Thymic positive and negative selection of developing T lymphocytes confronts us with a paradox: How can a T-cell antigen receptor (TCR)-major histocompatibility complex (MHC)/peptide interaction in the former process lead to transduction of signals allowing for cell survival and in the latter induce programmed cell death or a hyporesponsive state known as anergy? One of the hypotheses put forward states that the outcome of a TCR-MHC/peptide interaction depends on the cell type presenting the selecting ligand to the developing thymocyte. Here we describe the development and lack of self-tolerance of CD8+ T lymphocytes in transgenic mice expressing MHC class I molecules in the thymus exclusively on cortical epithelial cells. Despite the absence of MHC class I expression on professional antigen-presenting cells, normal numbers of CD8+ cells were observed in the periphery. Upon specific activation, transgenic CD8+ T cells efficiently lysed syngeneic MHC class I+ targets in vitro and in vivo, indicating that thymic cortical epithelium (in contrast to medullary epithelium and antigen-presenting cells of hematopoietic origin) is incapable of tolerance induction. Thus, compartmentalization of the antigen-presenting cells involved in thymic positive selection and tolerance induction can (at least in part) explain the positive/negative selection paradox.
Introduction
Because of the random nature of T-cell receptor alpha (TCRα) and TCRβ gene rearrangements, the developing T-cell repertoire needs to undergo positive and negative selection processes. Thymic positive selection is thought to enrich for T cells expressing a clonotypic TCRαβ capable of interaction with self-major histocompatibility complex (MHC) heterodimers, although alternative views exist. Thymic negative selection eliminates or inactivates self-reactive T lymphocytes through induction of apoptosis and anergy. Although negative selection probably rids the developing T-cell repertoire of the majority of self-reactivity, peripheral tolerance mechanisms survey remaining self-specific T-cell clones. The resulting peripherally circulating T-cell repertoire is capable of recognition of foreign, but not self-peptides presented by self-MHC molecules.1-5
Because thymic positive and negative selection both involve TCR-MHC/peptide interactions, an important issue is what determines the outcome of such an interaction. Initially, studies have focused on the thymic stromal cell types involved in thymic selection. It has been known that MHC/peptide complexes expressed on cortical epithelium are capable of positive selection of conventional TCRαβ+thymocytes, whereas medullary epithelium as well as antigen-presenting cells (APCs) of hematopoietic origin cannot,6-8 although quantitatively minor exceptions to this rule have been reported.9,10 On the other hand, negative selection is known to be mediated primarily by APCs of hematopoietic origin (reviewed in Anderson et al1 and Laufer et al11).
Given the fact that thymic epithelium can induce positive selection while APCs cannot, it was conceivable that differences in the repertoire of presented peptides play a role in the outcome of a TCR-MHC/peptide interaction. Although peptide elution studies from epithelial cells and APCs have thus far failed to confirm this hypothesis,12 several lines of evidence indicate that thymic epithelial cells have specialized antigen presentation properties and may present different peptides from those presented by professional APC.13-18 Therefore, an interaction with MHC/peptide ligands expressed by thymic epithelial cells may allow for positive selection, and these cells would subsequently not be negatively selected because the same ligand is not expressed on cells capable of negative selection. However, in mice expressing MHC class II molecules loaded with a single peptide CD4+ T lymphocytes develop,19-23 demonstrating that possible differences in the repertoire of peptides presented by positively and negatively selecting cell types alone cannot fully explain the positive/negative selection paradox.
An alternative hypothesis on the mechanism of thymic positive/negative selection states that the intensity of TCR triggering determines the selection outcome. A high avidity interaction would lead to negative selection, a very weak signaling to death by neglect, whereas an intermediate TCR-MHC/peptide avidity would allow for positive selection. In fetal thymus organ cultures, it has been shown that the low level expression of agonist MHC/peptide ligand allows the survival of developing thymocytes, supporting the avidity model.24,25 However, when tested, the surviving mature T cells could not be activated by using the same ligand, suggesting that even low avidity interactions do not allow for functional positive selection.26,27 Recent data on the capacity of modified peptide ligands to differentially induce certain, but not other effector functions, suggested that these ligands may also play a role in positive selection (reviewed by Jameson et al28 and Germain and Stefanova29). Studies on TCR-transgenic fetal thymus organ cultures supplemented with modified peptides have suggested a role for these peptides in positive selection.30 However, the fact that altered peptide ligands can also inhibit positive selection,31 and an unexpected MHC/modified peptide ligand-induced mismatch between MHC class specificity and CD4/CD8 lineage outcome32 has complicated the interpretation of altered peptide studies. Finally, a differentiation-state dependent “interpretation” of TCR-mediated signals has been proposed to play a role in positive/negative selection.33 34
In an alternative approach to the paradox of positive/negative selection, we and others have initiated a dissection of the 2 selection processes.8 35-38 We have previously generated irradiation bone marrow chimeras in which radioresistant cells (which are required for positive selection) express MHC molecules, but APCs of hematopoietic origin do not. Although a 2- to 3-fold increase in the rate of development of mature thymocytes was observed, syngeneic reactivity of chimera-derived T cells was rather limited, implying that a significant level of negative selection was still operating in the absence of MHC expression on APCs. Here we report data on transgenic mice expressing MHC class I ligands exclusively on thymic cortical epithelial cells. Although positive selection seems to occur normally in these transgenic mice, no evidence for any negative selection could be observed using in vitro and in vivo assays. Therefore, positive and negative selection seem to be mediated by different cell types expressing self-MHC/peptide ligands.
Materials and methods
Mice
C57BL/6 and DBA/2 mice were obtained from Harlan Netherlands (Zeist, The Netherlands), whereas H-2Kb mutant B6.C-H2bm1- and β2-microglobulin (β2m)-deficient C57BL/6-β2m° mice39 were purchased from Jackson Laboratories (Bar Harbor, ME). The construct used to generate K14-β2m transgenic mice was made as follows: A 0.6-kilobase (kb)SalI/NsiI β2m complementary DNA (cDNA) fragment (generously provided by Dr D. Margulies, National Institutes of Health, Bethesda, MD) was inserted into the BamHI site of pG3Z.K14 (generously provided by Dr E. Fuchs, Howard Hughes Medical Institute, University of Chicago, IL), a vector containing the human keratin 14 (K14) promoter, βglobin intron, and the K14 poly A addition signal.40 The 3.6-kb transgene was excised withKpnI and HindIII and microinjected into (B6 β2m° x C57BL/6)F1 zygotes. Transgenic founders were backcrossed to C57BL/6-β2m° mice to obtain K14-β2m transgenic, endogenous β2m-deficient mice.
Immunohistology
Thymi were snap frozen in liquid nitrogen and subsequently placed in OCT (Miles, Elkhard, IN). Cryostat sections (5 μm) were air-dried on frosted microscope slides for at least 2 hours, placed in acetone at room temperature (RT) for 5 minutes, and rehydrated in phosphate-buffered saline (PBS). Blocking was performed for 15 minutes with antibody diluent (Dako, Glostrup, Denmark). Sections were then incubated with monoclonal antibody 6C3 (Pharmingen, San Diego, CA) at a saturating concentration for 30 minutes. After washing with PBS/0.01% Tween20 for 30 minutes, slides were incubated with FITC-labeled antirat IgG F(ab′)2 antibody fragment (Immunotech, Marseille, France) for 30 minutes and subsequently washed for 30 minutes. After blocking with mouse Ig (Pierce, Rockford, IL), slides were incubated with biotinylated H-2Kb and H-2Db specific antibody 28-8-6 (Pharmingen), washed, and finally incubated with rhodamine-conjugated streptavidine (Immunotech). After extensive washing, slides were mounted in Faramount (Dako) and analyzed by immunofluorescence microscopy.
Flow cytometric analysis
Single cell suspensions were prepared from thymus, spleen, and pooled mesenteric, brachial, inguinal, and axillary lymph nodes isolated from K14-β2m mice and β2m+/° littermates, as well as from bone marrow from hematopoietic chimeras. Cells were preincubated with 2.4G2 culture supernatant to block Fc receptors,41 washed, and subsequently incubated with antibody for 30 minutes in PBS/2% fetal calf serum (FCS). The following antibodies were used: PE-conjugated anti-CD62L and anti-B220 (Caltag, Burlingame, CA); FITC-conjugated anti-TCRβ and anti-H-2Kb, PE-conjugated anti-CD4, anti-CD44, anti-CD69, anti-Mac1, and anti-CD11c, and Cy-chrome–labeled anti-CD8 (all from Pharmingen). After 2 washes in PBS/2% FCS, cells were analyzed with a FACScan flow cytometer (Becton Dickinson, San Jose, CA).
Cytotoxic T lymphocyte assays
Unseparated splenocytes derived from K14-β2m transgenic or control (C57BL/6, B6.C-H2bm1 or DBA/2) mice were cultured for 6 days in the presence of T-cell–depleted (anti-Thy1 antibody AT8342 plus complement) irradiated (1000 Rad γ) splenocytes in the presence of 30 U/mL IL-2 (EL4 supernatant43). For lysis of RMA and EL-4 targets (C57BL/6 origin43,44), C57BL/6 APC-stimulated effector cells were used, whereas P815 (DBA/2 origin45) lysis assays were performed by using T cells stimulated with DBA/2 APCs. Targets (2000 cells per well) were labeled with 51Cr, extensively washed, and mixed with effector cells in duplicate at effector (viable cell)-to-target (E/T) ratios indicated. 51Cr release in the supernatant was measured 4 hours later. Specific lysis is51Cr release above background as a percentage of maximum (as determined by acid lysis of targets). For antibody-blocking experiments, effectors were preincubated (30 minutes) with the indicated concentrations of anti-CD4 (GK1.546) or anti-CD8 (H3547) antibodies, then mixed with51Cr-labeled targets (2000 cells per well) at an E/T ratio required for half maximum lysis.
Limiting dilution assays
Splenocytes from K14-β2m and control (C57BL/6 and B6.C-H2bm1) mice were preincubated with 2.4G2 supernatant,41 and subsequently doubly stained with PE-conjugated anti-CD8 and FITC-conjugated anti-TCRβ antibodies (Pharmingen). CD8+TCR+ cells were then sorted (with a FACStar Plus sorter, Becton Dickinson, San Jose, CA) directly into 96-well plates containing 2.5 × 106 irradiated (1000 Rad γ) C57BL/6 splenocytes per well. Cultures were maintained for 2 weeks in 200 μL Dulbecco modified Eagle medium supplemented with 30 U/mL exogenous IL2 (EL4 supernatant). Half (100 μL) of the cultures were used for standard cytotoxic assays, performed with51Cr-labeled RMA targets. Lysis was considered positive if51Cr release exceeded mean + 3 SD of spontaneous release (measured in 48 control wells containing targets and APCs). The frequency of RMA lysing precursors was calculated as described.48
Hematopoietic chimeras
Irradiation bone marrow chimeras were prepared essentially as described previously.49 Briefly, anti-NK1.1 antibody-treated hosts (100 μg PK136 intraperitoneally50) were lethally irradiated (1000 Rad γ) using a 137Cs source and intravenously reconstituted with 107 C57BL/6 plus C57BL/6-β2m° bone marrow cells (ratio 1:1) that had previously been depleted of T cells using anti-Thy1 antibody AT8342 plus complement. Unseparated lymph node cells (107) from transgenic or control mice were coinjected. In some experiments, transgenic lymph node cells were depleted of CD4+ and/or CD8+ T cells before transfer by using anti-CD4 antibody RL172.451 or anti-CD8 antibody 3.16852 plus complement. Chimeras were kept on antibiotic-containing drinking water (0.2% Bactrim, Roche, Basel, Switzerland) for the duration of the experiment (2 weeks).
Results
Major histocompatibility complex class I expression in K14-β2m transgenic mice
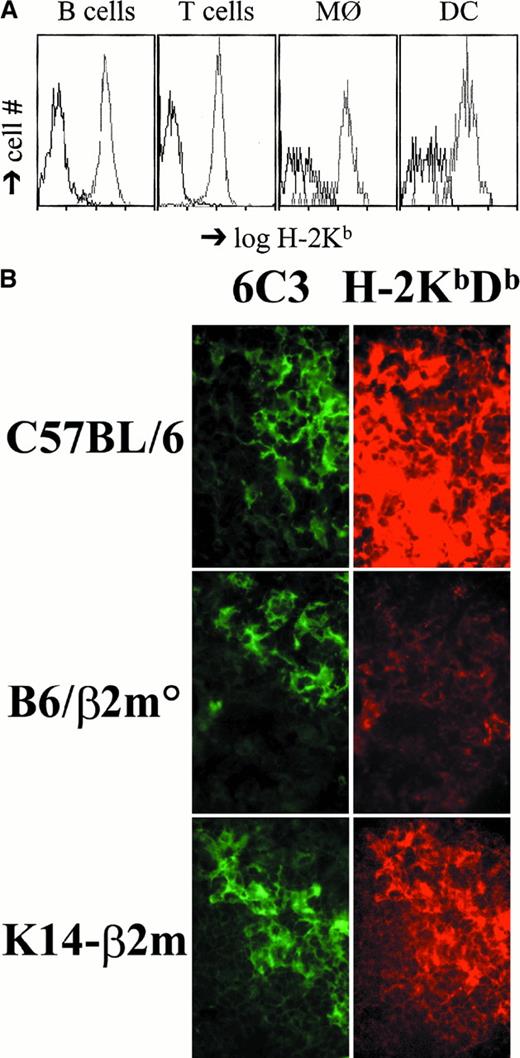
We have generated transgenic mice that express murine β2m under the control of the human keratin K14 promoter known to be exclusively active in the basal layer of stratified squamous epithelia.40,53 Transgenic mice were crossed to C57BL/6 β2m-deficient animals39 and K14-β2m transgenic, endogenous β2m-deficient mice identified (K14-β2m). Flow cytometric analysis of K14-β2m T cells, B cells, macrophages, and dendritic cells from bone marrow, spleen, liver, and thymus demonstrated that these cells do not express detectable levels of MHC class I molecules (Figure 1A; data not shown). In contrast, cortical (but not medullary) epithelial cells in K14-β2m transgenic thymi express MHC class I molecules at levels approaching those observed in wild-type controls (Figure 1B).
MHC class I expression in K14-β2m mice.
(A) Lack of H-2Kb expression on K14-β2m splenic B cells, T cells, macrophages, and dendritic cells. Splenocytes from K14-β2m mice (black lines) and β2m+/° littermates (gray lines) were stained with anti–H-2Kb combined with anti-B220, anti-TCRβ, anti–Mac-1, or anti-CD11c antibodies. Histograms of H-2Kb expression on electronically gated B220+ (B cells), TCRβ+ (T cells), Mac-1+ (macrophages), or CD11c+ (dendritic cells) subsets are representative of 3 independent experiments. (B) Thymic cortical epithelial expression of MHC class I in K14-β2m mice. Sections of wild-type C57BL/6, C57BL/6-β2m°, and K14-β2m thymi were doubly stained with FITC-conjugated antibody 6C3, which detects cortical epithelial cells, and rhodamine-labeled H-2Kb– and H-2Db–specific antibody 28-8-6. Green and red fluorescence of the same fields is shown.
MHC class I expression in K14-β2m mice.
(A) Lack of H-2Kb expression on K14-β2m splenic B cells, T cells, macrophages, and dendritic cells. Splenocytes from K14-β2m mice (black lines) and β2m+/° littermates (gray lines) were stained with anti–H-2Kb combined with anti-B220, anti-TCRβ, anti–Mac-1, or anti-CD11c antibodies. Histograms of H-2Kb expression on electronically gated B220+ (B cells), TCRβ+ (T cells), Mac-1+ (macrophages), or CD11c+ (dendritic cells) subsets are representative of 3 independent experiments. (B) Thymic cortical epithelial expression of MHC class I in K14-β2m mice. Sections of wild-type C57BL/6, C57BL/6-β2m°, and K14-β2m thymi were doubly stained with FITC-conjugated antibody 6C3, which detects cortical epithelial cells, and rhodamine-labeled H-2Kb– and H-2Db–specific antibody 28-8-6. Green and red fluorescence of the same fields is shown.
Thymic cortical epithelial expression of major histocompatibility complex class I allows development of CD8+ T lymphocytes
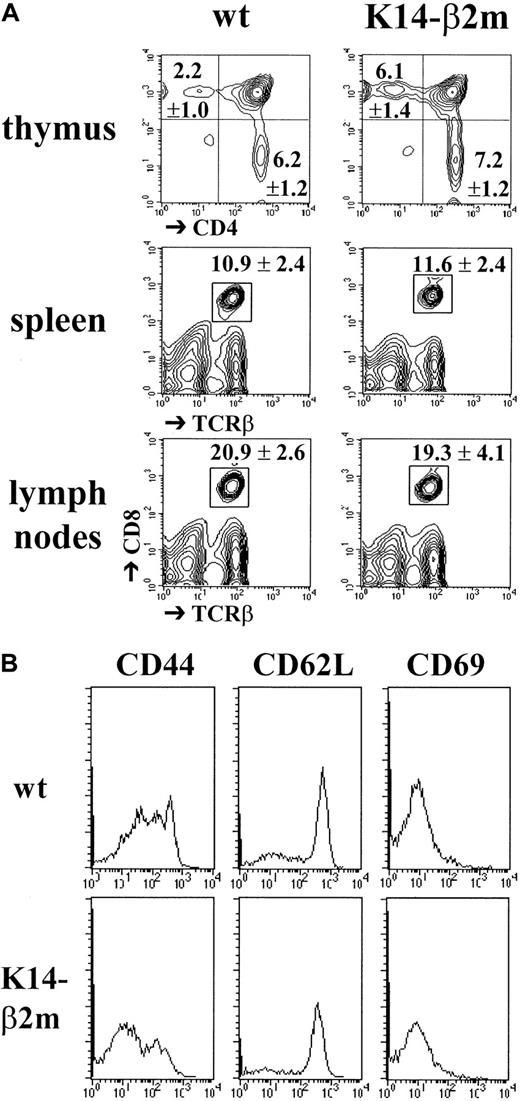
We next analyzed the development of mature CD8+ T lymphocytes in K14-β2m transgenic mice. Compared with wild-type controls, thymi from transgenic mice contained a 2- to 3-fold increased percentage and an absolute number of mature CD4−CD8+TCRhigh thymocytes (Figure2A). In K14-β2m lymph nodes and spleens, normal numbers of CD8+ T lymphocytes were detected (Figure 2A). Moreover, on the basis of the expression of the activation/memory markers CD44, CD62L, and CD69, most CD8+ splenocytes from transgenic as well as wild-type littermates had a naive phenotype (Figure 2B). This result is surprising in view of recent reports indicating that peripheral naive CD8+ T lymphocytes require TCR interactions with MHC class I for their survival.54 55
Development and peripheral maintenance of CD8+ T lymphocytes in K14-β2m mice.
(A) Thymocytes from K14-β2m mice and β2m+/° littermates were triply stained with anti-CD4, anti-CD8, and anti-TCRβ antibodies. Percentages (mean ± SD, n = 6) of CD4−CD8−, CD4+CD8+, CD4+CD8−TCRhigh and CD4−CD8+TCRhigh cells are indicated. Thymi from K14-β2m mice and β2m+/° littermates contained 127 ± 45 × 106 and 116 ± 25 × 106 cells, respectively. Spleen and lymph node cell suspensions were stained with anti-CD8 and anti-TCRβ antibodies (n = 3). K14-β2m mice and β2m+/° littermates had 98 ± 26 × 106 and 107 ± 25 × 106 splenocytes and 20 ± 6 × 106 and 28 ± 2 × 106 lymph node cells, respectively. (B) Expression of memory/activation markers by CD8+ splenocytes from K14-β2m mice and β2m+/° littermates. Cells were stained with anti-TCRβ, anti-CD8, and anti-CD44, anti-CD62L or anti-CD69 antibodies. Representative histograms of electronically gated TCRβ+CD8+ cells are shown.
Development and peripheral maintenance of CD8+ T lymphocytes in K14-β2m mice.
(A) Thymocytes from K14-β2m mice and β2m+/° littermates were triply stained with anti-CD4, anti-CD8, and anti-TCRβ antibodies. Percentages (mean ± SD, n = 6) of CD4−CD8−, CD4+CD8+, CD4+CD8−TCRhigh and CD4−CD8+TCRhigh cells are indicated. Thymi from K14-β2m mice and β2m+/° littermates contained 127 ± 45 × 106 and 116 ± 25 × 106 cells, respectively. Spleen and lymph node cell suspensions were stained with anti-CD8 and anti-TCRβ antibodies (n = 3). K14-β2m mice and β2m+/° littermates had 98 ± 26 × 106 and 107 ± 25 × 106 splenocytes and 20 ± 6 × 106 and 28 ± 2 × 106 lymph node cells, respectively. (B) Expression of memory/activation markers by CD8+ splenocytes from K14-β2m mice and β2m+/° littermates. Cells were stained with anti-TCRβ, anti-CD8, and anti-CD44, anti-CD62L or anti-CD69 antibodies. Representative histograms of electronically gated TCRβ+CD8+ cells are shown.
Activated CD8+ T lymphocytes from K14-β2m mice efficiently lyse syngeneic major histocompatibility complex class I expressing targets in vitro
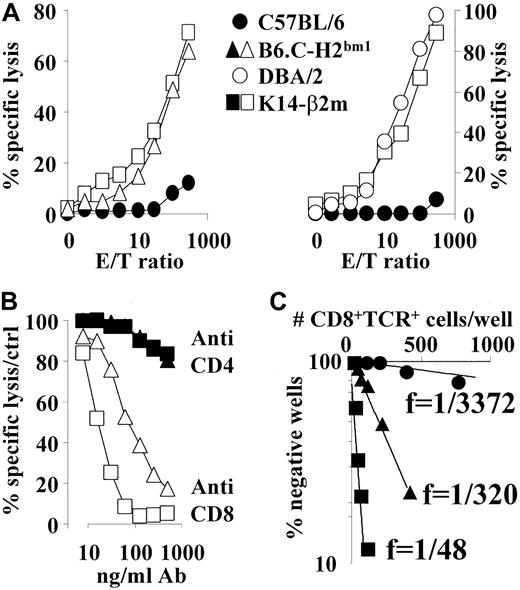
To analyze whether T lymphocytes derived from K14-β2m transgenic mice had undergone negative selection, splenocytes were cultured with irradiated syngeneic MHC class I and II expressing (C57BL/6) APCs for 6 days in the presence of exogenous IL-2, followed by an in vitro lysis assay that used C57BL/6-derived RMA or EL-4 lymphomas as targets. K14-β2m–derived effector T cells lysed syngeneic targets as efficiently as (allogeneic) B6.C-H2bm1- or DBA/2-derived T cells (Figure 3A). Similar results were obtained with T lymphocytes derived from a second independent K14-β2m transgenic mouse line (data not shown). Lysis by both K14-β2m– and B6.C-H2bm1–derived effector T cells was completely CD8-dependent, as shown by anti-CD8 antibody blocking (Figure3B).
Activated CD8+ T lymphocytes from K14-β2m mice lyse MHC class I+ syngeneic targets in vitro.
(A) Splenocytes from DBA/2, C57BL/6, B6.C-H2bm1 and K14-β2m mice were stimulated in vitro with irradiated C57BL/6 spleen cells in the presence of exogenous IL-2. After 6 days of culture, effector cells were assayed for cytolytic activity using C57BL/6-derived RMA lymphoma target cells at E/T ratios indicated. Data are representative of 3 independent experiments. Similar results were obtained by using C57BL/6-derived EL-4 thymoma cells as targets (data not shown). (B) In vitro–stimulated effector cells from K14-β2m and B6.C-H2bm1 mice were incubated for 30 minutes with titrated concentrations of anti-CD4 or anti-CD8 antibodies before addition of labeled RMA target cells. Data are depicted as percentage of lysis in the absence of antibody. (C) Precursor frequency analysis of syngeneic target lysing CD8+ T lymphocytes. Indicated numbers of CD8+TCRβ+ splenocytes were electronically sorted into 96-well plates seeded with C57BL/6 APCs. Lysis of RMA targets was assessed 2 weeks later.
Activated CD8+ T lymphocytes from K14-β2m mice lyse MHC class I+ syngeneic targets in vitro.
(A) Splenocytes from DBA/2, C57BL/6, B6.C-H2bm1 and K14-β2m mice were stimulated in vitro with irradiated C57BL/6 spleen cells in the presence of exogenous IL-2. After 6 days of culture, effector cells were assayed for cytolytic activity using C57BL/6-derived RMA lymphoma target cells at E/T ratios indicated. Data are representative of 3 independent experiments. Similar results were obtained by using C57BL/6-derived EL-4 thymoma cells as targets (data not shown). (B) In vitro–stimulated effector cells from K14-β2m and B6.C-H2bm1 mice were incubated for 30 minutes with titrated concentrations of anti-CD4 or anti-CD8 antibodies before addition of labeled RMA target cells. Data are depicted as percentage of lysis in the absence of antibody. (C) Precursor frequency analysis of syngeneic target lysing CD8+ T lymphocytes. Indicated numbers of CD8+TCRβ+ splenocytes were electronically sorted into 96-well plates seeded with C57BL/6 APCs. Lysis of RMA targets was assessed 2 weeks later.
We next analyzed the frequency of autospecific CD8+K14-β2m transgenic T lymphocytes by limiting dilution analysis. The minimal estimate of the H-2b reactive cytotoxic T lymphocyte (CTL) precursor frequency among K14-β2m–derived CD8 T lymphocytes was 1 of 48, ie, 7-fold higher than that observed among allogeneic B6.C-H2bm1 CTL (1 of 320), and almost 100-fold higher than that of syngeneic wild-type C57BL/6 cells (1 of 3372) (Figure 3C). These data confirm the high level of autoreactivity of K14-β2m–derived CTL.
CD8+ T cells from K14-β2m mice kill syngeneic major histocompatibility complex class I–expressing hematopoietic targets in vivo
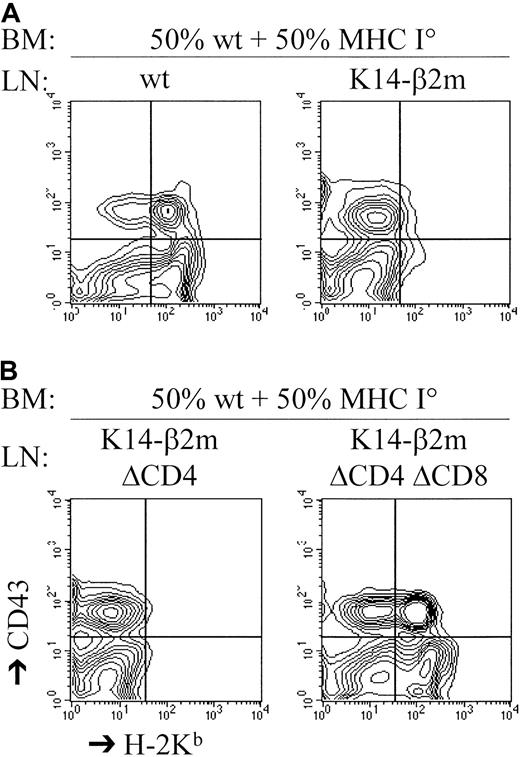
T lymphocytes that can be activated by syngeneic MHC ligands in vitro are not necessarily reactive to syngeneic cells in vivo.38 56-58 To assess whether K14-β2m–derived T cells were capable of lysing syngeneic targets in vivo, we cotransferred C57BL/6 and C57BL/6-β2m° bone marrow as well as K14-β2m or control C57BL/6 lymph node T cells into lethally irradiated C57BL/6 hosts. Survival of coinjected C57BL/6 and β2m° bone marrow cells was analyzed by flow cytometry 2 weeks after transfer. In K14-β2m T-cell–injected hosts, no MHC class I positive bone marrow precursor cells had survived, and only β2m° bone marrow had reconstituted the hosts (Figure 4A). Moreover, antibody-depletion experiments demonstrated that CD8+ T cells from the K14-β2m lymph node cell inoculum were capable of in vivo C57BL/6 bone marrow lysis (Figure 4B). Therefore, CD8+T lymphocytes developing in K14-β2m transgenic mice are capable of lysing targets that express syngeneic MHC class I ligands in vivo as well as in vitro.
CD8+ T lymphocytes from K14-β2m mice kill syngeneic MHC class I+ hematopoietic cells in vivo.
Lethally irradiated C57BL/6 hosts were reconstituted with a mixture of C57BL/6 and C57BL/6-β2m° bone marrow cells that were cotransferred with C57BL/6 or K14-β2m lymph node (LN) cells. Where indicated, the K14-β2m lymph node cells were depleted of either CD4+ (LN ΔCD4) or CD4+ plus CD8+ (LN ΔCD4ΔCD8) cells (by antibody plus complement treatment) before transfer. Reconstitution by C57BL/6 bone marrow cells was assessed 2 weeks later by 2-color flow cytometry of bone marrow with the use of anti-CD43 (detecting B cell precursors) and anti–H-2Kbantibodies.
CD8+ T lymphocytes from K14-β2m mice kill syngeneic MHC class I+ hematopoietic cells in vivo.
Lethally irradiated C57BL/6 hosts were reconstituted with a mixture of C57BL/6 and C57BL/6-β2m° bone marrow cells that were cotransferred with C57BL/6 or K14-β2m lymph node (LN) cells. Where indicated, the K14-β2m lymph node cells were depleted of either CD4+ (LN ΔCD4) or CD4+ plus CD8+ (LN ΔCD4ΔCD8) cells (by antibody plus complement treatment) before transfer. Reconstitution by C57BL/6 bone marrow cells was assessed 2 weeks later by 2-color flow cytometry of bone marrow with the use of anti-CD43 (detecting B cell precursors) and anti–H-2Kbantibodies.
Discussion
Analysis of the mechanisms responsible for thymic positive and negative selection would be greatly facilitated by the dissection of these processes. Here we have reported data on mice expressing MHC class I molecules under control of the human K14 promoter. In the thymus, MHC class I expression was limited to cortical epithelial cells, and no expression by medullary epithelial cells, T or B lymphocytes, dendritic cells, and macrophages was observed. CD8+ T lymphocytes developed apparently normally and populated peripheral lymphoid organs. These cells could be stimulated to lyse syngeneic targets in vitro as well as in vivo. Therefore, dissection of the thymic positive and negative selection mechanisms can be achieved by using transgenic mice that express MHC class I molecules exclusively on cortical epithelial cells.
Expression of transgenic β2m under control of the human K14 promoter in the thymus was limited to cortical epithelial cells, consistent with the results obtained by Laufer and colleagues8 who used transgenic mice that expressed the I-Aβ chain under control of the same promoter construct. In transgenic mice expressing the costimulatory molecule B7-1 under control of a human K14 promoter construct, the expression pattern seemed very different: Medullary (but not cortical) epithelial cells expressed the transgene.59However, the promoter construct was not identical to the one used by us and by Laufer and colleagues.8,60 Finally, analysis of murine K14 expression in the thymus revealed its exclusive localization in medullary regions.61 Taken together, these data suggest that cortical targeting achieved by us and by Laufer and colleagues critically depends on the promoter construct and is not an inherent characteristic of K14 expression.
Thymic cortical epithelial expression of MHC class I molecules in our transgenic mice allowed for efficient positive selection of mature CD8+ thymocytes. Similarly, MHC class II expression by thymic cortical epithelial cells leads to positive selection of mature CD4+ thymocytes.6,8 In contrast, in mice in which MHC class II expression was limited to thymic medullary epithelium, no positive selection of CD4+ T lymphocytes occurred.6 Moreover, MHC class I expression by APCs leads to positive selection of only a minute population of cytotoxic T cells, whereas MHC class II expression by APCs does not allow detectable positive selection of CD4+ T cells.7,9,10 37It therefore appears that only thymic cortical epithelium supports positive selection of significant numbers of both CD4+6,8and CD8+ T lymphocytes (our results).
In our K14-β2m mice, thymic cortical epithelial expression of MHC class I led to the development of 2- to 3-fold increased numbers of mature CD8+ thymocytes. In the absence of MHC class I expression by professional APCs (and therefore of an important fraction of thymic clonal deletion), this increase in mature CD8+thymocytes was to be expected. Indeed, we have previously shown that in bone marrow chimeras expressing MHC by radioresistant thymic epithelial cells, but lacking MHC expression by APCs, a 2- to 3-fold increase in the rate of generation of mature CD8+ thymocytes occurs.37
Because peripheral naive CD8+ T lymphocytes are believed to require TCR interactions with MHC class I for their survival,54,55 the persistence of normal numbers of peripheral K14-β2m CD8+ T cells in the absence of MHC class I expression by professional APCs was rather unexpected. In contrast to naive T cells, memory CD8+ T cells are thought to survive in the absence of continuous TCR-MHC class I interactions,55 though alternative views exist.54 Nevertheless, flow cytometric analysis of K14-β2m peripheral CD8+ T cells revealed that the vast majority expressed a naive resting phenotype. Therefore, it appears that even in the absence of MHC class I expression by professional APCs,62 a quantitatively normal CD8+ T-cell repertoire is maintained. The peripheral CD8+ T lymphocyte pool in K14-β2m transgenic mice may be maintained by increased thymic egress of mature cells, or alternatively may persist because of TCR interactions with MHC class I expressed extrathymically on epithelial cells.40 53 Additional experiments will be required to distinguish between these 2 possibilities.
A significant proportion of peripheral CD8+ T lymphocytes from K14-β2m transgenic mice were autospecific and could be induced to lyse syngeneic MHC-expressing target cells in vitro. In bone marrow chimeras lacking MHC class I (J.V.M.V.M. and H.R.M.D., unpublished data, 1997) or MHC class I and II expression38 by hematopoietic cells, but expressing MHC on radioresistant elements (eg, thymic cortical and medullary epithelium), T lymphocytes lysing host-type MHC-expressing target cells in vitro could readily be generated as well. Syngeneic H-2b targets were approximately 10-fold less efficiently lysed by chimera-derived CD8+ T lymphocytes than by allogeneic DBA/2 T cells (our unpublished results, 1997, and van Meerwijk and MacDonald38). In contrast, K14-β2m and allogeneic DBA/2 or B6.C-H2bm1 CTL lysed H-2b targets equally efficiently. Moreover, although K14-β2m transgenic CTL lysed H-2b targets in vitro as well as in vivo, in the case of P→F1 and MHC°→wild-type chimeras, as well as in transgenic mice expressing tolerizing ligands exclusively on medullary epithelium, in vitro T-cell reactivity to host or transgene-type MHC was accompanied by in vivo tolerance, a phenomenon termed “split tolerance.”38,56-58 Taken together, these data confirm a role for medullary epithelium in negative selection.16,17,58,63,64 Moreover, because mature CD4+ T lymphocytes that had artificially developed in the absence of any MHC-TCR interaction (and therefore MHC-dependent positive or negative selection) were equally reactive to H-2b and H-2d APCs,65 the fact that H-2b targets are lysed equally efficiently by K14-β2m as by allogeneic B6.C-H2bm1– and DBA/2-derived T cells strongly suggest that cortical epithelium does not support negative selection.
Our data indicating that cortical epithelial cells are incapable of CD8+ T lymphocyte tolerance induction are apparently contradictory to earlier reports showing that in mice expressing transgenic MHC class I restricted TCRs autospecific (cortical) CD4+CD8+ thymocytes were absent (reviewed by Stockinger66). However, deletion observed in these mice is not necessarily due to MHC expressed by cortical epithelial cells and may be mediated by cortical macrophages, a hypothesis consistent with the relatively poor TCR-transgenic CD4+CD8+thymocyte deletion by ligand-expressing thymic epithelial cells.67 Data suggesting that other mechanisms may be involved in the depletion of autospecific TCR transgenic CD4+CD8+ thymocytes have also been reported.68 69
The frequency of autoreactive CTL precursors in K14-β2m mice (as determined by limiting dilution analysis) was 1 of 48, a value similar to that obtained by Laufer and colleagues for autoreactive CD4+ T-cell precursors.8 These data suggest that only 2% of positively selectable thymocytes normally undergo tolerance induction, a value well below the 50% to 70% obtained earlier by us and by others analyzing T-hybrid specificity and kinetics of mature CD4+ or CD8+ thymocyte development.22,37 65 However, values obtained by limiting dilution analysis are necessarily minimal estimates, and (in the absence of a valid correction for plating efficiency) cannot be compared with kinetic and T-hybrid data.
In conclusion, our data indicate that MHC class I expressed by cortical epithelial cells cannot induce negative selection of CD8+ T lymphocytes, a conclusion consistent with earlier data on reactivity of CD4+ T cells that had developed in mice expressing MHC class II exclusively on cortical epithelium.8,35Therefore, cortical epithelial cells appear to be specialized in thymic positive selection, whereas medullary epithelial cells and intrathymic APCs of hematopoietic origin are specialized in negative selection. Although certain in vitro cultured thymic epithelial cell lines are capable of both positive and negative selection in vitro and when injected intrathymically,70,71 the physiologic relevance of these findings remains unclear. Whatever the explanation, the data presented here and elsewhere8 35 on K14-MHC transgenic mice clearly point to a general lack of tolerance induction by cortical epithelial cells. The ability to dissect thymic positive and negative selection by using these mice should facilitate analysis of the responsible mechanisms.
We thank Dr E. Fuchs for the K14 promoter construct, Dr D. Margulies for β2m cDNA, Dr C. L. Blanchard for constructing the K14-β2m vector, Dr M. Guttinger for helpful suggestions, and R. Lees and G. Enault for expert technical help.
Supported in part by grants from ARC (#7287), Région Midi Pyrénées (RECH/97001940), FRM (10000121-10), and by institutional funds from INSERM and University Toulouse III.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Joost P. M. van Meerwijk, INSERM U395 CHU Purpan, BP 3028, 31024 Toulouse Cedex 3, France; e-mail:joost.van-meerwijk@purpan.inserm.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal