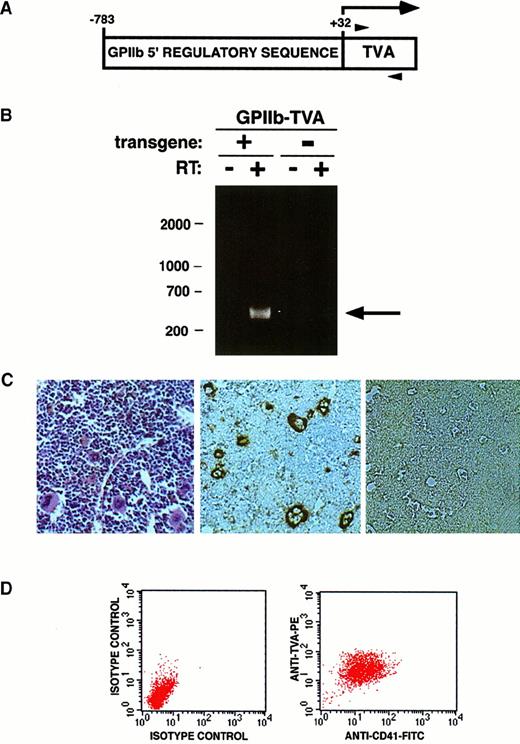
(A) Schematic of the transgene cassette used to express TVA, indicating the position of the 5′ (−783) and 3′ (+32) ends of the humanGPIIb regulatory sequence ligated to the 950 bptv-a cDNA. Numbering is assigned relative to 0 for the native GPIIb transcription start site. The horizontal arrow indicates the transcribed message, and translation begins with a methionine at the 5′ end of the tv-a cDNA. Arrowheads indicate the positions of primers used for RT-PCR. (B) A founder mouse (9552) positive for the transgene by Southern blot was bred with nontransgenic littermates. RNA isolated from the bone marrow of a transgene-containing offspring (+) and a nontransgenic littermate (−) was subjected to RT-PCR without (−) and with (+) reverse transcriptase in the reaction. The 420-bp band indicative of TVA mRNA (arrow) is only seen when reverse transcriptase is used (lane 1 vs lane 2). Bone marrow from mice lacking the transgene does not express TVA mRNA (lanes 3, 4). DNA size markers (bp) are indicated on the left. (C) Hematoxylin and eosin stain of bone marrow from a TVA-expressing mouse demonstrates megakaryocytes (left panel). Immunohistochemical staining demonstrates TVA expression on megakaryocytes in the bone marrow of TVA-expressing 9552 offspring (center panel) but no TVA expression in bone marrow from nontransgenic littermates (right panel). (D) Independent FACS analysis using isotype controls plotted against forward scatter was used to identify a generous gate that encompasses all cells stained by either anti-CD41 or anti-TVA antibody. Two-dimensional FACS analysis using anti-CD41 and anti-TVA antibodies demonstrates dual staining of essentially all gated cells.
Generation of TVA-expressing transgenic mice.
(A) Schematic of the transgene cassette used to express TVA, indicating the position of the 5′ (−783) and 3′ (+32) ends of the humanGPIIb regulatory sequence ligated to the 950 bptv-a cDNA. Numbering is assigned relative to 0 for the native GPIIb transcription start site. The horizontal arrow indicates the transcribed message, and translation begins with a methionine at the 5′ end of the tv-a cDNA. Arrowheads indicate the positions of primers used for RT-PCR. (B) A founder mouse (9552) positive for the transgene by Southern blot was bred with nontransgenic littermates. RNA isolated from the bone marrow of a transgene-containing offspring (+) and a nontransgenic littermate (−) was subjected to RT-PCR without (−) and with (+) reverse transcriptase in the reaction. The 420-bp band indicative of TVA mRNA (arrow) is only seen when reverse transcriptase is used (lane 1 vs lane 2). Bone marrow from mice lacking the transgene does not express TVA mRNA (lanes 3, 4). DNA size markers (bp) are indicated on the left. (C) Hematoxylin and eosin stain of bone marrow from a TVA-expressing mouse demonstrates megakaryocytes (left panel). Immunohistochemical staining demonstrates TVA expression on megakaryocytes in the bone marrow of TVA-expressing 9552 offspring (center panel) but no TVA expression in bone marrow from nontransgenic littermates (right panel). (D) Independent FACS analysis using isotype controls plotted against forward scatter was used to identify a generous gate that encompasses all cells stained by either anti-CD41 or anti-TVA antibody. Two-dimensional FACS analysis using anti-CD41 and anti-TVA antibodies demonstrates dual staining of essentially all gated cells.
Screening transgenic mice
Distal tail segments were dissolved overnight as described, and the precipitated DNA was resuspended in 200 μL sterile ddH2O.27 Fifteen microliters ofNcoI-digested tail DNA was used in Southern blots probed with a radiolabeled 620-bp EagI–EcoRItv-a probe as described.27Tv-a mRNA expression in bone marrow was identified by reverse transcription–polymerase chain reaction (RT-PCR): cDNA copies of total mRNA (TRIzol reagent; Gibco-BLR, Rockville, MD) were generated using the SuperScript reverse transcription system (Gibco-BRL) according to the manufacturer's recommendations, followed by 30 cycles of PCR (30 seconds at 95°C, 30 seconds at 58°C, and 30 seconds at 72°C) using primers TVA1 (5′-TGCTGCCCGGTAACGTGA-3′) and TVA2 (5′-GGCAGAGCAGTTCAGTCC-3′) to generate a unique 420-bp product.
Cell lines
Chicken fibroblast DF1 cells were cultured in DMEM-21 containing 10% fetal bovine serum (Gibco-BRL), 2 mM L-glutamine, 100 U penicillin G sodium/mL, and 0.1 mg streptomycin sulfate/mL. Baf/3-TVA cells are immortalized pre-B cells that express TVA under the control of a cytomegalovirus promoter. Baf/3-TVA cells are grown in RPMI supplemented with 10% fetal bovine serum (Gibco-BRL), 2 mM L-glutamine, 100 U penicillin G sodium/mL, 0.1 mg streptomycin sulfate/mL, and 10% WEHI-conditioned media.
Antibodies and cytokines
Fluorescein isothiocyanate (FITC)-conjugated F4/80 is from Serotec (Raleigh, NC), and anti-Flag M2 monoclonal antibody is from Sigma (St Louis, MO). FITC-conjugated antibodies to CD41, Gr-1, CD3, and B220 and phycoerythrin-conjugated antibodies to Ter-119, and all respective isotype controls, are from Pharmingen (San Diego, CA). Antibodies to detect phosphorylated Erk 1/2 (p44/42), total Erk 1/2 (p44/42), phosphorylated (Tyr705) Stat-3, total Stat-3, phosphorylated (Ser473) Akt, and total Akt are from New England Biolabs (Beverly, MA). Antibody to Stat-5b, which also detects Stat-5a, is from Santa Cruz Biotechnology (Santa Cruz, CA). Horseradish peroxidase (HRP)-conjugated donkey antirabbit antibody is from Amersham Pharmacia (Piscataway, NJ), and HRP-conjugated swine antigoat antibody is from Boehringer Mannheim (Indianapolis, IN). Murine stem cell factor (SCF), murine IL-3 (IL-3), and murine IL-11 (IL-11) are from PeproTech (Rocky Hill, NJ). Murine IL-6 (IL-6) is from R&D Systems (Minneapolis, MN). Genentech (San Francisco, CA) generously provided human thrombopoietin (h-TPO).
Plasmid constructs
Murine c-mpl cDNA was removed from plasmid pSK-c-mpl28 as a 2-kb EcoR1–Bcl1 fragment and ligated into the pCla12 shuttle vector.29 The FLAG sequence (DYKDDDDK) was generated by PCR and inserted between Mpl residues 26 and 27, immediately downstream of the amino-terminal Mpl leader sequence, generating pCla12-FLAGMpl. UsingCla1 sites, the 2-kb FLAGMpl sequence was cloned into the replication competent avian retroviral vector RCAS.29 Mpl-Y582F was generated using a 2-step PCR procedure. Primer pairs were: 5′-CCG AGC CTG CAC TGG AGG GA-3′ and 5′-CAG TCC TCT GAA GTC CAT CTG-3′; and 5′-CAG ATG GAC TTC AGA GGA CTG-3′ and 5′-ATA AGC TTG GGC TGC AGG-3′. The final PCR product was digested with AvrII and NcoI to generate a 209-bp fragment containing the Y582F mutation that was cloned into pCla12-FLAGMpl to generate pCla12-FLAGMpl-Y582F. Mpl-Δ50 was also generated using PCR mutagenesis. The oligo pair was 5′-CCG AGC CTG CAC TGG AGG GA-3′ and 5′-CAT GCC ATG GTT ATC AGG GAC ACA GAG GTA AAG GAG-3′, which introduced 3 consecutive stop codons after Mpl residue 575. TheBlp1-Nco1 Mpl fragment from plasmid pMT21myc-c-mplΔ7 (kindly provided by Marion Dorsch and Stephen P. Goff, Columbia University, New York, NY) was introduced into pCla12-FLAGMpl and pCla12-FLAGMpl-Y582F to generate pCla12-FLAGMpl-Δ7 and pCla-12FLAGMpl-Δ7, Y582F, respectively. All Mpl sequences were transferred into the avian RCAS vector using the Cla1 cloning site to generate RCAS-Mpl, RCAS-Mpl-Δ7, RCAS-Mpl-Y582F, RCAS-Mpl-Δ50, RCAS-Mpl-Δ7, Y582F. All Mpl mutations were confirmed by DNA sequencing. The FLAG sequence is present on all Mpl sequences in RCAS to facilitate immunologic detection with anti-FLAG antibody, but the FLAG designation is left off the names of the RCAS vectors to simplify the nomenclature. RCAS-GFP expresses an enhanced green fluorescent protein, and RCAS-PURO expresses the puromycin resistance gene.27
Virus production
DF1 cells at 40% to 60% confluence were transfected with RCAS vectors using the calcium phosphate precipitation method as described.27 Fresh media were added 12 to 16 hours after transfection. After 5 days in culture, the virus-producing DF1 cells were subjected to FACS analysis for the expression of each FLAG-tagged Mpl construct or GFP. The RCAS system typically produces viral titers of 5 × 106 or greater.29
Bone marrow cultures and infections
Murine bone marrow cells were harvested using standard procedures and cultured in Iscove modified Dulbecco medium (IMDM) supplemented with 10% horse serum (Gibco-BRL), 100 U penicillin G sodium/mL, and 0.1 mg streptomycin sulfate/mL as described.27 For cells from anmpl+/+ background, the media were supplemented with 10 ng/mL SCF, 10 ng/mL IL-3, and 250 ng/mL h-TPO, whereas h-TPO was not included in the media for cells harvested from anmpl−/− background. Unfractionated bone marrow cells were cultured for 48 hours before they were infected with avian retrovirus expressing either GFP, the puromycin-selectable marker, or one of the Mpl constructs by co-culture of virus-producing DF1 cells in 6-well plates. Infections were performed for 8 hours daily for 3 days. At 48 hours after infection, cells were selected in 250 or 500 ng/mL h-TPO when Mpl constructs were introduced or in 1.5 μg/mL puromycin after infection with RCAS-PURO. To assess TVA expression on megakaryocytes from mpl−/− mice, maximal megakaryocyte-lineage maturation was achieved by culturing the bone marrow in a serum-free medium supplemented with IL-11 (10 ng/mL), IL-6 (10 ng/mL), and h-TPO (50 ng/mL).30
FACS analysis and cell staining
FACS analysis for surface markers, immunohistochemical staining for CD41 and von Willebrand factor (vWf), and Wright-Giemsa staining were performed as previously described.27 Isotype controls were used in all experiments.
Immunoprecipitation and Western blot analysis
For 14 to 16 hours, 1 to 2 × 107 cells were starved of serum and cytokines and then exposed for 10 minutes to 250 ng/mL h-TPO (stimulated) or buffer (unstimulated). Cells were then immediately lysed in 300 μL lysis buffer (50 mM Tris, pH 8.1, containing 150 mM NaCl, 1% NP-40, 1 μg/mL each of leupeptin, pepstatin, and aprotinin, 1 mM phenylmethylsulfonyl fluoride, and 2 mM sodium orthovanadate). Lysates were centrifuged for 10 minutes at 10 000g. For Western blots, 20 μg of clarified total protein (Bradford assay; Bio-Rad, Hercules, CA) was mixed with 2 × sodium dodecyl sulfate (SDS) sample buffer, incubated at 100°C for 5 minutes, and subjected to SDS–polyacrylamide gel electrophoresis (PAGE). For immunoprecipitations, 4 to 5 μg primary antibody was incubated with 1 mg clarified total protein and incubated overnight at 4°C. This was followed by a 2-hour incubation with protein G-coated Sepharose beads, boiling in SDS sample buffer and then SDS-PAGE. Proteins were transferred to a nitrocellulose membrane using an E&K semi-dry blotter at 1- to 2-mA/cm2 gel for 1 hour. Membranes were washed once in phosphate-buffered saline containing 0.1% Tween-20 and were blocked for 1 hour at room temperature. Membranes were incubated with primary antibodies for 1 hour at RT or O/N at 4°C, washed 3 × with 0.1% Tween-20 in phosphate-buffered saline, incubated for 45 to 60 minutes at room temperature with a 1:10 000 dilution of the appropriate HRP-conjugated secondary antibody, washed three times, and developed using Supersignal West Pico Chemiluminescent substrate kit (Pierce, Rockford, IL).
Cell proliferation
RCAS-infected cells expressing the Mpl constructs were washed and plated at 1 × 105 cells/mL in a 12-well culture plate in the presence of the indicated cytokines. Cells were plated in triplicate for each cytokine condition. Cell counts were determined by hemocytometry.
Apoptosis assay
Cells expressing the Mpl constructs were cultured at a starting density of 1 × 106 cell/mL in a 12-well tissue culture plate in IMDM containing varying h-TPO concentrations. Apoptosis was determined after 3 days in culture using the Annexin V-FITC Apoptosis Detection kit (Oncogene Research Products) according to the manufacturer's protocol. Apoptotic cells were defined by FACS analysis as those that stained positively for propidium iodide, annexin V-FITC, or both.
Results
Human GPIIb regulatory sequence directs megakaryocyte-lineage–restricted TVA expression
The tv-a transgene was detected by Southern blot analysis of tail DNA from one (9552) of 13 pups born from fertilized oocytes injected with a GPIIb-tva950 expression cassette (Figure 1A). RT-PCR of bone marrow RNA from transgenic and nontransgenic 9552 offspring demonstrated a 420-bp fragment indicative of tv-a mRNA only in mice carrying the transgene (Figure 1B, lanes 1 and 2 vs lanes 3 and 4). The 420-bp fragment did not result from DNA contaminating the RNA preparation, as evidenced by the absence of a band when reverse transcriptase was not included in the RT-PCR cDNA synthesis reaction (Figure 1B, lane 1 vs lane 2). Peripheral blood counts from the transgenic mice did not differ from their nontransgenic littermates, and overt health problems were not observed in mice followed up for 1 year.
Immunohistochemical staining demonstrated TVA expression on bone marrow megakaryocytes from GPIIb-tva+ mice but not from their nontransgenic littermates (Figure 1C). TVA was not detected on mature erythrocytes or leukocytes or on their readily identifiable precursors, but occasional cells smaller than mature megakaryocytes stained positively for TVA (Figure 1C). Total bone marrow grown for 5 days in IL-3, SCF, and TPO, and then dual-stained for CD41 and TVA, demonstrated that essentially all (more than 97%) CD41-expressing cells also expressed TVA (Figure 1D). We could not convincingly detect TVA expression in the absence of CD41 expression, and all mature megakaryocytes expressed TVA. TVA expression is readily detected on splenic megakaryocytes, but we were unable to detect TVA in the splenic lymphoid tissue, the liver, small intestine, heart, and kidney of transgenic mice (data not shown).
To further clarify whether functional TVA expression was limited to the megakaryocyte-lineage cells, total bone marrow was harvested and infected with RCAS-PURO, an avian retroviral vector that expresses a puromycin-resistance gene. FACS analysis of cells after RCAS-PURO infection and puromycin selection demonstrate a surface phenotype consistent with a pure megakaryocyte lineage. They express CD41 but do not express Ter119, CD3, B220, Gr-1, or F4/80. Taken together, these data strongly suggest that the 815-bp regulatory sequence of the human GPIIb gene used in our transgene construct directed megakaryocyte-lineage restricted, functional TVA expression.
Virally introduced Mpl confers TPO-responsive proliferation inmpl−/−megakaryocyte-lineage cells
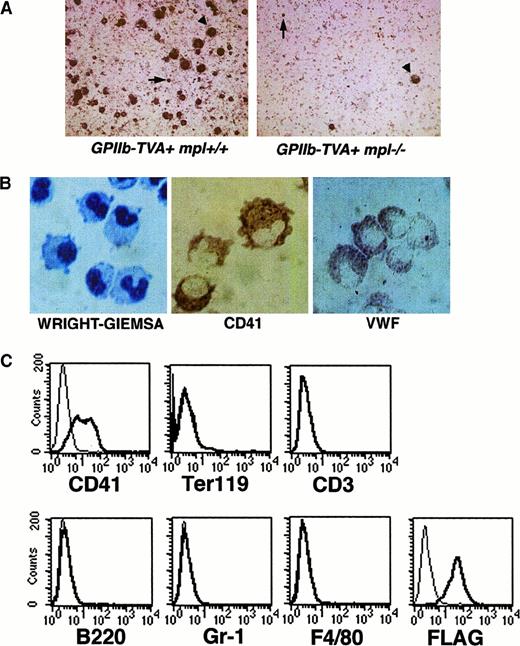
To determine whether the GPIIb-tva+ mice could be used to study Mpl signaling in primary megakaryocyte-lineage cells that lack endogenous Mpl expression, theGPIIb-tva+ mice were crossed to anmpl−/− background. Cultured bone marrow fromGPIIb-tva+mpl−/− mice demonstrated the expected approximately 10-fold reduction in megakaryocytes and TVA-expressing cells compared with marrow fromGPIIb-tva+ mice (Figure2A).
Phenotype of primary bone marrow cells from
GPIIb-tva+mpl−/− mice after infection with RCAS-Mpl and selection in TPO. (A) TVA staining of bone marrow from GPIIb-tva+mpl+/+mice and GPIIb-tva+mpl−/−mice cultured as described in “Materials and methods” demonstrates the anticipated reduction in TVA-expressing cells in thempl−/−background. Arrowheads indicate mature megakaryocytes, and arrows indicate TVA-expressing, small, early megakaryocyte-lineage cells. (B) Wright-Giemsa stain demonstrates the irregular cell shape and the folded nuclei (left panel), and immunohistochemical staining demonstrates surface CD41 (center panel) and intracellular vWF (right panel) inGPIIb-tva+mpl−/−bone marrow cells after infection with RCAS-Mpl and selection in TPO. (C) FACS analysis for surface antigen expression on the cells in panel B demonstrates expression of the megakaryocyte marker CD41 but no expression of erythroid (Ter119), T-lymphocyte (CD3), B-lymphocyte (B220), granulocyte (Gr-1), or macrophage (F4/80) markers. The FLAG-tagged Mpl introduced by RCAS-Mpl is readily detected on the cell surface with an anti-FLAG antibody. Thin lines, fluorochrome-conjugated isotype controls; thick lines, fluorochrome-conjugated antigen-specific antibody.
Phenotype of primary bone marrow cells from
GPIIb-tva+mpl−/− mice after infection with RCAS-Mpl and selection in TPO. (A) TVA staining of bone marrow from GPIIb-tva+mpl+/+mice and GPIIb-tva+mpl−/−mice cultured as described in “Materials and methods” demonstrates the anticipated reduction in TVA-expressing cells in thempl−/−background. Arrowheads indicate mature megakaryocytes, and arrows indicate TVA-expressing, small, early megakaryocyte-lineage cells. (B) Wright-Giemsa stain demonstrates the irregular cell shape and the folded nuclei (left panel), and immunohistochemical staining demonstrates surface CD41 (center panel) and intracellular vWF (right panel) inGPIIb-tva+mpl−/−bone marrow cells after infection with RCAS-Mpl and selection in TPO. (C) FACS analysis for surface antigen expression on the cells in panel B demonstrates expression of the megakaryocyte marker CD41 but no expression of erythroid (Ter119), T-lymphocyte (CD3), B-lymphocyte (B220), granulocyte (Gr-1), or macrophage (F4/80) markers. The FLAG-tagged Mpl introduced by RCAS-Mpl is readily detected on the cell surface with an anti-FLAG antibody. Thin lines, fluorochrome-conjugated isotype controls; thick lines, fluorochrome-conjugated antigen-specific antibody.
Unfractionated bone marrow fromGPIIb-tva+mpl−/− mice was cultured in media containing IL-3 and SCF and was infected with either RCAS-GFP or RCAS-Mpl. Forty-eight hours later, the cells were washed extensively and plated in media containing TPO as the only added cytokine. In multiple independent experiments, the RCAS–GFP-infected cells failed to survive, whereas the RCAS–Mpl-infected cells grew in a TPO-dependent fashion. The cells had a folded nucleus on Wright-Giemsa staining, and immunohistochemical staining demonstrated surface CD41 expression and intracellular vWF (Figure 2B). FACS analysis of the Mpl-expressing cells demonstrated surface CD41 expression but no detectable Ter119, CD3, B220, Gr-1, or F4/80 surface markers for erythroid, T-lymphocyte, B-lymphocyte, granulocyte, and macrophage lineages, respectively (Figure 2C). The introduced Mpl was readily detectable on the surface using an antibody directed against the amino-terminal FLAG sequence (Figure 2C). Having demonstrated that we could introduce biologically active Mpl into primary megakaryocyte-lineage cells fromGPIIb-tva+mpl−/− mice, we next used such primary cells to determine the effect of selected Mpl mutations on TPO-induced proliferation (Figure3).
Schematic maps of Mpl constructs.
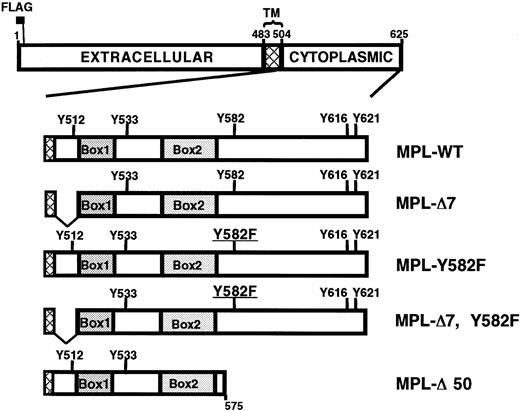
The top drawing depicts the 625-amino acid wild-type murine Mpl with the extracellular, intracellular, and transmembrane (TM; cross-hatch) domains indicated. The N-terminal FLAG epitope is located immediately downstream of the leader sequence. Intracellular domains of the 5 constructs used in this report are schematized showing the location of Box1, Box2, and the 5 intracellular tyrosines. Mpl-Δ7 is missing the 10 intracellular residues (505-514) immediately adjacent to the cell membrane. Mpl-Δ50 lacks the 50 C-terminal amino acids, residues 576 to 625.
Schematic maps of Mpl constructs.
The top drawing depicts the 625-amino acid wild-type murine Mpl with the extracellular, intracellular, and transmembrane (TM; cross-hatch) domains indicated. The N-terminal FLAG epitope is located immediately downstream of the leader sequence. Intracellular domains of the 5 constructs used in this report are schematized showing the location of Box1, Box2, and the 5 intracellular tyrosines. Mpl-Δ7 is missing the 10 intracellular residues (505-514) immediately adjacent to the cell membrane. Mpl-Δ50 lacks the 50 C-terminal amino acids, residues 576 to 625.
The membrane-proximal intracellular MPL sequence is required for TPO-induced proliferation in primary megakaryocyte-lineage cells
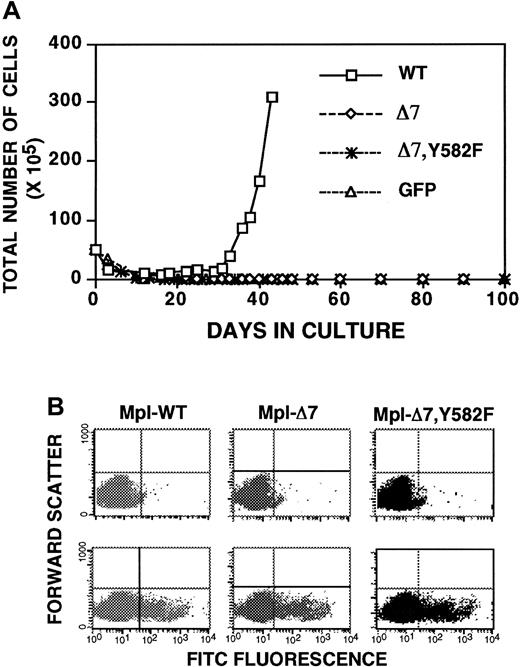
Deletion of the membrane-proximal 10 amino acids of the Mpl intracellular domain (Mpl-Δ7; Figure 3) unmasks a JAK/STAT-independent TPO-induced proliferation in immortalized, nonmegakaryocyte-lineage cells.31,32 To better understand the biologic significance of these observations, we sought to determine whether the same Mpl mutant has a similar phenotype in primary megakaryocyte-lineage cells. Unfractionated bone marrow fromGPIIb-tva+mpl−/− mice was infected with RCAS-Mpl, RCAS-Mpl-Δ7, and RCAS-GFP, and cells were selected in TPO. Unlike what happens in immortalized cells,31,32Mpl-Δ7 failed to confer a TPO-induced proliferative signal in primary megakaryocyte-lineage cells (Figure 4A), even at h-TPO concentrations up to 500 ng/mL. Because others have described enhanced TPO-stimulated proliferation associated with an Mpl Y582F mutation,14 we added a Y582F mutation to Mpl-Δ7 to ask whether the doubly mutated Mpl transmits a TPO-induced proliferative signal in primary megakaryocyte-lineage cells. Bone marrow cells from GPIIb-tva+mpl−/−mice infected with RCAS-Mpl-Δ7, Y582F (Figure 3) also failed to proliferate in response to TPO (Figure 4A).
Membrane-proximal 10 amino acids from the Mpl intracellular domain are required for TPO-induced proliferation in primary megakaryocyte-lineage cells.
(A) Growth curves of unfractionatedGPIIb-tva+mpl−/−bone marrow after infection with RCAS-Mpl, RCAS-MplΔ7, and RCAS-MplΔ7, Y582F, and selection in TPO demonstrate a loss of TPO-induced proliferation when the membrane-proximal 10 amino acids are missing from the Mpl intracellular domain. (B) All virally expressed Mpl have an N-terminal FLAG epitope. FACS analysis using isotype control (top panels) and anti-FLAG antibody (lower panels) demonstrates similar levels of surface Mpl expression for Baf/3-TVA cells infected with the same virus stocks used in panel A.
Membrane-proximal 10 amino acids from the Mpl intracellular domain are required for TPO-induced proliferation in primary megakaryocyte-lineage cells.
(A) Growth curves of unfractionatedGPIIb-tva+mpl−/−bone marrow after infection with RCAS-Mpl, RCAS-MplΔ7, and RCAS-MplΔ7, Y582F, and selection in TPO demonstrate a loss of TPO-induced proliferation when the membrane-proximal 10 amino acids are missing from the Mpl intracellular domain. (B) All virally expressed Mpl have an N-terminal FLAG epitope. FACS analysis using isotype control (top panels) and anti-FLAG antibody (lower panels) demonstrates similar levels of surface Mpl expression for Baf/3-TVA cells infected with the same virus stocks used in panel A.
To exclude the possibility that the failure of Mpl-Δ7 and Mpl-Δ7, Y582F to confer TPO-inducible proliferation in primary megakaryocyte-lineage cells was due to defective virus stocks, identical viral stocks used to infect primary bone marrow cells were also used to infect Baf/3-TVA cells. FACS analysis using an anti-FLAG antibody demonstrated similar levels of Mpl surface expression for RCAS-Mpl, RCAS-Mpl-Δ7, and RCAS-Mpl-Δ7, Y582F infected Baf/3-TVA cells (Figure 4B). Notably, when these cells were selected in TPO, we found TPO-dependent growth similar to prior reports for Baf/3 cells expressing Mpl-Δ732 (data not shown).
Mpl tyrosine-582 has an antiproliferative function in primary megakaryocyte-lineage cells
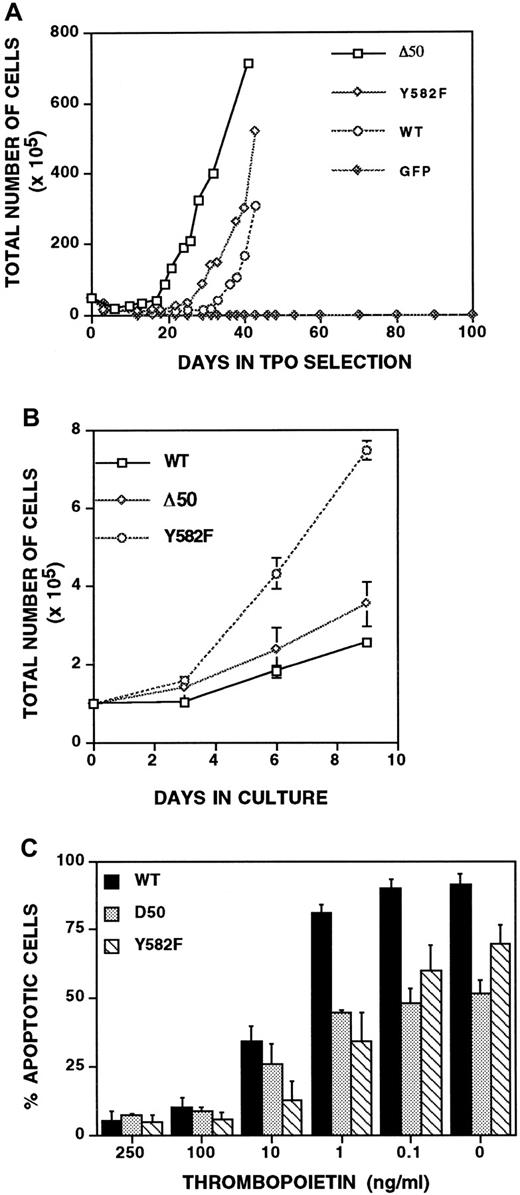
Studies using immortalized, nonmegakaryocyte-lineage cells suggest that Mpl residue Y582 plays a critical role in a roughly 20-amino acid antiproliferative domain within the intracellular portion of Mpl.13 14 To determine whether this is true in primary megakaryocyte-lineage cells, we infected bone marrow fromGPIIb-tva+mpl−/− mice with RCAS-Mpl, RCAS-MplY582F, and RCAS-Mpl-Δ50, the latter mutant lacking Mpl residues 576 to 625 (Figure 3). All 3 Mpl constructs conferred TPO-dependent proliferation in TVA-expressing primary bone marrow cells from GPIIb-tva+mpl−/− mice (Figure5A). To directly compare their proliferative responses to TPO, equal numbers of cells expressing wild-type or mutant Mpl were cultured, and the total cell number was monitored over time. Compared with wild-type Mpl, Mpl-Y582F imparts a marked TPO-dependent proliferative advantage, whereas cells expressing Mpl-Δ50 proliferate similarly to those expressing wild-type Mpl (Figure 5B).
MplY582F has a proliferative advantage over wild-type Mpl.
(A) Growth curves of unfractionatedGPIIb-tva+mpl−/−bone marrow grown in TPO-containing media after infection with RCAS-Mpl, RCAS-MplY582F, RCAS-MplΔ50, or RCAS-GFP. (B) Plating equal numbers of TPO-responsiveGPIIb-tva+mpl−/−bone marrow cells expressing wild-type Mpl, MplY582F, or MplΔ50 demonstrates a clear proliferative advantage imparted by the Y582F mutation. (C) Percentage of apoptotic cells at various h-TPO concentrations was determined for cells expressing Mpl, MplY582F, and MplΔ50. The percentage of apoptotic cells includes all cells that stain with FITC-conjugated anti-annexin V antibody, annexin V+/PI− and annexin V+/PI+ cells. Results are the average and standard deviation of 3 to 5 independent experiments for each Mpl construct.
MplY582F has a proliferative advantage over wild-type Mpl.
(A) Growth curves of unfractionatedGPIIb-tva+mpl−/−bone marrow grown in TPO-containing media after infection with RCAS-Mpl, RCAS-MplY582F, RCAS-MplΔ50, or RCAS-GFP. (B) Plating equal numbers of TPO-responsiveGPIIb-tva+mpl−/−bone marrow cells expressing wild-type Mpl, MplY582F, or MplΔ50 demonstrates a clear proliferative advantage imparted by the Y582F mutation. (C) Percentage of apoptotic cells at various h-TPO concentrations was determined for cells expressing Mpl, MplY582F, and MplΔ50. The percentage of apoptotic cells includes all cells that stain with FITC-conjugated anti-annexin V antibody, annexin V+/PI− and annexin V+/PI+ cells. Results are the average and standard deviation of 3 to 5 independent experiments for each Mpl construct.
To determine whether changes in apoptosis can explain the proliferative advantage provided by the point mutation Y582F (Figure5B), cells were grown at varying TPO concentrations and assayed for apoptosis using propidium iodide and annexin V staining. At h-TPO concentrations of 250 ng/mL, the concentration used in Figure 5A, and h-TPO concentrations of 100 ng/mL, the percentage of apoptotic cells (annexin V+) was indistinguishable among the different Mpl constructs (Figure 5C). The Mpl-Y582F proliferative advantage in Figure5B was, therefore, not due to a decrease in apoptosis. Interestingly, at 1 ng/mL or less h-TPO, Mpl-Y582F and Mpl-Δ50 had significantly fewer apoptotic cells than does wild-type Mpl (Figure 5C), an effect seen even in the absence of TPO (Figure 5C). Of note, no cell proliferation occurred for any of the Mpl constructs at 1 ng/mL or less h-TPO (data not shown).
The synergistic effect of SCF on TPO-induced megakaryocyte-lineage proliferation is independent of the C-terminal 50 amino acids of Mpl
SCF is synergistic with TPO for colony-forming unit–megakaryocyte colony size and number when bone marrow is cultured in vitro.33 To determine whether SCF is synergistic with TPO in stimulating the proliferation of primary megakaryocyte-lineage cells in our system, GPIIb-tva+mpl−/−cells expressing wild-type Mpl, Mpl-Δ50, or MplY582F were grown in media supplemented with either SCF, TPO, SCF and TPO, or no added cytokines (Figure 6). Although SCF failed to provide an independent proliferative signal in any of the cells tested, it provided a similar and marked synergistic effect on TPO-induced proliferation for all 3 Mpl constructs. The synergistic effect was therefore independent of any Mpl-stimulated intracellular signaling that originated in the C-terminal 50 amino acids of the 121-amino acid Mpl intracellular domain. Of note,GPIIb-tva+mpl−/− cells expressing Mpl-Δ7 and Mpl-Δ7, Y582F not only failed to proliferate in response to TPO, as described above, they also failed to proliferate in media containing both TPO and SCF (data not shown).
Stem cell factor lacks an independent proliferative effect yet is synergistic with TPO-induced proliferation in primary megakaryocyte-lineage cells.
TPO-responsive GPIIb-tva+mpl−/−bone marrow cells expressing Mpl, MplY582F, and MplΔ50 were grown in the presence of 10 ng/mL SCF and 250 ng/mL h-TPO, 250 ng/mL h-TPO, 10 ng/mL SCF, or no added cytokine, and total cell counts were recorded on the days indicated. Each point represents the average of 3 independent experiments.
Stem cell factor lacks an independent proliferative effect yet is synergistic with TPO-induced proliferation in primary megakaryocyte-lineage cells.
TPO-responsive GPIIb-tva+mpl−/−bone marrow cells expressing Mpl, MplY582F, and MplΔ50 were grown in the presence of 10 ng/mL SCF and 250 ng/mL h-TPO, 250 ng/mL h-TPO, 10 ng/mL SCF, or no added cytokine, and total cell counts were recorded on the days indicated. Each point represents the average of 3 independent experiments.
Others have described a synergistic effect between TPO and IL-11 and between TPO and erythropoietin (EPO) when total bone marrow is used to generate megakaryocyte-containing colonies in semisolid (agar) media.33 Neither IL-11 nor EPO was synergistic with TPO in proliferation assays using the early megakaryocyte-lineage cells we describe in this report, and neither cytokine provided a proliferative signal on its own.
Primary megakaryocyte-lineage cells provide a new model for studies of Mpl-related intracellular signaling
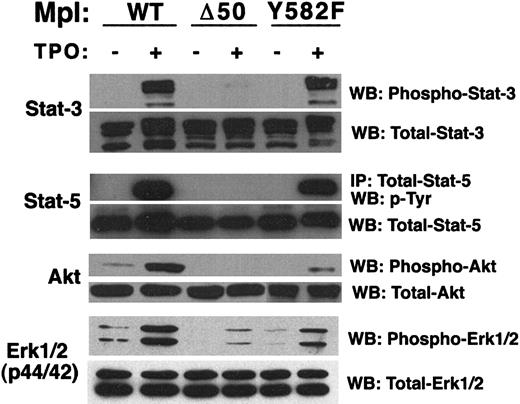
The ability to perform Mpl structure–function studies in a pure population of primary megakaryocyte-lineage cells should help clarify and advance our understanding of MPL-related intracellular signaling and, thereby, our understanding of megakaryocytopoiesis. Hence, we studied TPO-induced activation of 3 intracellular signaling pathways inGPIIb-tva+mpl−/− cells expressing wild-type Mpl, Mpl-Δ50, or MplY582F. Although all 3 Mpl constructs conferred TPO-inducible proliferation (Figure 6), Mpl-Δ50 uniquely proliferated in the absence of detectable Stat-5 phosphorylation and the near-absence of detectable Stat-3 phosphorylation (Figure7). The point mutation Y582F had no measurable effect on this signaling cascade. Similar to studies in cell lines14 and an Mpl knock-in mouse,17phosphorylation of the PI3 kinase substrate Akt required the Mpl C-terminus. Furthermore, Mpl tyrosine 582 contributes to Akt phosphorylation (Figure 7).
Immunodetection of TPO-induced activation of intracellular signaling molecules.
Western blot (WB) or immunoprecipitation (IP) followed by Western blot was used to determine TPO-stimulated activation of Stat-3, Stat-5, Akt, and Erk1/2 (p44/42) in primary megakaryocyte-lineage cells expressing wild-type or mutant Mpl. TPO-stimulated cells (+) are compared to nonstimulated cells (−) for each Mpl construct. Equal protein loading was verified by the use of antibodies that detect total (phosphorylated and nonphosphorylated) forms of each signaling molecule tested.
Immunodetection of TPO-induced activation of intracellular signaling molecules.
Western blot (WB) or immunoprecipitation (IP) followed by Western blot was used to determine TPO-stimulated activation of Stat-3, Stat-5, Akt, and Erk1/2 (p44/42) in primary megakaryocyte-lineage cells expressing wild-type or mutant Mpl. TPO-stimulated cells (+) are compared to nonstimulated cells (−) for each Mpl construct. Equal protein loading was verified by the use of antibodies that detect total (phosphorylated and nonphosphorylated) forms of each signaling molecule tested.
TPO-stimulation of wild-type Mpl also activates the Ras-Raf-MEK-Erk intracellular signaling cascade in cell lines and primary megakaryocytes (reviewed in Drachman et al16). To further characterize our system, we assayed for TPO-induced Erk activation–phosphorylation in primary megakaryocyte-lineage cells expressing virally introduced wild-type Mpl, Mpl-Y582F, and Mpl-Δ50. Similar to published findings, including knock-in mice expressing Mpl lacking the C-terminal 60 amino acids,17 we saw markedly reduced but detectable Erk activation in cells expressing Mpl-Δ50 (Figure 7). At most, only a small diminution of Erk phosphorylation was seen with the point mutant Mpl-Y582F (Figure 7).
Discussion
Immortalized, nonmegakaryocyte-lineage cell lines have been central to our current understanding of the mechanism of action of various hematopoietic cytokine receptors, including Mpl.10-16,31,32 However, cytokine receptor structure-function studies, including those designed to help understand the role of intracellular signaling in specific developmental and biologic processes, should ideally be performed in primary cells that naturally express the receptor of interest. This would help overcome the recurring problem of cell-line dependent findings13,15,31 and of differences between primary cells and cell lines,14,17 18 and it would allow study of the interaction of multiple cytokines that naturally act on the cell type of interest. The GPIIb-tva+mpl−/−mice described here provide the first system in which to perform Mpl structure–function studies in primary, megakaryocyte-lineage cells lacking endogenous Mpl expression without the need to make numerous transgenic or knock-in mice.
Human GPIIb regulatory sequences can direct megakaryocyte-lineage–restricted functional transgene expression
As part of our interest in establishing murine systems that allow for the introduction and expression of genes in primary megakaryocyte-lineage cells,27 815 bp of human GPIIb 5′ regulatory sequence was used to generate transgenic mice with megakaryocyte-lineage–restricted TVA expression. Immunohistochemical analysis of multiple tissues using an anti-TVA antibody detected TVA expression only in the bone marrow and spleen. Although bone marrow TVA expression is readily apparent on megakaryocytes, small cells expressing TVA were also detected (Figure 1C). When total bone marrow is infected with RCAS-PURO—an avian retrovirus that confers resistance to puromycin—the puromycin-resistant cells express surface CD41, but they do not express surface markers for lymphoid (CD3, B220), granulocyte (Gr-1), erythroid (Ter119), or macrophage (F4/80) lineages. In addition, essentially all TVA-expressing cells co-express CD41 (Figure 1D). The small TVA-expressing bone marrow cells are therefore most consistent with early megakaryocyte-lineage cells that would be predicted to express TVA if transgene expression paralleled murine GPIIb expression.
One major experimental use for the GPIIb-tva+mice is to cross them to genetically null backgrounds to study genes important for megakaryocyte-lineage development and megakaryocyte biology. We therefore generatedGPIIb-tva+mpl−/− mice. RCAS-Mpl infection of unfractionated bone marrow fromGPIIb-tva+mpl−/− mice yields TPO-responsive cells that express CD41 on their surfaces, lack surface markers of other hematopoietic lineages, and express intracellular vWF (Figure 2). Taken together, our findings support the conclusion that functional TVA expression is restricted to megakaryocyte-lineage cells in the GPIIb-tva+ and theGPIIb-tva+ mpl−/− mice, and theGPIIb-tva+ mpl−/− mice provide a novel system for introducing Mpl into primary megakaryocyte-lineage cells that lack endogenous Mpl expression.
Some reports have questioned the long-held view that GPIIb, also called integrin αΙΙb, is expressed exclusively on megakaryocyte-lineage cells and platelets. GPIIb expression was detected on avian intra-embryonic and post-natal multilineage progenitors.34 Human GPIIb 5′-regulatory sequence (800 bp) was used to express herpes simplex virus thymidine kinase transgene in megakaryocyte-lineage cells, but low-level expression in multilineage progenitors was suspected based on the effect of acyclovir treatment on the transgenic bone marrow.35 Similar results were obtained when a 2.7-kb fragment of the murine GPIIb promoter was used to express thymidine kinase in transgenic mice.36 The role of a cryptic promoter within the thymidine kinase coding sequence, already known to function in testes,37 cannot be excluded as contributing to extra-megakaryocyte–lineage expression. These reports suggest that the murine or human GPIIb promoter may result in nonmegakaryocyte-lineage transgene expression in transgenic mice. However, they do not detract from our conclusion that the 815-bp human GPIIb 5′-regulatory sequence in the GPIIb-tva+mice directs TVA expression that allows for megakaryocyte-lineage–restricted infection by subgroup A, avian retroviruses.
GPIIb-tva+mpl−/−mice allow for Mpl structure–function studies in primary megakaryocyte-lineage cells
Deletion of the membrane-proximal 10 amino acids from the Mpl intracellular domain (Mpl-Δ7) results in JAK/STAT-independent TPO-inducible proliferation in the Baf/3, 32D, and UT-7 immortalized cell lines engineered to express Mpl-Δ7.31 32 Although we confirmed the published results in immortalized Baf/3 cells, we found that Mpl-Δ7 fails to confer a TPO-dependent proliferative signal in primary megakaryocyte-lineage cells fromGPIIb-tva+mpl−/− mice (Figure 4).
Other reports of cell lines engineered to express various Mpl mutants show a tight correlation between Stat-3 or Stat-5 activation, or both, and TPO-stimulated proliferation.10,11,14 However, though TPO-stimulation of wild-type Mpl robustly phosphorylates Stat-3 and Stat-5 in primary megakaryocyte-lineage cells, TPO stimulation of Mpl-Δ50 stimulates proliferation of these cells with only minimally detectable Stat-3 phosphorylation and no detectable Stat-5 phosphorylation (Figure 7). This conflicts with findings from Baf/3 cells expressing Mpl truncation mutants, in which TPO-induced proliferation correlates with Stat-5 phosphorylation.14However, our data agree with findings fromStat-5−/− mice in which bone marrow megakaryocyte and circulating platelets numbers are essentially unaltered.38 It is difficult to know whether the trace residual Stat-3 phosphorylation seen with Mpl-Δ50 fulfills a Stat-3 requirement for TPO-induced proliferation or whether it supports a model in which neither Stat-3 nor Stat-5 is required for TPO-induced proliferation of early, primary megakaryocyte-lineage cells. Regardless, our findings underscore the importance of performing receptor signaling studies in primary, physiologically relevant cells, and they demonstrate the potential of our system for better understanding the mechanism(s) underlying the proliferation of megakaryocyte-lineage cells.
Numerous studies have reported the activation of STAT family members after TPO stimulation.18,39-44 The somewhat confusing nature of the data are cogently summarized by a model in which Stat-3 and Stat-5 are the key STAT family members activated by TPO-engagement of Mpl, but the specific STAT family member activated changes during the developmental continuum that defines megakaryocytopoiesis.18 The TPO-induced Stat-3 phosphorylation demonstrated in platelets from knock-in mice expressing a C-terminal truncated Mpl17 appears greater than what we see with Mpl-Δ50. However, the primary cells studied in our system uniquely represent megakaryocyte-lineage committed, megakaryocyte precursors. Such differences may reflect the above-mentioned model in which different STAT family members are differentially activated or support different functions at different times during megakaryocytopoiesis and thrombopoiesis.18
When expressed in cell lines, Mpl Y582 contributes to an antiproliferative domain within the intracellular portion of Mpl.13 14 We have demonstrated for the first time that such a domain functions in primary megakaryocyte-lineage cells (Figure5B). Although we have not yet identified the molecular mechanism underlying the Mpl-Y582F phenotype, it is not attributable to an increase in Akt activation (Figure 7) or to a general increase in anti-apoptotic signaling (Figure 5C). However, the Y582F mutation imparts a constitutively active anti-apoptotic phenotype that remains present at low TPO concentrations (1 ng/mL or less) that may more closely reflect the TPO concentration seen by megakaryocyte-lineage cells in a natural physiologic setting (Figure 5C). These findings provide a cogent reminder of the need to study the effect of Mpl mutations over a range of TPO concentrations. The highly similar apoptosis data for Mpl-Y582F and Mpl-Δ50 suggest that the Mpl-Δ50 phenotype seen at low TPO concentrations may simply reflect the loss of Y582 (Figure 5C). However, the proliferative differences between Mpl-Y582F and Mpl-Δ50 (Figure 5B) suggest that the Mpl-Y582F proliferation advantage requires not only the loss of a tyrosine at residue 582 but also the presence of some or all of the other 49 amino acids missing from the Mpl C-terminus in Mpl-Δ50.
The system described allows one to study the interplay of various physiologically relevant cytokines involved in megakaryocytopoiesis. For example, we demonstrated that SCF cannot independently stimulate the proliferation of primary megakaryocyte-lineage cells, but it has a marked synergistic effect on TPO-induced proliferation (Figure 6). The similarity of this finding to what is seen with cultured murine bone marrow33 helps validate our system. Because we could introduce Mpl mutants into the primary megakaryocyte-lineage cells fromGPIIb-tva+mpl−/− mice, we were also able to demonstrate that the SCF-TPO synergism is independent of intracellular signaling that originates from the Mpl C-terminal 50 amino acids (Figure 6). Others have demonstrated a synergistic effect for IL-11 and TPO, and for EPO and TPO in CFU-Mk formation from total bone marrow,33 but neither of these 2 cytokine combinations is synergistic for expanding the early megakaryocyte-lineage cells that proliferate after RCAS-Mpl-WT infection of GPIIb-TVA+mpl−/− bone marrow. This difference suggests that the cells responding to the synergistic effect in CFU-Mk formation may be at a different developmental stage than the megakaryocyte-lineage committed cells assayed in our system.
The expression of wild-type and mutant Mpl protein inGPIIb-TVA+mpl−/− bone marrow cells provides a model system in which to conduct Mpl structure–function studies in early megakaryocyte-lineage cells (Figures 4-6). However, a similar expansion of early megakaryocyte-lineage cells is not seen when uninfected bone marrow from mpl+/+ mice is grown under similar conditions. Our finding that early megakaryocyte-lineage cells do not proliferate in TPO-containing media after RCAS-GFP infection of GPIIb-TVA+mpl−/− bone marrow cells (Figure 4) suggests that the RCAS virus itself does not directly contribute to the proliferation of RCAS–Mpl-infectedGPIIb-TVA+mpl−/− cells. We speculate that the RCAS virus may somehow impair megakaryocyte-lineage maturation, thereby allowing for the extended proliferation of early megakaryocyte-lineage cells. We are undertaking studies to directly address this hypothesis and to identify viral proteins that may have such an effect on megakaryocyte-lineage maturation.
The GPIIb-tva+ mice described are one of interest of a series of transgenic mice we have generated for introducing genes into primary megakaryocyte-lineage cells. We previously described mice in which the GP-Ibα promoter expressed TVA restricted to the megakaryocyte.27GPIIb-TVA+mpl−/− andGPIbα-TVA+mpl−/− mice yield similar results in the type of Mpl replacement studies reported here. Although we focused in this report on studies of Mpl-associated proliferation by early megakaryocyte-lineage cells, we are developing conditions in which to apply the system to study megakaryocyte development. Crossing GPIIb-tva+ orGPIbα-tva+ mice to other biologically relevant knockout mice, such as NF-E2−/−mice,45 should provide new models for the study of biologic processes related to megakaryocytopoiesis and megakaryocyte function. Moreover, the general method we describe should be applicable to any hematopoietic lineage of interest.
We thank John Scarborough (Oregon Health Sciences University, Portland) for performing the oocyte injections; Paul Bray (Baylor College of Medicine, Houston, TX), Paul Bates (University of Pennsylvania, Philadelphia), Phil Leder (Harvard Medical School, Cambridge, MA), Steve Hughes (National Cancer Institute, Frederick, MD), and Marion Dorsch and Stephen P. Goff (Columbia University, New York, NY), for plasmids, and the staff at Genentech (San Francisco, CA) for recombinant human TPO and mpl−/− mice.
Supported by grant P50 HL54476 from the National Institutes of Health (NHLBI) Transfusion Medicine Specialized Center for Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Andrew D. Leavitt, Departments of Laboratory and Internal Medicine, University of California, San Francisco, 505 Parnassus Ave, Rm L-514, Box 0100, San Francisco, CA 94143-0100; e-mail: leavitt@pangloss.ucsf.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal