Abstract
Experimental autoimmune encephalomyelitis (EAE) is a demyelinating disease of the central nervous system (CNS) that serves as a model for multiple sclerosis (MS) in humans. In mice, EAE is mediated by Th1 type CD4+ T cells specific for various myelin proteins which migrate from the periphery to the CNS. Removal or blocking of CD4+ cells before or shortly after disease induction was shown to prevent disease onset and/or disease progression but also results in general immune suppression. Most treatment regimens for autoimmune diseases currently rely on general suppression of the T-cell compartment most commonly by steroids. In this paper, an experimental, gene therapy-based model is presented in which susceptible mice are made resistant to EAE induction by specifically down-regulating an autoreactive T-cell population. By using a retroviral gene transfer protocol, normal B cells were genetically modified to constitutively express the SJL-specific proteolipid (PLP) encephalitogenic determinant and then adoptively transferred into syngeneic hosts. To ensure appropriate presentation of the exogenous encephalitogenic peptide in association with MHC class II, the encephalitogenic sequence was fused to a lysosomal targeting sequence. Adoptive transfer of syngeneic B cells expressing the PLP encephalitogenic determinant into normal, naive, genetically susceptible mice induced PLP-specific unresponsiveness and completely protected the majority (62% and 83% using an intermediate and a high titer retroviral vector, respectively) of the animals from EAE induction. The remaining animals had a delayed disease onset and/or lower disease severity. All protected mice expressed the exogenous gene in the spleen as detected by reverse transcriptase-polymerase chain reaction.
Introduction
Experimental autoimmune encephalomyelitis (EAE) is an experimental animal model for T-cell–mediated demyelinating disease of the central nervous system (CNS).1,2 The disease is readily induced in experimental animals by immunization with purified myelin proteins such as myelin basic protein (MBP),3myelin proteolipid protein (PLP),4 and myelin oligodendrocyte glycoprotein (MOG),5 or peptides spanning the various encephalitogenic determinants of these proteins. EAE is characterized by perivascular leukocytic infiltration into the CNS6,7 causing local inflamation, followed by focal demyelination. Although direct evidence for an autoimmune mechanism in multiple sclerosis (MS) is lacking, the histopathology is characteristic of an immune-mediated tissue damage that greatly resembles EAE.1 Therefore, EAE is currently the animal model most widely used to test novel therapeutic protocols.2
It is widely accepted that both EAE and MS have a cellular pathogenesis and are initiated by MHC class II-restricted Th1 CD4+ T cells specific for antigenic determinants on MBP,8-11PLP,12,13 and MOG.14,15 T cells that induce EAE are called encephalitogenic T cells, and the antigenic determinants to which they are specific are called encephalitogenic determinants. Numerous studies have demonstrated that blocking or eliminating CD4+ cells could prevent the induction and progression of EAE. For example, blocking activation of CD4+ cells by the administration of monoclonal antibodies (mAbs) specific for CD4,16-20 MHC class II antigens,21-23 and certain members of the VLA adhesion molecules24strongly inhibits the induction and progression of EAE in vivo. However, these treatments are not antigen specific and target the entire CD4+ population. They are therefore immunosuppressive and symptomatic treatments in nature and cannot be considered as a long-term therapy.
A more useful and clinically applicable approach would be to induce the specific elimination and/or inactivation of the encephalitogenic T-cell clone(s) that mediate EAE or MS in the host. This approach became feasible because of recent advancements in the understanding of the mechanisms leading to T-cell activation versus T-cell unresponsiveness and the development of efficient retroviral-mediated gene transfer techniques. Recent studies demonstrated that a productive activation event for Th1-type cells requires 2 simultaneous signals. The first is the engagement of the T-cell receptor by its specific ligand (antigen in association with MHC class II) and the second is a costimulatory signal delivered mainly by binding of B7-1 and B7-2 expressed on the surface of the same antigen-presenting cell (APC) to CD28, which is expressed on the surface of the responding T cell.25-28 If engagement of the T-cell receptor occurs in the absence of a costimulatory signal, the responding T cells will be inactivated and will remain refractive even to a complete set of activation signals. This state of immunologic unresponsiveness is very stable and long-lasting.25-27 Physiologically, this occurs whenever antigens are presented by APCs that do not express costimulatory molecules and are presumably an important safety mechanism to ensure that immune responses (mostly to self antigens) are not easily initiated. Therefore, costimulatory molecules, for the most part, are not expressed on resting (nonactivated) APCs.26,28 B cells are a typical example of such APCs. Although they constitutively express MHC class II antigens, they do not express costimulatory molecules in their resting stage.28-30 It could therefore be expected that surface antigens expressed in resting B cells would lead to antigen-specific T-cell unresponsiveness. Indeed, this was shown to be the case in various experimental systems for several antigens, including the encephalitogenic determinant of MBP.30-34 In the latter case, specific B-cell targeting was achieved by chemically coupling the antigens to anti-IgD antibodies, which results only in transient cell surface expression.33 34
On the basis of these findings, we hypothesized that if normal, resting B cells could be genetically modified to constitutively express a particular autoantigen, they would continuously down-regulate all T-cell clones specific for this antigen. To test this, we used a rapid and highly efficient gene transfer protocol previously developed in our laboratory35 to introduce a gene coding for the PLP encephalitogenic determinant into syngeneic B cells in a way that the encephalitogenic peptide will be constitutively expressed in conjunction with MHC class II antigens. This was achieved by constructing a chimeric gene containing a portion of the PLP gene sequence that spans the SJL-specific encephalitogenic determinant and a lysosomal targeting sequence. Lysosomal targeting is required to ensure expression of the exogenous encephalitogenic determinant in conjunction with MHC class II molecules.36-39
The results presented in this paper strongly support this hypothesis. We show that the adoptive transfer of syngeneic B cells expressing a PLP-derived encephalitogenic determinant into genetically susceptible mice protects the majority of the treated animals from induction of EAE by direct immunization. Expression of the exogenous gene was detected in the spleen of all protected animals and correlated with the level of protection. If this treatment proves to be efficacious when administered after the onset of clinical symptoms, it could provide a working model for the treatment of T-cell–mediated autoimmune diseases.
Materials and methods
Mice
Female (BALB/c × SJL)F1 mice purchased from the Jackson Laboratory (Bar Harbor, ME) were used between 8 and 14 weeks of age.
Retroviral vectors and virus-producer cell lines
The pSNV-MPL (Figure 1A) was made by replacing the AsADA gene of pAsADA40 with the spleen necrosis virus U3 promoter (SNV) and MPL (miniPLP–lysosomal acid phosphatase [LAP]) cassette. The MPL cassette (Figure2A) was constructed by inserting the PLP 100-154 coding sequence (miniPLP), (which was amplified from PLP complementary DNA [cDNA], provided by W. B. Macklin), into theBglII site of the lysosomal targeting vector pM6PR-LAP36 (provided by C. Peters). In pMPL-PURO and pMML-PURO (Figure 3A,B), MPL, and MML (miniMBP–LAP) cassettes were placed upstream of an encephalomyocarditis virus internal ribosome entry site (IRES) (provided by J. Zhang), which was linked to the puromycin resistance gene (puro). The MML cassette was made by fusing the transmembrane domain (TM) and lysosomal targeting sequences from pM6PR-LAP at the SacI site of the chimeric geneLM4341 (created by C. Benoist), which contains hen egg lysozyme (HEL) and part of myelin basic protein (miniMBP) genes. pN2 (provided by E. Gilboa) contains only theneo marker gene driven directly by MoMLV LTR (Figure 1B).
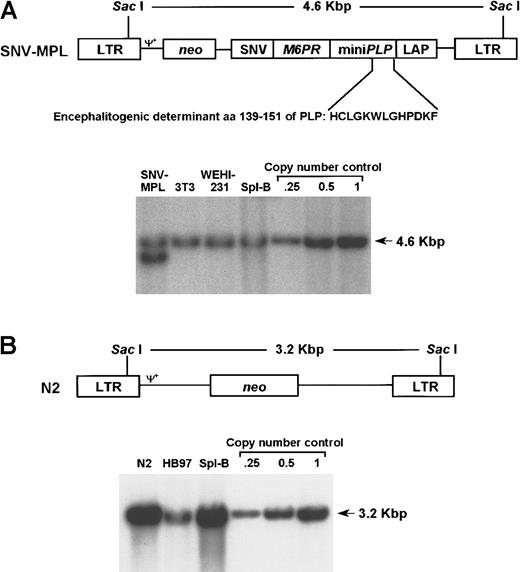
Retroviral vectors SNV-MPL, N2, and gene transfer efficiency.
Upper panels depict diagrams of the MoMLV-derived vectors used in these studies. The relevant restriction enzymes and the distance between them are indicated. Ψ+ represents the packaging signal. Lower panels illustrate the gene transfer efficiency obtained with each vector as assayed by Southern blotting. (A) pSNV-MPL contains the MPL cassette under the transcriptional control of the spleen necrosis virus U3 promoter (SNV) and the neo marker gene. Southern blotting was performed on genomic DNA extracted from SNV-MPL producer cells, SNV-MPL-infected 3T3 cells, SNV-MPL- infected WEHI 231 B-cell line, SNV-MPL cocultivated primary, spleen B cells, and copy number controls as indicated above each lane. (B) pN2 (provided by E. Gilboa) contains only the neo marker gene driven directly by MoMLV LTR. Southern blotting analysis was performed on genomic DNA extracted from N2 producer cells, N2-infected HB97 B-cell line, N2 cocultivated primary, spleen B cells, and copy number controls as indicated above each lane.
Retroviral vectors SNV-MPL, N2, and gene transfer efficiency.
Upper panels depict diagrams of the MoMLV-derived vectors used in these studies. The relevant restriction enzymes and the distance between them are indicated. Ψ+ represents the packaging signal. Lower panels illustrate the gene transfer efficiency obtained with each vector as assayed by Southern blotting. (A) pSNV-MPL contains the MPL cassette under the transcriptional control of the spleen necrosis virus U3 promoter (SNV) and the neo marker gene. Southern blotting was performed on genomic DNA extracted from SNV-MPL producer cells, SNV-MPL-infected 3T3 cells, SNV-MPL- infected WEHI 231 B-cell line, SNV-MPL cocultivated primary, spleen B cells, and copy number controls as indicated above each lane. (B) pN2 (provided by E. Gilboa) contains only the neo marker gene driven directly by MoMLV LTR. Southern blotting analysis was performed on genomic DNA extracted from N2 producer cells, N2-infected HB97 B-cell line, N2 cocultivated primary, spleen B cells, and copy number controls as indicated above each lane.
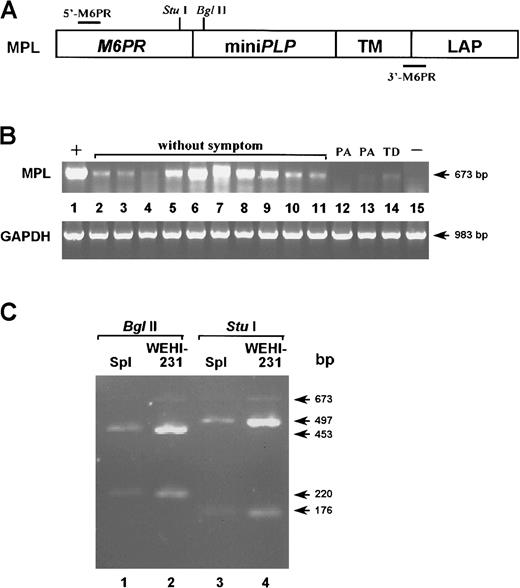
Exogenous gene expression in mice treated with primary B cells infected with SNV-MPL vector virus expressing PLP.
(A) A diagram of the MPL cassette is depicted. Recognition sites for the restriction enzymes StuI and BglII are indicated as well as the positions of the primers 5′-M6PR and 3′-M6PR used for RT-PCR. (B) RT-PCR amplification was performed on total RNA extracted from the spleens of protected (lanes 2-11) and unprotected (lanes 12-14) mice reconstituted with SNV-MPL–infected B cells. PA and TD indicate paralysis and tail drop, respectively. Lane 1 represents SNV-MPL infected, neo-selected WEHI 231 cells as positive control and lane 15 represents spleen from an untreated normal control mouse. The amplification of the housekeeping gene GAPDH was performed in each sample to ensure that equal levels of cDNA were used. (C) To confirm that the amplified sequence corresponds to the exogenous sequence, some of the amplified products were purified and analyzed by restriction enzyme digestion. Lanes 1 and 3 represent the DNA from a protected mouse and lanes 2 and 4 represent DNA from the SNV-MPL–infected WEHI 231 cell line. Lanes 1 and 2 were digested withBglII and lanes 3 and 4 were digested with StuI. Both enzymes cut once within the amplified sequence and yielded the expected size bands.
Exogenous gene expression in mice treated with primary B cells infected with SNV-MPL vector virus expressing PLP.
(A) A diagram of the MPL cassette is depicted. Recognition sites for the restriction enzymes StuI and BglII are indicated as well as the positions of the primers 5′-M6PR and 3′-M6PR used for RT-PCR. (B) RT-PCR amplification was performed on total RNA extracted from the spleens of protected (lanes 2-11) and unprotected (lanes 12-14) mice reconstituted with SNV-MPL–infected B cells. PA and TD indicate paralysis and tail drop, respectively. Lane 1 represents SNV-MPL infected, neo-selected WEHI 231 cells as positive control and lane 15 represents spleen from an untreated normal control mouse. The amplification of the housekeeping gene GAPDH was performed in each sample to ensure that equal levels of cDNA were used. (C) To confirm that the amplified sequence corresponds to the exogenous sequence, some of the amplified products were purified and analyzed by restriction enzyme digestion. Lanes 1 and 3 represent the DNA from a protected mouse and lanes 2 and 4 represent DNA from the SNV-MPL–infected WEHI 231 cell line. Lanes 1 and 2 were digested withBglII and lanes 3 and 4 were digested with StuI. Both enzymes cut once within the amplified sequence and yielded the expected size bands.
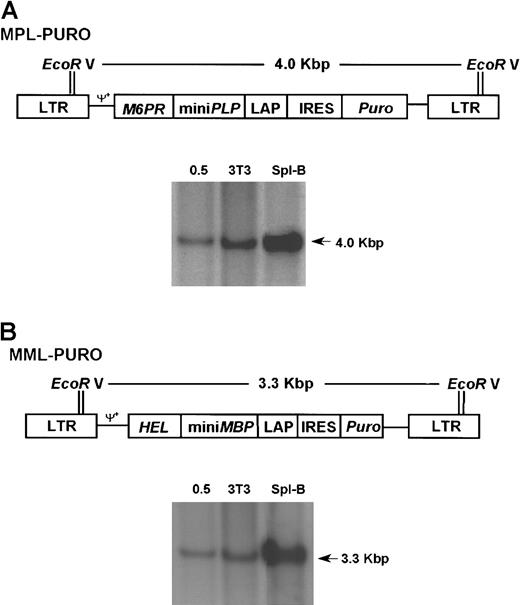
Retroviral vectors MPL-PURO, MML-PURO, and gene transfer efficiency.
(A) pMPL-PURO contains the same MPL cassette used in SNV-MPL with the difference that in this vector the cassette is under the transcriptional control of the viral LTR. The puromycin resistance gene (puro) is translated from an IRES (provided by J. Zhang) and was placed 3′ of the MPL cassette. Southern blotting was performed on genomic DNA from MPL-PURO–infected 3T3 cells, MPL-PURO cocultivated primary spleen B cells, and a 0.5 copy number control as indicated. (B) pMML-PURO contains the MML chimeric gene under the transcriptional control of the viral LTR and the puro gene, which is translated from IRES. Southern blotting was performed on genomic DNA extracted from MML-PURO–infected 3T3 cells, MML-PURO cocultivated primary spleen B cells, and a 0.5 copy number control as indicated.
Retroviral vectors MPL-PURO, MML-PURO, and gene transfer efficiency.
(A) pMPL-PURO contains the same MPL cassette used in SNV-MPL with the difference that in this vector the cassette is under the transcriptional control of the viral LTR. The puromycin resistance gene (puro) is translated from an IRES (provided by J. Zhang) and was placed 3′ of the MPL cassette. Southern blotting was performed on genomic DNA from MPL-PURO–infected 3T3 cells, MPL-PURO cocultivated primary spleen B cells, and a 0.5 copy number control as indicated. (B) pMML-PURO contains the MML chimeric gene under the transcriptional control of the viral LTR and the puro gene, which is translated from IRES. Southern blotting was performed on genomic DNA extracted from MML-PURO–infected 3T3 cells, MML-PURO cocultivated primary spleen B cells, and a 0.5 copy number control as indicated.
Virus producer cells SNV-MPL, N2, MPL-PURO, and MML-PURO were made in the MLV-based producer cell line GP + E86 cells by techniques described previously.40 The retroviral titer for SNV-MPL ranged from 5 × 105 to 2 × 106 CFU/mL and 1 to 2 × 107 for N2, MPL-PURO, and MML-PURO. Production of replication competent viruses by the producer cells was assayed as previously described35 and found to be negative for all cell lines.
Cell preparations, retroviral mediated gene transduction, and Southern blotting analysis
Spleen cells were obtained from (BALB/c x SJL)F1 mice. Enriched populations of splenic B lymphocytes were prepared by antibody plus complement-mediated killing of T cells using a cocktail of anti-Thy1, anti-CD4, and anti-CD8 antibodies, followed by discontinuous Percoll density gradient (60%, 65%, and 70% layers) purification. This procedure routinely yields more than 85% B cells from the spleen, as analyzed by flow cytometry. Retroviral transduction was performed by cocultivation: 8 × 106 stimulated spleen B cell populations were plated at a density of 1 × 106 cells/mL in RPMI media, onto a confluent lawn of irradiated (1600 rad) virus-producing cells in 100-mm tissue culture plates in media containing 6 μg/mL polybrene and 50 μg/mL lipopolysaccharide (LPS). The target cells were harvested 24 hours later, and 40 × 106 cells (on average) were adoptively transferred into syngeneic normal (BALB/c x SJL)F1 mice.
For assessment of gene transfer efficiency, genomic DNA was extracted either from 107 retroviral transduced target cells cultured for an additional 24 hours in fresh media or from infected WEHI-231 B cell line, HB97 B cell hybridoma line, and 3T3 cells. Southern blotting was performed according to standard procedures. For detection of theneo marker gene, 10 μg per lane ofSacI-digested genomic DNA were screened using a32P-labeled probe, EagI-AvaI fragment from the neo coding region; whereas for the detection ofpuro marker, genomic DNA was digested with EcoRV and probed with 32P-labeled NcoI fragment from the puro coding region.
Experimental autoimmune encephalomyelitis induction and scoring
EAE was induced 7 to 14 days after gene transfer by direct immunization with 200 μg PLP 139-151 emulsified in CFA fortified with 4 mg/mL of heat-killed H37 RA Mycobacterium tuberculosis. Pertussis toxin was injected intravenously via the lateral tail vein at the time of priming and 48 hours after priming. EAE was scored on an arbitrary scale as follows: tail drop (limp tail) −1, hind limbs paralysis −2, full paralysis without the ability to move −3, and death −4.
Reverse transcriptase-polymerase chain reaction amplification
Whole spleen RNA was extracted from gene transferred and control mice using Trizol (Life Technologies, Grand Island, NY). First strand complementary DNA (cDNA) was synthesized using 2 μg of total RNA, which was primed with oligo dT, then reversed-transcribed using Superscript II reverse transcriptase (Life Technologies) at 42°C for 90 minutes. Duplicate reactions without reverse transcriptase were performed as negative control (data not shown). Construct MPL (Figure2A) specific oligonucleotide primers 5′-M6PR (5′-CTTGCGACTTGGTAGGAGAA-3′) and 3′-M6PR (5′-CTCTGTGTCTGCAGACGTGA-3′) were used to generate a 673-base pair (bp) product. Glyceraldehde 3-phosphate dehydrogenase (GAPDH) cDNA was synthesized and amplified with 5′-primer (5′-TGAAGGTCGGTGTGAACGGATTTGGC -3′) and 3′-primer (5′-CATGTAGGCCATGAGGTCCACCAC-3′), which generated a 983-bp PCR product, to serve as internal control of the polymerase chain reactions (PCRs). The MML-specific primers were 5′-HEL (5′-GAAGCGTCACGGACTTGATA-3′) and 3′-M6PR, generating a 346-bp PCR product. The cycling parameters were 3 minutes at 94°C, followed by 35 cycles of 40 seconds at 94°C, 40 seconds at 55°C, and 1 minute at 72°C. Restriction digestion and agarose gel electrophoresis were used to analyze the PCR products after amplification.
T-cell proliferation assay and measurement of interleukin-2 protein levels
Mice were primed with either PLP peptide (p139-151) or keyhole limpet hemocyanin (KLH) emulsified in complete Freund adjuvant (CFA) in the hind footpads. Nine days later, cell suspensions prepared from the draining popliteal lymph node (LN) (4 × 105 cells per well in 96-well plates) were restimulated in vitro with the corresponding antigen for 24 hours. One set of cells was pulsed with 0.0185 MBq (0.5 μCi) of3HTdR for an additional 10 hours and then harvested. Radioactivity was measured in a scintillation counter. Interleukin-2 (IL-2) protein levels were measured in culture supernatants from a second, identical set of cells using a sandwich enzyme-linked immunosorbent assay (ELISA). The capture and detecting antibody pair and the murine IL-2 standard were purchased from BD Biosciences (San Diego, CA), and the assay was performed according to the manufacturer's supplied protocol.
Interleukin-2–specific messenger RNA quantitation
LN cells were prepared as previously described but were cultured in 24-well plates, 4 × 106 cells per well. Total RNA was extracted using Trizol (Life Technologies) and the IL-2–specific messenger RNA (mRNA) quantitation was performed with a “Quantikine mRNA kit” (R&D, Minneapolis, MN) according to the manufacturer-supplied procedure.
Statistical analysis
The nature of the in vivo experiments are such that they do not allow for a simple conventional t (or similar) significance test unless average severity scores are used. On the basis of statistician's advice, we decided to use the Kruskal-Wallis nonparametric significance test for the in vivo experiments.
Results
Retroviral vectors and packaging cell lines
The retroviral vector used in the first set of experiments contained the MPL chimeric gene (Figure 1A), comprising from the 5′ to 3′ direction the sequences encoding aa 1-183 of the mannose 6 phosphate receptor (M6PR), aa 100-154 of PLP, aa 184-197 of M6PR, and aa 379-423 of the lysosomal acid phosphatase (LAP), which includes the transmembrane and cytoplasmic domains. This gene was inserted into a Moloney murine leukemia virus (MoMLV)–based vector under transcriptional control of the SNV U3. The M6PR gene supplies the leader sequence required for endoplasmic reticulum (ER) targeting, and the LAP sequence was used for lysosomal targeting,36 which is a prerequisite for expression of a protein in conjunction with MHC class II.37 A construct containing the M6PR gene fused to LAP was shown by others to effectively target M6PR into the lysosomal compartment.36 Moreover, routing an antigen to the lysosomal compartment was shown to greatly enhance antigen presentation.38 39 The neo gene was also included in the vector to allow for selection of vector virus–producing lines and as a marker gene for the detection of infected cells at various times after adoptive transfer. The N2 vector (which only contains the neo gene) was used as a control vector (Figure 1B). The SNV-MPL packaging cell line produced a titer of 5 × 105 to 2 × 106 CFU/mL.
Syngeneic B cells infected with the spleen necrosis virus-myeloproliferative leukemia vector virus confer resistance to experimental autoimmune encephalomyelitis
Highly enriched splenic B cells were infected by cocultivation with the SNV-MPL packaging cell line with an infection efficiency of 0.3 copies per cell as ascertained by gel densitometry scanning (Figure 1A). On average, 40 × 106 infected B cells were then adoptively transferred into syngeneic, nonirradiated (BALB/c x SJL)F1 mice. The same number of cells infected with the N2 control vector were injected into control animals. EAE was induced in the 2 groups of mice as well as in normal, untreated control animals 7 to 14 days after adoptive transfer by direct immunization with PLP peptide (p139-151) in fortified CFA and pertussis. As shown in Table1, 10 of 16 animals adoptively transferred with B cells infected with the SNV-MPL vector were completely protected from EAE induction. The remaining 6 animals had a much milder disease, with a delayed onset (16.8 vs 13.7 days). In contrast, all the control mice (either untreated or mice injected with B cells infected with the N2 vector) developed severe EAE (Table 1).
B cells expressing the proteolipid protein encephalitogenic determinant protect (BALB/c × SJL) F1 mice from induction of EAE by direct immunization
| Vectors . | No. of mice . | EAE . |
|---|---|---|
| SNV-MPL* | 10 | No symptoms |
| 3 | Tail drop | |
| 3 | Paralysis | |
| N2† | 1 | Tail drop |
| 4 | Paralysis | |
| No vector‡ | 2 | Tail drop |
| 5 | Paralysis | |
| 4 | Death |
| Vectors . | No. of mice . | EAE . |
|---|---|---|
| SNV-MPL* | 10 | No symptoms |
| 3 | Tail drop | |
| 3 | Paralysis | |
| N2† | 1 | Tail drop |
| 4 | Paralysis | |
| No vector‡ | 2 | Tail drop |
| 5 | Paralysis | |
| 4 | Death |
Significantly different from
and
(P < .05).
Furthermore, the protected animals remained asymptomatic through the duration of the experiment (3 months), whereas nearly half (5 of 12) of the surviving control mice relapsed at least once during this period.
Protected mice express the exogenous chimeric gene
To verify that B cells from mice that were protected from EAE induction indeed expressed the exogenous gene, RT-PCR utilizing an M6PR-specific 5′ primer (5′-M6PR) and an M6PR-LAP junction-specific 3′ primer (3′-M6PR, Figure 2A) was performed by using total RNA extracted from whole spleens 3 months after disease onset. Amplified RT-PCR products from an SNV-MPL–infected, neo-selected WEHI 231 B-cell line and from a spleen of an untreated mouse were used as positive and negative controls, respectively (Figure 2B). RT-PCR product of the expected size was amplified from total spleen RNA isolated from 10 protected mice and 3 unprotected, surviving mice that did show clinical symptoms (2 paralyzed and one tail drop). As seen in Figure 2B, higher expression of the transduced gene was detected in protected mice than in mice with clinical symptoms. To confirm that the amplified sequence corresponded to the exogenous sequence, the amplified DNA products were purified and analyzed by restriction enzyme digestion with BglII and StuI, both of which cut once within the amplified sequence and yielded the expected size bands (Figure 2C).
High titer retroviral vectors
Although the SNV promoter was shown to yield high levels of expression in various cell lines,42 and a good protection level was achieved with the SNV-MPL vector virus, this vector could only be produced in relatively low titers. We therefore constructed a different vector (MPL-PURO) in which both the chimeric gene (containing the PLP encephalitogenic determinant) and the puromycin resistance marker gene (puro) were driven by the viral LTR promoter (Figure 3A). The MLV LTR was shown by others to be a strong promoter in lymphocytes.43 Translation of puro was controlled by the encephalomyocarditis virus IRES (Figure 3A). A vector containing the encephalitogenic determinant of MBP rather than the PLP determinant was also constructed and was used as a control vector (MML-PURO, Figure 3B), which should not protect against PLP-induced disease. The packaging cell lines for these vectors produced more than 10-fold higher vector virus titers (2 × 107 CFU/mL), which resulted in a higher infection efficiency of 1.0 to 2.0 copies per cell, respectively, (Figure 3A,B) compared with 0.3 copies per cell for the SNV-MPL vector (Figure 1A). Expression of the MPL-PURO and the MML-PURO viral RNA in transduced primary B cells 48 hours after transduction was assessed by Northern blot analysis and was shown to be positive (data not shown).
B cells infected with the MPL-PURO vector virus block experimental autoimmune encephalomyelitis induction
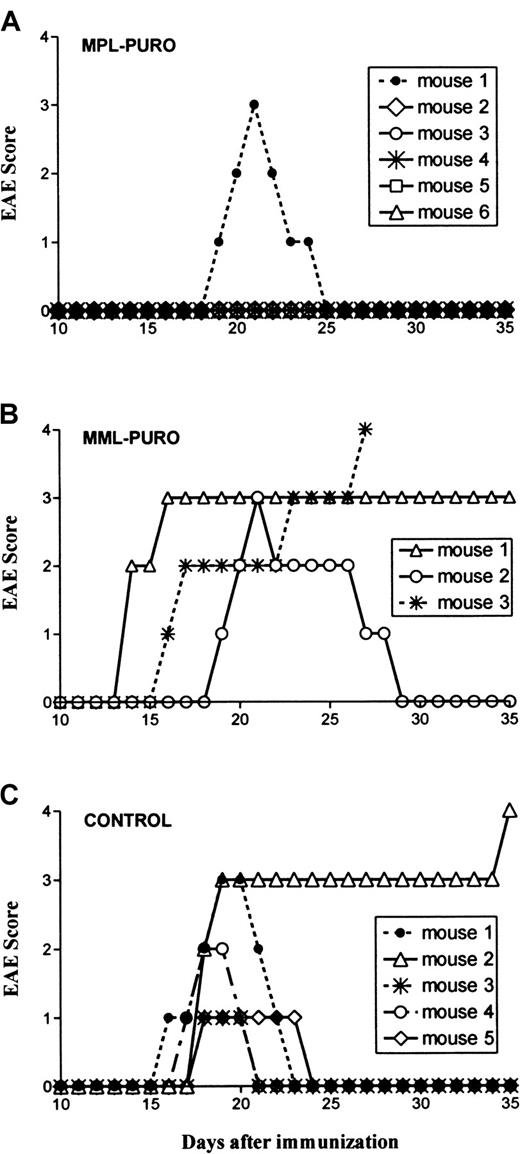
The in vivo experiments using B cells infected with the MPL-PURO and the MML-PURO control vector viruses were performed as described for the first virus producer cell, SNV-MPL. Five of the 6 mice reconstituted with B cells infected with the MPL-PURO vector were completely protected from EAE induction by direct immunization (Figure4A). In contrast, all 3 control mice injected with B cells transduced with MML-PURO and the 5 untreated controls developed severe EAE (Figure 4B,C). All mice were followed for 40 days after disease onset. All the protected mice remained asymptomatic, whereas 2 of the 6 surviving control animals relapsed once during this period.
Protection from EAE induction by B cells transduced with the MPL-PURO vector virus.
Each symbol represents an individual mouse. Mice were treated with transduced B cells 7 to 10 days before induction of EAE. EAE was scored as described in “Material and methods.” (A) Progression of EAE in 6 mice that were treated with MPL-PURO–infected spleen B cells, which express the PLP encephalitogenic determinant. (B) Progression of EAE in 3 mice treated with B cells transduced with MML-PURO, which express the encephalitogenic determinant of MBP. (C) Progression of EAE in 5 untreated syngeneic-sex and age-matched control mice. Group A is significantly different from groups B and C (P < .05).
Protection from EAE induction by B cells transduced with the MPL-PURO vector virus.
Each symbol represents an individual mouse. Mice were treated with transduced B cells 7 to 10 days before induction of EAE. EAE was scored as described in “Material and methods.” (A) Progression of EAE in 6 mice that were treated with MPL-PURO–infected spleen B cells, which express the PLP encephalitogenic determinant. (B) Progression of EAE in 3 mice treated with B cells transduced with MML-PURO, which express the encephalitogenic determinant of MBP. (C) Progression of EAE in 5 untreated syngeneic-sex and age-matched control mice. Group A is significantly different from groups B and C (P < .05).
To verify that protected mice contained B cells expressing the exogenous PLP gene, RT-PCR using MPL-specific primers was performed on total RNA isolated from the spleens of the treated animals 40 days after disease onset. As shown in Figure5A, 4 of 5 protected animals expressed high levels of the chimeric gene. The fifth protected animal showed a lower level of expression. In contrast, the sole surviving unprotected mouse had barely detectable MPL expression (Figure 5A, lane 7). As expected, mice adoptively transferred with MML-PURO–transduced B cells had no detectable MPL expression (Figure 5A, lanes 8,9). However, these mice expressed a high level of the MBP encephalitogenic determinant as detected by RT-PCR using MML-specific primers (Figure 5B). Because these MML-PURO–transduced mice developed severe EAE to PLP (Figure4B), we concluded that the protection provided by MPL-PURO was antigen specific. Expression of GAPDH, a control housekeeping gene, was detected with equal intensity in all mice (Figure 5A,B), suggesting that the level of expression of the PLP encephalitogenic determinant and/or the number of transduced B cells correlated with protection from disease induction.
Exogenous gene expression in mice treated with primary B cells infected with MPL-PURO vector virus-expressing PLP.
(A) RT-PCR was performed on total RNA extracted from the spleens of mice treated with MPL-PURO–infected B cells (lanes 2-7) and mice treated with B cells infected with the control vector MML-PURO (lanes 8 and 9). An untreated mouse was used as negative control (lane 10), and a MPL-PURO–infected WEHI 231 B-cell line was used as positive control (lane 1). PA indicates paralysis. (B) RT-PCR with the use of MML-specific primers was performed on total RNA extracted from the spleens of mice treated with MML-PURO–infected B cells, lanes 1 and 2. RNA extracted from the spleen of an untreated mouse (lane 3) and from MPL-PURO–infected WEHI 231 B cells (lane 4) were used as negative controls. For all samples, amplification of the housekeeping geneGAPDH was performed to ensure that equal levels of total RNA were used.
Exogenous gene expression in mice treated with primary B cells infected with MPL-PURO vector virus-expressing PLP.
(A) RT-PCR was performed on total RNA extracted from the spleens of mice treated with MPL-PURO–infected B cells (lanes 2-7) and mice treated with B cells infected with the control vector MML-PURO (lanes 8 and 9). An untreated mouse was used as negative control (lane 10), and a MPL-PURO–infected WEHI 231 B-cell line was used as positive control (lane 1). PA indicates paralysis. (B) RT-PCR with the use of MML-specific primers was performed on total RNA extracted from the spleens of mice treated with MML-PURO–infected B cells, lanes 1 and 2. RNA extracted from the spleen of an untreated mouse (lane 3) and from MPL-PURO–infected WEHI 231 B cells (lane 4) were used as negative controls. For all samples, amplification of the housekeeping geneGAPDH was performed to ensure that equal levels of total RNA were used.
MPL-PURO–treated mice are unresponsive to PLP
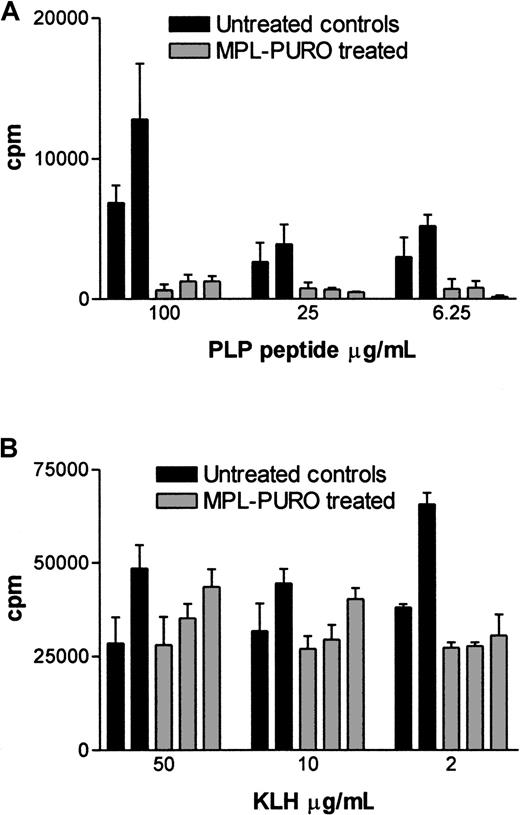
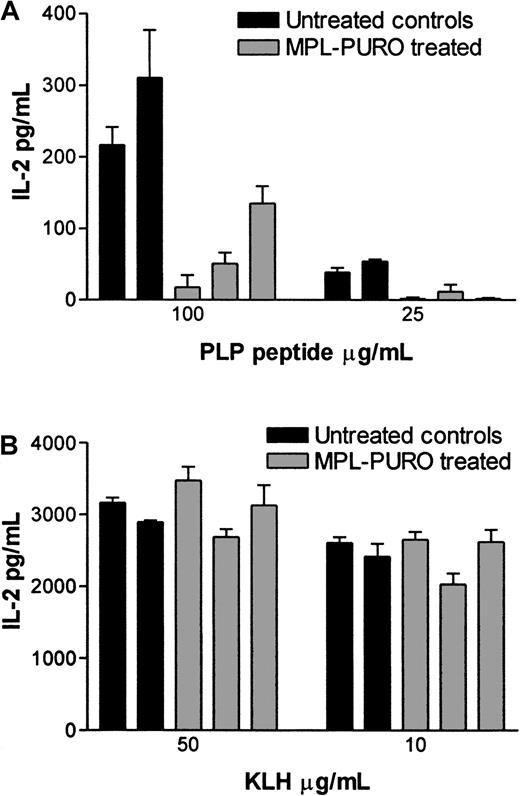
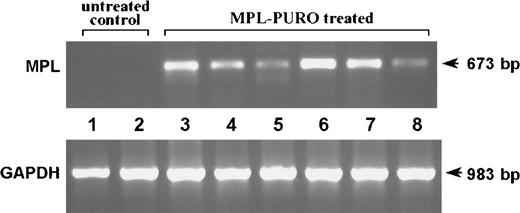
To evaluate the immunologic responses of mice adoptively transferred with B cells expressing the PLP encephalitogenic determinant (MPL-PURO), treated animals were primed to PLP p139-151 or KLH (a control antigen) 4 weeks after adoptive transfer. Nine days later, their responses to PLP and KLH were evaluated by antigen-specific T-cell proliferation and IL-2 production. As shown in Figure 6A, treated animals responded very poorly to the encephalitogenic PLP peptide compared with untreated controls, whereas responses to KLH were similar in both groups (Figure6B). Responses to PLP and KLH were also assessed by measuring the levels of IL-2 protein in culture supernatants. The levels of IL-2 found in cultures stimulated with PLP were significantly lower in treated animals compared with controls (Figure7A). In contrast, no difference in IL-2 levels were found in KLH-stimulated cultures prepared from KLH primed, treated, and control animals (Figure 7B). The levels of mRNA in stimulated cells from parallel cultures (as determined by a quantitative mRNA ELISA) corresponded to the levels of IL-2 protein (data not shown). Expression of the exogenous MPL chimeric gene in all treated animals used for the experiments described previously were confirmed by RT-PCR. As shown in Figure8, expression of the exogenous gene was detected in all adoptively transferred animals. These results strongly suggest that the expression of the the PLP encephalitogenic determinant in peripheral B cells leads to the induction of antigen-specific unresponsiveness.
T-cell proliferation in response to the stimulations of PLP peptide and KLH in control and MPL-PURO–transferred mice.
MPL-PURO–treated and untreated control mice were primed with PLP p139-151/CFA or KLH/CFA in the hind footpads, and the draining LNs were removed 9 days later. Doses of 4 × 105 LN cells were cultured in vitro with or without the presence of the specific antigens. 3H-thymidine was added 24 hours after incubation, and cells were harvested 10 hours later. The data show mean ± SD CPM derived from triplicate cultures. Responses to PLP p139-151 are shown in (A) and to KLH in (B). Each bar represents one mouse; the black bars represent the untreated control mice, and the gray ones represent MPL-PURO–treated mice. Concentrations of the stimulating antigen are indicated in the x-axis.
T-cell proliferation in response to the stimulations of PLP peptide and KLH in control and MPL-PURO–transferred mice.
MPL-PURO–treated and untreated control mice were primed with PLP p139-151/CFA or KLH/CFA in the hind footpads, and the draining LNs were removed 9 days later. Doses of 4 × 105 LN cells were cultured in vitro with or without the presence of the specific antigens. 3H-thymidine was added 24 hours after incubation, and cells were harvested 10 hours later. The data show mean ± SD CPM derived from triplicate cultures. Responses to PLP p139-151 are shown in (A) and to KLH in (B). Each bar represents one mouse; the black bars represent the untreated control mice, and the gray ones represent MPL-PURO–treated mice. Concentrations of the stimulating antigen are indicated in the x-axis.
IL-2 production in response to the stimulations of PLP peptide and KLH in control and MPL-PURO–transferred mice.
IL-2 production in untreated control mice and MPL-PURO–treated mice were measured using the same preparation of LN cells used in the T-cell proliferation assay (“Materials and methods,” Figure 6). Supernatants were harvested 24 hours after initiation of cultures and IL-2 levels were determined by ELISA. IL-2 production in response to PLP p139-151 is shown in (A) and to KLH in (B). Each bar represents one mouse; the black bars represent the untreated control mice, and the gray bars represent MPL-PURO–treated mice. The concentrations of specific antigen used for stimulation are indicated in the x-axis.
IL-2 production in response to the stimulations of PLP peptide and KLH in control and MPL-PURO–transferred mice.
IL-2 production in untreated control mice and MPL-PURO–treated mice were measured using the same preparation of LN cells used in the T-cell proliferation assay (“Materials and methods,” Figure 6). Supernatants were harvested 24 hours after initiation of cultures and IL-2 levels were determined by ELISA. IL-2 production in response to PLP p139-151 is shown in (A) and to KLH in (B). Each bar represents one mouse; the black bars represent the untreated control mice, and the gray bars represent MPL-PURO–treated mice. The concentrations of specific antigen used for stimulation are indicated in the x-axis.
Expression of MPL chimeric gene in mice unresponsive to PLP p139-151.
RT-PCR was performed on total RNA extracted from the spleens of mice reconstituted with MPL-PURO–infected B cells (lanes 3 to 8) and untreated control mice (lanes 1 and 2). The amplification of the housekeeping gene GAPDH was performed in each sample to ensure that equal levels of cDNA were used. Mice shown in lanes 6 to 8 were primed with PLP p139-151 and mice shown in lanes 3 to 5 were primed with KLH.
Expression of MPL chimeric gene in mice unresponsive to PLP p139-151.
RT-PCR was performed on total RNA extracted from the spleens of mice reconstituted with MPL-PURO–infected B cells (lanes 3 to 8) and untreated control mice (lanes 1 and 2). The amplification of the housekeeping gene GAPDH was performed in each sample to ensure that equal levels of cDNA were used. Mice shown in lanes 6 to 8 were primed with PLP p139-151 and mice shown in lanes 3 to 5 were primed with KLH.
Discussion
Currently, there are no specific treatments for any T-cell–mediated autoimmune disease in humans. All treatments, most notably steroids, are based on general suppression of the entire T-cell compartment and therefore only offer temporary symptomatic relief and cannot be used for prolonged periods. Other treatments such as interferon β and copolymer 1 (copaxon) are not general immune suppressants but are much less efficacious and, as yet, do not offer long-term benefits.44-47 In animals, more experimental treatments were reported, but most were again based on nonspecific suppression of immune responses. For example, temporary removal or blocking of the CD4+ T-cell compartment by monoclonal antibodies directed to CD4, MHC class II, and various adhesion molecules were shown to block the induction and progression of EAE, but at the same time suppress general immune responses.17-24Other, more specific treatments such as the use of antibodies to a specific T-cell receptor produced mixed results48-50 and are only useful in the case of highly restricted T-cell responses such as the response to MBP in SJL mice. Induction of “oral tolerance” was encouraging at first but later proved to be limited and in some cases deleterious.51,52 A more promising approach taken by several laboratories involved the induction of specific unresponsiveness to an encephalitogenic determinant by the intravenous injection of soluble encephalitogenic protein or peptide or the administration of peptide-coupled syngeneic splenocytes.53-56 Although protection from EAE induction was achieved, these treatments are transient in nature and therefore do not offer a long-term benefit.
In this paper, we present a gene therapy-based model for the prevention of T-cell–mediated autoimmune diseases by specifically down-regulating encephalitogenic T-cell clones without affecting general immune responses. The model is based on the premise that antigens presented on the cell surface of normal resting B cells induce a long-lasting, antigen-specific T-cell unresponsiveness. Tolerance induction by resting B cells to antigens expressed on their surface is well documented.25,30-34 Perhaps the most striking demonstration of this is the induction of tolerance to the MMTV-derived superantigen Mls (minor lymphocyte stimulatory antigen), which is expressed predominantly on B cells. Adoptive transfer of B cells from an Mls+ strain to an MHC identical, Mls− mice resulted in a long-lasting state of immune tolerance to this potent superantigen.57
To achieve similar tolerance to an autologous encephalitogenic determinant, our strategy was to introduce an exogenous gene coding for the encephalitogenic determinant of PLP into normal syngeneic B cells such that it will be constitutively expressed in association with MHC class II. The 2 retroviral vectors, SNV-MPL and MPL-PURO, both contained a fusion protein comprising the PLP determinant and lysosomal targeting sequence to confer lysosomal localization.36This is required because the association of MHC class II molecules with peptides presented by them occurs in the lysosomal compartment.37-39 B cells transduced with either of these vectors protected the majority (62% with SNV-MPL and 83% with MPL-PURO) of recipient animals from induction of EAE by direct immunization with the corresponding encephalitogenic peptide in CFA. Moreover, the protected animals remained asymptomatic throughout the experiment (3 months for the experiments using the SNV-MPL vector and 40 days for the MPL-PURO vector), whereas nearly half of the control mice relapsed at least once during this period. The higher titer vector virus had a slightly better efficacy than the lower titer vector (83% vs 62%), although other differences between the 2 groups of experiments could have contributed to this effect.
Analysis of exogenous RNA levels by RT-PCR suggests that there is some correlation between gene expression and protection from disease. In the first set of experiments, 5 of the 10 protected mice had high levels of mRNA expression, 4 had moderate levels, and one had low but detectable levels of expression. In contrast, all 3 surviving, treated, unprotected animals had barely detectable levels of MPL expression. In the second set of experiments using the MPL-PURO vector, 4 of the 5 treated, protected mice had high expression levels of the exogenous gene and one had low levels, whereas one treated but unprotected animal had barely detectable levels of MPL expression. More mice would have to be evaluated to confirm this trend.
Although in this study, EAE was always induced 7 to 14 days after adoptive transfer, we and others35,58 59 have shown that a substantial portion of resting B cells are very long lived (at least a year), and we therefore predict that mice will remain protected for long periods after adoptive transfer. This point, as well as the determination of the optimal number of cells required to induce protection, are currently under investigation.
Why were some adoptively transferred mice unprotected? Although we do not have a direct answer to this question, we believe that the treated mice that did develop clinical signs had a lower number of transduced B cells at the time of disease induction. This is supported by the RT-PCR data, although one of the protected animals also showed low expression levels. It is not yet clear why the “take” of the adoptively transferred B cells is lower in some mice.
Two additional points deserve consideration. The first is the effect of the in vitro manipulation of the transduced B cells on their survival and function after adoptive transfer. We have previously shown that genetically modified B cells persisted in reconstituted hosts for at least a year after adoptive transfer with no reduction in expression levels of the exogenous genes.35 The second is the use of LPS in the gene transfer protocol to activate the targeted B cells before cocultivation with the vector virus-producing cells that is necessary for onco-retroviral integration. Although LPS is a potent B-cell activator, it has been shown that expression of B7 molecules after B-cell activation is relatively short (a few hours).28 29 The lack of expression of B7-1 and B7-2 was verified by flow cytometry before adoptive transfer (40 to 44 hours after stimulation, data not shown).
As for the underlying mechanism responsible for the observed protection, the results presented in Figures 6 and 7 strongly suggest that antigen-specific T-cell unresponsiveness is being induced in treated animals. This is demonstrated by greatly reduced PLP-specific T-cell proliferation and IL-2 production in PLP primed, treated animals. Both of these parameters are hallmarks of a state of T-cell unresponsiveness known as T-cell anergy and is well documented in the literature.25,30-32,60,61 Although at this point, we cannot directly show that this state of anergy is induced by the genetically modified antigen-presenting B cells because they lack costimulatory molecules, we can, however, rule out the possibility that tolerance is induced by a soluble, secreted peptide. By using a monoclonal antibody specific for the encephalitogenic determinant of PLP in a sensitive ELISA assay, we could not detect any secreted peptide in the supernatant of the WEHI-231 B-cell line expressing high levels of MPL mRNA (data not shown). This was an expected result because the fusion protein produced by the exogenous gene contains a transmembrane domain that has been shown by others to strongly inhibit secretion.62
We believe that the model presented here could serve as a foundation for a clinically relevant protocol for the treatment of MS. Experiments are now under way to determine whether the same type of genetically modified B cells can also affect the progression of EAE when injected after the onset of clinical symptoms. The fact that the few treated mice that did develop clinical signs did not relapse is encouraging. Although in humans the encephalitogenic determinants are not yet defined, the approach used in these studies could still be used if expression of the entire PLP, MBP, orMOG genes or of a gene encoding a fusion protein containing multiple encephalitogenic determinants is achieved. Expression of multiple determinants in one chimeric gene would also address concerns raised by the phenomenon of epitope spreading described in some cases of EAE in mice.63 Epitope spreading, as the name implies, is when the immune response against one antigen or a particular antigenic epitope facilitates the response against another antigen or epitope that was not involved in the initial pathogenic process.
Supported in part by National Institute of Health Grants 5 R01 NS38272 (Y.R.), RO1-NS38272, and RO1-CA50777 (J.P.D.), the National Multiple Sclerosis Society Grant 2626A2/1 (Y.R.), a Milstein Foundation Grant (Y.R and J.P.D.), a National Research Service Award T32AI07403 (C.-C.C.), and an Individual National Service Award, GM19331 (A.R.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Yacov Ron, Department of Molecular Genetics and Microbiology, University of Medicine and Dentistry of New Jersey, Robert Wood Johnson Medical School, 675 Hoes Ln, Piscataway, NJ 08854; e-mail: yron@umdnj.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal