Advances in chemotherapy have led to a favorable long-term prognosis in approximately 50% of patients with aggressive non-Hodgkin lymphoma (NHL). However, the remaining patients do not enjoy such prolonged survival after standard treatment. New prognostic factors are needed to define this poor-prognosis group and to plan an appropriate treatment strategy. It has been reported that serum nm23-H1 protein may be a new prognostic factor for aggressive NHL. In the present study involving multiple institutions and a large number of patients, the level of nm23-H1 protein was compared among different types of lymphoma; it was lowest for indolent lymphoma, followed by aggressive lymphoma and then highly aggressive lymphoma. In addition, patients with aggressive NHL and higher nm23-H1 levels had worse overall and progression-free survival rates than those with lower nm23-H1 levels. The nm23-H1 level was also compared between patients with diffuse large B-cell lymphoma and patients with peripheral T-cell lymphoma. The results suggest that the level of nm23-H1 could serve as a prognostic factor in both groups. Moreover, the prognosis of lymphoma patients could be ascertained even more precisely by combining soluble interleukin-2 receptor or soluble CD44 and nm23-H1 levels. A multivariate analysis confirmed that the nm23-H1 level is an independent and important prognostic factor in aggressive NHL. Therefore, it may provide useful information for clinicians to determine the appropriate therapy for each type of lymphoma.
Introduction
Combination chemotherapy has improved the outcome of patients with aggressive non-Hodgkin lymphoma (NHL).1-3The treatment strategy for aggressive NHL is based on the 5 independent prognostic factors of the International Prognostic Index (IPI), namely age, performance status (PS), number of extranodal lesions, Ann Arbor stage, and serum lactate dehydrogenase (LDH) level.4 It is important to analyze the prognostic factors for NHL at the time of the initial medical examination and to apply the above classification to the treatment plan. The standard therapy,5,6 in which the CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) regimen plays a central role, is used in patients with an index of 0 or 1 (low-risk [L] group) or 2 (low-intermediate [L-I] risk group). However, high-intermediate (H-I) and high-risk (H) patients do not respond well to the CHOP therapy, and thus more potent treatments have been used on an experimental basis.7
In addition, various biologic prognostic factors have been studied recently, including serum cytokines such as soluble interleukin-2 receptor (sIL-2R),8 IL-6,9IL-10,10 intercellular adhesion molecule-1,11and soluble CD4412; and the p53 gene mutation,13 bcl-2 protein,14 and the bcl-6 gene alteration.15 By examining information derived from lymphoma biology and novel prognostic factors, or by combining various prognostic factors and investigating additional prognostic information, which should be added to the existing basic system, we can anticipate the establishment of treatment strategies based on each NHL prognosis-predicting factor.
The nm23 proteins are involved in tumor metastasis regulation and have nucleoside diphosphate kinase enzyme activity.16,17 Six isotypes of the human nm23 gene (nm23-H1, nm23-H2, DR-nm23, nm23-H4, nm23-H5, and nm23-H6) have been identified. Immunohistochemical studies have reported that patients with high-grade NHL and Hodgkin lymphoma exhibited significantly higher levels of nm23-H1 expression than did those with low-grade NHL.18 Recently, we established an enzyme-linked immunosorbent assay (ELISA) technique to determine the serum level of nm23-H1 protein. We previously reported that the levels of nm23-H1 in aggressive NHL were significantly higher than those in controls and that the prognosis for patients with nm23-H1 levels of 80 ng/mL and above was poor.19 Therefore, measurement of nm23-H1 protein might be useful for selecting the treatment method. In the present study, a more detailed analysis with a large number of patients at multiple institutions, we examined the nm23-H1 level in each histopathologic type of NHL and confirmed that the nm23-H1 level could be used as an independent prognostic factor in patients with diffuse large B-cell lymphoma (DLBCL) and peripheral T-cell lymphoma (PTCL). In addition, the present study was conducted on 4 individual IPI groups. The prognostic significance of this protein was enhanced when examined in combination with sIL-2R or sCD44. The present results suggest that nm23-H1 measurement may be useful for planning a treatment strategy in combination with IPI and histopathologic analysis.
Patients, materials, and methods
Patients
We measured nm23-H1 levels in a consecutive series of 606 untreated patients whose clinical course was being managed by the Adult Lymphoma Treatment Study Group in Japan from 1988 to 1999. (None of the patients at Toho University School of Medicine included in the previously published study in Blood19were included in the present study.) Of these 606 patients, 548 had NHL, including 108 with the indolent type and 440 with aggressive lymphoma according to the revised European-American classification of lymphoid neoplasms (REAL) schema.20 Indolent lymphoma was of the B-cell lineage in all patients, including 76 with low-grade B-cell lymphoma (LGBL), 12 with marginal-zone B-cell lymphoma (MZBL), and 20 with mantle cell lymphoma (MCL). Aggressive lymphoma was of B-cell lineage in 364 patients and of T-cell lineage in 76. The aggressive B-cell lymphoma was follicular large cell lymphoma (FLCL) in 32, DLBCL in 314, lymphoblastic lymphoma (LBL) in 7, and small non–cleaved cell lymphoma (SNCL) in 11. The aggressive T-cell type was PTCL in 63 patients, angioimmunoblastic lymphoma (AIL) in 8, anaplastic large cell lymphoma (T- and null-cell types) in 1, and nasal and nasal-type NK/T-cell lymphoma (NK/T) in 4. In addition, 40 patients had Hodgkin disease and 18 others had adult T-cell leukemia/lymphoma (ATLL). The median age was 66 years (range, 18-80 years).
Clinical staging was performed according to the Ann Arbor classification system.21 Evaluation included a complete history and physical examination: chest roentgenography; bone marrow aspiration and biopsy; computed tomography of the chest, abdomen, and pelvis; hemogram and differential counts; and routine biochemistry tests. Laparotomy was not performed for staging. IPI was determined in all NHL cases.4 Patients with aggressive NHL and disseminated disease were treated with cyclophosphamide, vincristine, prednisolone, bleomycin, doxorubicin, and procarbazine (COP-BLAM)22; cyclophosphamide, doxorubicin, methotrexate, bleomycin, vincristine, etoposide, ifosfamide, and prednisolone (CAMBO-VIP)23; or another anthracycline-containing combination chemotherapy regimen. Indolent lymphoma was usually treated with a single agent or cyclophosphamide, vincristine, and prednisone (COP). In addition to the chemotherapy, 386 patients received megavoltage radiotherapy. Patients were followed up at intervals of a few months. Reevaluation included physical examination; hemogram and differential counts; biochemistry tests; and computed tomography of the chest, abdomen, and pelvis. The median follow-up time was 63 months (range, 5-121 months), and the final follow-up examination was performed in April 2000. Serum samples were obtained from 45 healthy volunteers with a mean age of 57 years (range, 18-84 years) for comparison. An approval was obtained from the institutional review board at the Toho University School of Medicine for these studies. Informed consent was provided according to the Declaration of Helsinki.
ELISA for human nm23-H1
We previously established an ELISA procedure to determine nm23-H1 protein levels in the serum.19,24 The measurements were made at the Department of Immunology, SRL, Tokyo, Japan. Soluble IL-2R was measured by sandwich ELISA as described in the literature.8 Soluble CD44 was measured by sandwich ELISA using the sCD44std ELISA kit (Bender Medsystems, Vienna, Austria).
Statistical analysis
The patients' characteristics were compared using χ2 tests. Complete remission (CR) was defined as an absence of detectable disease on clinical, radiologic criteria. Judgment criteria used for the analysis were progression-free survival (PFS) and overall survival (OS). Progression was defined as a progression of the lymphoma in nonresponding patients or partial-response patients, a relapse for complete-response patients, or death from any cause without progression. PFS was calculated as the duration from randomization to the date of the first progression. Patients who did not experience progression at the time of analysis were censored at the most recent date of disease assessment or at the stop date if the most recent date was later. OS was measured from the date of randomization to the date of death, regardless of cause. Patients who had not died at the time of analysis were censored at the most recent date when they were known to be alive or at the stop date if the most recent date was later. Survival analysis was performed according to the Kaplan-Meier method.25 The statistical significance of differences in survival was determined by the log-rank and generalized Wilcoxon tests.26 Differences between groups were evaluated by the Mann-Whitney U-test (nonparametric analysis),27 and P < .05 was taken to indicate statistical significance. Multivariate analysis of the prognosis was performed using Cox's proportional-hazards regression model.28 All calculations were performed with SAS software (version 6.10; SAS Institute, Cary, NC).
Results
Effect of freezing on nm23-H1 protein levels of serum samples
The effect of freeze-thawing was studied in 20 serum samples. Before freezing, serum nm23-H1 protein levels of the 20 treated samples ranged from 0 to 112 ng/mL (median, 32.1 ng/mL), whereas after freezing and thawing, the range was from 0 to 110 ng/mL (median, 31.9 ng/mL). Hence, freezing and thawing of the serum samples did not have any marked effect on serum nm23-H1 protein levels.
Examination of nm23-H1 protein levels in each histologic type of NHL and healthy controls
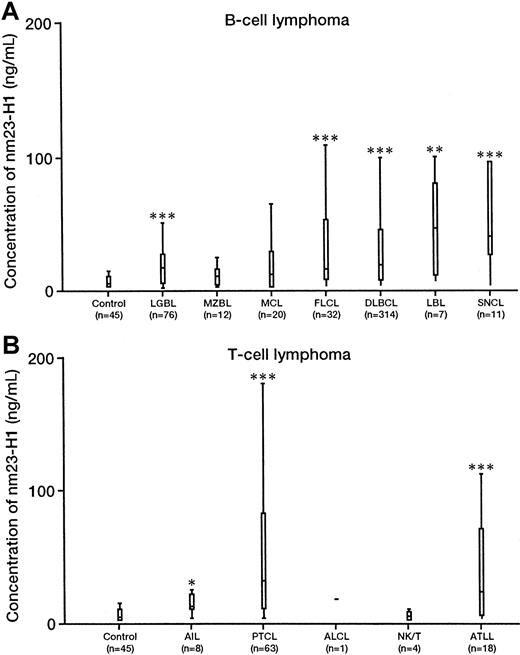
The serum level of nm23-H1 (mean ± SD) was significantly elevated in patients with malignant lymphoma (n = 606, 34.61 ± 53.35 ng/mL) compared with that in healthy controls (n = 45, 3.89 ± 4.06 ng/mL; P = .00001). The serum levels of nm23-H1 in Hodgkin disease (n = 40, 24.23 ± 23.13 ng/mL,P = .00001); indolent NHL (n = 108, 23.40 ± 42.82 ng/mL, P = .00001); aggressive NHL (n = 440, 38.33 ± 52.47 ng/mL, P = .00001); and ATLL (n = 18, 40.82 ± 50.36 ng/mL, P = .00027) were significantly higher than those in healthy controls. Next, we examined B-cell lymphoma (Figure 1A). We compared the nm23-H1 levels (mean ± SD) in the following types with the control levels: LGBL (n = 76, 19.6 ± 24.2 ng/mL,P = .00001); MZBL (n = 12, 9.1 ± 8.1 ng/mL, not significant [NS]); MCL (n = 20, 40.0 ± 85.2 ng/mL, NS); FLCL (n = 32, 26.9 ± 29.9 ng/mL, P = .00024); DLBCL (n = 314, 35.2 ± 46.1 ng/mL, P = .00001); LBL (n = 7, 43.0 ± 37.9 ng/mL, P = .00839); and SNCL (n = 11, 71.62 ± 75.59 ng/mL, P = .00018). Among the various histologic types, the level of nm23-H1 in SNCL was significantly higher than the levels in LGBL (P = .028) and MZBL (P = .02). There were no significant differences among other histologic types, but the level of nm23-H1 was highest in SNCL, followed by DLBCL, FLCL, LGBL, MZBL, and MCL. Hence, the level of nm23-H1 was lowest for indolent lymphoma, followed by aggressive lymphoma and highly aggressive lymphoma. For T-cell lymphoma (Figure 1B), we compared nm23-H1 levels in the following types to control levels: AIL (n = 8, 12.6 ± 7.7 ng/mL,P = .03323); PTCL (n = 63, 59.35 ± 79.17 ng/mL,P = .00001); NK/T (n = 4, 3.75 ± 4.11 ng/mL, NS); and ATLL (n = 18, 40.81 ± 50.36 ng/mL, P = .00038). Although there were no significant differences in the level of nm23-H1 among the different histologic types of T-cell lymphoma, it was higher in PTCL and ATLL.
Serum levels of nm23-H1 protein in non-Hodgkin lymphoma and healthy controls.
Upper and lower lines indicate the 10th and 90th percentiles; boxes indicate the 25th and 75th percentiles. The line through each box indicates the median. ALCL indicates anaplastic large cell lymphoma. Non-Hodgkin lymphoma; (n = 606). Healthy controls; (n = 45). (P = .0001; Wilcoxon test). *P < .05; **P < .01; ***P < .0001.
Serum levels of nm23-H1 protein in non-Hodgkin lymphoma and healthy controls.
Upper and lower lines indicate the 10th and 90th percentiles; boxes indicate the 25th and 75th percentiles. The line through each box indicates the median. ALCL indicates anaplastic large cell lymphoma. Non-Hodgkin lymphoma; (n = 606). Healthy controls; (n = 45). (P = .0001; Wilcoxon test). *P < .05; **P < .01; ***P < .0001.
Correlation of nm23-H1 levels to other serum markers in NHL
The correlation between nm23-H1 and other known serum markers was examined in aggressive NHL. There was a slight correlation between nm23-H1 and LDH (r = 0.417, P = .0001), but not with sIL-2R (r = 0.234, P = .0001), β2-microglobulin (β2-MG) (r = 0.275, P = .0001), or sCD44 (r = 0.190, P = .0001) in patients with aggressive B cell–NHL (B-NHL). We next examined aggressive T cell–NHL (T-NHL) and also observed a weak correlation between nm23-H1 and LDH (r = 0.392, P = .0005), but no correlation with sIL-2R (r = 0.217, NS), β2-MG (r = 0.197, NS), or sCD44 (r = −0.06, NS).
Relation between serum nm23-H1 protein levels and clinicopathologic data in NHL
The relation between nm23-H1 protein levels and clinicopathologic factors was investigated in 440 patients with aggressive NHL. When the median nm23-H1 concentration was used as the cutoff value, a high serum level of nm23-H1 before treatment was strongly associated with poor prognostic features, such as greater age, poor PS, elevated serum LDH level, higher Ann Arbor stage, greater number of extranodal sites, and B symptoms (P < .01 for all comparisons, Mann-Whitney U-test or Kruskal-Wallis test). The assessment of each risk group classified according to IPI revealed significantly higher levels of nm23-H1 in the H-I and H risk groups than in the L and L-I risk groups (P = .0001, Kruskal-Wallis test). CR was achieved in 346 (78.6%) of the 440 patients, and the mean serum level of nm23-H1 in these 346 patients was 20.25 ± 24.65 ng/mL at the time of diagnosis. This value was significantly lower than that in patients who showed a partial remission or failed to respond (92.34 ± 98.56 ng/mL, P = .0001), suggesting a close relation between the serum level of nm23-H1 and therapeutic response. We also examined the 314 DLBCL patients and found that the percentage of patients with higher nm23-H1 was greater (P < .01 for all comparisons, Mann-Whitney U-test or Kruskal-Wallis test) among those aged 60 or above and those with PS 2 to 4, elevated serum LDH level, more advanced Ann Arbor stage, and 2 or more extranodal sites. Moreover, nm23-H1 was significantly higher in non-CR than in CR patients (P = .001). A previous study19involving 149 patients with aggressive NHL did not indicate significant correlations between the level of nm23-H1 and age, serum LDH level, or B symptoms, but such correlations were found in the present analysis.
Correlation of nm23-H1 with survival and PFS
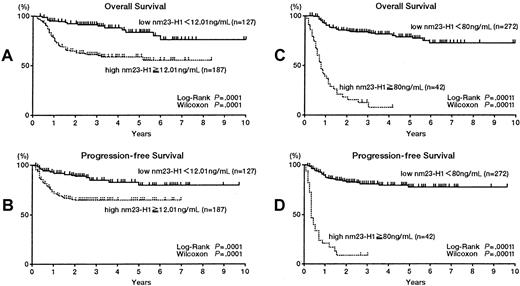
In this multiple-institution study, we examined the survival of 440 patients with aggressive NHL and found that the 5-year survival rates for the high nm23-H1 group (≥ 12.01 ng/mL, n = 268) and low nm23-H1 group (< 12.01 ng/mL, n = 172) were 53.3% and 77.1%, respectively (P = .0001 for both the log-rank test and generalized Wilcoxon test). Respective PFS values were 60.9% and 80.8% (P = .0001). We set various cutoff points over 12.01 ng/mL, which was the upper limit in control serum (3.89 + 2 SD). We also examined patients with a cutoff point of 80 ng/mL for nm23-H1. The 4-year survival rates for the high nm23-H1 group (≥ 80 ng/mL, n = 65) and low nm23-H1 group (< 80 ng/mL, n = 375) were 6.0% and 71.5%, respectively (P = .0001), with PFS values of 10.0% and 76.3% (P = .0001).
Next we analyzed DLBCL (Table 1) and PTCL patients. The 314 patients with DLBCL were divided into 2 groups according to different serum nm23-H1 levels. All cutoff values showed significant prognostic value (Table 1). Figures2A and B show the results using a cutoff value of 12.01 ng/mL. The 5-year survival rates for the high and low nm23-H1 groups were 58.7% and 83.6%, respectively (P = .0001), with respective PFS values of 64.8% and 79.5% (P = .0001). Figures 2C and D show the results using a cutoff value of 80 ng/mL. The 4-year survival rates for the high and low nm23-H1 groups were 5.5% and 71.4%, respectively (P = .0001), with PFS values of 7.9% and 77.8% (P = .0001). We also examined PTCL and found that the 5-year survival rates were 37.6% if nm23-H1 was 12.01 ng/mL or above and 93.3% if nm23-H1 was below 12.01 ng/mL (P = .0009 for the log-rank test and P = .0013 for the generalized Wilcoxon test). Respective PFS values were 46.3% and 87.4% (P = .006 for the log-rank test and P = .006 for the generalized Wilcoxon test) (Figures 3A,B). The 3-year survival rates were 0% for nm23-H1 of 80 ng/mL or higher and 81.1% for nm23-H1 below 80 ng/mL (P = .0001), with respective PFS values of 0% and 75.8% (P = .0001) (Figures3C,D).
Prognostic factors in a univariate analysis of diffuse large B-cell lymphoma
| Factor . | No. of patients . | 5-year survival (%) . | Relative risk (95% CI) . | P value . | 5-year PFS (%) . | Relative risk (95% CI) . | P value . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wilcoxon test . | Log-rank test . | Wilcoxon test . | Log-rank test . | |||||||||||||||
| International Prognostic Index | ||||||||||||||||||
| L + L-I | 192 | 85.9 | 7.18 | .0001 | .0001 | 80.1 | 3.77 | .0001 | .0001 | |||||||||
| H-I + H | 122 | 39.2 | (4.39-11.77) | 52.2 | (2.36-6.01) | |||||||||||||
| WHO performance status | ||||||||||||||||||
| 0-1 | 237 | 80.5 | 4.58 | .0001 | .0001 | 75.8 | 2.84 | .0001 | .0001 | |||||||||
| 2-4 | 77 | 35.5 | (3.01-6.99) | 50.4 | (1.78-4.53) | |||||||||||||
| Serum LDH level | ||||||||||||||||||
| Normal | 117 | 89.5 | 5.00 | .0001 | .0001 | 82.0 | 2.90 | .0001 | .0001 | |||||||||
| Above normal | 197 | 53.1 | (2.71-9.24) | 63.4 | (1.66-5.05) | |||||||||||||
| Ann Arbor stage | ||||||||||||||||||
| I, II | 139 | 81.0 | 3.05 | .0001 | .0001 | 82.8 | 2.87 | .0001 | .0001 | |||||||||
| III, IV | 175 | 57.3 | (1.86-4.99) | 59.3 | (1.70-4.83) | |||||||||||||
| Extranodal sites | ||||||||||||||||||
| 0-1 | 280 | 72.1 | 3.74 | .0001 | .0001 | 72.6 | 2.92 | .0001 | .0002 | |||||||||
| > 1 | 34 | 35.8 | (2.28-6.12) | 48.3 | (1.63-5.22) | |||||||||||||
| B symptoms | ||||||||||||||||||
| Absent | 231 | 74.2 | 2.25 | .0002 | .0001 | 73.4 | 1.83 | .0072 | .0120 | |||||||||
| Present | 83 | 51.1 | (1.47-3.46) | 61.5 | (1.13-2.94) | |||||||||||||
| Bulky disease | ||||||||||||||||||
| Absent | 266 | 70.3 | 1.30 | .5777 | .3522 | 72.0 | 1.41 | .5623 | .2404 | |||||||||
| Present | 46 | 56.5 | (0.74-2.26) | 56.8 | (0.79-2.52) | |||||||||||||
| Age at diagnosis (yr) | ||||||||||||||||||
| < 60 | 124 | 83.5 | 3.18 | .0001 | .0001 | 83.0 | 2.46 | .0008 | .0006 | |||||||||
| ≥ 60 | 190 | 57.9 | (1.87-5.40) | 61.2 | (1.44-4.17) | |||||||||||||
| Sex | ||||||||||||||||||
| Male | 164 | 69.2 | 1.06 | .7366 | .7913 | 69.6 | 1.00 | .9428 | .9940 | |||||||||
| Female | 150 | 63.7 | (0.70-1.61) | 71.5 | (0.64-1.58) | |||||||||||||
| nm23-H1 level (ng/mL) | ||||||||||||||||||
| Control + 2SD ≤ 12.01 | 127 | 83.6 | 3.87 | .0001 | .0001 | 79.5 | 2.72 | .0001 | .0001 | |||||||||
| Control + 2SD > 12.01 | 187 | 58.7 | (2.24-6.68) | 64.8 | (1.60-4.63) | |||||||||||||
| Median ≤ 17.00 | 158 | 83.2 | 4.33 | .0001 | .0001 | 80.4 | 3.32 | .0001 | .0001 | |||||||||
| Median > 17.00 | 156 | 53.9 | (2.62-7.16) | 60.3 | (2.00-5.49) | |||||||||||||
| point ≤ 32.50 | 209 | 81.6 | 5.22 | .0001 | .0001 | 79.5 | 4.11 | .0001 | .0001 | |||||||||
| point > 32.50 | 105 | 42.0 | (3.35-8.13) | 50.8 | (2.59-6.52) | |||||||||||||
| ¾ point ≤ 45.00 | 236 | 81.0 | 7.02 | .0001 | .0001 | 79.7 | 6.06 | .0001 | .0001 | |||||||||
| ¾ point > 45.00 | 78 | 53.9 | (4.55-10.83) | 37.7 | (3.83-9.60) | |||||||||||||
| ≤ 80.00 | 272 | 77.9 | 12.12 | .0001 | .0001 | 77.8 | 14.34 | .0001 | .0001 | |||||||||
| > 80.00 | 42 | 5.5 | (7.73-18.99) | 7.9 | (8.73-23.58) | |||||||||||||
| sIL-2 receptor level (U/mL) ≤ 1000 | 137 | 86.7 | 4.45 | .0001 | .0001 | 85.4 | 3.34 | .0001 | .0001 | |||||||||
| > 1000 | 177 | 53.4 | (2.58-7.66) | 57.5 | (1.96) | |||||||||||||
| sCD44 level (ng/mL) | ||||||||||||||||||
| Median ≤ 464.0 | 111 | 77.4 | 1.82 | .0248 | .0247 | 77.4 | 1.68 | .0557 | .0688 | |||||||||
| Median > 464.0 | 110 | 58.8 | (1.07-3.09) | 63.3 | (0.98-2.89) | |||||||||||||
| Control + 2SD ≤ 598.8 | 141 | 76.3 | 1.86 | .0291 | .0174 | 74.0 | 1.68 | .0401 | .0528 | |||||||||
| Control + 2SD > 598.8 | 80 | 53.6 | (1.11-3.11) | 63.5 | (0.99-2.66) | |||||||||||||
| ≤ 500 | 120 | 78.3 | 2.03 | .0093 | .0071 | 78.3 | 1.89 | .0243 | .0177 | |||||||||
| > 500 | 101 | 55.8 | (1.20-3.43) | 60.8 | (1.11-3.23) | |||||||||||||
| Factor . | No. of patients . | 5-year survival (%) . | Relative risk (95% CI) . | P value . | 5-year PFS (%) . | Relative risk (95% CI) . | P value . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wilcoxon test . | Log-rank test . | Wilcoxon test . | Log-rank test . | |||||||||||||||
| International Prognostic Index | ||||||||||||||||||
| L + L-I | 192 | 85.9 | 7.18 | .0001 | .0001 | 80.1 | 3.77 | .0001 | .0001 | |||||||||
| H-I + H | 122 | 39.2 | (4.39-11.77) | 52.2 | (2.36-6.01) | |||||||||||||
| WHO performance status | ||||||||||||||||||
| 0-1 | 237 | 80.5 | 4.58 | .0001 | .0001 | 75.8 | 2.84 | .0001 | .0001 | |||||||||
| 2-4 | 77 | 35.5 | (3.01-6.99) | 50.4 | (1.78-4.53) | |||||||||||||
| Serum LDH level | ||||||||||||||||||
| Normal | 117 | 89.5 | 5.00 | .0001 | .0001 | 82.0 | 2.90 | .0001 | .0001 | |||||||||
| Above normal | 197 | 53.1 | (2.71-9.24) | 63.4 | (1.66-5.05) | |||||||||||||
| Ann Arbor stage | ||||||||||||||||||
| I, II | 139 | 81.0 | 3.05 | .0001 | .0001 | 82.8 | 2.87 | .0001 | .0001 | |||||||||
| III, IV | 175 | 57.3 | (1.86-4.99) | 59.3 | (1.70-4.83) | |||||||||||||
| Extranodal sites | ||||||||||||||||||
| 0-1 | 280 | 72.1 | 3.74 | .0001 | .0001 | 72.6 | 2.92 | .0001 | .0002 | |||||||||
| > 1 | 34 | 35.8 | (2.28-6.12) | 48.3 | (1.63-5.22) | |||||||||||||
| B symptoms | ||||||||||||||||||
| Absent | 231 | 74.2 | 2.25 | .0002 | .0001 | 73.4 | 1.83 | .0072 | .0120 | |||||||||
| Present | 83 | 51.1 | (1.47-3.46) | 61.5 | (1.13-2.94) | |||||||||||||
| Bulky disease | ||||||||||||||||||
| Absent | 266 | 70.3 | 1.30 | .5777 | .3522 | 72.0 | 1.41 | .5623 | .2404 | |||||||||
| Present | 46 | 56.5 | (0.74-2.26) | 56.8 | (0.79-2.52) | |||||||||||||
| Age at diagnosis (yr) | ||||||||||||||||||
| < 60 | 124 | 83.5 | 3.18 | .0001 | .0001 | 83.0 | 2.46 | .0008 | .0006 | |||||||||
| ≥ 60 | 190 | 57.9 | (1.87-5.40) | 61.2 | (1.44-4.17) | |||||||||||||
| Sex | ||||||||||||||||||
| Male | 164 | 69.2 | 1.06 | .7366 | .7913 | 69.6 | 1.00 | .9428 | .9940 | |||||||||
| Female | 150 | 63.7 | (0.70-1.61) | 71.5 | (0.64-1.58) | |||||||||||||
| nm23-H1 level (ng/mL) | ||||||||||||||||||
| Control + 2SD ≤ 12.01 | 127 | 83.6 | 3.87 | .0001 | .0001 | 79.5 | 2.72 | .0001 | .0001 | |||||||||
| Control + 2SD > 12.01 | 187 | 58.7 | (2.24-6.68) | 64.8 | (1.60-4.63) | |||||||||||||
| Median ≤ 17.00 | 158 | 83.2 | 4.33 | .0001 | .0001 | 80.4 | 3.32 | .0001 | .0001 | |||||||||
| Median > 17.00 | 156 | 53.9 | (2.62-7.16) | 60.3 | (2.00-5.49) | |||||||||||||
| point ≤ 32.50 | 209 | 81.6 | 5.22 | .0001 | .0001 | 79.5 | 4.11 | .0001 | .0001 | |||||||||
| point > 32.50 | 105 | 42.0 | (3.35-8.13) | 50.8 | (2.59-6.52) | |||||||||||||
| ¾ point ≤ 45.00 | 236 | 81.0 | 7.02 | .0001 | .0001 | 79.7 | 6.06 | .0001 | .0001 | |||||||||
| ¾ point > 45.00 | 78 | 53.9 | (4.55-10.83) | 37.7 | (3.83-9.60) | |||||||||||||
| ≤ 80.00 | 272 | 77.9 | 12.12 | .0001 | .0001 | 77.8 | 14.34 | .0001 | .0001 | |||||||||
| > 80.00 | 42 | 5.5 | (7.73-18.99) | 7.9 | (8.73-23.58) | |||||||||||||
| sIL-2 receptor level (U/mL) ≤ 1000 | 137 | 86.7 | 4.45 | .0001 | .0001 | 85.4 | 3.34 | .0001 | .0001 | |||||||||
| > 1000 | 177 | 53.4 | (2.58-7.66) | 57.5 | (1.96) | |||||||||||||
| sCD44 level (ng/mL) | ||||||||||||||||||
| Median ≤ 464.0 | 111 | 77.4 | 1.82 | .0248 | .0247 | 77.4 | 1.68 | .0557 | .0688 | |||||||||
| Median > 464.0 | 110 | 58.8 | (1.07-3.09) | 63.3 | (0.98-2.89) | |||||||||||||
| Control + 2SD ≤ 598.8 | 141 | 76.3 | 1.86 | .0291 | .0174 | 74.0 | 1.68 | .0401 | .0528 | |||||||||
| Control + 2SD > 598.8 | 80 | 53.6 | (1.11-3.11) | 63.5 | (0.99-2.66) | |||||||||||||
| ≤ 500 | 120 | 78.3 | 2.03 | .0093 | .0071 | 78.3 | 1.89 | .0243 | .0177 | |||||||||
| > 500 | 101 | 55.8 | (1.20-3.43) | 60.8 | (1.11-3.23) | |||||||||||||
CI indicates confidence interval; PFS, progression-free survival; L, low risk; L-I, low-intermediate risk; H-I, high-intermediate risk; H, high risk; WHO, World Health Organization.
Overall survival and progression-free survival of patients with diffuse large B-cell lymphoma.
(A,B) Patients with high nm23-H1 (≥ 12.01 ng/mL; n = 187) had a worse prognosis than patients with low nm23-H1 (< 12.01 ng/mL; n = 127). (C,D) Patients with high nm23-H1 (≥ 80 ng/mL; n = 42) had a worse prognosis than patients with low nm23-H1 (< 80 ng/mL; n = 272).
Overall survival and progression-free survival of patients with diffuse large B-cell lymphoma.
(A,B) Patients with high nm23-H1 (≥ 12.01 ng/mL; n = 187) had a worse prognosis than patients with low nm23-H1 (< 12.01 ng/mL; n = 127). (C,D) Patients with high nm23-H1 (≥ 80 ng/mL; n = 42) had a worse prognosis than patients with low nm23-H1 (< 80 ng/mL; n = 272).
Overall survival (A,C) and progression-free survival (B,D) of patients with peripheral T-cell lymphoma.
(A,B) Patients with high nm23-H1 (≥ 12.01 ng/mL; n = 45) had a worse prognosis than patients with low nm23-H1 (< 12.01 ng/mL; n =18). (C,D) Patients with high nm23-H1 (≥ 80 ng/mL; n = 16) had a worse prognosis than patients with low nm23-H1 (< 80 ng/mL; n = 47).
Overall survival (A,C) and progression-free survival (B,D) of patients with peripheral T-cell lymphoma.
(A,B) Patients with high nm23-H1 (≥ 12.01 ng/mL; n = 45) had a worse prognosis than patients with low nm23-H1 (< 12.01 ng/mL; n =18). (C,D) Patients with high nm23-H1 (≥ 80 ng/mL; n = 16) had a worse prognosis than patients with low nm23-H1 (< 80 ng/mL; n = 47).
Significance of nm23-H1 in the OS and PFS of patients in various IPI groups
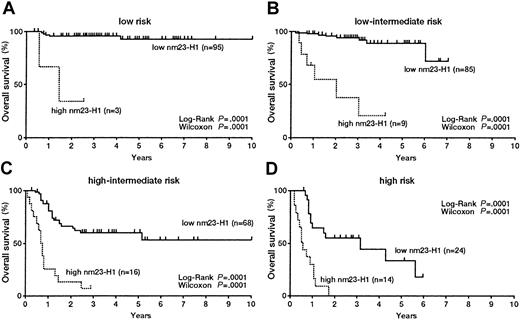
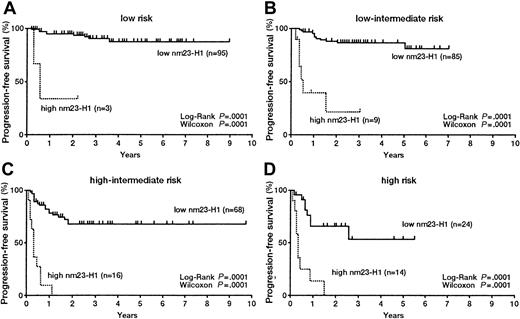
We evaluated the significance of serum nm23-H1 levels in DLBCL patients classified according to the IPI. In the L risk group, both OS (Figure 4A) and PFS (Figure5A) were worse for patients with an nm23-H1 level of 80 ng/mL or higher than for patients (n = 3, 3-year survival 33.3%) with a level lower than 80 ng/mL (n = 95, 10-year survival 92.3%) (P = .0001). These results indicate that the therapeutic outcome was worse when the nm23-H1 level was high. The prognosis for patients with nm23-H1 of 80 ng/mL or higher was also poor in the L-I, H-I, and H risk groups (P = .0001) (Figures 4B,C,D). We examined the PFS with a cutoff value for nm23-H1 of 80 ng/mL and obtained the following results: in the L risk group, high nm23-H1 (n = 3, 2-year PFS 33.3%) and low nm23-H1 (n = 95, 9-year PFS 87.0%); in the L-I risk group, high nm23-H1 (n = 9, 3-year PFS 19.1%) and low nm23-H1 (n = 85, 6-year PFS 79.0%); in the H-I risk group, high nm23-H1 (n = 16, 1-year PFS 8.3%) and low nm23-H1 (n = 68, 9-year PFS 68.0%); and in the H risk group, high nm23-H1 (n = 14, 2-year PFS 0%) and low nm23-H1 (n = 24, 6-year PFS 51.8%). The PFS of patients with high nm23-H1 was poor in all of these risk groups (P = .0001) (Figures 5A-D). We also examined aggressive NHL as a whole by dividing the patients into 2 groups according to an nm23-H1 level of at least 80 ng/mL and below 80 ng/mL. The examination of OS revealed significant differences (P = .0001) between high nm23-H1 (n = 5, 3-year survival 40.0%) and low nm23-H1 (n = 126, 10-year survival 89.4%) in the L risk group; high nm23-H1 (n = 18, 4-year survival 14.9%) and low nm23-H1 (n = 122, 10-year survival 72.0%) in the L-I risk group; high nm23-H1 (n = 23, 3-year survival 4.8%) and low nm23-H1 (n = 97, 10-year survival 56.2%) in the H-I risk group; and high nm23-H1 (n = 19, 2-year survival 0.0%) and low nm23-H1 (n = 122, 6-year survival 15.5%) in the H risk group. The results were similar when we examined PFS. There were significant differences (P = .0001) between high and low nm23-H1, as follows: high nm23-H1 (n = 5, 3-year PFS 40.0%) and low nm23-H1 (n = 126, 10-year PFS 86.0%) in the L risk group; high nm23-H1 (n = 18, 4-year PFS 16.2%) and low nm23-H1 (n = 122, 10-year PFS 76.9%) in the L-I risk group; high nm23-H1 (n = 23, 1-year PFS 9.2%) and low nm23-H1 (n = 97, 9-year PFS 67.6%) in the H-I risk group; and high nm23-H1 (n = 19, 2-year PFS 0.0%) and low nm23-H1 (n = 122, 6-year PFS 15.5%) in the H risk group. Therefore, we might be able to predict the therapeutic outcome in each IPI risk group of aggressive NHL by using an nm23-H1 cutoff value of 80 ng/mL at diagnosis.
Overall survival curves of patients with diffuse large B-cell lymphoma.
(A) Low risk; (B) low-intermediate risk; (C) high-intermediate risk; and (D) high-risk groups based on the International Prognostic Index.
Overall survival curves of patients with diffuse large B-cell lymphoma.
(A) Low risk; (B) low-intermediate risk; (C) high-intermediate risk; and (D) high-risk groups based on the International Prognostic Index.
Progression-free survival curves of patients with diffuse large B-cell lymphoma.
(A) Low risk; (B) low-intermediate risk; (C) high-intermediate risk; and (D) high-risk groups based on the International Prognostic Index.
Progression-free survival curves of patients with diffuse large B-cell lymphoma.
(A) Low risk; (B) low-intermediate risk; (C) high-intermediate risk; and (D) high-risk groups based on the International Prognostic Index.
Assessment of the survival of patients with aggressive NHL using nm23-H1 and other serum markers
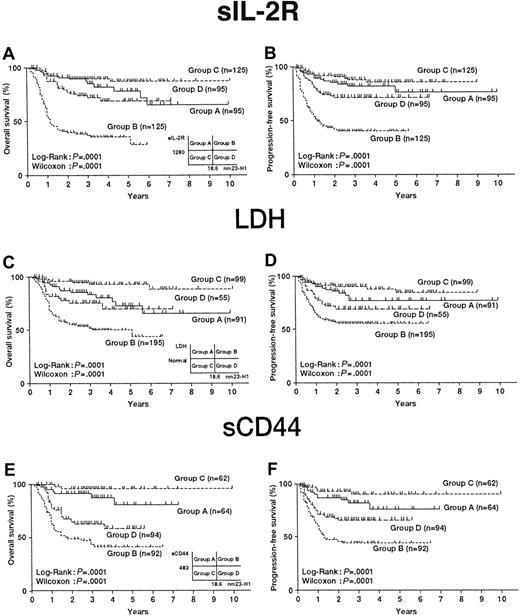
We investigated the possibility of obtaining a more accurate prognosis by combining nm23-H1 and other known prognostic factors of aggressive NHL (sIL-2R, LDH, and sCD44). The combination of sIL-2R (median, 1280 U/mL) and nm23-H1 (median, 18.6 ng/mL) is shown in Figure6A. The serum levels of nm23-H1 and sIL-2R in aggressive NHL were plotted and divided into 4 groups (group A, high sIL-2R and low nm23-H1; group B, high sIL-2R and high nm23-H1; group C, low sIL-2R and low nm23-H1; and group D, low sIL-2R and high nm23-H1). The OS values for groups A, B, C, and D were 65.1%, 28.1%, 87.3%, and 68.7%, respectively. An analysis of survival probability among these groups showed that group B had a significantly shorter survival than the other groups (P = .0001). The PFS values for groups A, B, C, and D were 76.4%, 40.0%, 85.6%, and 71.3%, respectively, and the prognosis for group B was again significantly worse than those in the other groups (P = .0001) (Figure 6B).
Classification of 440 patients with aggressive non-Hodgkin lymphoma according to the 2 important independent prognostic factors.
(A,C,E) Overall survival curves; (B,D,F) Progression-free survival curves. Categories included: high nm23-H1 (≥ 18.6 ng/mL) and low nm23-H1 (< 18.6 ng/mL); high sIL-2R (≥ 1019 U/mL) and low sIL-2R (< 1019 U/mL); high LDH (≥) and low LDH (< normal); and high sCD44 (≥ 483 ng/mL) and low sCD44 (≥ 483 ng/mL).
Classification of 440 patients with aggressive non-Hodgkin lymphoma according to the 2 important independent prognostic factors.
(A,C,E) Overall survival curves; (B,D,F) Progression-free survival curves. Categories included: high nm23-H1 (≥ 18.6 ng/mL) and low nm23-H1 (< 18.6 ng/mL); high sIL-2R (≥ 1019 U/mL) and low sIL-2R (< 1019 U/mL); high LDH (≥) and low LDH (< normal); and high sCD44 (≥ 483 ng/mL) and low sCD44 (≥ 483 ng/mL).
Next, the combination of LDH (below and above the reference value) and nm23-H1 (median, 18.6 ng/mL) was evaluated. Four groups were created as described above, and the OS values for groups A, B, C, and D were 65.2%, 43.5%, 88.3%, and 69.3%, respectively. An analysis of survival probability among these groups showed that group B had a significantly shorter survival than the other groups (P = .0001) (Figure 6C). The PFS values for groups A, B, C, and D were 77.3%, 55.6%, 84.9%, and 69.2%, respectively. Similar results were obtained in the analysis of PFS (P = .0001) (Figure 6D).
The combination of sCD44 (median, 483 ng/mL) and nm23-H1 (median, 18.6 ng/mL) was also tested. The OS values for groups A, B, C, and D were 81.8%, 41.6%, 96.1%, and 58.9%, respectively. An analysis of survival probability among these groups showed that group B had a significantly shorter survival than the other groups (P = .0001) (Figure 6E). The PFS values for groups A, B, C, and D were 75.9%, 44.3%, 89.9%, and 65.6%, respectively. The prognosis for group B was significantly poorer in PFS (P = .0001) (Figure 6F). These results indicate that the combination of sIL-2R or sCD44 and nm23-H1 may be a useful prognostic factor even when the level of nm23-H1 protein is lower than 80 ng/mL.
Univariate and multivariate analyses of OS and PFS in patients with aggressive NHL
Among patients with DLBCL, the OS was significantly worse for patients with the following characteristics: the World Health Organization (WHO) PS of 2 to 4, elevated serum LDH level, Ann Arbor stage of III/IV, more than one extranodal site, B symptoms, 60 years of age or older, a member of the IPI H-I + H risk group, sIL-2R level of 1000 U/mL or higher, nm23-H1 level of 12.01 ng/mL or higher, and sCD44 level of 500 ng/mL or higher. The PFS rate was significantly lower in patients with the following characteristics: PS of 2 to 4, normal or raised serum LDH level, Ann Arbor stage of III/IV, more than one extranodal site, B symptoms, 60 years of age or older, a member of the IPI H-I + H risk group, sIL-2R level of 1000 U/mL or higher, nm23-H1 level of 12.01 ng/mL or higher, and sCD44 level of 500 ng/mL or higher (Table 1).
The 10 prognostic factors (nm23-H1 level, age, stage, B symptoms, PS, extranodal sites, LDH level, IPI score, sIL-2R level, and sCD44 level) that were statistically significant according to a univariate analysis were further evaluated for their association with survival by a multivariate analysis using a Cox proportional hazards model. This analysis showed that in patients with aggressive NHL, the serum nm23-H1 level was the most important independent prognostic factor (Table2). An elevated serum nm23-H1 level was also identified as the most important prognostic determinant for a poor PFS (Table 2). An additional multivariate analysis revealed that the 5 prognostic factors used to calculate the IPI score and the serum nm23-H1 level were associated with OS and PFS (data not shown). These results indicate that the nm23-H1 level is an independent prognostic factor that may predict both the OS and PFS. This was also true for ascertaining the OS and PFS in patients with aggressive NHL.
Multivariate analysis by Cox proportional hazards model on diffuse large B-cell lymphoma
| Category . | Estimate (value ± SE) . | P value . | Hazards ratio . |
|---|---|---|---|
| Overall survival | |||
| nm23-H1 (< 12.01/≥ 12.01 ng/mL) | 1.51 ± 0.45 | .0007 | 4.571 |
| Soluble IL-2R (< 1000/≥ 1000 U/mL) | 0.72 ± 0.41 | .0810 | 2.038 |
| IPI (L, L-I/H-I, H) | 0.66 ± 0.49 | .1797 | 1.941 |
| Performance status (0, 1/2-4) | 0.56 ± 0.33 | .0841 | 1.757 |
| Extranodal sites (≤ 1/> 1) | 0.47 ± 0.39 | .2370 | 1.592 |
| LDH (normal/>normal) | 0.43 ± 0.42 | .3066 | 1.544 |
| Stage (I, II/III, IV) | 0.41 ± 0.41 | .3103 | 1.514 |
| Age (< 60/≥ 60 yr) | 0.38 ± 0.38 | .3130 | 1.468 |
| Soluble CD44 (< 500/≥ 500 ng/mL) | 0.20 ± 0.30 | .4948 | 1.224 |
| B symptoms | −0.55 ± 0.34 | .1061 | 0.578 |
| Progression-free survival | |||
| nm23-H1 (< 12.01/≥ 12.01 ng/mL) | 0.86 ± 0.36 | .0157 | 2.373 |
| Soluble IL-2R (< 1000/≥ 1000 U/mL) | 0.67 ± 0.38 | .0778 | 1.945 |
| Stage (I, II/III, IV) | 0.59 ± 0.41 | .1502 | 1.797 |
| LDH (normal/> normal) | 0.48 ± 0.42 | .2480 | 1.617 |
| Extranodal sites (≤ 1/> 1) | 0.38 ± 0.45 | .4090 | 1.455 |
| Age (< 60/≥ 60 yr) | 0.35 ± 0.37 | .3361 | 1.421 |
| Soluble CD44 (< 500/≥ 500 ng/mL) | 0.29 ± 0.29 | .3125 | 1.343 |
| IPI (L, L-I/H-I, H) | 0.27 ± 0.47 | .6320 | 1.254 |
| Performance status (0, 1/2-4) | 0.11 ± 0.35 | .7554 | 1.116 |
| B symptoms | −0.33 ± 0.34 | .3306 | 0.717 |
| Category . | Estimate (value ± SE) . | P value . | Hazards ratio . |
|---|---|---|---|
| Overall survival | |||
| nm23-H1 (< 12.01/≥ 12.01 ng/mL) | 1.51 ± 0.45 | .0007 | 4.571 |
| Soluble IL-2R (< 1000/≥ 1000 U/mL) | 0.72 ± 0.41 | .0810 | 2.038 |
| IPI (L, L-I/H-I, H) | 0.66 ± 0.49 | .1797 | 1.941 |
| Performance status (0, 1/2-4) | 0.56 ± 0.33 | .0841 | 1.757 |
| Extranodal sites (≤ 1/> 1) | 0.47 ± 0.39 | .2370 | 1.592 |
| LDH (normal/>normal) | 0.43 ± 0.42 | .3066 | 1.544 |
| Stage (I, II/III, IV) | 0.41 ± 0.41 | .3103 | 1.514 |
| Age (< 60/≥ 60 yr) | 0.38 ± 0.38 | .3130 | 1.468 |
| Soluble CD44 (< 500/≥ 500 ng/mL) | 0.20 ± 0.30 | .4948 | 1.224 |
| B symptoms | −0.55 ± 0.34 | .1061 | 0.578 |
| Progression-free survival | |||
| nm23-H1 (< 12.01/≥ 12.01 ng/mL) | 0.86 ± 0.36 | .0157 | 2.373 |
| Soluble IL-2R (< 1000/≥ 1000 U/mL) | 0.67 ± 0.38 | .0778 | 1.945 |
| Stage (I, II/III, IV) | 0.59 ± 0.41 | .1502 | 1.797 |
| LDH (normal/> normal) | 0.48 ± 0.42 | .2480 | 1.617 |
| Extranodal sites (≤ 1/> 1) | 0.38 ± 0.45 | .4090 | 1.455 |
| Age (< 60/≥ 60 yr) | 0.35 ± 0.37 | .3361 | 1.421 |
| Soluble CD44 (< 500/≥ 500 ng/mL) | 0.29 ± 0.29 | .3125 | 1.343 |
| IPI (L, L-I/H-I, H) | 0.27 ± 0.47 | .6320 | 1.254 |
| Performance status (0, 1/2-4) | 0.11 ± 0.35 | .7554 | 1.116 |
| B symptoms | −0.33 ± 0.34 | .3306 | 0.717 |
IPI indicates International Prognostic Index; L, low risk; L-I, low-intermediate risk; H-I, high-intermediate risk; H, high risk.
Discussion
We previously reported that a nondifferentiating mouse myeloid leukemia cell line produced differentiation-inhibiting factors. Suppression of the production of these inhibitory factors caused the nondifferentiating leukemic cells to become sensitive to differentiation inducers. One of these factors was purified as a homologue of nm23.24,29,30 The nm23-H1 gene was isolated on the basis of its reduced expression in highly metastatic counterparts.16 Expression of this gene was found to be inversely correlated with the tumor's metastatic potential in experimental rodent cells and in certain human tumors.31nm23 inhibits the differentiation of murine and human myeloid leukemia cells, and nm23 expression is greatly increased during blast formation in normal lymphocytes. These findings suggest that nm23 genes play a role in the growth and differentiation of normal and malignant hematopoietic cells. On the basis of the biologic activity of nm23 proteins as differentiation inhibitory factors, we previously investigated the relative levels of nm23-H1 and nm23-H2 transcripts in bone marrow and peripheral blood samples from patients with acute myelogenous leukemia (AML) cells. We determined the correlation between the expression of nm23 and clinical data and evaluated the importance of the nm23 mRNA expression level as a prognostic factor in AML.32,33 In a previous study, we measured the serum nm23-H1 protein levels by ELISA in 102 AML patients and found that serum nm23-H1 protein levels were high in AML patients compared with controls, and that patients with high levels exhibited a poor prognosis.34
There is also a close correlation between malignancy and the level of nm23-H1 protein expression in malignant lymphoma. We previously reported that in malignant lymphoma, nm23-H1 levels are significantly higher than those in controls, and that because patients with high levels of nm23-H1 exhibit a poor prognosis, nm23-H1 levels can be useful for selecting the course of treatment.19 In this study, a large number of patients were examined at multiple institutions, and the histopathologic types defined by the REAL classification were used. The level of nm23-H1 was significantly correlated with PS, stage, LDH, and number of extranodal lesions; therefore, nm23-H1 may be related to lymphoma dissemination or tumor burden. In the present multiple-institution study with a greater number of patients, the results confirmed the previous findings that the level of nm23-H1 is an independent prognostic factor and that potent therapy will be necessary to treat lymphoma patients with serum nm23-H1 levels of 80 ng/mL or higher. Because a recent trend in lymphoma therapy is to design different treatments for each histologic type, the serum level of nm23-H1 was precisely analyzed in DLBCL patients, who account for 40% to 50% of all NHL cases.
DLBCL is an aggressive malignancy of mature B lymphocytes, and patients with DLBCL show a highly variable clinical course. Although most patients respond initially to chemotherapy, fewer than half of all patients achieve a durable remission.35 Therefore, because patients with DLBCL are frequently encountered and can be treated, we need to quickly establish a treatment method that is not excessive. We also need to remain cognizant of problems associated with late-stage complications resulting from extensive treatment, as seen in long-term survivors, especially the development of secondary cancers. Currently, IPI is commonly used as the standard approach for separating patients into 2 groups and, in many cases, CHOP therapy is routinely applied to L and L-I risk groups, whereas more potent chemotherapy is required in H-I and H risk groups. However, a poor prognosis has been observed in L and L-I groups, and a good prognosis has been observed in H-I and H groups. By establishing a poor-prognosis group in L and L-I risk patients and a good-prognosis group among H-I and H risk patients, we can select an appropriate treatment by further classification of patient groups.
Some intensive studies of biologic prognostic factors have been performed recently, with p53 mutation13 and DNA microarray assay36 proving to be the prognostic factors for aggressive B-cell lymphoma. These genetic analyses will be conducted on lymph nodes and lymphatic tissues to identify prognostic factors at the gene level in the near future. However, the serum level of nm23-H1 can be measured quickly and easily using a small amount of serum before the start of therapy. The serum level of this protein will still be useful for selecting an appropriate treatment for different types of lymphoma. In the present study of DLBCL, nm23-H1 protein levels were elevated in patients with a PS score of 2 to 4, above-normal LDH, more advanced Ann Arbor stage, 2 or more extranodal sites, and more advanced IPI. The nm23-H1 protein level was lower in CR than in non-CR patients, and the prognosis was poor with regard to both OS and PFS in patients with an nm23-H1 level of greater than 80 ng/mL. After dividing the subjects into 4 groups according to their IPI values (L, L-I, H-I, and H risk groups), we investigated the effect of nm23-H1 protein levels (above and below 80 ng/mL) on OS and PFS (Figures 4 and 5). The results showed that prognosis was poorer for those with an nm23-H1 protein level of more than 80 ng/mL in all risk groups. Hence, CHOP therapy may not be sufficient, and more potent therapy may be needed for L risk patients with an nm23-H1 protein level of more than 80 ng/mL. Furthermore, standard therapy should be sufficient for H-I and H risk patients with low nm23-H1 protein levels. In the study of each IPI, we observed significant differences in all of the risk groups. Multivariate analysis demonstrated that the nm23-H1 level could be an independent prognostic factor for DLBCL. On the basis of our findings, we propose that the nm23-H1 protein level is a significant and independent prognostic factor for DLBCL and that it will be important in the future in planning the treatment strategy for DLBCL. With regard to PTCL, patients with high nm23-H1 also exhibited a poor prognosis. However, future studies for each risk group of IPI should be performed on a greater number of patients, and nm23-H1 may become a prognostic factor not only for DLBCL but also for PTCL. The p53 mutation and the DNA microarray assay can ascertain the prognosis only of DLBCL, whereas nm23-H1 may ascertain the prognosis of both DLBCL and PTCL. Moreover, we found that it may be possible to ascertain the prognosis by combining nm23-H1 and other known prognostic factors of aggressive NHL. The results indicated that the combination of nm23-H1 and sIL-2R or sCD44 could determine the prognosis more accurately, even when the nm23-H1 level was less than 80 ng/mL, suggesting that it will be possible to provide more appropriate therapy for each type of lymphoma by combining various independent prognostic factors.
The nm23-H1 protein level, which can be measured quickly and easily using a small serum sample, is useful for determining a suitable treatment strategy because it makes it possible to predict the prognosis before treatment. In the future, large prospective studies will be needed to establish the nm23-H1 protein level as a prognostic factor for planning treatment strategy.
We thank the Department of Immunology, SRL Inc, Tokyo, Japan for measuring the serum nm23-H1 protein levels.
Supported in part by grants from the Ministry of Health and Welfare and Grants-in Aid for Scientific Research Group C and Cancer Research from The Ministry of Education, Science, Sports and Culture, Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Nozomi Niitsu, 1-8-12-S1307, Omori-honcho, Ota-ku, Tokyo 143-0011, Japan; e-mail:nniitsu@med.kitasato-u.ac.jp.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal