Abstract
In most human cells, the average length of telomere repeats at the ends of chromosomes provides indirect information about their mitotic history. To study the turnover of stem cells in patients with bone marrow failure syndromes, the telomere length in peripheral blood granulocytes and lymphocytes from patients with aplastic anemia (AA, n = 56) and hemolytic paroxysmal nocturnal hemoglobinuria (n = 6) was analyzed relative to age-matched controls by means of fluorescence in situ hybridization and flow cytometry. The telomere lengths in granulocytes from patients with AA were found to be significantly shorter than those in age-adjusted controls (P = .001). However, surprisingly, telomere length in granulocytes from AA patients who had recovered after immunosuppressive therapy did not differ significantly from controls, whereas untreated patients and nonresponders with persistent severe pancytopenia showed marked and significant telomere shortening. These results support extensive proliferation of hematopoietic stem cells in subgroups of AA patients. Because normal individuals show significant variation in telomere length, individual measurements in blood cells from AA patients may be of limited value. Whether sequential telomere length measurements can be used as a prognostic tool in this group of disorders remains to be clarified.
Introduction
Most acquired aplastic anemia (AA) is thought to result from immune-mediated damage to the stem and progenitor cell compartment. Pancytopenia, the hallmark of the disease, correlates well with the decreased numbers of immature hematopoietic cells measured either as CD34+ cells or as colony-forming cells.1-3 That the stem cell compartment is also affected by the pathologic process has been inferred from the finding that very primitive human hematopoietic cells measured in long-term culture–initiating cell (LTC-IC) assays or cobblestone area–forming cell assays are also reduced in number.4,5Although some recovery of LTC-IC numbers may occur in patients successfully treated with immunosuppression, in most patients a significant deficit in stem cell numbers persists despite apparent hematologic recovery4 (also, J.P.M. et al, unpublished data, 1999). Similar findings have been reported by others,1,5,6 and a sustained deficit in LTC-ICs has been observed up to 5 years after bone marrow transplantation for AA and hematologic malignancies.6-9 Possibly, reconstitution of hematopoiesis from a relatively small number of stem cells does not fully restore the size of the stem cell compartment.
Telomeres play an important role in maintaining chromosomal integrity,10,11 and shortening of telomeres is associated with genetic instability (for review, see de Lange12). Several experimental findings suggest that telomere length measurements can be used as a marker of stem cell turnover in vitro and in vivo. In most normal human somatic cells, telomere repeats are gradually lost with replication and age13,14 owing to the inability of conventional DNA polymerase to fully replicate the 3′ end of DNA; this is also known as the “end-replication problem.”15,16Human telomerase is a reverse transcriptase enzyme capable of counteracting replicative telomere shortening by adding single-stranded telomeric DNA to the 3′ ends of chromosomes (for review, see Greider17). Telomerase is constitutively expressed in cells of the germ line and is present at variable levels in normal hematopoietic progenitor cells, activated T cells, and germinal center B lymphocytes as well as in the majority of human cancers.18-20 Studies of telomerase knock-out mice have provided evidence in support of a functional link between replicative life span, genetic instability, and telomere length.21-23In addition, overexpression of telomerase was reported to result in the extension of telomeres and immortalization of normal human epithelial cells and fibroblasts.24,25 Hematopoietic cells from different stages in ontogeny differ in proliferative potential,26 and these functional differences correlate with differences in telomere length.27 Telomeres in donor-derived hematopoietic cells from transplant recipients show accelerated telomere shortening following allogeneic bone marrow transplantation as compared with cells in the donor,28with the degree of telomere shortening inversely correlated with the number of infused stem cells.29
On the basis of these previous studies, the measurement of telomere length seems a reasonable approach to the study of stem cell dynamics in bone marrow failure syndromes of different etiology. Indeed, in a recent study, this approach was used to study patients with AA, and a significant shortening of telomeres in AA compared with age-matched controls was reported.30 In patients with persistent cytopenia, a correlation between telomere loss and disease duration was found, whereas in patients whose blood counts became normal after therapy, the rate of telomere loss was stabilized. No differences in age-adjusted telomere length were found between patients with active AA and patients who responded to immunosupressive therapy.30 However, that study did not take into account the telomere length in different leukocyte subpopulations, ie, lymphocytes and granulocytes.
We recently introduced a novel technique for measurement of the average telomere length in cells using fluorescence in situ hybridization (FISH) with peptide nucleic acid (PNA) probes and flow cytometry (flow-FISH).31 Using this technique, we have analyzed the telomere length kinetics in subpopulations of unseparated peripheral blood leukocytes (PBLs) in a large population of healthy donors.32 In this study, it was found that both granulocytes and naive T lymphocytes show a rapid decline in telomere length in early childhood and a much more gradual but steady decline thereafter, most likely reflecting the turnover of their common precursors: hematopoietic stem cells.
One of the attractive features of flow-FISH is the ability to measure the telomere fluorescence in different cell types from the same patient. With such comparisons, it is possible to compensate to some extent for the pronounced heritable variation in telomere length observed in comparable cell types from normal individuals of the same age.32,33 The value of this approach was recently illustrated in studies of normal T cells and malignant myeloid cells from the blood of patients with chronic myeloid leukemia.34 Here, we describe our initial studies of telomere length dynamics in blood leukocyte subsets from patients with AA.
Patients, materials, and methods
Patient characteristics
We analyzed peripheral blood samples from 67 patients who presented clinically with AA (n = 61) or paroxysmal nocturnal hemoglobinuria (PNH, n = 6), ranging in age from 1 to 76 years (Table1). Informed consent for blood sampling was obtained according to a protocol approved by the Institutional Review Board of the National Heart, Lung and Blood Institute. Telomere length analysis was performed without knowledge of demographic or clinical characteristics, and sequential samples from the same patient were excluded from the analysis. The diagnosis of AA was established by bone marrow biopsy and white blood cell (WBC) count criteria according to the International Study of Aplastic Anemia and Agranulocytosis; severity was classified by the criteria of Camitta et al.35 Severe AA (sAA, n = 4) was defined by the presence of a hypocellular bone marrow and at least 2 of 3 peripheral blood criteria: absolute neutrophil count (ANC) lower than 500/μL, platelet count lower than 20 000/μL, and reticulocyte count lower than 60 000/μL. Moderate AA (mAA, n = 15) was defined as AA with the presence of a hypocellular bone marrow, absence of other hematologic disease, and depression of at least 2 of 3 blood counts with ANC lower than 1200/μL, platelet count below 70 000/μL, and anemia with hemoglobin lower than 8.5 g/dL, but not fulfilling the criteria for severe disease. Patients with hemolytic PNH without significant cytopenia were analyzed separately. The diagnosis of PNH was established on the basis of the presence of glycosyl phosphatidylinositol (GPI)-anchor–deficient red blood cell count and/or leukocytes in peripheral blood and/or positive Hams test.36
Clinical and demographic parameter of patients participating in the study
| Diagnosis . | No. . | Age (y) . | Duration (mo) . | ANC (/mm3) . | Hb (g/dL) . | Plts (×103/mm3) . | Therapy . |
|---|---|---|---|---|---|---|---|
| sAA | 4 | 28 ± 10.7 | 5.3 ± 2.5 | 0.9 ± 0.3 | 8.3 ± 0.7 | 25.5 ± 15.6 | Not previously treated except for transfusions |
| mAA | 15 | 33.7 ± 3.3 | 77.1 ± 25.2 | 1.4 ± 0.2 | 10 ± 0.6 | 56.2 ± 9.9 | Supportive, occasional transfusions, 1 patient received danazol |
| sAANR | 19 | 29.7 ± 4.1 | 30.8 ± 8 | 1.0 ± 0.3 | 9 ± 1.0 | 35.4 ± 6.2 | 2 patients treated with cytoxan/CsA; 14 with ATG/CsA; 2 patients treated with ATG/CsA and subsequent cytoxan/CsA; 2 treated with 2 courses of ATG/CsA; of these patients, 3 patients on G-CSF, 1 patient on G-CSF/SCF; 1 patient on G-CSF/EPO |
| recAA | 23 | 39.5 ± 4.2 | 54.4 ± 10.5 | 2.1 ± 0.2 | 11.2 ± 0.5 | 117 ± 13.9 | All patients treated with ATG/CsA; 2 had 2 courses of ATG/CsA; 5 of these patients currently on CsA; in 3 of these patients CsA was restarted; 3 also received androgens |
| PNH | 6 | 39 ± 5.5 | 46 ± 13 | 1.7 ± 0.3 | 8.9 ± 1.6 | 103 ± 29 | Transfusions, courses of steroids |
| Diagnosis . | No. . | Age (y) . | Duration (mo) . | ANC (/mm3) . | Hb (g/dL) . | Plts (×103/mm3) . | Therapy . |
|---|---|---|---|---|---|---|---|
| sAA | 4 | 28 ± 10.7 | 5.3 ± 2.5 | 0.9 ± 0.3 | 8.3 ± 0.7 | 25.5 ± 15.6 | Not previously treated except for transfusions |
| mAA | 15 | 33.7 ± 3.3 | 77.1 ± 25.2 | 1.4 ± 0.2 | 10 ± 0.6 | 56.2 ± 9.9 | Supportive, occasional transfusions, 1 patient received danazol |
| sAANR | 19 | 29.7 ± 4.1 | 30.8 ± 8 | 1.0 ± 0.3 | 9 ± 1.0 | 35.4 ± 6.2 | 2 patients treated with cytoxan/CsA; 14 with ATG/CsA; 2 patients treated with ATG/CsA and subsequent cytoxan/CsA; 2 treated with 2 courses of ATG/CsA; of these patients, 3 patients on G-CSF, 1 patient on G-CSF/SCF; 1 patient on G-CSF/EPO |
| recAA | 23 | 39.5 ± 4.2 | 54.4 ± 10.5 | 2.1 ± 0.2 | 11.2 ± 0.5 | 117 ± 13.9 | All patients treated with ATG/CsA; 2 had 2 courses of ATG/CsA; 5 of these patients currently on CsA; in 3 of these patients CsA was restarted; 3 also received androgens |
| PNH | 6 | 39 ± 5.5 | 46 ± 13 | 1.7 ± 0.3 | 8.9 ± 1.6 | 103 ± 29 | Transfusions, courses of steroids |
Values are mean ± SEM.
ANC indicates absolute neutrophil count; Hb, hemoglobin; sAA, severe aplastic anemia (AA); mAA, moderate AA; sAANR, patients with sAA who relapsed or did not respond to initial therapy; recAA, AA patients who improved after therapy; PNH, paroxysmal nocturnal hemoglobinuria; Plts, platelets; CsA, cyclosporine; ATG, antithymocyte globulin; G-CSF, granulocyte–colony stimulating factor; SCF, stem cell factor; EPO, erythropoietin.
Treated AA patients were subdivided into those who improved after therapy with antithymocyte globulin and cyclosporine (CsA) or cyclophosphamide and CsA in combination (recAA group, n = 23). All patients treated with these drugs had initially presented with severe disease. Hematopoietic recovery was defined as substantial improvement in at least 2 lineages, decrease in transfusion requirements or transfusion-independence, and/or elevation of absolute neutrophil counts above 5 × 107/L. We also analyzed sAA patients who had relapsed or who did not respond to initial therapy, all of whom continued to fulfill the severity criteria (sAANR group, n = 19). In one patient with recAA, we did not recover enough cells for analysis. In another patient with recAA, as well as in 3 patients with sAANR, gating on granulocytes was not possible because of insufficient numbers of granulocytes and/or failure to discriminate between granulocytes and lymphocytes by flow cytometry after hybridization.
Results are indicated as the mean ± SEM unless indicated otherwise. As controls, we used telomere fluorescence values after gating on the granulocyte or lymphocyte population of total PBLs obtained from healthy individuals, as described previously.32
Sample preparation
Erythrocytes were lysed by incubating peripheral blood with ammonium chloride solution (Stemcell Technologies, Vancouver, BC, Canada) in a 1:4 ratio 3 times for 10 minutes on ice. After lysis, the cells were washed with phosphate-buffered saline (PBS) with 0.1% bovine serum albumin (BSA) and counted by means of a hemocytometer.
Telomere fluorescence in situ hybridization and flow cytometry
The average length of telomere repeats at chromosome ends in individual peripheral blood leukocytes was measured by flow-FISH as previously reported.32 Analysis of the PBLs of one patient with sAANR after gating on lymphocytes and granulocytes is shown in Figure 1. Briefly, after gating on diploid cells on the basis of staining with propidium iodide (PI) (R1 as shown in Figure 1A), granulocytes (R2 in Figure 1B) and lymphocytes (R3 in Figure 1B) were discriminated on the basis of size and granularity. Analysis was performed with and without fluorescein isothiocyanate (FITC)–labeled telomere-specific PNA probe (dark and light gray peaks in Figure 1C-D, respectively) to allow subtraction of autofluorescence of cells in the same light scatter window (Figure 1B) from telomere fluorescence (horizontal bars in Figure 1C-D). In 5% of patients, insufficient numbers of white blood cells were obtained. In around 10% of the samples analyzed, the distinction between granulocytes and lymphocytes was not possible. If fewer than 5% of the 5000 events acquired did not correspond to previously established gates for granulocytes or lymphocytes,32 the values were not used in the analysis. FITC-labeled fluorescent beads (QuantumTM-24 Premixed; Flow Cytometry Standards, San Juan, Puerto Rico) were used to correct for daily shifts in the linearity of the flow cytometer and fluctuations in the laser intensity and alignment.37 At the beginning of each experiment, the fluorescence signals from the 4 different populations of FITC-labeled microbeads suspended in PBS with 0.1% BSA were acquired. Voltage and amplification of FL1 parameter were set in such a way that blank, 5579, 15 842, and 36 990 molecular equivalents of soluble fluorochrome (MESF) units per bead corresponded to channel numbers 25, 162, 456, and 942, respectively, in the FL1 channel on a linear scale. The resulting calibration curve (y = 0.02604x) was then used to convert telomere fluorescence into molecular equivalents of MESF units, allowing comparison of results among experiments. To estimate the telomere length (in kilobases [kb]) from telomere fluorescence in MESF units, the slope of the calibration curve described previously31 was used (y = 0.019x) in the following equation: kb = MESF × 0.02604 × 0.019. In order to analyze the day-to-day variation in hybridization efficiency, we analyzed aliquots of the same frozen lymphoma cells in each experiment as reported previously.31
Flow-FISH analysis of PBLs from a 14-year-old patient with sAANR 24 months after diagnosis.
(A) The cells were gated on region 1 (diploid cells, R1) on the basis of propidium iodide (PI) fluorescence and forward light scatter (FSC). (B) Additional regions 2 (lymphocyte gate, R2) and 3 (granulocyte gate, R3) were selected within R1 from FSC versus side scatter dot plot histograms. (C, D) Telomere fluorescence in the lymphocyte (panel C) and granulocyte (panel D) subfraction of total PBL was analyzed following hybridization with or without FITC–(C3TA2)3 PNA (dark gray and light gray histograms in panels C and D, respectively). At the time of analysis, the patients' blood counts were as follows: Hb, 8 g/dL; platelets, 19 000/μL; neutrophils, 800/μL; the difference from the age-adjusted normal telomere length for granulocytes (deltaTELgran) was − 6700 soluble fluorochrome (MESF).
Flow-FISH analysis of PBLs from a 14-year-old patient with sAANR 24 months after diagnosis.
(A) The cells were gated on region 1 (diploid cells, R1) on the basis of propidium iodide (PI) fluorescence and forward light scatter (FSC). (B) Additional regions 2 (lymphocyte gate, R2) and 3 (granulocyte gate, R3) were selected within R1 from FSC versus side scatter dot plot histograms. (C, D) Telomere fluorescence in the lymphocyte (panel C) and granulocyte (panel D) subfraction of total PBL was analyzed following hybridization with or without FITC–(C3TA2)3 PNA (dark gray and light gray histograms in panels C and D, respectively). At the time of analysis, the patients' blood counts were as follows: Hb, 8 g/dL; platelets, 19 000/μL; neutrophils, 800/μL; the difference from the age-adjusted normal telomere length for granulocytes (deltaTELgran) was − 6700 soluble fluorochrome (MESF).
Statistics
The following variables were used for statistical analysis of data: fluorescence values after gating on granulocyte and lymphocyte subpopulations of PBLs, peripheral blood counts at the time the sample was obtained, disease duration and duration after initiation of therapy in AA patients, and the patient's age. To avoid a mixture of cross-sectional and serial measurements, only the first sample analyzed was included in the analysis if sequential samples were available from one individual. Age-matched controls were from a cohort of 301 individuals analyzed previously.32 Individual patient measurements were age-corrected by subtraction from the linear regression line previously established for lymphocytes and granulocytes from normal individuals32; the resulting difference from the age-adjusted normal telomere length (deltaTEL) for a particular cell type (deltaTELgran or deltaTELlymph) was also expressed in MESF units. Statistical comparison of patient samples with controls (Figure 2) was performed after a maximum likelihood estimation of the parameters for the controls based on the assumption that telomere length values are normally distributed and that the means and the standard deviations depend linearly on age. The comparison between the patient groups among themselves and with the control was based on the age standardized values.
Age-adjusted telomere length in granulocytes from patients with AA.
Age adjustments were made on the basis of data from a cohort reported on previously.32 The specific telomere fluorescence was analyzed after gating on granulocytes as shown in Figure 1B,D. Mean and SE were expressed for each subgroup. Note that patients with sAANR and sAA/mAA showed significantly shorter telomeres compared with age-adjusted controls, whereas deltaTELgran in patients with recAA did not differ significantly from controls.
Age-adjusted telomere length in granulocytes from patients with AA.
Age adjustments were made on the basis of data from a cohort reported on previously.32 The specific telomere fluorescence was analyzed after gating on granulocytes as shown in Figure 1B,D. Mean and SE were expressed for each subgroup. Note that patients with sAANR and sAA/mAA showed significantly shorter telomeres compared with age-adjusted controls, whereas deltaTELgran in patients with recAA did not differ significantly from controls.
Results
Telomere length analysis of granulocytes derived from subgroups of patients with AA and hemolytic PNH
Telomere fluorescence in peripheral blood granulocytes from patients with AA was compared with measurements made previously in a population of 301 normal healthy controls.32 As shown in Figure 2, the age-adjusted granulocyte telomere fluorescence (deltaTELgran) in all patients with AA was significantly lower than expected (− 1500 ± 500 MESF,P = .001). Average deltaTELgran in samples from patients with hemolytic PNH without cytopenia (mean + SE: − 200 ± 2300 MESF, n = 6; data not shown) did not differ significantly from age-adjusted normal controls32 (range: 7100 to −6300 MESF, data not shown).
To further analyze telomere shortening in patients with AA, we subdivided the patients on the basis of the severity of the disease and their response to clinical intervention. There were significant differences in deltaTELgran, dependent on the clinical stage of the disease (Figure 2). Patients with untreated severe or moderate AA (sAA/mAA) as well as patients who had failed treatment and manifested continued severe pancytopenia (sAANR) showed shorter telomere length as compared with healthy individuals (− 1700 ± 800 MESF, P = .046; and − 2600 ± 800 MESF,P = .005, respectively). Strikingly, granulocytes from patients who showed hematologic improvement and stable counts after immunosuppressive therapy (recAA group) did not differ in telomere length from age-adjusted controls (deltaTELgran: − 400 ± 700 MESF). However, when compared directly, the difference in standardized deltaTELgran values between samples from patients with sAANR compared to patients with recAA did not reach statistical significance (P = .1), most likely because the number of patients in each group was too small. Patients with moderate AA (mAA group) showed significant telomere shortening (P = .01). Analysis of disease duration within this group revealed a long history of low counts in mAA (mean disease duration: 77.1 ± 25.2 months). Given the moderate depression in blood counts, these patients were, in general, managed conservatively. Disease duration was longer for recAA as compared with sAANR (54.4 ± 10.5 vs 30.8 ± 8 months). Therefore, we also correlated disease duration with the granulocyte telomere fluorescence within the various groups of patients with AA. However, in contrast to previous reports by others,30 no significant correlation of disease duration or time after initiation of treatment with peripheral blood counts or granulocyte telomere fluorescence was detected in either group. Furthermore, no correlation between time to remission and granulocyte telomere fluorescence was found in the recAA group (data not shown).
Telomere length dynamics with age in different subgroups of patients with AA
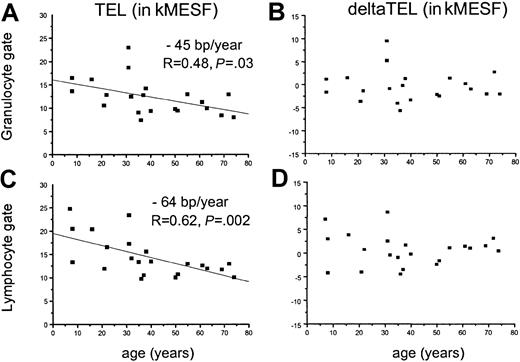
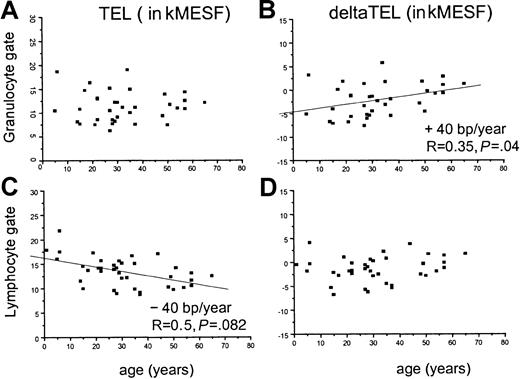
In normal individuals, both the average length of telomeres in nucleated blood cells and the rate of telomere shortening have been found to vary with age.32,38 We thus used linear regression analysis to examine the telomere fluorescence as a function of age in samples obtained from patients with recAA (Figure3) and in samples from patients who belonged to either the sAANR or the untreated sAA/mAA group (Figure4). Comparisons were performed separately for gated granulocytes (Figure 3A-B; Figure 4A-B) and lymphocytes (Figure 3C-D; Figure 4C-D) in view of the substantial differences in the age-related telomere length dynamics in those populations.32 When telomere fluorescence values (TEL, Figure 3A,C; Figure 4A,C) were plotted separately for each group, a significant decline in telomere length with age was found in granulocytes (− 45 base pairs (bp)/y, Figure 3A) and lymphocytes (− 64 bp/y, Figure 3C) from patients with recAA. The rate of telomere shortening in this group was not different from the control population, as shown by the distribution of the age-adjusted deltaTEL values (Figure 3B,D). In contrast, in untreated or nonresponding AA patients, no significant decline in calculated telomere length with age was found in the granulocyte gate (Figure 4A). This effect translated into a significantly positive slope of the linear regression line (+ 40 bp/y) when deltaTELgran was plotted versus age (Figure 4B). Thus, although as a group patients with sAANR or untreated AA show mostly negative deltaTELgran values throughout the entire age range, this relative telomere length deficit appeared to be most pronounced in young patients and to decline with age. In the same patients, the rate of telomere loss per year in lymphocytes was slightly lower than in normal controls (− 45 bp/y, Figure4C).
Linear regression analysis of absolute and age-adjusted telomere fluorescence in granulocytes and lymphocytes from recAA patients.
A linear regression analysis of absolute (panels A and C) and age-adjusted (panels B and D) telomere fluorescence in granulocytes (panels A and B) and lymphocytes (panels C and D) from patients with recovered AA as measured by flow-FISH. Note that absolute telomere length declines with age (panels A and C) and that telomere length and the rate of telomere shortening are not different from controls (panels B and D), indicating no significant differences in telomere length kinetics in patients with AA who respond to immunosuppressive therapy.
Linear regression analysis of absolute and age-adjusted telomere fluorescence in granulocytes and lymphocytes from recAA patients.
A linear regression analysis of absolute (panels A and C) and age-adjusted (panels B and D) telomere fluorescence in granulocytes (panels A and B) and lymphocytes (panels C and D) from patients with recovered AA as measured by flow-FISH. Note that absolute telomere length declines with age (panels A and C) and that telomere length and the rate of telomere shortening are not different from controls (panels B and D), indicating no significant differences in telomere length kinetics in patients with AA who respond to immunosuppressive therapy.
Linear regression analysis of absolute and age-adjusted telomere fluorescence in granulocytes and lymphocytes from patients with untreated or refractory AA.
Linear regression analysis of absolute (panels A and C) and age-adjusted (panels B and D) telomere fluorescence in granulocytes (panels A and B) and lymphocytes (panels C and D) from patients with untreated (sAA/mAA) or refractory (sAANR) AA measured by flow FISH. Note that no significant decline was found in absolute telomere length with age in granulocytes (panel A) whereas absolute lymphocyte telomere length declined at slightly lower rate than in controls (panel C) and that age-adjusted telomere length in granulocytes amounts to approximately − 5000 MESF at birth but increases with age (panel B).
Linear regression analysis of absolute and age-adjusted telomere fluorescence in granulocytes and lymphocytes from patients with untreated or refractory AA.
Linear regression analysis of absolute (panels A and C) and age-adjusted (panels B and D) telomere fluorescence in granulocytes (panels A and B) and lymphocytes (panels C and D) from patients with untreated (sAA/mAA) or refractory (sAANR) AA measured by flow FISH. Note that no significant decline was found in absolute telomere length with age in granulocytes (panel A) whereas absolute lymphocyte telomere length declined at slightly lower rate than in controls (panel C) and that age-adjusted telomere length in granulocytes amounts to approximately − 5000 MESF at birth but increases with age (panel B).
Correlation of peripheral blood counts with telomere length in patients with AA and PNH
We next addressed the question of whether age-adjusted telomere length correlated with peripheral blood counts, which are used as an indicator of disease severity in patients with AA and PNH. As shown in Table 1, corresponding peripheral blood parameters were obtained for samples of all subgroups analyzed. Average peripheral blood counts for all samples were as follows: WBCs, 3.2 (0.2 × 103/μL; absolute neutrophil count (Gran), 1.5 (0.1 × 103/μL; absolute lymphocyte count (Lymph) 1.3 (0.1 × 103/μL; absolute monocyte count (Mono) 0.3, 0.2 × 103/μL; platelet count (Plts) 70.7, 7.7 × 103/μL; and hemoglobin (Hb) 10.3 (0.5 g/dL). Using linear regression analysis, we found significant positive correlations with deltaTELgran for WBC (P = .0003), Gran (P = .006), Plts (P = .002), Mono (P = .02), and Hb (P = .04) (Figure 5).
Correlation of age-adjusted granulocyte telomere length (deltaTELgran) in all patients with AA and PNH with peripheral blood counts.
Note that deltaTELgran is correlated with cell counts in all subpopulations of peripheral blood cells except for unseparated lymphocytes in support of the notion that disease severity and state are correlated with telomere length decline in granulocytes.
Correlation of age-adjusted granulocyte telomere length (deltaTELgran) in all patients with AA and PNH with peripheral blood counts.
Note that deltaTELgran is correlated with cell counts in all subpopulations of peripheral blood cells except for unseparated lymphocytes in support of the notion that disease severity and state are correlated with telomere length decline in granulocytes.
Discussion
In this study, we measured the telomere length in nucleated blood cells from a cohort of patients with bone marrow failure syndromes using a newly introduced technique combining FISH and flow cytometry (flow-FISH).31 32 Measurements for different cell types were expressed as absolute telomere fluorescence values or as the difference in telomere fluorescence relative to age-adjusted controls (deltaTEL). We found significantly decreased deltaTEL values in granulocytes derived from patients with AA. In addition, we found that in patients who responded to immunosuppressive therapy, the estimated telomere length was not significantly different from controls, and a relatively normal rate of telomere shortening with age was observed in this group of patients.
The current study was based on the predicate that, when hereditary differences in telomere length are taken into account, the telomere length in mature circulating leukocytes directly reflects the divisional history of the hematopoietic stem cells from which the leukocytes are derived. One important underlying assumption is that the number of cell divisions required for differentiation from a stem cell to a mature blood cell is both independent of patient age and also relatively constant for a particular differentiation lineage even in patients with marrow failure syndromes. Although these propositions are difficult to test directly, they are in agreement with telomere length measurement obtained with purified normal bone marrow cells,27 blood cells from bone marrow transplant recipients,28,29 and granulocytes and T-cell populations from normal individuals31,32 and patients with chronic myeloid leukemia.34 We furthermore hypothesized that patients with marrow failure syndromes would show variable telomere shortening in circulating cells relative to normal controls, depending on the extent and the duration of the damage to the stem cell compartment and the number of compensatory stem cell divisions. On the basis of these considerations, cytopenia per se does not have to be associated with a decrease in telomere length (eg, upon acute toxicity to stem cells or in situations of increased demand for mature blood cells but suppressed stem cell turnover) whereas normal blood counts can be associated with a decrease in telomere length (increased demand on production of blood cells matched by increased stem cell turnover, or normal demand, matched by a reduced number of stem cells with a higher number of accumulated cell divisions). Of course, other factors than stem cell turnover also determine the telomere length in peripheral blood cells and the results of flow-FISH analysis. First, there are marked hereditary differences in telomere length among normal individuals of the same age.32,33 However, method-related variability (estimated to be up to 15% of telomere fluorescence values) and, possibly, telomerase and/or other regulators of telomere length in human cells, as has been suggested by others,38may also have contributed to variation in measurements that is unrelated to actual differences in the mitotic history of nucleated blood cells and their precursors.
Although recovered patients showed some improvement in the LTC-IC numbers, a profound deficit in the number of these functional progenitor cells persists in these patients even in the presence of a normal blood count.42 In view of these findings, the observation that the telomere length in granulocytes and the rate of telomere shortening with age in this group of patients did not differ significantly from controls is of interest. One would perhaps have expected that extensive damage to the stem cell pool would have required increased mitotic activity in residual stem cells accompanied by a measurable decline in telomere length. However, to date, it is not clear how many hematopoietic stem cell clones are actually contributing to steady-state normal (physiologic) or stressed (pathophysiologic) hematopoiesis at any given point in time. Therefore, one possible explanation for the normal telomere length in patients with recAA is that the damage to the stem cell pool was insufficient to require detectable compensatory cell divisions in remaining stem cells. A possible reduction in stem cell numbers and functional reserve in patients with recAA might become apparent upon increased hematopoietic stress, eg, cytostatic chemotherapy or infection. Alternatively, the primary targets of immune destruction in these patients may not have been stem cells but progenitor cells. In this case, the number of additional, compensatory cell divisions from stem cells may also have been too small to result in a measurable change in granulocyte telomere length. Importantly, in patients with moderate depression of blood counts but a long history of the disease, a significant decrease in granulocyte telomere length was found. Most likely, this finding reflects a gradual and more general depletion of stem cell numbers with progressively fewer stem cell clones being forced to divide more frequently than normal to sustain hematopoiesis. A similar postulate may apply to patients with severe refractory AA in whom the telomere length was most significantly depressed.
In a previous study on total peripheral blood leukocytes using conventional Southern blot analysis, no difference in telomere length was found in a group of patients with active AA compared with a group of patients with recovered AA.30 Both subgroups showed a similar average amount of telomere shortening (approximately 0.8 kb), which is comparable to our findings for patients with severe refractory AA (estimated to be approximately 1.2 kb). This discrepancy might be explained by the fact that the differences in telomere lengths seen in the granulocyte compartment in our study were hidden because Ball et al30 used unfractionated peripheral blood cells as a source for the telomere length analysis. Since considerable differences in telomere length in lymphocytes and granulocytes had been reported previously,32 differences solely in the cellular composition within the leukocyte fraction in the 2 groups could prevent these differences from being detectable. Interestingly, Ball et al found deltaTEL to progressively decrease with the duration of the disease at an additional rate of 216 bp/y in the “active AA group” whereas no such correlation was found in patients with fully recovered blood counts.30 Early therapeutic intervention may have prevented substantial losses of stem cells, allowing a full reconstitution of hematopoiesis in our recovered AA patient population.
The telomere length in granulocytes of patients with untreated or refractory AA did not seem to decline significantly with age as observed in normal individuals. As a result, an increase in deltaTELgran with age was observed in this subgroup (Figure4). A potential explanation for this observation is that the stem cell compartment in younger patients is capable of compensating losses of stem cells for a longer period of time by increasing the turnover of remaining stem cells. However, at the time of clinical presentation, this capacity may have been exhausted. The lack of a decline in telomere length with age in the granulocytes from this patient group supports this hypothesis and argues in favor of a critical telomere length threshold that needs to be reached before patients fail to regenerate hematopoiesis. This threshold may be reached earlier in older patients who have shorter telomeres to start with than in younger patients.
Analysis of telomere length in lymphocyte populations did not reveal significant differences in telomere length dynamics between both subgroups of AA patients and controls. A more detailed analysis of lymphocyte subpopulations is required to discriminate between possible underlying immune or autoimmune phenomena and to study potential shifts in subpopulations of naive/memory/CD4/CD8 cells in AA. In contrast, the positive correlation between age-adjusted telomere length in granulocytes with blood cell counts in all leukocyte subpopulations (except lymphocytes) appears to reflect the severity of the disease.
No consistent shortening of telomere length was reported in patients with PNH in an earlier study.30 On the basis of these findings, the authors concluded that oligoclonality may not be required for the evolution of PNH. In our study, the telomere length in the granulocytes from a limited number of PNH patients was also highly variable, which supports the previous conclusion and perhaps reflects heterogeneity of the associated bone marrow disease. A normal or even increased telomere length was observed in cells from patients in whom the majority of leukocytes displayed the PNH phenotype. As GPI-deficient cells arise from a mutation in a single hematopoietic stem cell, lack of significant telomere shortening in the cells derived from this clone is unexpected and difficult to explain. On the basis of the observation that purified candidate stem cells show asymmetric cell divisions in vitro, we previously postulated the existence of an extensive hierarchy within the stem cell compartment on the basis of differences in replicative history between individual stem cells.39,40 Perhaps GPI-deficient clones are sometimes derived from a stem cell that has divided fewer times than the majority of the stem cells contributing to hematopoiesis. Alternatively, the molecular defect in PNH may result in altered regulation of telomere length in affected cells. We prefer the replicative hierarchy model because telomere-mediated replicative senescence could explain the apparent exhaustion of PNH clones in (rare) patients with spontaneous remission of the disease.41
Future studies performed with sequential samples will help to circumvent some of the limitations of cross-sectional studies that are associated with the pronounced interindividual variation in telomere length. These studies are warranted to validate the use of telomere length in granulocytes as a measure of the mitotic history of stem cells.
Supported by National Institutes of Health grant AI29524 and by a grant from the National Cancer Institute of Canada with funds from the Terry Fox Run as well as a grant from the Deutsche Forschungsgemeinschaft (T.H.B.).
T.H.B. and J.P.M. contributed equally to this article.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Peter M. Lansdorp, Terry Fox Laboratory, British Columbia Cancer Agency, 601 West 10th Ave, Vancouver, BC, V5Z 1L3, Canada; e-mail: plansdor@bccancer.bc.ca.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal