The diagnosis of invasive aspergillosis (IA) in patients with hematologic disorders is not straightforward; lack of sensitive and specific noninvasive diagnostic tests remains a major obstacle for establishing a precise diagnosis. In a series of 362 consecutive high-risk treatment episodes that were stratified according to the probability of IA based on recently accepted case definition sets, the potential for diagnosis of serial screening for circulating galactomannan (GM), a major aspergillar cell wall constituent was validated. After incorporating postmortem findings to allow a more accurate final analysis, this approach proved to have a sensitivity of 89.7% and a specificity of 98.1%. The positive and negative predictive values equaled 87.5% and 98.4%, respectively. False-positive reactions occurred at a rate of 14%, although this figure might be overestimated due to diagnostic uncertainty. More or less stringent criteria of estimation could highly influence sensitivity, which ranged from 100% to 42%; the impact on other test statistics was far less dramatic. All proven cases of IA, including 23 cases confirmed after autopsy only, had been detected before death, although serial sampling appeared to be necessary to maximize detection. The excellent sensitivity and negative predictive value makes this approach suitable for clinical decision making. Unfortunately, given the species-specificity of the assay, some emerging non-Aspergillus mycoses were not detected. In conclusion, serial screening for GM, complemented by appropriate imaging techniques, is a sensitive and noninvasive tool for the early diagnosis of IA in high-risk adult hematology patients.
Introduction
Invasive aspergillosis (IA) is an emerging opportunistic infection among many categories of immunocompromised hosts.1 The high mortality rate results partly from difficulties in establishing a reliable diagnosis, particularly in patients who receive cytoreductive or marrow-ablative therapy.2,3 Although gold diagnostic standards exist, they usually require invasive procedures to obtain specimens for histological examination and culture.4 Unfortunately, such aggressive procedures are often precluded by cytopenia or by the critical condition of these patients. Hence, definite diagnosis is infrequently established before death or before fungal proliferation becomes overwhelming and therapy may no longer be successful.5 6
In an attempt to overcome this diagnostic obstacle, clinicians have tried to estimate the probability—even possibility—of IA based on sets of objective clinical, radiological, and microbiological criteria. However, lack of standardization has always impaired the comparison of data between centers.7 Recently, a consensus committee composed of members from the Invasive Fungal Infections Co-operative Group of the European Organization for Research and Treatment of Cancer (EORTC-IFICG), Brussels, Belgium, and the Mycoses Study Group of the National Institutes of Allergy and Infectious Diseases (NIAID-MSG), Bethesda, MD, has taken a lead in developing standardized definitions of invasive fungal infection in cancer patients and stem cell transplantation recipients for use in research studies.7
The incorporation of microbiological findings may improve the specificity of these case definitions, thereby reducing the number of false-positive classifications. Unfortunately, with respect to IA, culturing of body fluids has a low diagnostic yield and does not always discriminate between invasive disease, colonization, and contamination.8 Newer diagnostic approaches have focused on the detection of surrogate markers such as circulating fungal antigens or metabolites.9 One such component is galactomannan (GM), a major aspergillar cell-wall constituent released during invasive disease. Recently a commercially available sandwich enzyme-linked immunosorbent assay (ELISA) for the detection of GM was introduced.10,11 The assay employs the rat monoclonal antibody EB-A2 and recognizes the 1 → 5-β-D-galactofuranoside side chains of the GM molecule. By using the same antibody as both capture and detector antibody, the threshold for detection could be lowered to 1.0 ng/mL−1 serum,10 11 whereas the earlier latex agglutination test had a 15 ng/mL−1 threshold.
An excellent sensitivity and specificity of the assay was recently demonstrated in hematology patients. However, many of the studies are hampered by their retrospective design, the limited number of proven cases, the heterogeneity of the population under study, the use of low-risk control groups, and the verification of biases toward negative test results. In addition, every comparison between studies remains impossible due to differences and overlaps in case definitions.12-17
To investigate the expression of this exo-antigen and to validate its diagnostic potential in hematology patients, we conducted a prospective trial in patients at high risk for IA. Treatment episodes were classified according to the EORTC/MSG case definitions for invasive fungal infections and were validated by necropsy in a large number of fatalities.
Patients, materials, and methods
Study population and design
From January 1997 through February 2000, serum GM levels were prospectively measured in a consecutive series of adult patients with hematological disorders who were at risk for developing IA. Eligible patients were (1) receiving chemotherapy with an expected duration of neutropenia (less than 500 cells per μL) of at least 10 days because of de novo or relapsed acute leukemia (AL), chronic myeloid leukemia (CML) undergoing AL-like induction, lymphoblastic non-Hodgkin lymphoma, or high-risk myelodysplasia (refractory anemia with excess blasts [RAEB], RAEB in transformation [RAEBt], or acute myeloid leukemia [sAML]) or (2) undergoing allogeneic bone marrow or peripheral blood stem cell transplantation. We excluded those patients who were undergoing autologous transplantation, had aplastic anemia, or were less than 16 years of age.
We collected 5 mL whole blood at least twice weekly, starting at admission, until death or discharge from hospital. The group of allogeneic transplantation recipients was further screened at least once weekly as outpatients until cessation of immunosuppressive therapy. ELISA (Platelia Aspergillus; Sanofi Diagnostics Pasteur, Marnes-La-Coquette, France) was performed once weekly by the same technicians, as described previously.12 An optical density (OD) index of 1.0 or more was considered positive; a result was considered “true-positive” when 2 consecutive samples for the same patient tested positive.
All patients were hospitalized in single reverse-isolation rooms in a unit equipped with high-efficiency particulate air filters until recovery of the neutrophils above 500 cells per μL. Antifungal prophylaxis with 200-400 mg/d itraconazole capsules or a combination of 10 mg aerosolized and 10 mg low-dose intravenous (IV) amphotericin B was given twice a day throughout the period of neutropenia; itraconazole was continued in patients receiving prolonged immunosuppressive therapy.
Broad-spectrum antibiotics were initiated at the first bout of neutropenic fever according to published guidelines.18 In patients who remained febrile after 48-72 hours, a glycopeptide drug was added. Criteria for initiating antifungal therapy (0.7-1.0 mg/kg amphotericin B or 200 mg IV itraconazole) included (1) persistent fever after 5-7 days of adequate IV antibiotic treatment; (2) development of new pulmonary infiltrates on chest x-ray while receiving antibacterial therapy; (3) computed tomography (CT) scan abnormalities with characteristic features of invasive mycosis (IM); (4) isolation of molds from upper or lower respiratory tract; (5) sudden intracranial events compatible with IM; or (6) fever relapsing after an afebrile interval of at least 48 hours in neutropenic patients still receiving broad-spectrum antibacterial therapy.
During hospitalization, patients were surveyed for the development of sinopulmonary signs and symptoms. Diagnostic procedures included daily physical examination; conventional chest (and sinus) x-ray at admission, followed by weekly chest x-rays, which increased to 2-3 times weekly in cases of persistent fever; repeated blood and sputum cultures as clinically indicated; weekly surveillance cultures on stool and urine samples; and oral washes for bacterial and fungal growth. In cases of clinical suspicion of invasive fungal disease, a diagnostic work-up was initiated including high-resolution pulmonary CT scan followed by guided bronchoalveolar lavage (BAL) if feasible. Lavage samples were submitted for bacterial, fungal, mycobacterial, andLegionella cultures; direct immunofluorescent staining forPneumocystis carinii; acid-fast staining; and viral cultures. No endobronchial biopsies were taken in cytopenic patients. Other diagnostic procedures were performed on clinical indication.
Necropsy was pursued in all fatal cases unless there was an explicit refusal by the patient or his family. Specimens of the lungs were sent for fungal culture. Specimens were stained with periodic acid-Schiff or Gomori methenamine silver stain for the detection of hypha invasion.
Case definitions and classification
Treatment episodes were classified according to the EORTC/MSG case definitions; ELISA results were excluded as microbiological criterion. At least one of the following host factors was present for all patients: prolonged neutropenia, graft-versus-host disease (GVHD), and/or prolonged use of steroids. Proven IA referred to the histopathological evidence of tissue invasion (needle aspiration or biopsy and/or autopsy specimen) by filamentous fungi that disclosed septated, acutely branching hyaline hyphae and a culture yielding theAspergillus species. The isolation of Aspergillusspecies from a normally sterile, but clinically infected, body site (excluding BAL fluid and sinus aspirate) obtained by a sterile procedure was also defined as proven IA. Positive histopathology without species identification was classified as proven invasive fungal infection (IFI).
Probable invasive pulmonary aspergillosis referred to the presence of a positive culture or cytology for Aspergillusspecies from sputum or BAL fluid together with one major (halo sign, air-crescent sign, or cavity within an area of consolidation on CT imaging) or 2 of 3 minor clinical criteria. The criteria included (1) symptoms of lower respiratory tract infection such as cough, pleuritic chest pain, dyspnea, or hemoptysis; (2) pleural rub; or (3) any new infiltrate not fulfilling the major radiological criteria without an alternative diagnosis. Probable sinonasal aspergillosis was defined by a positive culture or cytology for Aspergillus species from sinus aspirate together with radiological evidence of invasive infection in the sinuses or at least 2 of 5 clinical criteria (upper respiratory symptoms; nose ulceration, eschar, and epistaxis; periorbital swelling; maxillary tenderness; or necrotic lesions or perforation of hard palate). Identification of a non-Aspergillus mold in identical settings was considered as probable IFI. Possible IFI (including possible IA) was defined by the above-mentioned host factors and either (1) a positive microbiological criterion or (2) one major (or 2 minor) criteria from the clinical section. Study patients without any reference to IA or with proven non-Aspergillus fungal infections were not suspected of having IA. Patients could be upgraded on the basis of a later surgical procedure or necropsy.
Statistical analysis
The objective of this study was to assess the diagnostic potential of serial screening for GM in a consecutive cohort of adult hematology patients at high risk for IA. But, because the histopathologic confirmation of fungal tissue invasion is still considered the reference diagnostic test, the true status of disease of many study patients remains unknown in the absence of tissue specimen.19 In this setting, the sensitivity and specificity of any noninvasive diagnostic test remain ill-defined and may dramatically be influenced by the proposed case definitions.20 Therefore, several different estimates were used to calculate sensitivity, specificity, and predictive values from 2 × 2 tables. Cases without any clue referring to IA and those with proven non-Aspergillus fungal infections were considered true negative for all estimates. Method A calculated the statistical values based on episodes that are known to be truly positive and truly negative. Method B assumed that all episodes of proven and probable IA were true-positive episodes and that the uninfected cases were true-negative ones. Methods C and D provided extreme values, estimating that all cases of possible IFI are either true-positive (method C) or true-negative (method D).
Trying to partially overcome serious verification bias, an unselected group of autopsied fatalities was analyzed separately. Sensitivity was calculated from the results of proven cases; specificity was calculated from the results of the negative group. Positive and negative predictive values were estimated from the combination of both groups. Finally, we integrated the autopsy findings in the entire group analysis and reassessed the findings via methods A-D. It is likely that these estimates are the most accurate for clinical practice.
Results
Study episodes
Following the EORTC/MSG criteria, 362 consecutive treatment episodes in 191 patients were classified as proven IA (n = 7), probable IA (n = 30), probable IFI (n = 2), possible IFI (n = 97), and no IA (n = 226). A total of 4300 sera samples were analyzed; 502 (11.7%) sera samples tested were repeatedly positive in 44 (12.1%) episodes. The number of patients, demographic data, distribution of underlying disorders, host factors for IA, number of samples, distribution of positive treatment episodes, and antifungal use according to classification are summarized in Table1. In 12 (3.3%) episodes, positivity was limited to a single positive sample, which exceeded an OD value of 1.5 only once. Persistent fever (n = 30) or documented non-Aspergillus fungal infection (3 Candidainfections, 1 disseminated Fusarium infection, and 1Alternaria alternata sinusitis) provoked the initiation of antifungal therapy in 15% of episodes without IA.
Patient characteristics and distribution of serum samples based on premortem classification of treatment episodes
| . | Proven IA . | Probable IA . | Probable IFI . | Possible IFI . | No IA . | Total . |
|---|---|---|---|---|---|---|
| Patients, no. | 7 | 29 | 2 | 83 | 132 | 191 |
| Age, y | ||||||
| Mean | 45.7 | 50 | 55 | 44.8 | 41.4 | 44.4 |
| Median | 46 | 52 | 55 | 45 | 40 | 45 |
| Range | 26-69 | 27-79 | NA | 17-82 | 16-76 | 16-82 |
| Sex, M/F | 5/2 | 17/12 | 1/1 | 57/26 | 90/42 | 131/60 |
| Episodes, no. (%) | 7 (1.9) | 30 (8.3) | 2 (0.5) | 97 (26.8) | 226 (62.4) | 362 (100) |
| AML | 1 | 9 | 1 | 43 | 69 | 123 (33.9) |
| ALL | 1 | 4 | 7 | 30 | 42 (11.6) | |
| Total acute leukemia | 165 (45.5) | |||||
| Lymphoblastic lymphoma | — | 7 | 5 | 10 | 22 (6) | |
| Myelodysplasia | 1 | 2 | 1 | 14 | 34 | 52 (14.3) |
| Allogeneic SCT | — | 3 | 18 | 45 | 66 (18.2) | |
| Follow-up | 4 | 5 | 8 | 29 | 46 (12.7) | |
| CML | — | — | 2 | 9 | 11 (3) | |
| Neutropenic episodes, no. (%) | 2 | 26 | 2 | 87 | 193 | 310 (85.6) |
| Duration, d | ||||||
| Mean | 35 | 25.3 | NA | 20.5 | 20 | 20.7 |
| Median | 35 | 19 | NA | 23.9 | 19 | 19 |
| Range | 35-69 | 6-64 | 10-22 | 3-75 | 2-57 | 2-75 |
| Episodes with corticosteroids, no. (%) | 5 (71.4) | 15 (50) | 1 (50) | 31 (31.9) | 63 (27.8) | 115 (31.7) |
| Episodes with bacteremia, no. (%) | 3 (42.8) | 12 (40) | 2 (100) | 55 (56.7) | 109 (48.2) | 181 (50) |
| Samples per episode, no. | 256 | 529 | 12 | 1119 | 2384 | 4300 |
| Mean | 36.5 | 17.6 | NA | 11.5 | 10.5 | 11.8 |
| Median | 33 | 14.5 | NA | 9.5 | 8.5 | 9 |
| Range | 26-65 | 4-62 | 4-8 | 2-66 | 3-56 | 2-66 |
| Pos episodes, no. (%) | 7 (100) | 20 (66.6) | 0 | 15 (15.5) | 2 (0.9) | 44 (12.1) |
| Pos samples, no. (%) | 116 (45.1) | 218 (41.2) | 0 | 157 (14) | 11 (0.5) | 502 (11.7) |
| Per pos episodes, no. | ||||||
| Mean | 19 | 10.9 | NA | 10.5 | 5.5 | 11.2 |
| Median | 13.5 | 7 | NA | 5.5 | 5.5 | 9.5 |
| Range | 2-50 | 2-54 | NA | 2-52 | 4-7 | 2-54 |
| Single sample with pos episodes, no. (%) | 0 | 2 | 0 | 3 | 7 | 12 (3.3) |
| Episodes with antifungals, no. (%) | 7 (100) | 30 (100) | 2 (100) | 84 (86.6) | 35 (15.4) | 158 (43.6) |
| . | Proven IA . | Probable IA . | Probable IFI . | Possible IFI . | No IA . | Total . |
|---|---|---|---|---|---|---|
| Patients, no. | 7 | 29 | 2 | 83 | 132 | 191 |
| Age, y | ||||||
| Mean | 45.7 | 50 | 55 | 44.8 | 41.4 | 44.4 |
| Median | 46 | 52 | 55 | 45 | 40 | 45 |
| Range | 26-69 | 27-79 | NA | 17-82 | 16-76 | 16-82 |
| Sex, M/F | 5/2 | 17/12 | 1/1 | 57/26 | 90/42 | 131/60 |
| Episodes, no. (%) | 7 (1.9) | 30 (8.3) | 2 (0.5) | 97 (26.8) | 226 (62.4) | 362 (100) |
| AML | 1 | 9 | 1 | 43 | 69 | 123 (33.9) |
| ALL | 1 | 4 | 7 | 30 | 42 (11.6) | |
| Total acute leukemia | 165 (45.5) | |||||
| Lymphoblastic lymphoma | — | 7 | 5 | 10 | 22 (6) | |
| Myelodysplasia | 1 | 2 | 1 | 14 | 34 | 52 (14.3) |
| Allogeneic SCT | — | 3 | 18 | 45 | 66 (18.2) | |
| Follow-up | 4 | 5 | 8 | 29 | 46 (12.7) | |
| CML | — | — | 2 | 9 | 11 (3) | |
| Neutropenic episodes, no. (%) | 2 | 26 | 2 | 87 | 193 | 310 (85.6) |
| Duration, d | ||||||
| Mean | 35 | 25.3 | NA | 20.5 | 20 | 20.7 |
| Median | 35 | 19 | NA | 23.9 | 19 | 19 |
| Range | 35-69 | 6-64 | 10-22 | 3-75 | 2-57 | 2-75 |
| Episodes with corticosteroids, no. (%) | 5 (71.4) | 15 (50) | 1 (50) | 31 (31.9) | 63 (27.8) | 115 (31.7) |
| Episodes with bacteremia, no. (%) | 3 (42.8) | 12 (40) | 2 (100) | 55 (56.7) | 109 (48.2) | 181 (50) |
| Samples per episode, no. | 256 | 529 | 12 | 1119 | 2384 | 4300 |
| Mean | 36.5 | 17.6 | NA | 11.5 | 10.5 | 11.8 |
| Median | 33 | 14.5 | NA | 9.5 | 8.5 | 9 |
| Range | 26-65 | 4-62 | 4-8 | 2-66 | 3-56 | 2-66 |
| Pos episodes, no. (%) | 7 (100) | 20 (66.6) | 0 | 15 (15.5) | 2 (0.9) | 44 (12.1) |
| Pos samples, no. (%) | 116 (45.1) | 218 (41.2) | 0 | 157 (14) | 11 (0.5) | 502 (11.7) |
| Per pos episodes, no. | ||||||
| Mean | 19 | 10.9 | NA | 10.5 | 5.5 | 11.2 |
| Median | 13.5 | 7 | NA | 5.5 | 5.5 | 9.5 |
| Range | 2-50 | 2-54 | NA | 2-52 | 4-7 | 2-54 |
| Single sample with pos episodes, no. (%) | 0 | 2 | 0 | 3 | 7 | 12 (3.3) |
| Episodes with antifungals, no. (%) | 7 (100) | 30 (100) | 2 (100) | 84 (86.6) | 35 (15.4) | 158 (43.6) |
IA indicates invasive aspergillosis; IFI, invasive fungal infection; NA, not applicable; M, male; F, female; ALL, acute lymphoblastic leukemia; SCT, stem cell transplantation; and pos, positive.
Analysis based on antemortem classification
All 7 (100%) patients who were diagnosed with proven IA repeatedly tested positive (median, 13.5 positive sera samples per episode). Multiple consecutive positive results (7 of 56 and 4 of 30 tested sera samples) were found in 2 (0.88%) of 226 treatment episodes that made up the negative control group. Both episodes occurred late in the course of allogeneic stem cell transplantation recipients who were receiving steroids for GVHD. Of 30 episodes of probable IA, 20 (66.6%) episodes repeatedly tested positive (median, 7 sera samples per episode), whereas 10 episodes (152 sera samples) remained constantly negative. Two episodes of probable IFI were identified; Alternaria and Fusarium species were isolated from sputum in one ELISA− patient each. These patients were considered as cases of probable invasive alternariosis and fusariosis and were excluded from the analysis.
Finally, 15 (15.5%) of 97 episodes of possible IFI yielded repeatedly ELISA+ results (median, 5.5 sera samples per episode). Based on the antemortem stratification in proven and probable IA versus no IA (estimate B), the sensitivity and specificity of serial GM monitoring was 73% and 99.1%, respectively. The positive predictive value of the test was 93.1%, the negative predictive value was 95.7%, and the efficacy was 95.4%. However, as depicted in Table2, alternative estimates can dramatically change sensitivity (range, 31.3% to 100%) and predictive values.
Sensitivity, specificity, predictive values, and efficacy according to different estimates
| Analysis based on premortem stratification . | HDG . | Final analysis after incorporating autopsy findings . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate, % . | A . | B . | C . | D . | A . | B . | C . | D . | |
| Sensitivity | 100 | 72.9 | 31.3 | 72.9 | 91 | 100 | 89.7 | 41.9 | 89.7 |
| Specificity | 99.1 | 99.1 | 99.1 | 94.7 | 91.5 | 98.1 | 98.1 | 98.1 | 97.1 |
| PPV | 77.7 | 93.1 | 95.4 | 61.3 | 88.2 | 85.7 | 87.5 | 88.6 | 79.5 |
| NPV | 100 | 95.7 | 70.8 | 96.8 | 93.5 | 100 | 98.4 | 82.7 | 98.7 |
| Efficacy | 99.1 | 95.4 | 73.9 | 92.5 | 91.2 | 98.3 | 97.0 | 83.5 | 96.3 |
| Analysis based on premortem stratification . | HDG . | Final analysis after incorporating autopsy findings . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate, % . | A . | B . | C . | D . | A . | B . | C . | D . | |
| Sensitivity | 100 | 72.9 | 31.3 | 72.9 | 91 | 100 | 89.7 | 41.9 | 89.7 |
| Specificity | 99.1 | 99.1 | 99.1 | 94.7 | 91.5 | 98.1 | 98.1 | 98.1 | 97.1 |
| PPV | 77.7 | 93.1 | 95.4 | 61.3 | 88.2 | 85.7 | 87.5 | 88.6 | 79.5 |
| NPV | 100 | 95.7 | 70.8 | 96.8 | 93.5 | 100 | 98.4 | 82.7 | 98.7 |
| Efficacy | 99.1 | 95.4 | 73.9 | 92.5 | 91.2 | 98.3 | 97.0 | 83.5 | 96.3 |
HDG indicates analysis of histologically documented group; PPV, positive predictive value; and NPV, negative predictive value.
Analysis of a histologically documented subgroup
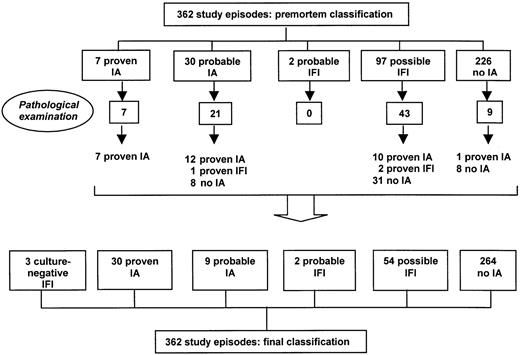
Following postmortem examination in 76 (92.6%) of 82 fatalities, 23 cases could be upgraded, resulting in 30 proven cases of IA (Figure1). These additional cases had previously been categorized as probable IA (n = 12), possible IFI (n = 10), and no IA (n = 1); they all repeatedly tested positive before death (367 of 683 sera samples; 54%). Autopsy also revealed pulmonary tissue invasion by hyphae in 3 patients who constantly tested negative (1 probable IA and 2 possible IFI episodes). However, fungal cultures from autopsy specimens remained negative in the 2 possible cases and yieldedC krusei in the probable case. Although histopathology is more sensitive than culture for diagnosing fungal infection, it does not allow unequivocal confirmation of the diagnosis of an invasive Aspergillus infection because several other fungi may reveal similar microscopic appearance.19 Given the species-specificity of the ELISA, we therefore considered these cases as proven IFI, not proven IA. Signs of invasive mycosis were absent in the remaining 47 autopsies, although galactomannan had been detected in 4 of these episodes (3 probable IA and 1 possible IFI episode) in 5 of 17, 5 of 7, 7 of 10, and 16 of 20 samples, respectively.
Classification of study episodes before and after postmortem examination.
Based on the results of this subgroup and considering the 3 cases of proven IFI as “unconfirmed” IA, the sensitivity and specificity of serial screening for GM was 91% and 91.5%, respectively; the positive predictive value equaled 88.2%; the negative predictive value, 93.5%; and the efficacy, 91.2%. The lower specificity and positive predictive value resulted from the 4 positive episodes without evidence of fungal invasion on autopsy. However, these latter patients had received prolonged antifungal therapy, including granulocyte transfusions in 2 instances, followed by a gradual decline and clearance of antigenemia before autopsy. The proven case in the premortem “no IA” group was a steroid-treated transplantation recipient diagnosed withToxoplasma encephalitis, Klebsiella pneumoniaebacteremia, and C albicans peritonitis. Autopsy demonstrated pulmonary toxoplasmosis and also involvement of filamentous fungi, later confirmed to be A fumigatus. Excluding the 3 patients with proven IFI, the results were a sensitivity of 100%; specificity, 91.5%; positive predictive value, 88.2%; and negative predictive value, 100%.
Final analysis of the entire study population
The incorporation of these postmortem findings should decrease the level of diagnostic uncertainty and allow more accurate analysis of the entire population under study (Figure 1). At the end, 30 episodes could be classified as proven IA (from 1.9% premortem to 8.3% postmortem); 3 episodes, proven IFI (0% to 0.8%, respectively); 9, probable IA (8.3% to 2.5%, respectively); 2 episodes, probable IFI (0.5% to unchanged, respectively); 54 episodes, probable IFI (26.8% to 14.9%, respectively); and 264 episodes, without evidence of IA (62.4% to 72.9%, respectively). The number of treatment episodes with confirmed disease status (proven IA and no IA) increased from 233 (64.4%) to 294 (81.2%) episodes. With the exception of 2 episodes that were caused byA flavus, all Aspergillus isolates were identified as A fumigatus.
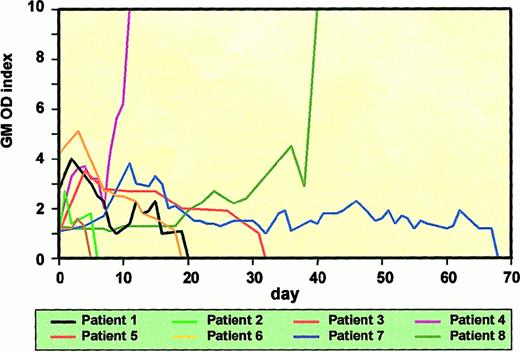
The ELISA results are summarized in Table3. All 30 (100%) patients with proven IA tested positive for GM (median, 9.5 positive sera samples per episode). There were no “false-negative” episodes detected. However, in one transplantation recipient with paralumbar Aspergillusabscess, GM was not detected until the development of pulmonary infiltrates. The detection of GM preceded the initiation of broad-spectrum antifungals (criteria outlined in “Patients, materials, and methods”) in 18 of 29 treated patients (median, 6 days; range, 1-27 days) and coincided with the start of antifungal therapy in 4 patients. In 7 (24%) patients, there was a positive ELISA after the start of antifungal therapy (median, 5.5 days; range, 1-58 days). In 27 (90%) patients, GM was detected before the sampling day of the first positive culture (mean, 10.5 days; range, 1-100 days). Galactomannanemia preceded the development of new pulmonary infiltrates on chest x-ray (nodules, diffuse shadows, or cavities) in 19 (68%) of 28 evaluable patients (median, 5 days; range, 1-27 days) and coincided in 4 patients. Only 5 (18%) patients developed infiltrates before the detection of positive sera (median, 1 day; range, 1-4 days). GM detection preceded the establishment of definite diagnosis in 29 (96%) of 30 proven cases by a median of 17 days (range, 2-110 days). Transient positivity was not observed. All 24 patients with persisting or raising titers of GM eventually died of or with IA. Six patients cleared their GM (optical density index was less than 0.9 on at least 3 consecutive sera samples): 4 of the patients survived the infection, including a patient with cerebral involvement; the 2 remaining patients died of fatal hemoptysis and bacterial pneumonia, respectively. Quantification of some of the GM ELISA results is presented in Figure 2.
Distribution of serum samples after incorporating autopsy findings in the initial stratification
| . | Proven IA . | Probable IA . | Probable IFI . | Possible IFI . | No IA . | Total . |
|---|---|---|---|---|---|---|
| Episodes, no. (%) | 30 (8.3) | 9 (2.5) | 2 (0.5) | 54 (14.9) | 264 (72.9) | 362 (100) |
| Samples, no. | 676 | 149 | 12 | 668 | 2748 | 4300 |
| Per episode | ||||||
| Mean | 22.5 | 16.5 | NA | 12.3 | 10.4 | 11.8 |
| Median | 17.5 | 18 | NA | 10 | 9 | 9 |
| Range | 5-65 | 7-24 | 4-8 | 5-66 | 3-56 | 2-66 |
| Pos episodes, no. (%) | 30 (100) | 5 (55.5) | 0 | 4 (7.4) | 5 (1.9) | 44 (12.1) |
| Pos samples, no. (%) | 365 (54) | 22 (14.7) | 0 | 75 (11.2) | 40 (1.4) | 502 (11.7) |
| Per pos episodes, no. | ||||||
| Mean | 12.1 | 4.4 | NA | 18.7 | 8 | 11.2 |
| Median | 9.5 | 3 | NA | 10 | 7 | 9.5 |
| Range | 2-54 | 2-8 | NA | 2-52 | 5-16 | 2-54 |
| Single sample with Pos episodes, no. (%) | 0 | 1 | 0 | 2 | 9 | 12 (3.3) |
| . | Proven IA . | Probable IA . | Probable IFI . | Possible IFI . | No IA . | Total . |
|---|---|---|---|---|---|---|
| Episodes, no. (%) | 30 (8.3) | 9 (2.5) | 2 (0.5) | 54 (14.9) | 264 (72.9) | 362 (100) |
| Samples, no. | 676 | 149 | 12 | 668 | 2748 | 4300 |
| Per episode | ||||||
| Mean | 22.5 | 16.5 | NA | 12.3 | 10.4 | 11.8 |
| Median | 17.5 | 18 | NA | 10 | 9 | 9 |
| Range | 5-65 | 7-24 | 4-8 | 5-66 | 3-56 | 2-66 |
| Pos episodes, no. (%) | 30 (100) | 5 (55.5) | 0 | 4 (7.4) | 5 (1.9) | 44 (12.1) |
| Pos samples, no. (%) | 365 (54) | 22 (14.7) | 0 | 75 (11.2) | 40 (1.4) | 502 (11.7) |
| Per pos episodes, no. | ||||||
| Mean | 12.1 | 4.4 | NA | 18.7 | 8 | 11.2 |
| Median | 9.5 | 3 | NA | 10 | 7 | 9.5 |
| Range | 2-54 | 2-8 | NA | 2-52 | 5-16 | 2-54 |
| Single sample with Pos episodes, no. (%) | 0 | 1 | 0 | 2 | 9 | 12 (3.3) |
For abbreviations, see Table 1.
Quantification of GM ELISA results.
Time course of antigenemia in 8 selected patients. Six patients, including 4 survivors, cleared GM. Patients nos. 4 and 8 represent a larger group of patients with rising antigen titers; they all died of or with IA.
Quantification of GM ELISA results.
Time course of antigenemia in 8 selected patients. Six patients, including 4 survivors, cleared GM. Patients nos. 4 and 8 represent a larger group of patients with rising antigen titers; they all died of or with IA.
In contrast, none of 3 patients with proven IFI tested positive. Of the remaining 9 episodes of probable IA, 4 (44.4%) episodes were constantly negative. All 4 antigen-negative patients survived and were discharged from hospital. The number of antigen-positive episodes of possible IFI decreased from 15.5% to 7.4% (4 of 54 episodes). Two of these latter patients (with 12 of 15 and 52 of 66 positive samples) died of proven IA during subsequent allogeneic transplantation. The third episode (3 of 60 sera episodes) occurred in a febrile transplantation recipient with multiple pulmonary nodules; open lung biopsy demonstrated signs of bronchiolitis obliterans with organizing pneumonia without fungal involvement. Both fever and antigenemia disappeared after withdrawal of the Hickman intravenous line, and unexpectedly, culture of the catheter tip yieldedPenicillium species. In the fourth patient (8 of 10 positive sera episodes), permission for autopsy was not obtained. Following postmortem examination, the number of “false-positive” episodes in the “no IA group” doubled from 0.9% to 1.9% (5 of 264 episodes); 40 (1.4%) sera samples tested positive. In these 5 patients, serum antigenemia declined gradually over time and became undetectable during antifungal therapy, which suggests a possible cure. There was no correlation between ELISA titers and true-positive versus “false-positive” episodes.
Based on this classification, the sensitivity and specificity of serial GM monitoring according to estimate B was 89.7% and 98.1%, respectively. The positive predictive value of the test was 87.5%; negative predictive value, 98.4%; and efficacy, 97%. Results from alternative estimates based on higher (estimate from method A) and lower (estimates from methods C-D) levels of certainty are depicted in Table 2.
Table 4 represents the value of antigenemia according to the number of positive sera samples in different groups of IA. The presence of 2 (or more) consecutive positive sera samples increased the specificity and positive predictive value of this assay in all subgroups, with a minimal decrease in sensitivity from 92.3% to 89.7%. Given these findings, the absolute value of a single sample appears less informative than the demonstration of a rising or decreasing titer in antigenemia.
Galactomannan antigenemia in patient with invasive aspergillosis according to number of serum samples
| . | 1 positive sample, % . | 2 or more positive samples, % . | ||||||
|---|---|---|---|---|---|---|---|---|
| Sens . | Spec . | PPV . | NPV . | Sens . | Spec . | PPV . | NPV . | |
| Proven IA | 100 | 94.6 | 68.1 | 100 | 100 | 98.1 | 85.7 | 100 |
| Probable IA | 66.6 | 94.6 | 30 | 98.8 | 55.5 | 98.1 | 50 | 98.4 |
| Proven + probable IA | 92.3 | 94.6 | 72 | 98.8 | 89.7 | 98.1 | 87.5 | 98.4 |
| Possible IFI | 11.1 | 94.6 | 30 | 83.8 | 7.4 | 98.1 | 44.4 | 83.8 |
| . | 1 positive sample, % . | 2 or more positive samples, % . | ||||||
|---|---|---|---|---|---|---|---|---|
| Sens . | Spec . | PPV . | NPV . | Sens . | Spec . | PPV . | NPV . | |
| Proven IA | 100 | 94.6 | 68.1 | 100 | 100 | 98.1 | 85.7 | 100 |
| Probable IA | 66.6 | 94.6 | 30 | 98.8 | 55.5 | 98.1 | 50 | 98.4 |
| Proven + probable IA | 92.3 | 94.6 | 72 | 98.8 | 89.7 | 98.1 | 87.5 | 98.4 |
| Possible IFI | 11.1 | 94.6 | 30 | 83.8 | 7.4 | 98.1 | 44.4 | 83.8 |
Proven IFI and probable IFI were excluded from the analysis.
Discussion
Diagnosing IA in hematology patients remains frustratingly difficult, and absolute certainty is seldom attained before death due to the nature of the reference standard. Insisting on irrefutable evidence of deep tissue infection before any therapeutic intervention exposes many patients to invasive, potentially life-threatening tests and results in an unacceptable delay of potentially lifesaving therapy. On the other hand, a substantial diagnostic certainty is still required to avoid over-treatment.21 In an attempt to increase and standardize that level of certainty for clinical research, the EORTC/MSG has recently generated sets of criteria for case classification.7 The specificity of these definitions, which initially depend on host and clinical characteristics, can be improved by incorporating culture-based and nonculture-based microbiological criteria.22 This is the first prospective study that analyses the diagnostic contribution and accuracy of the GM ELISA test while using these newly proposed case definitions.
Although serial sampling was necessary to maximize detection, we could demonstrate the presence of antigenemia in all patients with proven IA. Conversely, GM antigenemia was expressed in less than 2% of study episodes that carried a high risk but demonstrated no evidence of IA. The excellent sensitivity and absence of false-negative test results in proven cases contrasts with previous observations12-17; however, differences in case definitions and in study population may account for this discrepancy. Less stringent definitions will not discriminate between invasive disease and colonization, whereas differences in degree of immunodeficiency and underlying host factors may have significant impact on the detection of circulating antigens, as demonstrated for the detection of enolase in invasive candidiasis.23
In the present analysis, the population under study, including all negative cases, was confined to a relevant group of adult patients receiving acute leukemia–based regimens or undergoing allogeneic transplantation. Previous evaluations may have been hampered by the enrollment of lower risk categories (eg, low-grade lymphoma or myeloma) and/or by using a heterogeneous population with a low likelihood of developing IA as negative controls (eg, autologous transplantation recipients). Nevertheless, truly false-negative results of the assay have been reported, eg, in a nonneutropenic child with chronic granulomatous disease and proven Aspergillus abscess, comparable with the pattern seen in one of our transplantation patients.24 We can only speculate on plausible explanations for these false-negative measurements such as limited angio-invasion or encapsulation of the process, use of prophylaxis, or low-level release of GM. Infrequent sampling appears to be an unlikely cause because transient or alternating positivity was never observed in our proven cases. In this analysis we observed 3 cases of ELISA−, but with biopsy-proven invasive mycosis. Considering these cases as “false-negative proven IA” reduced the sensitivity and NPV from 100% to 91% and 93.5%. Unfortunately, in the absence of culture identification, histopathology alone cannot discriminate between the Aspergillus species and other fungal pathogens with similar histologic appearance such as theScedosporium or Fusarium species.19Finally, although A fumigatus and A flavus were the only identified pathogens, it seems unlikely that this assay would fail to detect any other Aspergillus species.25
The improved sensitivity of the sandwich ELISA (compared with the latex-agglutination assay) might be counterbalanced by a loss of specificity due to a greater occurrence of false-positive results. Considering only proven cases and control episodes after incorporating autopsy findings, we found a specificity of 98%, but a false-positivity rate of 14%. The occurrence of multiple positive assays and single-positive results frequently coincided with the period of mucositis following cytoreductive therapy; it has been postulated that translocation of dietary GM molecules via damaged gut mucosa could result in false-positive assays.26 Alternative explanations, such as cross-reactivity with blood products, cytotoxic agents, or bacterial exo-antigens, have not yet been convincingly demonstrated.12,25 With the exception of thePenicillium species, cross-reactivity with other fungal pathogens has not been observed.25 However, the true nature and significance of this false-positivity remains unknown; although necropsy failed to reveal IA in 4 of the cases that had been identified as probable, GM had been progressively cleared before autopsy. Knowing that GM correlates with the extent of fungal burden11 and seems to correspond to clinical response,27 this evolution perhaps reflects antemortem cure.
Because the currently available noninvasive diagnostic tools are limited, and in view of the high mortality rate of established fungal infection, empirical therapy is widely used in high-risk immunocompromised patients.28 However, this concept can be challenged for several reasons: not all patients carry the same risk, toxicity and cost may be high, and local epidemiology and resistance patterns may be altered. The real value of any new noninvasive diagnostic test with regard to IA will largely depend on its potential to discriminate “unproven” IA from alternative etiologies with similar clinical presentation (ie, cases normally classified as probable or possible).
In this series, autopsy performed in 50% (n = 64) of all episodes of probable IA and possible IFI revealed 22 additional cases of proven IA; all cases were identified by GM detection before death. Of the remaining 42 episodes without evidence of fungal involvement on autopsy, 38 episodes tested negative, whereas 4 episodes that tested positive were negative at the time of autopsy. In spite of the continuing uncertainty about the significance of these “false-positive” reactions, a sensitivity and negative predictive value of 100% makes this assay suitable for clinical decision making. A confirmed positive test result (especially raising titers) in a relevant clinical setting supports and should encourage clinicians to start (or change) antifungal therapy, even in the absence of other criteria that would normally warrant therapy for mold infections (see “Patients, materials, and methods”) or in the presence of alternative explanations (eg, our transplantation recipient with toxoplasmosis). Besides, in our series GM detection coincided with or preceded the initiation of antifungal therapy in 76% of proven cases of IA. And conversely, persistently negative results do not support the tentative diagnosis of IA, even in the presence of a positive isolate. Also demonstrated in our series, 8 of 30 probable cases (positive isolate) had no evidence of IA following pathological examination; negative tests should convince treating physicians to look for alternative explanations including non-Aspergillus mold infections.
A strategy simply based on antigenemia would reduce the use of broad-spectrum antifungals to 12% compared to 43% antifungal use on the basis of classical clinical criteria. However, the species-specificity of the assay cannot exclude the involvement of fungal pathogens with similar clinical presentation, including some of the emerging species such as Alternaria,Fusarium, and Mucorales, and does not provide information about coinfections (eg, cytomegalovirus orCandida). In view of the emergence of these non-Aspergillus mold infections, withholding antifungal therapy based on negative ELISA results is not yet advisable, although the probability of incorrect withholding of therapy appears to be very low.29 At present, we believe that GM detection provides supportive evidence of the Aspergillus etiology of an infectious process in the right context. It does not replace other diagnostic tools (most importantly CT imaging30) in the work-up of unexplained fever and in the exploration of invasive fungal infections in general in high-risk hematology patients.
Based on these results, a confirmed positive ELISA result on serum should indeed be considered a useful diagnostic criterion. By incorporating GM as a microbiological criterion in the EORTC/ MSG proposal, 44 instead of 30 episodes of probable IA could be identified; 68% of which proved to be IA. Unfortunately, the true disease status of the remaining episodes could not be adequately determined. Therefore, detection of ELISA positivity, even when combined with suggestive CT imaging, does not replace tissue sampling for histology and culture as a gold-reference diagnostic standard.
We do realize that the study design shares some of the shortcomings and limitations that are commonly reported in evaluations of new diagnostic tests. Recently, Lijmer et al31 stated that a prospective comparison of the test under study and the reference test, performed in a consecutive series of patients from a relevant clinical population, remains the optimal design. Although we have prospectively included representative patients in a consecutive way, many negative and some of the positive test results have not, for obvious ethical reasons, been verified by the invasive reference test. Such a design inevitably fails to identify all false test results, both negative and positive ones, and can lead to an inadequate estimation of diagnostic accuracy. Therefore, patients may still remain incorrectly classified on the basis of these new case definitions.32 In addition, this validation may not apply to other risk categories of IA, such as solid organ transplantation recipients and acquired immunodeficiency syndrome (AIDS) patients. Finally, the study excluded pediatric patients, a subgroup known to present with a high incidence of false-positive test results.33
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Johan Maertens, Department of Hematology, University Hospital Gasthuisberg, Herestraat 49, B-3000 Leuven, Belgium; e-mail: johan.maertens@uz.kuleuven.ac.be.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal