The Dana-Farber Cancer Institute (DFCI) acute lymphoblastic leukemia (ALL) Consortium Protocol 91-01 was designed to improve the outcome of children with newly diagnosed ALL while minimizing toxicity. Compared with prior protocols, post-remission therapy was intensified by substituting dexamethasone for prednisone and prolonging the asparaginase intensification from 20 to 30 weeks. Between 1991 and 1995, 377 patients (age, 0-18 years) were enrolled; 137 patients were considered standard risk (SR), and 240 patients were high risk (HR). Following a 5.0-year median follow-up, the estimated 5-year event-free survival (EFS) ± SE for all patients was 83% ± 2%, which is superior to prior DFCI ALL Consortium protocols conducted between 1981 and 1991 (P = .03). There was no significant difference in 5-year EFS based upon risk group (87% ± 3% for SR and 81% ± 3% for HR, P = .24). Age at diagnosis was a statistically significant prognostic factor (P = .03), with inferior outcomes observed in infants and children 9 years or older. Patients who tolerated 25 or fewer weeks of asparaginase had a significantly worse outcome than those who received at least 26 weeks of asparaginase (P < .01, both univariate and multivariate). Older children (at least 9 years of age) were significantly more likely to have tolerated 25 or fewer weeks of asparaginase (P < .01). Treatment on Protocol 91-01 significantly improved the outcome of children with ALL, perhaps due to the prolonged asparaginase intensification and/or the use of dexamethasone. The inferior outcome of older children may be due, in part, to increased intolerance of intensive therapy.
Introduction
During the last decade investigators have reported favorable outcomes for children with acute lymphoblastic leukemia (ALL), with long-term event-free survival rates for unselected patient populations ranging from 60% to 80%.1-4 Advances in the treatment of childhood ALL have resulted from clinical trials conducted by several multi-institutional groups. The Dana-Farber Cancer Institute (DFCI) ALL Consortium has conducted such trials since 1981. Treatment strategies include intensive, multi-agent induction therapy; early intensification with weekly, high-dose asparaginase; frequent pulses of vincristine and corticosteroids during continuation therapy; and for high-risk (HR) patients, doxorubicin during intensification. We have previously reported a 7-year overall event-free survival (EFS) rate of 78% for children with ALL treated between 1985 and 1987.5 Acute toxicities from our treatment approach include asparaginase-related allergic events and pancreatitis.5,6 In long-term survivors we have observed asymptomatic echocardiographic changes related to anthracycline exposure7 as well as short stature and learning disabilities of varying severity, presumably secondary to central nervous system (CNS)–directed therapy.8
DFCI ALL Consortium Protocol 91-01 was open for enrollment between 1991 and 1995. The objective of the protocol was to improve outcome while minimizing toxicity. Based upon the reports of other investigators that high-dose intravenous (IV) 6-mercaptopurine (6-MP) improved the outcome of patients with B-lineage ALL,9,10 Protocol 91-01 randomized patients to receive pulses of either high-dose IV 6-MP or standard-dose oral 6-MP during the first year of post-remission therapy. In addition, for all patients, dexamethasone was substituted for prednisone during post-remission therapy based upon in vitro and in vivo data, which demonstrated that the former was associated with increased antileukemia activity and enhanced CNS penetration.11-14 Also, the duration of high-dose asparaginase intensification was extended from 20 weeks (in prior protocols) to 30 weeks. Patients participated in 3 additional randomizations designed to evaluate whether acute and late toxicities could be reduced. The randomizations included a comparison of (1) 2 asparaginase preparations, native and polyethylene-glycosylated (PEG) Escherichia coliasparaginase; (2) 2 doxorubicin infusion rates, continuous infusion and bolus; and (3) 2 dosing schedules of cranial radiation (once daily and twice daily fractionation). We report here the treatment outcome results for DFCI ALL Consortium Protocol 91-01 at 5 years of median follow-up.
Patients, materials, and methods
Patients
Between December 1991 and December 1995, 386 children (age, 0-18 years) with newly diagnosed ALL (excluding mature B-cell ALL) were enrolled on Protocol 91-01. Informed consent was obtained from parents or guardians prior to instituting therapy. Nine patients were ineligible because of incorrect diagnosis (mature B-cell leukemia, acute myelogenous leukemia, and no leukemia) (n = 6); pretreatment with other anti-leukemia therapy (n = 2); and absence of signed parental consent prior to therapy (n = 1). The remaining 377 patients were evaluable for results of treatment.
Patients were enrolled from the following consortium institutions: DFCI/Children's Hospital (Boston, MA), Hospital Saint Justine (Montreal, Quebec, Canada), University of Rochester Medical Center (Rochester, NY), McMaster University Medical Center (Hamilton, Ontario, Canada), San Jorge Children's Hospital (San Juan, Puerto Rico), Maine Children's Cancer Program (Portland, ME), University of Massachusetts (Worcester, MA), Mount Sinai Medical Center (New York, NY), Le Centre Hospitalier de L'Universite (Laval, Quebec, Canada) and Oschner Institutions (New Orleans, LA). The institutional review boards of each participating institution approved the protocol prior to patient enrollment.
Therapy
Three patient risk groups were used to select therapy: standard risk (SR), high risk (HR), and infant high risk (infant HR). Risk group was determined at the time of diagnosis. Infant HR included all patients who were less than 12 months of age at diagnosis. HR patients presented with one or more of the following pretreatment characteristics: (1) white blood cell (WBC) count of at least 20 × 109 cells per L (20 000 cells per μL); (2) age between one year and less than 2 years or at least 9 years; (3) presence of leukemia blasts in a cytocentrifuged cerebrospinal fluid (CSF) specimen regardless of CSF WBC count (CNS-2 or CNS-3); (4) presence of a mediastinal mass; or (5) T-cell immunophenotype. Patients with the Ph+ chromosome t(9;22)(q34;q11) were treated as HR patients, but they received an allogeneic bone marrow transplantation (BMT) during first remission. All other patients were classified as SR.
Details of therapy are shown in Table 1. For SR and HR patients, CNS therapy commenced during the first cycle of intensification therapy. During that cycle, asparaginase and dexamethasone were not administered. Cranial radiation was delivered to SR boys and all HR patients. The dose of radiation was 1800 cGy, except for those with CNS leukemia at diagnosis (CNS-2 or CNS-3), all of whom received 1980 cGy. SR girls did not receive cranial radiation. Infant HR patients received an additional month of intensive chemotherapy immediately after the remission induction phase, which included high-dose cytarabine and high-dose methotrexate.15 Cranial radiation was delayed until 12 months of age.
Therapy on DFCI ALL Consortium Protocol 91-01
| Phase of therapy (time period) . | Therapy . |
|---|---|
| Investigational window (3 d) | Steroid × 3 d (randomized): prednisone (40 mg/m2/d) or dexamethasone (6, 18, or 150 mg/m2/d) |
| IT cytarabine* (dosage by age × 1 dose, d 0) | |
| Induction | Vincristine (1.5 mg/m2/wk; maximum, 2 mg) |
| (4 wk) | Prednisone (40 mg/m2/d) |
| Doxorubicin (30 mg/m2 per dose; d 1 and 2) | |
| Methotrexate (4 gm/m2, 8-24 h after doxorubicin) with leucovorin rescue | |
| IT cytarabine* (dosage by age × 1 dose, d 17) | |
| CNS therapy (3 wk) | SR girls: IT methotrexate†/cytarabine* (× 4 doses for 2 wk, then q 18 wk) |
| SR boys and all HR patients: cranial XRT 1800 cGy (randomized): hyperfractionated (90 cGy bid) or conventional (180 cGy qd) with IT methotrexate/cytarabine (dosage as in SR patients) | |
| Intensification | q 3-wk cycles |
| (30 wk) | SR: vincristine (2.0 mg/m2 IV q 3 wk; maximum, 2 mg) |
| dexamethasone (6 mg/m2/d PO × 5 d) | |
| methotrexate (30 mg/m2 IV or IM q wk) | |
| 6-MP (randomized): | |
| high-dose: 1000 mg/m2 IV for 20 h, wk 1 and 2 or conventional: 50 mg/m2/d PO × 14 days | |
| asparaginase (randomized): | |
| PEG 2500 IU/m2 IM q 2 wk × 15 doses or E coli 25 000 IU/m2 IM q wk × 30 doses | |
| HR: same as SR patients, except: | |
| dexamethasone dose (18 mg/m2/d PO × 5 d) | |
| no methotrexate | |
| doxorubicin (30 mg/m2 q 3 wk; cumulative dose, 360 mg/m2) (randomized): | |
| continuous infusion for 48 h or IV bolus | |
| Continuation | q 3-wk cycles |
| (until 2 y CCR) | SR: same as intensification, except no asparaginase; all 6-MP given via conventional dose (50 mg/m2/d × 14 d) |
| HR: same as SR patients, except dexamethasone dose (as above) |
| Phase of therapy (time period) . | Therapy . |
|---|---|
| Investigational window (3 d) | Steroid × 3 d (randomized): prednisone (40 mg/m2/d) or dexamethasone (6, 18, or 150 mg/m2/d) |
| IT cytarabine* (dosage by age × 1 dose, d 0) | |
| Induction | Vincristine (1.5 mg/m2/wk; maximum, 2 mg) |
| (4 wk) | Prednisone (40 mg/m2/d) |
| Doxorubicin (30 mg/m2 per dose; d 1 and 2) | |
| Methotrexate (4 gm/m2, 8-24 h after doxorubicin) with leucovorin rescue | |
| IT cytarabine* (dosage by age × 1 dose, d 17) | |
| CNS therapy (3 wk) | SR girls: IT methotrexate†/cytarabine* (× 4 doses for 2 wk, then q 18 wk) |
| SR boys and all HR patients: cranial XRT 1800 cGy (randomized): hyperfractionated (90 cGy bid) or conventional (180 cGy qd) with IT methotrexate/cytarabine (dosage as in SR patients) | |
| Intensification | q 3-wk cycles |
| (30 wk) | SR: vincristine (2.0 mg/m2 IV q 3 wk; maximum, 2 mg) |
| dexamethasone (6 mg/m2/d PO × 5 d) | |
| methotrexate (30 mg/m2 IV or IM q wk) | |
| 6-MP (randomized): | |
| high-dose: 1000 mg/m2 IV for 20 h, wk 1 and 2 or conventional: 50 mg/m2/d PO × 14 days | |
| asparaginase (randomized): | |
| PEG 2500 IU/m2 IM q 2 wk × 15 doses or E coli 25 000 IU/m2 IM q wk × 30 doses | |
| HR: same as SR patients, except: | |
| dexamethasone dose (18 mg/m2/d PO × 5 d) | |
| no methotrexate | |
| doxorubicin (30 mg/m2 q 3 wk; cumulative dose, 360 mg/m2) (randomized): | |
| continuous infusion for 48 h or IV bolus | |
| Continuation | q 3-wk cycles |
| (until 2 y CCR) | SR: same as intensification, except no asparaginase; all 6-MP given via conventional dose (50 mg/m2/d × 14 d) |
| HR: same as SR patients, except dexamethasone dose (as above) |
In the table, d indicates day; IT, intrathecal; wk, week; h, hour; SR, standard risk; HR, high risk; CCR, continuous complete remission; IM, intramuscularly; IV, intravenously; q, every (quaue); bid, twice a day (bis in die); qd, every day (quaque die); and XRT, radiation therapy.
Indicates IT cytarabine dosage per age: less than one year: 15 mg; at least 1 year, but less than 2 years: 20 mg; at least 2 years, but less than 3 years: 30 mg; and at least 3 years: 40 mg. Patients with CNS leukemia at diagnosis received twice-weekly doses of IT cytarabine until CSF was clear of blasts cells on 3 consecutive examinations.
IT methotrexate dosage per age: less than one year: 6 mg; at least 1 year, but less than 2 years: 8 mg; at least 2 years, but less than 3 years: 10 mg; and at least 3 years: 12 mg.
Asparaginase preparation was switched after a mild allergic event (local reaction, rash). Patients receiving E coliasparaginase were switched to weekly PEG asparaginase, and those receiving PEG were switched to E coli asparaginase to complete 30 weeks of therapy. All patients were switched to twice-weekly Erwinia asparaginase (25 000 IU/m2per dose) if they experienced a subsequent allergic event. Asparaginase therapy was held until resolution of mild pancreatitis or deep venous thrombosis, and the therapy was permanently stopped after severe allergic events (bronchospasm and/or lip or tongue swelling), severe pancreatitis (abdominal pain for at least 72 hours with elevated pancreatic enzymes), CNS thrombosis, or mild allergic events to all 3 preparations (E coli, PEG, andErwinia). Therapy for all patients was discontinued after patients had achieved 24 months of continuous complete remission (CCR).
Randomizations
Patients were eligible to participate in the following 5 randomizations. All randomizations were performed centrally and occurred following enrollment, prior to the initiation of therapy.
Investigational window.
To determine differences in early leukemic cell kill, patients were randomized to receive one of 4 possible 3-day courses of corticosteroids prior to the initiation of multi-agent remission induction therapy: 40 mg/m2/d prednisolone or 6, 18, or 150 mg/m2/d dexamethasone.
Asparaginase.
To determine whether PEG asparaginase was associated with decreased toxicity, patients were randomized to receive either 2500 IU/m2 PEG asparaginase intramuscularly (IM) every other week for 15 doses or native 25 000 IU/m2E coliasparaginase IM every week for 30 doses during the intensification phase of therapy. Because PEG asparaginase was not available in Canada, children treated at Canadian institutions (n = 127) were not eligible for the asparaginase randomization and were directly assigned to receive E coli L-asparaginase during intensification.
6-MP.
To determine whether high-dose IV 6-MP would improve outcome, all patients were randomized to receive either (1) high-dose 1000 mg/m2 IV 6-MP delivered as a continuous infusion for 20 hours on weeks 1 and 2 of every 3-week chemotherapy cycle or (2) conventional 50 mg/m2/d oral 6-MP on days 1-14 of every 3-week cycle during the first year of post-remission therapy.
Doxorubicin.
To determine if continuous infusion doxorubicin was associated with a decreased incidence of late echocardiographic abnormalities, HR patients were randomized to receive doxorubicin either as a 48-hour continuous infusion or as a conventional IV bolus for 15 minutes during intensification.
Cranial radiation.
To determine whether hyperfractionated radiation was associated with a decreased incidence of neuropsychologic sequelae, those patients receiving cranial radiation (SR boys and all HR patients, including infants) were randomized to receive either twice-daily fractions of 90 cGy (hyperfractionated) or once-daily fractions of 180 cGy (conventional).
Immunophenotype and cytogenetics
BM cells from diagnostic aspirates were examined for cell surface antigens using standard indirect immunofluorescence assays and were cultured for cytogenetic analyses as previously described.15
Statistical analysis
Fisher exact tests were used to compare presenting characteristics among patient groups.16 Outcome events were death during induction therapy, failure to achieve complete remission (defined as persistent leukemia at day 52 after diagnosis), death during remission, and relapse. EFS was the time from complete remission to the first outcome event; induction failure and induction deaths were considered events at time zero. Leukemia-free survival (LFS) was the time from complete remission to relapse; induction failure was considered a relapse at time zero. Overall survival (OS) was the time from start of treatment to death from any cause. CNS LFS was the time from complete remission to a relapse involving the CNS (whether isolated or combined with other sites).
EFS, LFS, and OS were estimated using the Kaplan-Meier method,17 and the Greenwood formula was used to calculate SE.18 Univariate analyses of differences in EFS, LFS, and OS were conducted with log-rank tests.19 Multiple regression was conducted using Cox proportional hazards regression models to assess prognostic factors for EFS, LFS, and OS.20 To determine the impact of a patient's ability to tolerate asparaginase on outcome, a landmark analysis was conducted21 that included all patients with non-Ph+chromosome who were alive and event-free 40 weeks after diagnosis.
Primary randomized efficacy comparison was defined prospectively as the standard-dose versus high-dose 6-MP, and planned sample size was determined based upon this randomization. Recruitment and evaluation of 360 evaluable patients was required to detect a 45% reduction in the risk of an event with 80% power using a 2-sided 0.05 level test. Given smaller sample sizes available for other randomizations (lack of availability of PEG asparaginase in Canada, HR patients only in doxorubicin randomization, and SR girls excluded from radiation randomization), the other randomized comparisons were designed to detect reductions in toxicities. Specifically, the asparaginase randomization had 80% power to detect a reduction in the risk of toxic reaction from 20% to 8%. The doxorubicin randomization had an 80% power to detect a reduction in the incidence of echocardiographic abnormalities in long-term survivors from 50% to 25% (2-sided 0.05 level test for both). The radiation randomization had 80% power to detect a reduction in the incidence of neuropsychologic sequelae from 40% to 20% and 80% power to detect differences in CNS relapse rate (5% vs 13%) in HR patients (2-sided 0.05 level test for both).
Results
Patient characteristics
The presenting clinical characteristics of the 377 evaluable patients are summarized in Table 2. We classified 240 (64%) patients as HR (including 7 infants); the remaining 137 (36%) patients were classified as SR. Age at diagnosis ranged from 55 days to 17.9 years, with a median of 4.4 years. Initial WBC count ranged from 0.7 to 1076.4 × 109 cells per L (700 to 1 076 400 cells per μL), with a median leukocyte count of 9.8 × 109 cells per L (9800 cells per μL).
Patient characteristics and outcome on Protocol 91-01
| . | Total no. . | 5-y EFS ± SE, % . | P . |
|---|---|---|---|
| Patients | 377 | 83 ± 2 | |
| DFCI risk group | |||
| Standard risk | 137 | 87 ± 3 | .24 |
| High risk | 240 | 81 ± 3 | |
| NCI risk group | |||
| Good-risk pre-B | 243 | 85 ± 2 | .66 |
| Poor-risk pre-B | 99 | 82 ± 4 | |
| T-cell > 1 y old | 28 | 79 ± 8 | |
| Infants < 1 y old | 7 | 71 ± 17 | .03 |
| Age | |||
| 0-11 mo | 7 | 71 ± 17 | |
| 12-23 mo | 33 | 97 ± 3 | |
| 24 mo to < 9 y | 254 | 84 ± 2 | |
| 9-18 y | 83 | 77 ± 5 | .60 |
| WBC count, cells × 109/L | |||
| < 20 000 | 255 | 84 ± 2 | |
| 20 000 to 49 999 | 53 | 83 ± 5 | |
| 50 000 to 99 999 | 28 | 89 ± 6 | |
| ≥ 100 000 | 41 | 78 ± 6 | .75 |
| Sex | |||
| Male | 199 | 84 ± 3 | |
| Female | 178 | 83 ± 3 | .41 |
| Immunophenotype | |||
| Pre-B | 349 | 84 ± 2 | |
| T-cell | 28 | 79 ± 8 | .34 |
| CNS at diagnosis | |||
| Negative | 324 | 84 ± 2 | |
| Positive | 46 | 78 ± 6 | |
| Unknown | 7 | 71 ± 17 | |
| Ploidy (n = 207 assessable patients) | |||
| Hyperdiploid | |||
| ≥ 50 chromosomes | 48 | 83 ± 6 | .35 |
| < 50 chromosomes | 18 | 72 ± 11 | |
| Diploid | 105 | 87 ± 3 | |
| Pseudodiploid | 26 | 81 ± 8 | |
| Hypodiploid | 10 | 90 ± 9 | |
| Asparaginase tolerance (n = 352 assessable patients) | |||
| ≤ 25 wk | 43 | 73 ± 7 | <.01 |
| ≥ 26 wk | 309 | 90 ± 2 |
| . | Total no. . | 5-y EFS ± SE, % . | P . |
|---|---|---|---|
| Patients | 377 | 83 ± 2 | |
| DFCI risk group | |||
| Standard risk | 137 | 87 ± 3 | .24 |
| High risk | 240 | 81 ± 3 | |
| NCI risk group | |||
| Good-risk pre-B | 243 | 85 ± 2 | .66 |
| Poor-risk pre-B | 99 | 82 ± 4 | |
| T-cell > 1 y old | 28 | 79 ± 8 | |
| Infants < 1 y old | 7 | 71 ± 17 | .03 |
| Age | |||
| 0-11 mo | 7 | 71 ± 17 | |
| 12-23 mo | 33 | 97 ± 3 | |
| 24 mo to < 9 y | 254 | 84 ± 2 | |
| 9-18 y | 83 | 77 ± 5 | .60 |
| WBC count, cells × 109/L | |||
| < 20 000 | 255 | 84 ± 2 | |
| 20 000 to 49 999 | 53 | 83 ± 5 | |
| 50 000 to 99 999 | 28 | 89 ± 6 | |
| ≥ 100 000 | 41 | 78 ± 6 | .75 |
| Sex | |||
| Male | 199 | 84 ± 3 | |
| Female | 178 | 83 ± 3 | .41 |
| Immunophenotype | |||
| Pre-B | 349 | 84 ± 2 | |
| T-cell | 28 | 79 ± 8 | .34 |
| CNS at diagnosis | |||
| Negative | 324 | 84 ± 2 | |
| Positive | 46 | 78 ± 6 | |
| Unknown | 7 | 71 ± 17 | |
| Ploidy (n = 207 assessable patients) | |||
| Hyperdiploid | |||
| ≥ 50 chromosomes | 48 | 83 ± 6 | .35 |
| < 50 chromosomes | 18 | 72 ± 11 | |
| Diploid | 105 | 87 ± 3 | |
| Pseudodiploid | 26 | 81 ± 8 | |
| Hypodiploid | 10 | 90 ± 9 | |
| Asparaginase tolerance (n = 352 assessable patients) | |||
| ≤ 25 wk | 43 | 73 ± 7 | <.01 |
| ≥ 26 wk | 309 | 90 ± 2 |
DFCI indicates Dana-Farber Cancer Institute; NCI, National Cancer Institute; WBC, white blood cells; CNS, central nervous system; EFS, event-free survival.
Treatment results
The outcome of the 377 patients is presented in Table3. As of November 1999, median follow-up was 5.0 years. There were 370 (98%) patients who achieved CR. Of the 7 patients who failed to achieve CR, 5 had persistent leukemia, and 2 patients died of sepsis. Nine (2%) patients experienced a remission death during chemotherapy, including 4 of 137 SR patients (all of sepsis) and 5 of 240 HR/infant HR patients (sepsis, n = 3;Pneumocystis carinii pneumonia, n = 1; and unknown, n = 1). Three (1%) other patients died in CR due to BMT-related toxicities (Ph+ chromosome, n = 2, and one infant). All remission deaths occurred in patients aged less than 9 years. There were 46 (12%) patients who relapsed and 312 (84%) patients who remained in CCR.
Results of Protocol 91-01 for 377 children with ALL
| . | Total . | SR . | HR . |
|---|---|---|---|
| Patients | |||
| Total, no. | 377 | 137 | 240 |
| Total, % | 100 | 36 | 64 |
| Induction, no. | |||
| Failures | 5 | 0 | 5 |
| Deaths | 2 | 0 | 2 |
| Complete remissions | |||
| Total, no. | 370 | 137 | 233 |
| Total, % | 98 | 100 | 97 |
| Relapse, no. | 46 | 16 | 30 |
| BM only | 31 | 12 | 19 |
| CNS only | 4 | 1 | 3 |
| CNS + BM | 8 | 3 | 5 |
| Testis | 2 | 0 | 2 |
| Testis + BM | 1 | 0 | 1 |
| Remission, no. | |||
| Deaths | 12 | 4 | 8 |
| Continuous3-150 | 312 | 117 | 195 |
| 5-y ± SE, % | |||
| EFS | 83 ± 2 | 87 ± 3 | 81 ± 3 |
| LFS | 87 ± 2 | 90 ± 3 | 85 ± 2 |
| OS | 88 ± 2 | 92 ± 2 | 86 ± 2 |
| . | Total . | SR . | HR . |
|---|---|---|---|
| Patients | |||
| Total, no. | 377 | 137 | 240 |
| Total, % | 100 | 36 | 64 |
| Induction, no. | |||
| Failures | 5 | 0 | 5 |
| Deaths | 2 | 0 | 2 |
| Complete remissions | |||
| Total, no. | 370 | 137 | 233 |
| Total, % | 98 | 100 | 97 |
| Relapse, no. | 46 | 16 | 30 |
| BM only | 31 | 12 | 19 |
| CNS only | 4 | 1 | 3 |
| CNS + BM | 8 | 3 | 5 |
| Testis | 2 | 0 | 2 |
| Testis + BM | 1 | 0 | 1 |
| Remission, no. | |||
| Deaths | 12 | 4 | 8 |
| Continuous3-150 | 312 | 117 | 195 |
| 5-y ± SE, % | |||
| EFS | 83 ± 2 | 87 ± 3 | 81 ± 3 |
| LFS | 87 ± 2 | 90 ± 3 | 85 ± 2 |
| OS | 88 ± 2 | 92 ± 2 | 86 ± 2 |
Median follow-up time was 5.0 years.
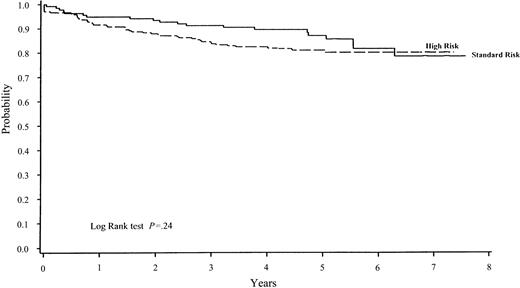
The estimated 5-year EFS ± SE was 83% ± 2% for the 377 children treated on Protocol 91-01 (Table 3 and Figure1). The 5-year EFS estimate according to the risk group was 87% ± 3% for SR patients and 81% ± 3% for HR patients (Figure 2). The difference in EFS between SR and HR patients was not statistically significant (P = .24). Excluding 7 infants and 6 patients with Ph+-chromosome disease, the 5-year EFS for the remaining 227 HR patients was 82% ± 3%. The estimated 5-year CNS LFS by risk group was 98% ± 1% for SR patients and 96% ± 1% for HR patients.
EFS for all children treated on 4 consecutive DFCI ALL Consortium protocols.
Consortium protocols shown are: 81-01 (1981-1985), 85-01 (1985-1987), 87-01 (1987-1991), and 91-01 (1991-1995). There was significant difference (P = .03) in EFS when comparing all 4 protocols, with most favorable results observed on Protocol 91-01 (5-year EFS ± SE, 83% ± 2%).
EFS for all children treated on 4 consecutive DFCI ALL Consortium protocols.
Consortium protocols shown are: 81-01 (1981-1985), 85-01 (1985-1987), 87-01 (1987-1991), and 91-01 (1991-1995). There was significant difference (P = .03) in EFS when comparing all 4 protocols, with most favorable results observed on Protocol 91-01 (5-year EFS ± SE, 83% ± 2%).
EFS on Protocol 91-01 by risk group.
The 5-year EFS ± SE for SR patients (n = 137) was 87% ± 3% and for HR patients (n = 240, including infants), 81% ± 3%. The difference between these 2 rates was not significant (P = .24).
EFS on Protocol 91-01 by risk group.
The 5-year EFS ± SE for SR patients (n = 137) was 87% ± 3% and for HR patients (n = 240, including infants), 81% ± 3%. The difference between these 2 rates was not significant (P = .24).
We also retrospectively analyzed outcome according to the age and leukocyte count criteria proposed by the Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute (NCI), Bethesda, MD.20 By those criteria, the estimated 5-year EFS for 243 patients with standard-risk B-precursor ALL (age, between 1 and 10 years, and initial WBC count, 50 × 109 cells per L [less than 50 000 cell per μL]) was 85% ± 2%, and the EFS for 99 patients with high-risk B-precursor ALL (age, at least 10 years, and/or initial WBC count, 50 × 109 cells per L [at least 50 000 cells per μL]) was 82% ± 4%. For 28 noninfant patients with T-lineage ALL, the estimated 5-year EFS was 79% ± 8%. For 7 infants, the estimated 5-year EFS was 71% ± 17%. There was no significant difference in EFS comparing the 4 groups based upon NCI risk group criteria (P = .66).
Special patient subgroups
All 6 patients with Ph+-chromosome ALL achieved CR and subsequently were treated with an allogeneic BMT in first CR. Two patients received transplantations from human leukocyte antigen (HLA)–matched sibling donors, one from a mismatched parent, and 3 from matched unrelated donors. Transplants occurred between 3-10 months after diagnosis. Two patients died of BMT-related toxicities, one patient relapsed after transplantation, and 3 patients remained alive in CCR. The estimated 5-year EFS for Ph+-chromosome patients was 50% ± 20%.
None of the 7 infants treated on Protocol 91-01 relapsed. One died in first CR of P carinii pneumonitis. Another was removed from protocol by his treating physician, received a matched unrelated donor BMT in first CR, and died of BMT-related toxicity. Five infants remained alive in CCR, with an estimated 5-year EFS of 71% ± 17%.
Prognostic factors
The results of the univariate analysis of prognostic factors for patients treated on Protocol 91-01 are presented in Table 2. Only age at diagnosis was a statistically significant prognostic factor (P = .03). The best outcome was achieved by those patients aged 12-23 months at diagnosis (n = 33), all of whom were classified and treated as HR, with a 5-year EFS of 97% ± 3%. The 5-year EFS of patients aged 12 months to less than 9 years was significantly superior to those aged 9-18 years (86% ± 2% vs 77% ± 5%;P = .03), as was the 5-year LFS (89% ± 2% vs 79% ± 5%; P = .01).
Risk group status (defined either by DFCI or NCI criteria), initial WBC count, sex, immunophenotype, and CNS status at the time of diagnosis were not statistically significant predictors of EFS. For the 207 patients on whom cytogenetic data were obtained, there was no significant difference in outcome based upon the number of chromosomes present in blast cells at diagnosis (ploidy). In multiple regression models, including age, WBC count, sex, and immunophenotype, age was the only significant factor predicting EFS, LFS, and OS (P = .02, P = .01, and P = .004, respectively).
EFS according to randomizations
There were no significant differences in presenting features among randomized patients compared with nonrandomized patients or between randomized groups (data not shown). As shown in Table4, there was no difference in EFS of randomized patients based upon investigational window corticosteroid (P = .73), asparaginase preparation (P = .29), 6-MP dosing (P = .90), or doxorubicin infusion rate (P = .23). For HR patients, the 5-year EFS and LFS were lower for those randomized to receive hyperfractionated cranial radiation (P = .05 andP = .02, respectively), although there was no difference in CNS LFS based upon randomized radiation schedule (P = .24). For SR males, there was no difference in EFS, LFS, or CNS LFS based upon CNS radiation randomization (P = .84, P = .79, and P = .36, respectively).
Outcome by randomizations
| Group . | Events/patients, no. . | 5-y EFS ± SE, % . | P . |
|---|---|---|---|
| Investigational window, | |||
| Dexamethasone (mg/m2/d) | |||
| 6 | 14/88 | 85 ± 4 | .73 |
| 18 | 18/89 | 80 ± 5 | |
| 150 | 17/86 | 79 ± 5 | |
| Prednisone (mg/m2/d) | |||
| 40 | 13/85 | 87 ± 4 | |
| Asparaginase preparation | |||
| E coli | 15/92 | 84 ± 4 | .29 |
| PEG | 24/106 | 78 ± 4 | |
| 6-MP dosing | |||
| Standard dose (PO) | 26/163 | 84 ± 3 | .90 |
| High dose (IV) | 26/159 | 85 ± 3 | |
| Doxorubicin infusion rate (HR only) | |||
| Bolus | 20/102 | 80 ± 4 | .23 |
| Continuous infusion for 48 h | 14/102 | 86 ± 4 | |
| CNS radiation fractionation | |||
| HR only | |||
| Conventional | 12/96 | 87 ± 3 | .05 |
| Hyperfractionation | 23/97 | 76 ± 4 | |
| SR only | |||
| Conventional | 5/31 | 90 ± 5 | .84 |
| Hyperfractionation | 4/28 | 85 ± 7 |
| Group . | Events/patients, no. . | 5-y EFS ± SE, % . | P . |
|---|---|---|---|
| Investigational window, | |||
| Dexamethasone (mg/m2/d) | |||
| 6 | 14/88 | 85 ± 4 | .73 |
| 18 | 18/89 | 80 ± 5 | |
| 150 | 17/86 | 79 ± 5 | |
| Prednisone (mg/m2/d) | |||
| 40 | 13/85 | 87 ± 4 | |
| Asparaginase preparation | |||
| E coli | 15/92 | 84 ± 4 | .29 |
| PEG | 24/106 | 78 ± 4 | |
| 6-MP dosing | |||
| Standard dose (PO) | 26/163 | 84 ± 3 | .90 |
| High dose (IV) | 26/159 | 85 ± 3 | |
| Doxorubicin infusion rate (HR only) | |||
| Bolus | 20/102 | 80 ± 4 | .23 |
| Continuous infusion for 48 h | 14/102 | 86 ± 4 | |
| CNS radiation fractionation | |||
| HR only | |||
| Conventional | 12/96 | 87 ± 3 | .05 |
| Hyperfractionation | 23/97 | 76 ± 4 | |
| SR only | |||
| Conventional | 5/31 | 90 ± 5 | .84 |
| Hyperfractionation | 4/28 | 85 ± 7 |
Toxicities
Asparaginase-related toxicities were observed in 29% of the 377 patients. The most frequent toxicities included allergic reactions (15%), pancreatitis (7%), and coagulopathy (defined as thromboses or clinical bleeding) (4.5%). One percent of the patients experienced a CNS thrombosis. Patients aged 9-18 years were more likely to experience an asparaginase-related toxicity compared with those less than 9 years (48% vs 24%; P < .01). Patients aged 9-18 years had a higher incidence of pancreatitis than younger patients (15% vs 5%;P < .01). The older patients also had a higher incidence of thromboses, including strokes (15% vs 2%; P < .01), but not allergic events (14% vs 16%; P = 1.00). Of the patients randomized to PEG asparaginase, 25% experienced a toxic reaction compared with 36% of E coli–randomized patients (P = .09). PEG asparaginase was associated with a lower incidence of mild allergic reactions (P = .02). There was no difference between the 2 preparations in the rates of dose-limiting toxicities such as severe allergic reaction (P = .22), severe pancreatitis (P = .78), or CNS thrombosis (P = 1.00).
There were 59 (16%) patients who experienced at least one bone fracture on therapy. There were no second malignancies or overt congestive heart failure observed in any patient. Follow-up time was insufficient to assess late CNS toxicity including growth parameters and neuropsychologic functioning as well as the incidence of late cardiac toxicity. Data regarding other toxicities, such as the incidence of seizures and osteonecrosis, were not prospectively collected.
Asparaginase intolerance and outcome
We analyzed the outcome of patients who were alive and in CR 40 weeks after diagnosis (and thus potentially could have received all 30 weeks of asparaginase intensification therapy) to determine whether asparaginase allergy and/or intolerance impacted subsequent outcome. Of the 352 patients included in the analysis, 54 (15%) patients experienced one or more allergic events. There was no difference in EFS when comparing those patients who developed an asparaginase allergy with those who did not (P = .31).
Of the 352 patients, 43 (12%) patients received less than 25 weeks of asparaginase. The remaining 308 (88%) patients received at least 26 weeks of asparaginase. Of the 43 patients who received less than 25 weeks of asparaginase, 37 (86%) patients experienced an asparaginase-related dose-limiting toxicity including pancreatitis (39% of 43 patients), allergy to one or more preparations (19%), CNS thrombosis/hemorrhage (12%), non-CNS deep venous thrombosis (7%), hyperglycemia (5%), hyperlipidemia (2%), and hepatitis (2%). Six (14%) patients received truncated therapy for other reasons including 2 patients with toxicities not clearly related to asparaginase (parasthesias and sepsis), 2 patients with non-protocol alteration in therapy, and 2 patients for unknown reasons. Asparaginase intolerance was associated with older age at diagnosis, but not with initial type of asparaginase (PEG or native E coli). Of 76 patients aged 9-18 years, 18 (24%) patients received less than 25 weeks of asparaginase compared with 25 (9%) of 276 patients less than 9 years of age (P < .01).
The 5-year EFS of patients who received less than 25 weeks of asparaginase therapy was significantly worse than those who received at least 26 weeks of asparaginase (73% ± 7% vs 90% ± 2%;P < .01) (Table 2). In multivariate Cox-proportional hazards model, including covariates for asparaginase intolerance, age, presenting WBC count, and immunophenotype, asparaginase intolerance was the only prognostically significant factor predicting EFS (P < .01).
Comparison with prior DFCI Consortium protocols
We compared EFS and LFS of patients treated on Protocol 91-01 with those achieved by patients treated on the 3 prior protocols conducted by the DFCI Consortium between 1981 and 1995. Details of Protocols 81-01 and 85-01 have been previously published.5 6 Risk group classification and presenting features were similar in all 4 protocols (Table 5). There was no significant difference in remission death rates (P = .74). EFS was significantly different when comparing the 4 protocols (P = .03), with the EFS and LFS for patients treated on Protocol 91-01 superior to those achieved by patients treated on prior DFCI ALL Consortium protocols conducted between 1981 and 1991 (P = .03 for EFS and P = .02 for LFS comparisons). The differences in EFS and LFS remained statistically significant when stratified by risk group (P = .03 andP = .02, respectively). EFS of Protocol 91-01 was significantly greater than Protocol 81-01 (P < .01), and there was a trend toward improved EFS on 91-01 that did not reach statistical significance when compared individually to Protocols 85-01 (P = .37) and 87-01 (P = .16).
Comparison of 4 consecutive DFCI Consortium Protocols (1981-1995)
| . | Protocols . | |||
|---|---|---|---|---|
| 81-01 . | 85-01 . | 87-01 . | 91-01 . | |
| Years conducted | 1981-1985 | 1985-1987 | 1987-1991 | 1991-1995 |
| Patients, no. | 289 | 220 | 369 | 377 |
| HR, % | 62 | 63 | 62 | 64 |
| Treatment differences | ||||
| Induction | ||||
| Methotrexate | high vs low | low | high vs low | high |
| Asparaginase | none | 1 dose | 1 dose | none |
| Weeks of therapy during intensification | 20 | 20 | 20 | 30 |
| CNS therapy | ||||
| SR, cGy | 1800 | 1800 | no XRT | no XRT (female), 1800 (male) |
| HR, cGy | 2800 | 2400 | 1800 | 1800 |
| Post-remission steroid | prednisone | prednisone | prednisone | dexamethasone |
| Remission death rate, % | 3.5 | 3.6 | 2.0 | 3.2 |
| 5-y ± SE, % | ||||
| EFS | 74 ± 3 | 78 ± 3 | 78 ± 2 | 83 ± 25-150 |
| LFS | 79 ± 3 | 82 ± 3 | 80 ± 2 | 87 ± 25-151 |
| . | Protocols . | |||
|---|---|---|---|---|
| 81-01 . | 85-01 . | 87-01 . | 91-01 . | |
| Years conducted | 1981-1985 | 1985-1987 | 1987-1991 | 1991-1995 |
| Patients, no. | 289 | 220 | 369 | 377 |
| HR, % | 62 | 63 | 62 | 64 |
| Treatment differences | ||||
| Induction | ||||
| Methotrexate | high vs low | low | high vs low | high |
| Asparaginase | none | 1 dose | 1 dose | none |
| Weeks of therapy during intensification | 20 | 20 | 20 | 30 |
| CNS therapy | ||||
| SR, cGy | 1800 | 1800 | no XRT | no XRT (female), 1800 (male) |
| HR, cGy | 2800 | 2400 | 1800 | 1800 |
| Post-remission steroid | prednisone | prednisone | prednisone | dexamethasone |
| Remission death rate, % | 3.5 | 3.6 | 2.0 | 3.2 |
| 5-y ± SE, % | ||||
| EFS | 74 ± 3 | 78 ± 3 | 78 ± 2 | 83 ± 25-150 |
| LFS | 79 ± 3 | 82 ± 3 | 80 ± 2 | 87 ± 25-151 |
Comparison of Protocol 91-01 with prior protocols:
EFS P = .03
LFS P = .02
Discussion
Children with ALL treated on Protocol 91-01 had a superior outcome to those treated on prior DFCI ALL Consortium protocols. The estimated 5-year EFS ± SE rate of 83% ± 2% is also superior to results reported by other investigators for similar groups of unselected patients with ALL.1-3 We also observed a significant improvement in LFS achieved on Protocol 91-01 compared to prior protocols, indicating that the superior outcome was not simply due to a reduction in remission deaths associated with advances in supportive care during the last decade.
We have previously demonstrated that early post-remission intensification with high-dose asparaginase improved outcome.22 On Protocol 91-01, duration of asparaginase intensification was extended from 20 to 30 weeks. Asparaginase intolerance (failure to receive at least 26 weeks of asparaginase) was an independent predictor of adverse outcome on multivariate analysis, suggesting that the prolongation of asparaginase therapy may have contributed to the improved outcome on Protocol 91-01. A prospective randomized trial comparing 20 versus 30 weeks of asparaginase intensification is necessary to confirm this finding. Although PEG asparaginase decreased the rate of mild allergic reactions, it was not associated with a decreased incidence of dose-limiting toxicities nor with improved outcome. Alternative asparaginase preparations or dosing strategies may mitigate severe toxicities, thereby allowing a greater proportion of patients to tolerate the extended intensification phase and improving outcome.
The other major differences between Protocol 91-01 and prior DFCI ALL Consortium protocols was the use of dexamethasone instead of prednisone during post-remission therapy and the inclusion of 5 randomized studies. None of the investigational arms of the randomized studies were associated with a statistically significant superior outcome, although, by design, only the 6-MP randomization (high versus low) was powered sufficiently to detect EFS differences.
The use of dexamethasone may have contributed to the improved outcome on Protocol 91-01, as other investigators have reported that dexamethasone has more potent in vitro antileukemic activity, higher free plasma levels, and enhanced CSF penetration, and dexamethasone may be more effective than prednisone or prednisolone in the treatment of childhood ALL.12,14,23,24 The relative toxicities of dexamethasone and prednisone have not been fully elucidated in patients with ALL. We have reported that the substitution of dexamethasone for prednisone during remission induction was associated with a high incidence of septic episodes and deaths25 and that post-remission dexamethasone may be associated with more severe neurocognitive late effects.26 The Children's Cancer Group recently reported a 9.3% incidence of osteonecrosis in patients treated with both prednisone and dexamethasone, with higher rates noted in patients receiving higher cumulative doses of dexamethasone.27 The incidence of osteonecrosis was not prospectively collected on Protocol 91-01, although we observed that 16% of patients experienced a fracture. We are currently conducting a prospective randomized comparison of dexamethasone and prednisone that could help clarify their relative efficacy and toxicity in the treatment of childhood ALL.
We observed a very low CNS relapse rate, with a 5-year CNS LFS rate of 98% in SR patients and 96% in HR patients. All patients except SR girls (80% of patients) received 1800 cGy cranial radiation. SR boys received cranial radiation because we had observed a higher-than-expected CNS relapse rate in this population when they were treated without radiation on a prior protocol. Other investigators have successfully used alternative CNS treatment strategies including the use of high-dose antimetabolite therapy and/or intensive intrathecal chemotherapy in lower risk patients4,23,28,29 and lower cranial radiation doses (12 Gy) in higher risk patients.30Although such treatments may offer comparable efficacy as conventionally fractionated 1800 cGy cranial radiation, the relative long-term neuropsychological sequelae of the various CNS treatment strategies remains unsettled.8,31 32
On Protocol 91-01 we investigated whether hyperfractionated cranial radiation might reduce late effects without compromising efficacy. While longer follow-up is needed to assess late effects, the use of hyperfractionated radiation therapy in HR patients was associated with an inferior outcome due to a higher marrow relapse rate, but not a higher CNS relapse rate. At this time we do not have an explanation for this unexpected finding. Currently, we are using intensive intrathecal therapy (without cranial radiation) in all SR patients. We have also adopted NCI age and WBC criteria to risk-stratify patients, thereby resulting in a higher proportion of patients being considered SR, so that only 40% of patients now considered HR receive cranial radiation. We are investigating whether the dose of cranial radiation can be decreased in HR patients without compromising efficacy, as has been reported by others.1
Other than ability to tolerate at least 26 weeks of asparaginase therapy, age at diagnosis was the only significant predictor of outcome on Protocol 91-01. The 2 age groups with relatively inferior prognoses were infants aged less than 1 year and children aged at least 9 years. The poor prognosis of infants with ALL has been reported by many investigators.33 We have previously demonstrated improved outcome for infants (50-month EFS, 54%) treated with therapy, which is nearly identical to that administered on Protocol 91-01, including the use of high-dose methotrexate and high-dose cytarabine.15Building upon these results and those of others suggesting that cytarabine may be an important component of therapy for infants with ALL, we are currently enrolling infants on an international collaborative protocol that includes high- and low-dose cytarabine during several phases of therapy.33
Several other studies have also reported that older children and adolescents with ALL fare worse than younger patients.1,2The inferior outcome of older children may be due to differences in the underlying biology of their disease, but also to an inability to tolerate the intensive 2-year regimen. While no patient aged 9-18 years experienced a remission death, older age at diagnosis was associated with an increased risk of developing a dose-limiting asparaginase-related toxicity, which may have negatively impacted subsequent outcome. Despite this increased vulnerability to dose-limiting toxicity, the outcome of older children on Protocol 91-01 (5-year EFS, 77%) compares favorably with recent reports by other investigators (EFS rates of 60% to 70% for similarly aged patients).1 2
There were no other statistically significant prognostic factors. For the first time, there was no significant difference in outcome based upon risk group status (P = .24). The results of Protocol 91-01 suggest that in the era of intensive multi-agent regimens, we are reaching the limits of prognostic significance of currently applied clinical risk factors. Other potentially important risk factors include early response to therapy,34,35 minimal residual disease levels at various time-points during therapy,36-38 and the presence of molecular abnormalities such as TEL-AML1 gene fusion.39-41 To meaningfully improve upon the results of Protocol 91-01, the prognostic significance of these and other factors needs to be established. In this way, more intensive regimens and novel therapies can be evaluated in children at highest risk of relapse, while attempts are made to diminish long-term toxicity in those who are successfully treated with current therapies.
We thank the patients, families, physicians, nurses, data managers, and all others who participated in this trial. We acknowledge the fundamental contributions of Kristin Barrett, Mia Donnelly, Jennifer Peppe, Molly Schwenn, Joyce Su, Sharon Thornhill, Stacy Waters, and Guangyong Zou.
Supported in part by grants CA 68484 and CA 06516 from the National Institutes of Health, Bethesda, MD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Lewis B. Silverman, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115; e-mail:lewis_silverman@dfci.harvard.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal