A form of autosomal dominant macrothrombocytopenia is characterized by mild or no clinical symptoms, normal platelet function, and normal megakaryocyte count. Because this condition has so far received little attention, patients are subject to misdiagnosis and inappropriate therapy. To identify the molecular basis of this disease, 12 Italian families were studied by linkage analysis and mutation screening. Flow cytometry evaluations of platelet membrane glycoproteins (GPs) were also performed. Linkage analysis in 2 large families localized the gene to chromosome 17p, in an interval containing an excellent candidate, the GPIbα gene. GPIbα, together with other proteins, constitutes the plasma von Willebrand factor (vWF) receptor, which is altered in Bernard-Soulier syndrome (BSS). In 6 of 12 families, a heterozygous Ala156Val missense substitution was identified. Platelet membrane GP studies were performed in 10 patients. Eight were distinguished by a reduction of GPs comparable to that found in a BSS heterozygous condition, whereas the other 2, without the Ala156Val mutation, had a normal content of platelet GPs. In conclusion, the current study provides evidence that most (10 of 12) patients with an original diagnosis of autosomal dominant macrothrombocytopenia shared clinical and molecular features with the heterozygous BSS phenotype. The remaining 2 affected subjects represented patients with “true” autosomal dominant macrothrombocytopenia; the GPIb/IX/V complex was normally distributed on the surface of their platelets. Thus, the diagnosis of heterozygous BSS must always be suspected in patients with inherited thrombocytopenia and platelet macrocytosis.
Introduction
Inherited thrombocytopenias were, in the past, considered exceedingly rare. Today the widespread diffusion of electronic cell counters has rendered the identification of these conditions more common, and many asymptomatic patients are discovered during routine blood analysis, often in adulthood. The hereditary nature of the illness may be missed, and patients are subject to misdiagnosis of autoimmune thrombocytopenic purpura and inappropriate therapy, such as steroid treatment and splenectomy.1-3
Different diagnostic parameters have been used to classify these disorders, including degree of bleeding, inheritance trait, platelet function and kinetics, and concomitant clinical abnormalities.4 The simplest classification relies on mean platelet volume (MPV), which separates macrothrombocytopenia from thrombocytopenia with normal MPV. Despite the lack of epidemiologic data, clinical practice suggests that the former are more frequent than the latter.
Patients with increased platelet size have been reported since the nineteenth century.5 In the first half of twentieth century, authors reported the genetic basis of some platelet macrocytoses associated with thrombocytopenia and described 2 distinct entities, Bernard-Soulier syndrome (BSS)6 and May-Hegglin anomaly (MHA).7,8 Over the following 5 decades, the molecular basis of BSS9 and MHA was clarified.10
Moreover, other forms of inherited macrothrombocytopenia have been described—gray platelet syndrome, platelet-type von Willebrand disease, Montreal platelet syndrome, macrothrombocytopenia with mitral valve insufficiency, familial macrothrombocytopenia with glycoprotein (GP) IV abnormality, Epstein, Fechtner, and Sebastian syndromes—all with their typical distinguishing features.11 However, these well-defined forms are rare, and most hereditary macrothrombocytopenias remain poorly characterized despite several case series. In 1975, von Behrens12 examined platelet count and MPV in 145 apparently healthy subjects from Italy and the Balkan peninsula, many of whom were affected by an undefined form of macrothrombocytopenia. Because this abnormality was not detected in control subjects from northern Europe, the condition was named Mediterranean macrothrombocytopenia. Subsequently, Najean et al2 retrospectively analyzed a series of patients with thrombocytopenia who underwent platelet kinetic studies and isolated 54 patients with chronic macrothrombocytopenia of unknown origin. This condition had an autosomal dominant transmission and was characterized by mild or no clinical symptoms, normal bone marrow megakaryocytosis and platelet survival, and normal in vitro platelet function. They named this condition genetic thrombocytopenia with autosomal dominant transmission. More recently, Fabris et al13 described 47 patients from 13 families with similar features who were reported as having chronic isolated hereditary macrothrombocytopenia.
The molecular mechanisms that cause hereditary giant platelet disorders are not known in detail except those for BSS. In this case, giant platelets are caused by defects in the platelet glycoprotein (GP) Ib/IX/V complex, a platelet receptor for von Willebrand factor (vWF) and the major membrane GP system that interacts with the platelet cytoskeleton.9 Patients with BSS were found to be homozygotes or compound heterozygotes for mutations in the GPIbα, GPIbβ, or GPIX genes, 3 of the 4 distinct gene products of the GPIb/IX/V complex. Only one family characterized by mild clinical symptoms was described as having autosomal dominant BSS.14
To identify the molecular defect of autosomal dominant hereditary macrothrombocytopenia, we studied 12 consecutive patients and their relatives. By evaluating platelet surface GPs and using molecular biology techniques, we concluded that macrothrombocytopenia was compatible with a heterozygous status of BSS in 10 patients. Of particular interest, a missense mutation of the GPIbα gene was responsible for the illness in 6 families.
Patients, materials, and methods
Clinical findings and laboratory evaluation of patients
Twelve consecutive patients (TP-1 to TP-12) who had hereditary macrothrombocytopenia that was transmitted through an autosomal dominant mechanism but who received no definitive diagnoses were enrolled in this study from January 1999 to March 2000 (Table1). The patients came from 12 unrelated families and required medical attention because of a mild bleeding diathesis, incidental discovery of thrombocytopenia, and platelet macrocytosis. Investigated subjects gave informed consent to the studies, which were carried out in accordance with the Principles of the Declaration of Helsinki.
Main features of the patients studied
| Family (patients in the family) . | Platelet count × 109/L mean (range) . | MPV fL mean (range) . | Clinics*(symptomatic patients) . | GPIb/IX/V complex† . | Bolzano mutation . |
|---|---|---|---|---|---|
| TP-1 (8) | 69 | 14.0 | P, E, M (4) | Defective | Yes |
| Figure 1 | (50-82) | (13.7-14.5) | |||
| TP-2 (9) | 88 | 12.9 | P, E (6) | Defective | Yes |
| Figure 1 | (21-127) | (12.2-13.6) | |||
| TP-3 (2) | 94 | 11.7 | E (2) | Defective | Yes |
| (77-111) | (11.5-11.9) | ||||
| TP-4 (2) | 74 | 12.7 | M (1) | nd | Yes |
| (68-80) | (12.3-13.2) | ||||
| TP-5 (3) | 49 | 16.1 | P (1) | Normal | No |
| (22-82) | (14.0-17.2) | ||||
| TP-6 (7) | 140 | 11.6 | No | Defective | No |
| (100-178) | (11.2-12.3) | ||||
| TP-7 (3) | 90 | 11.4 | E (2) | Normal | No |
| (68-135) | (10.4-11.9) | ||||
| TP-8 (3) | 58 | 13.9 | E, M (3) | Defective | Yes |
| (42-87) | (12.1-14.3) | ||||
| TP-9 (2) | 45 | 12.9 | P, E (2) | nd | Yes |
| (37-53) | (12.7-13.1) | ||||
| TP-10 (3) | 119 | 11.4 | No | Defective | No |
| (109-133) | (10.7-11.9) | ||||
| TP-11 (2) | 143 | 14.1 | No | Defective | No |
| (128-158) | (13.8-14.5) | ||||
| TP-12 (2) | 120 | 11.5 | E (1) | Defective | No |
| (116-125) | (11.2-11.8) |
| Family (patients in the family) . | Platelet count × 109/L mean (range) . | MPV fL mean (range) . | Clinics*(symptomatic patients) . | GPIb/IX/V complex† . | Bolzano mutation . |
|---|---|---|---|---|---|
| TP-1 (8) | 69 | 14.0 | P, E, M (4) | Defective | Yes |
| Figure 1 | (50-82) | (13.7-14.5) | |||
| TP-2 (9) | 88 | 12.9 | P, E (6) | Defective | Yes |
| Figure 1 | (21-127) | (12.2-13.6) | |||
| TP-3 (2) | 94 | 11.7 | E (2) | Defective | Yes |
| (77-111) | (11.5-11.9) | ||||
| TP-4 (2) | 74 | 12.7 | M (1) | nd | Yes |
| (68-80) | (12.3-13.2) | ||||
| TP-5 (3) | 49 | 16.1 | P (1) | Normal | No |
| (22-82) | (14.0-17.2) | ||||
| TP-6 (7) | 140 | 11.6 | No | Defective | No |
| (100-178) | (11.2-12.3) | ||||
| TP-7 (3) | 90 | 11.4 | E (2) | Normal | No |
| (68-135) | (10.4-11.9) | ||||
| TP-8 (3) | 58 | 13.9 | E, M (3) | Defective | Yes |
| (42-87) | (12.1-14.3) | ||||
| TP-9 (2) | 45 | 12.9 | P, E (2) | nd | Yes |
| (37-53) | (12.7-13.1) | ||||
| TP-10 (3) | 119 | 11.4 | No | Defective | No |
| (109-133) | (10.7-11.9) | ||||
| TP-11 (2) | 143 | 14.1 | No | Defective | No |
| (128-158) | (13.8-14.5) | ||||
| TP-12 (2) | 120 | 11.5 | E (1) | Defective | No |
| (116-125) | (11.2-11.8) |
P, petechias; E, epistaxis; M, mucosal bleeding; No, no clinical findings.
Defective means a reduction detected by flow cytometry; nd, nondetermined.
Platelet aggregation
For the study of in vitro platelet function, blood was collected in 3.8% (wt/vol) sodium citrate (blood–anticoagulant ratio 9:1). To minimize the loss of denser platelets, platelet-rich plasma (PRP) was obtained by sedimentation rate of blood at 1g for 20 to 30 minutes; platelet-poor plasma (PPP) was obtained by centrifugation of the remaining blood at 4500g for 20 minutes. The platelet count of PRP was adjusted to 150 × 109/L with PPP. Platelet aggregation was evaluated by the densitometric method of Born15 after stimulation with collagen (4 and 20 μg) (Mascia Brunelli, Milan, Italy), adenosine diphosphate (ADP, 5 and 20 μM), and ristocetin (1.5 and 3.0 mg/mL), both from Sigma Chemical, St Louis, MO, and the maximal extent of aggregation was recorded. Values obtained in 20 healthy subjects were used to identify the normal range.
Genome-wide search and linkage analysis of candidate loci
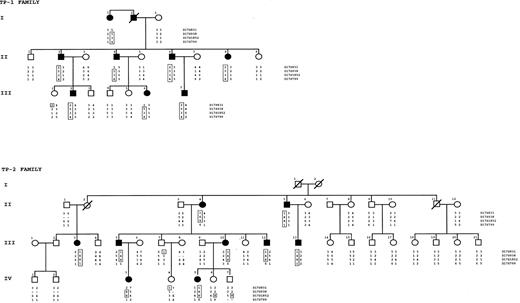
High-molecular–mass DNA was extracted from peripheral blood leukocytes in an automatic DNA extractor, according to the manufacturer's recommendations (ABI 341 GenePure; Applied Biosystems, Foster City, CA). For genome-wide search, genomic DNA samples from patients from 2 large families, TP-1 and TP-2, were used for linkage analysis (Figure 1). Genotyping was performed using an ABI Prism 377 DNA sequencer (ABI 377A) and the Linkage Mapping Set Version 2 (both Applied Biosystems). This set comprises 400 fluorescently labeled markers that define a 10-cM resolution human map. Prescreening was performed using only the 8 affected DNA samples from family TP-1 (Figure 1). Microsatellite mapping excluded most of the genome and identified a few loci at which patients had the same allele. The markers of candidate regions were then tested in all pedigree members, including those of family TP-2. Classic 2-point LOD score analysis was conducted in these pedigrees under the assumption of autosomal dominant inheritance and complete genetic penetrance. We used the MLink program included in the Linkage package (Applied Biosystems) to perform linkage analysis.16
Family pedigrees with haplotype reconstruction for informative markers on 17p.
The at-risk haplotype is boxed.
Family pedigrees with haplotype reconstruction for informative markers on 17p.
The at-risk haplotype is boxed.
Molecular analysis of the GPIbα gene
The GPIbα gene (GenBank accession numbers NM000173 andAC004771) was completely sequenced in one patient from families TP-1 and TP-2. The coding region, the untranslated exon 1, the donor and acceptor splicing sites,17 and the promoter18were amplified using the following oligonucleotide sets: 1F (5′-TGGAGAGGTTTTTAAAAGATG-3′) and 2R (5′-ACCTTGCTTCCATACGTAGAC-3′); 3F (5′-CCCCTGGTTATGCAACTGTG-3′) and 3R (5′-TGGATGCAAGGAGGAGGGCAT-3′); 4F (5′-GATTACTACCCAGAAGAGGACA-3′) and 5R (5′-CACAGGCTCTTCTCTCAAGG-3′). To analyze the region containing the VNTR polymorphism better, two new primers were synthesized, 7F (5′-ACACTTCACATGGAATCCAT-3′) and 7R (5′-GGATTCTAAGAGTGATACGGGT-3′). To separate the alleles, the polymerase chain reaction (PCR) product was electrophoresed on 3% agarose gel before sequencing.
PCR was performed in 50 μL containing 50 ng DNA, 15 pmol each primer, 2.5 mM MgCl2, 10 mM Tris-HCl (pH 7.5), 50 mM KCl, 0.01% Tween-20, 0.01% gelatin, 0.01% NP40, and 2 U Taq polymerase. Initial denaturation was 5 minutes at 94°C followed by amplification for 30 cycles with denaturation at 94°C for 30 seconds, annealing at 60°C for 45 seconds, and extension at 72°C for 45 seconds. After electrophoresis, PCR products were isolated and purified by GFX DNA and a Gel Band Purification Kit (Amersham, Pharmacia, Biotech, Buckinghamshire, England). Sequence analysis was performed using a fluorescence-labeled dideoxy-nucleotide termination method (dye terminator) in the automated DNA sequencer ABI 377A (Applied Biosystems). Both PCR oligonucleotides and specific internal primers were used in the sequencing reactions.
To confirm and detect the Ala156Val substitution, genomic DNA was amplified using primers 2F (5′-CACCCCATCTGTGAGGTCTCC-3′) and 3R (see above). Because the substitution creates a restriction enzyme site forHpaI,19 PCR products were purified, digested with the specific enzyme, and electrophoresed on 2% agarose gel.
Flow cytometry
The expression of platelet membrane GPs was investigated by flow cytometry with the following monoclonal antibodies (mAbs): SZ21 (Immunotech SA, Marseille, France), which recognizes GPIIIa (CD61); AP2 (Immunotech), which recognizes GPIIb/IIIa (CD41/61); MB45 (CLB, Amsterdam, The Netherlands) and SZ2 (Immunotech) against GPIbα (CD42b); FMC25 (kindly provided by Zola H, Adelaide, Australia) and SZ1 (Immunotech), which recognizes GPIX (CD42a); SW16 (CLB) against GPV (CD42d); FA6-152 and Gi9 (both from Immunotech) against GPIV (CD36) and GPIa (CD49b), respectively. MO2 (Coulter, Miami, FL) was used as the negative control. Fluorescein isothiocyanate-conjugated goat anti-mouse IgG (GAM-FITC) was also purchased from Coulter.
PRP and PPP were obtained from blood anticoagulated with EDTA (final concentration, 10 mM) as described above for the platelet aggregation studies. PRP was adjusted to 50 × 109 platelets/L with PPP and fixed with 0.2% paraformaldehyde for 5 minutes at room temperature. Samples of 100 μL were incubated for 30 minutes at room temperature with 10 μL of the working solution of each mAb. Platelets were then washed with phosphate-buffered saline (PBS) containing 3.0 mM EDTA and 5% (wt/vol) bovine serum albumin (Sigma), resuspended in 100 μL PBS and incubated for 30 minutes at room temperature in the dark with an equal volume of GAM-FITC diluted 1/50 in the same buffer. After an additional wash, cells were resuspended in 300 μL PBS containing 0.3 mM EDTA and 0.1% bovine serum albumin and were analyzed with an Epics XL flow cytometer (Coulter). Platelets were identified on the basis of their size (linear forward scatter) and granularity (log side scatter), and an electronic gate was drawn around the platelet cloud to exclude residual erythrocytes and white cells. Ten thousand platelet events were collected, and the value of mean fluorescence, expressed in arbitrary units, was recorded. A sample from a healthy donor was run with the patients' samples (the same healthy donor was used for all patients).
Results
Clinical and laboratory data
Twelve patients from unrelated Italian families affected by hereditary macrothrombocytopenia were enrolled in this study (Table 1). Clinical and laboratory investigations of their available relatives identified 34 additional subjects with quantitative or qualitative platelet defects. All had larger than normal platelets, and a platelet count lower than 150 × 109/L was observed in 29 of 34 subjects. The disease was transmitted within the families as an autosomal dominant trait (Figure 1).
The male–female ratio of the 46 affected subjects was 1:1, and their ages ranged from 1 to 70 years. They had platelet counts from 22 to 178 × 109/L. The mean value of MPV was 12.6 fL, with a range from 10.4 to 17.2 fL. In vitro platelet aggregation after ADP, collagen, and ristocetin was normal in all investigated patients (data not shown). Twenty-two patients had mild to moderate bleeding tendency consisting of frequent episodes of epistaxis, gingival bleeding, menorrhagia, easy bruising, or prolonged bleeding after dental surgery. Six patients from 5 families (TP-1, TP-2, TP-4, TP-5, and TP-7) underwent bone marrow examination that showed normal numbers of megakaryocytes. In some patients, however, the analysis did reveal mild and aspecific dysmegakaryocytopoietic phenomena, such as increased fraction of precursor cells and reduced megakaryocytes that appeared to be producing platelets.
Genome-wide search
To localize the gene(s) responsible for hereditary macrothrombocytopenia, we considered 2 large pedigrees, TP-1 and TP-2, for the genome-wide search (Figure 1). Under the assumption of complete genetic penetrance, the gene was mapped to chromosome 17q. Marker D17S938 gave a maximum 2-point LOD score of 7.51 at recombination fraction of 0.00.
Haplotype analysis was performed in both families (Figure 1). In TP-1, a telomeric recombination was evident between D17S831 and D17S938 in subjects I-1, II-4, III-1, and III-6. In TP-2, 2 recombination events in 2 healthy subjects, IV-6 and IV-7, established the centromeric limit of the disease region between D17S938 and D17S1852. Therefore, the candidate region was defined in an interval of approximately 18 cM between microsatellites D17S831 and D17S1852. Among possible disease candidates, GPIbα, one of the gene responsible for BSS, was of particular interest.
Mutation analysis
The GPIbα gene was completely sequenced in 2 probands from each of the TP-1 and TP-2 families. The screening identified a heterozygous C > T transition at position 515 in both patients (data not shown). This mutation, previously reported as the Bolzano variant, causes an Ala156Val amino acid substitution and creates a restriction enzyme site for HpaI.19 Restriction analysis in all family members showed that only the affected subjects were heterozygous for the missense mutation, indicating a full concordance within families between the phenotypic expression and the protein variant. Restriction analysis in the remaining 10 families allowed us to identify the same variant in the heterozygous state in 4 of them. In conclusion, 6 of 12 affected families had the Bolzano variant. The change was absent in 50 unaffected, unrelated control subjects.
Flow cytometry
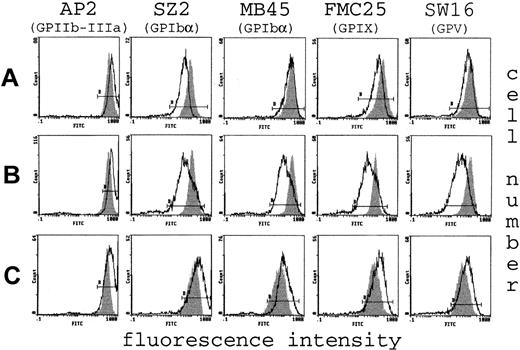
To investigate for possible defects of the platelet surface, the major membrane GPs were investigated in 10 of 12 patients and some of their relatives (the 2 unavailable patients were carriers of the Bolzano variant). The most abundant surface protein, GPIIb-IIIa, which acts as a receptor mainly for fibrinogen during platelet activation,20 was normal or increased in all subjects (Figure 2). Similar results were obtained for GPIa and GPIV, which are receptors for collagen21 and thrombospondin,22 respectively (data not shown). Conversely, the patterns of binding to the single components of the GPIb/IX/V complex differed between the affected subjects. Representative flow cytometry tracings are shown in Figure 2. Fluorescence was clearly reduced in 8 (including all those with the Bolzano variant) of 10 patients and in their respective affected relatives (Figure 2A-B). GPIb/IX/V complex was normal or increased in the other 2 patients (Figure 2C).
Flow cytometry analysis of platelet GPs.
Tracings of flow cytometry with mAbs against GPIIb/IIIa, GPIbα (SZ2 and MB45, the conformational-dependent and -independent mAb, respectively), GPIX, and GPV of 3 patients, each representative of a distinct group. At the genotype level patient A carries the Bolzano mutation, whereas patients B and C do not have the mutation. In each patient (white curve), the expression of GPIIb/IIIa on the platelet surface was increased compared to that of the healthy control (gray curve), as shown by shifting of the curves to the right. In contrast, single components of the GPIb/IX/V complex were variously reduced in patients A and B. These patients have a GP profile consistent with a heterozygous BSS phenotype. Of particular interest is the different fluorescence obtained with SZ2 and MB45 mAbs in the Bolzano patient, suggesting the presence of a conformationally changed GPIbα protein. Patient C shows a clear increase of all GP because of the larger volume of platelets and thus represents the “true” thrombocytopenia- affected patient.
Flow cytometry analysis of platelet GPs.
Tracings of flow cytometry with mAbs against GPIIb/IIIa, GPIbα (SZ2 and MB45, the conformational-dependent and -independent mAb, respectively), GPIX, and GPV of 3 patients, each representative of a distinct group. At the genotype level patient A carries the Bolzano mutation, whereas patients B and C do not have the mutation. In each patient (white curve), the expression of GPIIb/IIIa on the platelet surface was increased compared to that of the healthy control (gray curve), as shown by shifting of the curves to the right. In contrast, single components of the GPIb/IX/V complex were variously reduced in patients A and B. These patients have a GP profile consistent with a heterozygous BSS phenotype. Of particular interest is the different fluorescence obtained with SZ2 and MB45 mAbs in the Bolzano patient, suggesting the presence of a conformationally changed GPIbα protein. Patient C shows a clear increase of all GP because of the larger volume of platelets and thus represents the “true” thrombocytopenia- affected patient.
Among platelets with reduced fluorescence, alternative mAbs against GPIbα reacted differentially. In particular, binding of SZ2 was lower than that of MB45 in the 4 patients with Bolzano-type BSS (Figure 2A), whereas it was similarly reduced in the others (Figure 2B). In fact, SZ2, a conformational-dependent mAb, is specific for an N-terminal epitope not recognized when GPIbα undergoes a conformational change due to variants, including the Bolzano protein.23,24 In contrast, the binding of MB45 is less sensitive to these changes and better defines the surface content of GPIbα, even if conformationally altered.25 On the basis of these results, we confirm that Bolzano BSS platelets had both qualitative and quantitative defects in GPIbα.19 23 Therefore, the Bolzano protein is expressed, albeit with a reduced number of copies, on the platelet surface, but it is unable to bind vWF because of a conformational change.
On the basis of these studies, we grouped patients into 3 categories. The first group consisted of subjects with the Bolzano mutation and typical Bolzano platelet GP profile (8 patients from 4 families). The second group consisted of patients without the Bolzano variant but with a defective GPIb/IX/V complex characterized by a BSS profile of fluorescence (12 subjects from 4 families). The third group consisted of patients without the Bolzano mutation and a non-BSS profile defined by normal or increased binding of anti-GPIb/IX/V mAbs (3 subjects from 2 families).
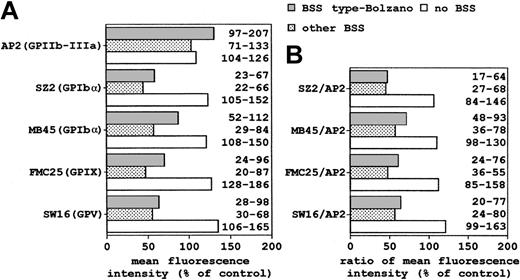
The mean of the fluorescence measurements obtained from patients of the 3 distinct groups was normalized to that from control subjects (Figure3A). The histogram showed a clear difference in vWF receptor content in heterozygous subjects with BSS and in thrombocytopenic patients without the BSS phenotype. The GPIb/IX/V defect appeared to be more significant when we normalized the data to the GPIIb/IIIa (Figure 3B).
Mean of flow cytometry results of platelet membrane GPs from patients of each group (see legend to Figure 2).
(A) Mean fluorescence intensity (arbitrary units) obtained with mAbs against GPIIb/IIIa, GPIbα, GPIX, and GPV was normalized to the mean from control platelets. (B) Mean values for single components of the GPIb/IX/V complex were normalized to those of GPIIb/IIIa and expressed as percentage of control platelets. Numbers on the right of histograms refer to the range of values.
Mean of flow cytometry results of platelet membrane GPs from patients of each group (see legend to Figure 2).
(A) Mean fluorescence intensity (arbitrary units) obtained with mAbs against GPIIb/IIIa, GPIbα, GPIX, and GPV was normalized to the mean from control platelets. (B) Mean values for single components of the GPIb/IX/V complex were normalized to those of GPIIb/IIIa and expressed as percentage of control platelets. Numbers on the right of histograms refer to the range of values.
Discussion
To understand the molecular basis of chronic macrothrombocytopenia transmitted as an autosomal dominant trait, we studied 12 consecutive Italian patients and their relatives. Family investigation identified another 34 subjects with either macrothrombocytopenia or platelet macrocytosis. Clinical manifestations were absent or mild; when they were present, common clinical findings were excessive ecchymoses, frequent epistaxis, gingival bleeding, prolonged menstrual periods, or prolonged bleeding after tooth extraction. No patients or their relatives reported serious surgical or obstetric hemorrhagic complication.
Genome-wide search in 2 large families localized a gene responsible for macrothrombocytopenia in a region of chromosome 17p containing the gene for GPIbα. This platelet GP forms a membrane complex with GPIbβ, GPIX, and GPV. Its amino terminus globular domain binds vWF, whereas its cytoplasmic tail contains a binding site for actin-binding protein.26 The former interaction initiates the arrest of platelets at sites of vascular injury, whereas the latter anchors membrane-associated cytoskeleton and is possibly involved in normal megakaryocytic maturation, demarcation membrane system production, and platelet size.11 A quantitative or qualitative defect of GPIb-IX-V complex, deriving from a homozygous defect of GPIbα, GPIbβ, or GPIX gene, is responsible for BSS, an illness characterized by a bleeding diathesis, thrombocytopenia, and large platelets that fail to interact with vWF.9
On the basis of linkage analysis, we hypothesized that autosomal dominant macrothrombocytopenia could be a mild form of BSS deriving from mutations of GPIbα. In 6 of 12 families, a heterozygous C > T transition at position 515 was identified. This mutation was previously reported as the Bolzano variant in 2 Italian patients with BSS patients—one was homozygous19 and the other was compound heterozygous.24 In the homozygous patient, the GPIb-IX-V complex was found to be expressed on the platelet surface, albeit with a reduced number of copies, but GPIbα was unable to bind vWF, probably because of an abnormal conformation of the expressed molecule deriving from the Ala156-Val substitution. It is unclear how this point mutation in the extracytoplasmic, leucine-rich repeat region leads to a defect in the mechanism of thrombopoiesis. However, it has been hypothesized that the altered conformation of this domain may be responsible for an abnormal interaction of the cytoplasmic tail of GPIbα with the cytoskeleton, resulting in abnormalities of platelet number and morphology.19 As expected, quantitative and qualitative defects of the GPIb/IX/V complex were also observed in our patients. Because of the heterozygous condition, the residual amount of normal GPIbα was sufficient to support vWF-mediated platelet aggregation after ristocetin stimulation (data not shown).
Among the patients without the Bolzano variant, flow cytometry studies identified a defect of the GPIb/IX/V complex in 4 of 6. In these 4 patients, the clinical findings, the hereditary pattern, and the reduction of the GPIb/IX/V complex were consistent with a heterozygous form of BSS. The screening for mutations of GPIbα, GPIbβ, GPIV, and GPV is in progress. However, preliminary results in some patients indicate the absence of variations, at least in the coding region of these genes, suggesting that other genes might be responsible for this disease (Savoia, personal communication).
In conclusion, 10 of 12 patients, originally diagnosed as affected by autosomal dominant macrothrombocytopenia, had a heterozygous BSS phenotype, as determined by platelet GP analysis. In 6 patients, the diagnosis was supported by the presence of a mutation in the GPIbα gene. The analysis excluded a molecular defect of the GPIb/IX/V complex in the remaining 2 patients, who therefore represent the only individuals affected by “true” autosomal dominant macrothrombocytopenia.
Because BSS is classically described as a recessive disorder, heterozygous subjects are expected to be asymptomatic. Although little attention has so far been devoted to these subjects, a careful search in the literature allowed us to collect data on 38 heterozygous relatives of BSS patients.27-37 Some were indeed asymptomatic, whereas others had from mild to moderate bleeding diatheses. Platelet count was reduced (lowest value 80 × 109/L) in 9 of 23 subjects, MPV was increased in 17 of 23, and a defect of GPIb-IX-V complex was observed in 25 of 36. Moreover, one white family has been reported in whom a mild form of BSS was transmitted with an autosomal dominant mechanism.14Literature data and our experience suggest that most patients with heterozygous BSS have a reduced platelet count, recognizable defect of GPIb-IX-V complex, or increased MPV—or any combination of these. In some patients these abnormalities are severe enough to elicit a bleeding diathesis.
There are 2 possible explanations for the high prevalence of the Bolzano variant in our sample. First, the mutation has such a detrimental action on platelet production that its effect is detectable in the heterozygous state. Second, this allele has a relatively high frequency in the Italian population. However, because qualitative and quantitative platelet abnormalities were previously described in heterozygous carriers for other BSS mutations27-37 and 3 of 6 Italian BSS chromosomes (all those characterized for mutations) carried the Bolzano variant,19,23 25 we favor the second hypothesis. On this basis, the results of our investigation are most relevant to Italian populations, and we cannot exclude that different results would be obtained in other countries.
Heterozygous patients with BSS patients, like homozygous patients,9 show a wide spectrum of clinical and platelet findings. The heterogeneity is likely due to several factors, such as the effect of different mutations and the genetic background, which influence variability not only among families but also among members of the same family. Therefore, the severity of BSS ranges from irrelevant to moderate and from moderate to severe in heterozygous and homozygous patients, respectively. The current classification of BSS as a recessive disorder hampers the diagnosis of those symptomatic, heterozygous patients whose illness is transmitted as a dominant trait.
Based on data reported concerning European, North American, and Japanese populations, the frequency of homozygous BSS has been estimated to be approximately 1 in 1 million,9 and, according to the Hardy-Weinberg law, the frequency of heterozygotes is 1 in 500. However, the proportion of heterozygous subjects with low platelet count, bleeding tendency, or both is unknown. In fact, the diagnosis is almost always missed when there are no homozygous BSS patients in the family. Therefore, a heterozygous BSS condition must be suspected in patients with autosomal dominant thrombocytopenia or platelet macrocytosis.
We thank Prof Mario Cazzola (Institute of Haematology, IRCCS San Matteo-University of Pavia) for referring to us one of the investigated families and Dr Maurizio Margaglione (IRCCS Hospital CSS) for providing some of the GPIbα primers.
Supported by grants from the Italian Ministry of Health (A.S., C.B., A.I.), MURST, and 60% University of Bari (A.I.). M.S. is the recipient of a fellowship from the Italian Foundation of Cancer Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Carlo Balduini, Medicina Interna, IRCCS San Matteo, Piazzale Golgi, 27100 Pavia, Italy; e-mail:c.balduini@smatteo.pv.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal